Transferrin Modified GSH Sensitive Hyaluronic Acid Derivative Micelle to Deliver HSP90 Inhibitors to Enhance the Therapeutic Efficacy of Brain Cancers
Abstract
Simple Summary
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials
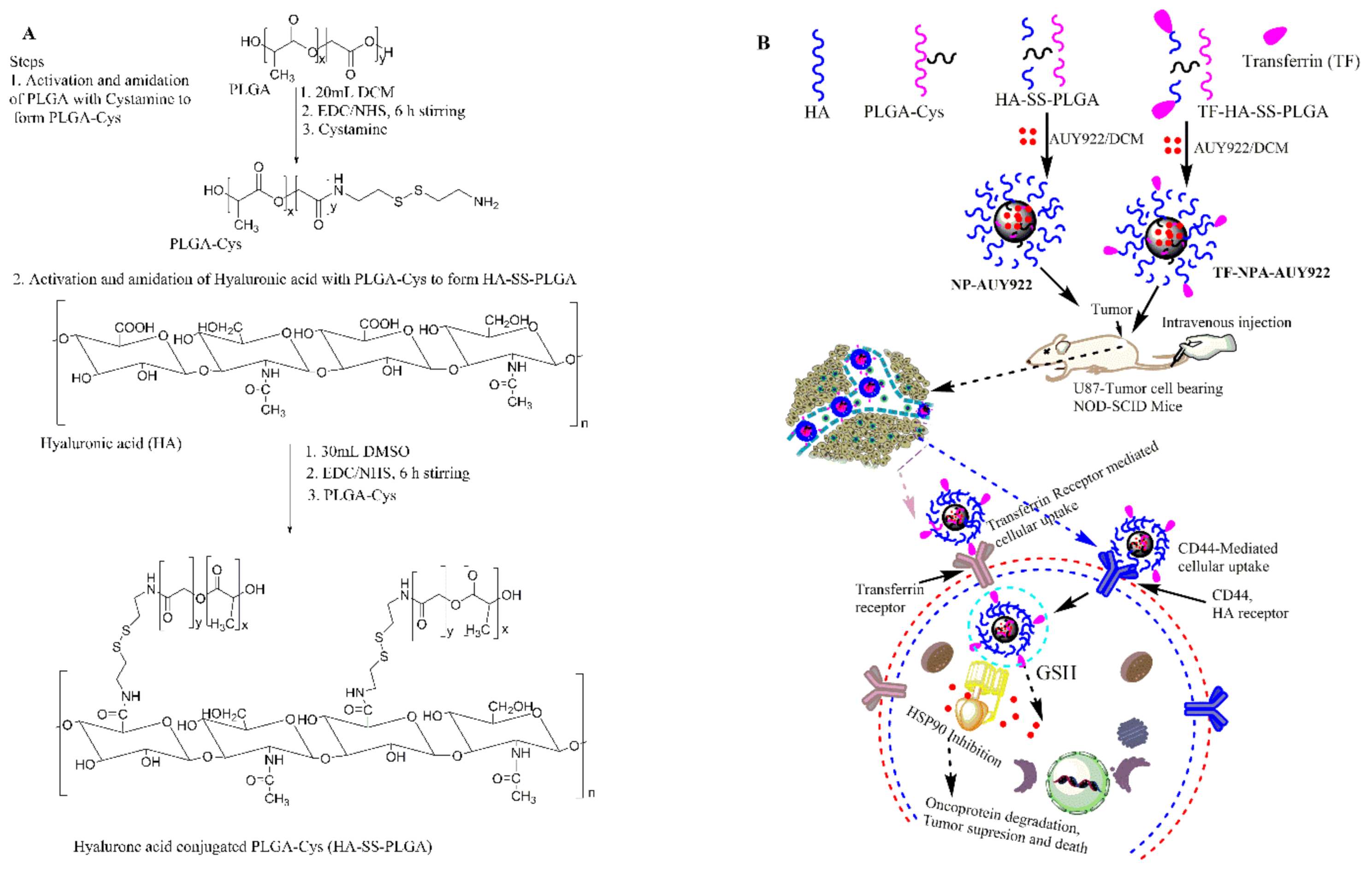
2.2. Synthesis and Characterization of GSH Responsive HA-SS-PLGA
2.3. Transferrin-Conjugated HA-SS-PLGA (TF-HA-SS-PLGA) Synthesis
2.4. Micelle Synthesis and AUY922 Loading
2.5. In Vitro Drug Release
2.6. In Vitro Stability
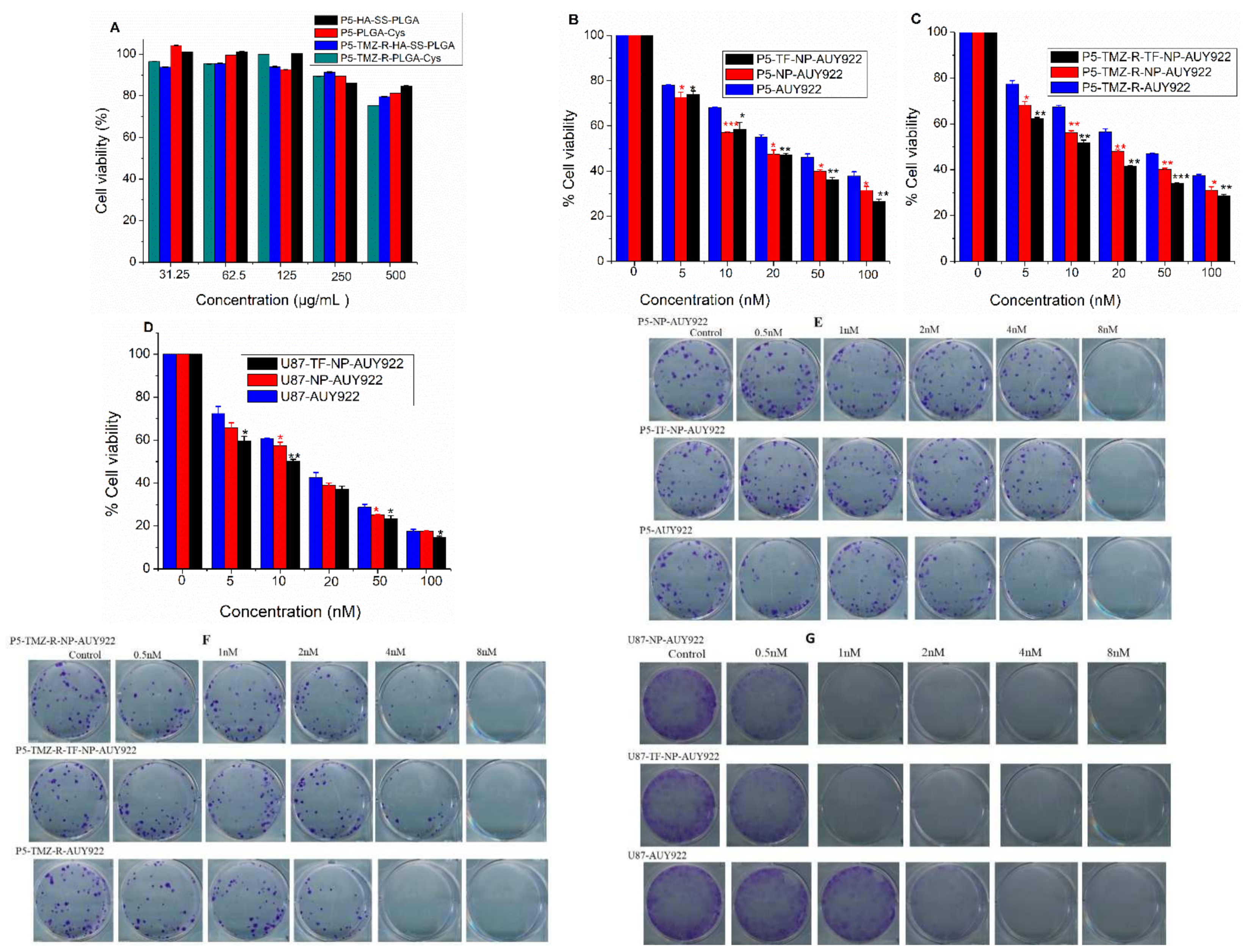
2.7. In Vitro Cytotoxicity
2.8. Colony Assay
2.9. Apoptosis Assay
2.10. Cell Cycle Analysis
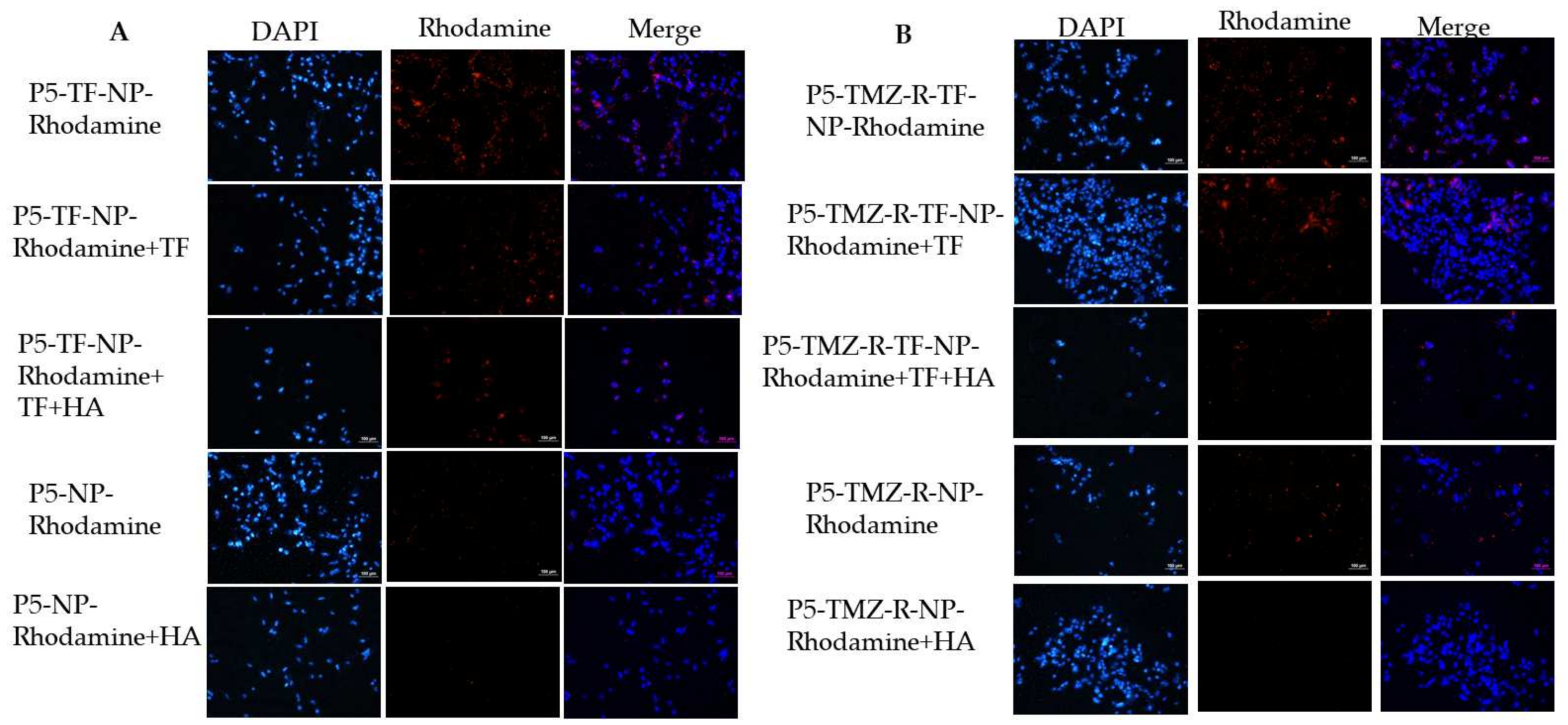
2.11. Cellular Uptake and Competitive Inhibition Study
2.12. Western Blot Analysis
2.13. In Vivo and Ex Vivo Biodistribution Study
2.14. In Vivo Anti-Tumor Efficacy Study and Biochemical Index Analysis
2.15. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of PLGA-Cys and HA-SS-PLGA Conjugates
3.2. Synthesis and Characterization of AUY922-Loaded Micelle
3.3. In Vitro Cytotoxicity Studies
3.4. Apoptosis and Cell Cycle Analysis
3.5. Cellular Uptake Studies
3.6. Free AUY922 and Nanoformulated AUY922 Inhibits HSP90 and Downregulates HSP90 Client Proteins in P5, P5-TMZ-R, and U87 Brain Cancer Cells
3.7. In Vivo and Ex-Vivo Biodistribution Studies
3.8. In Vivo Therapeutic Studies
3.9. Organ Function Test and H&E Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Am. Soc. Prev. Oncol. 2014, 23, 1985–1996. [Google Scholar] [CrossRef]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee Sh, U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. 2017, 18, 3–9. [Google Scholar] [CrossRef]
- Rock, K.; McArdle, O.; Forde, P.; Dunne, M.; Fitzpatrick, D.; O’Neill, B.; Faul, C. A clinical review of treatment outcomes in glioblastoma multiforme--the validation in a non-trial population of the results of a randomised Phase III clinical trial: Has a more radical approach improved survival? Br. J. Radiol. 2012, 85, e729–e733. [Google Scholar] [CrossRef]
- Cai, X.; Sughrue, M.E. Glioblastoma: New therapeutic strategies to address cellular and genomic complexity. Oncotarget 2017, 9, 9540–9554. [Google Scholar] [CrossRef]
- Frosina, G. DNA Repair and Resistance of Gliomas to Chemotherapy and Radiotherapy. Mol. Cancer Res. 2009, 7, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Haar, C.P.; Hebbar, P.; Wallace, G.C.T.; Das, A.; Vandergrift, W.A., 3rd; Smith, J.A.; Giglio, P.; Patel, S.J.; Ray, S.K.; Banik, N.L. Drug resistance in glioblastoma: A mini review. Neurochem. Res. 2012, 37, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Brada, M.; Judson, I.; Beale, P.; Moore, S.; Reidenberg, P.; Statkevich, P.; Dugan, M.; Batra, V.; Cutler, D. Phase I dose-escalation and pharmacokinetic study of temozolomide (SCH 52365) for refractory or relapsing malignancies. Br. J. Cancer 1999, 81, 1022–1030. [Google Scholar] [CrossRef]
- Carter, T.C.; Medina-Flores, R.; Lawler, B.E. Glioblastoma Treatment with Temozolomide and Bevacizumab and Overall Survival in a Rural Tertiary Healthcare Practice. Biomed. Res. Int. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Lee, S.Y. Temozolomide resistance in glioblastoma multiforme. Genes Dis. 2016, 3, 198–210. [Google Scholar] [CrossRef]
- Alexiou, G.A.; Kyritsis, A.P. HSP90 inhibitors for high-grade glioma treatment. Transl. Cancer Res. 2017, 20, S1–S2. [Google Scholar]
- Zhu, H.; Woolfenden, S.; Bronson, R.T.; Jaffer, Z.M.; Barluenga, S.; Winssinger, N.; Rubenstein, A.E.; Chen, R.; Charest, A. The novel Hsp90 inhibitor NXD30001 induces tumor regression in a genetically engineered mouse model of glioblastoma multiforme. Mol. Cancer 2010, 9, 2618–2626. [Google Scholar] [CrossRef]
- Chatterjee, S.; Burns, T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef]
- Zhang, L.; Fok, J.H.L.; Davies, F.E. Heat shock proteins in multiple myeloma. Oncotarget 2014, 5, 1132–1148. [Google Scholar] [CrossRef]
- Mahalingam, D.; Swords, R.; Carew, J.S.; Nawrocki, S.T.; Bhalla, K.; Giles, F.J. Targeting HSP90 for cancer therapy. Br. J. Cancer 2009, 100, 1523–1529. [Google Scholar] [CrossRef]
- Bagatell, R.; Whitesell, L. Altered Hsp90 function in cancer: A unique therapeutic opportunity. Mol. Cancer Ther. 2004, 3, 1021–1030. [Google Scholar] [PubMed]
- Ciocca, D.R.; Calderwood, S.K. Heat shock proteins in cancer: Diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones 2005, 10, 86–103. [Google Scholar] [CrossRef] [PubMed]
- Sauvageot, C.M.-E.; Weatherbee, J.L.; Kesari, S.; Winters, S.E.; Barnes, J.; Dellagatta, J.; Ramakrishna, N.R.; Stiles, C.D.; Kung, A.L.-J.; Kieran, M.W.; et al. Efficacy of the HSP90 inhibitor 17-AAG in human glioma cell lines and tumorigenic glioma stem cells. Neuro Oncol. 2009, 11, 109–121. [Google Scholar] [CrossRef]
- Den, R.B.; Lu, B. Heat shock protein 90 inhibition: Rationale and clinical potential. Adv. Med. Oncol. 2012, 4, 211–218. [Google Scholar] [CrossRef]
- Jhaveri, K.; Ochiana, S.O.; Dunphy, M.P.; Gerecitano, J.F.; Corben, A.D.; Peter, R.I.; Janjigian, Y.Y.; Gomes-DaGama, E.M.; Koren, J., 3rd; Modi, S.; et al. Heat shock protein 90 inhibitors in the treatment of cancer: Current status and future directions. Expert Opin. Investig. Drugs 2014, 23, 611–628. [Google Scholar] [CrossRef]
- Jensen, M.R.; Schoepfer, J.; Radimerski, T.; Massey, A.; Guy, C.T.; Brueggen, J.; Quadt, C.; Buckler, A.; Cozens, R.; Drysdale, M.J.; et al. NVP-AUY922: A small molecule HSP90 inhibitor with potent antitumor activity in preclinical breast cancer models. Breast Cancer Res. BCR 2008, 10, R33. [Google Scholar] [CrossRef]
- Okui, T.; Shimo, T.; Hassan, N.M.; Fukazawa, T.; Kurio, N.; Takaoka, M.; Naomoto, Y.; Sasaki, A. Antitumor effect of novel HSP90 inhibitor NVP-AUY922 against oral squamous cell carcinoma. Anticancer Res. 2011, 31, 1197–1204. [Google Scholar] [PubMed]
- Gaspar, N.; Sharp, S.Y.; Eccles, S.A.; Gowan, S.; Popov, S.; Jones, C.; Pearson, A.; Vassal, G.; Workman, P. Mechanistic Evaluation of the Novel HSP90 Inhibitor NVP-AUY922 in Adult and Pediatric Glioblastoma. Mol. Cancer Ther. 2010, 9, 1219–1233. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Anghel, A.; Rogers, A.M.; Desai, A.J.; Kalous, O.; Conklin, D.; Ayala, R.; O’Brien, N.A.; Quadt, C.; Akimov, M.; et al. Inhibition of HSP90 with AUY922 induces synergy in HER2-amplified trastuzumab-resistant breast and gastric cancer. Mol. Cancer 2013, 12, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Hasegawa, H.; Sasaki, D.; Ando, K.; Sawayama, Y.; Imanishi, D.; Taguchi, J.; Imaizumi, Y.; Hata, T.; Tsukasaki, K.; et al. Heat shock protein 90 inhibitor NVP-AUY922 exerts potent activity against adult T-cell leukemia-lymphoma cells. Cancer Sci. 2014, 105, 1601–1608. [Google Scholar] [CrossRef]
- Menezes, D.L.; Taverna, P.; Jensen, M.R.; Abrams, T.; Stuart, D.; Yu, G.K.; Duhl, D.; Machajewski, T.; Sellers, W.R.; Pryer, N.K.; et al. The Novel Oral Hsp90 Inhibitor NVP-HSP990 Exhibits Potent and Broad-spectrum Antitumor Activities In Vitro and In Vivo. Mol. Cancer Ther. 2012, 11, 730–739. [Google Scholar] [CrossRef]
- Trivedi, R.; Kompella, U.B. Nanomicellar formulations for sustained drug delivery: Strategies and underlying principles. Nanomedicine 2010, 5, 485–505. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Hassan, P.A. Self assembled materials: Design strategies and drug delivery perspectives. Phys. Chem. Chem. Phys. 2013, 15, 17016–17028. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Cho, H.; Bae, J.; Garripelli, V.K.; Anderson, J.M.; Jun, H.-W.; Jo, S. Redox-sensitive polymeric nanoparticles for drug delivery. Chem. Commun. 2012, 48, 6043–6045. [Google Scholar] [CrossRef]
- Quinn, J.F.; Whittaker, M.R.; Davis, T.P. Glutathione responsive polymers and their application in drug delivery systems. Polym. Chem. 2017, 8, 97–126. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Synthesis and characterization of redox-sensitive heparin-β-sitosterol micelles: Their application as carriers for the pharmaceutical agent, doxorubicin, and investigation of their antimetastatic activities in vitro. Mater. Sci. Eng. C 2017, 75, 1326–1338. [Google Scholar] [CrossRef]
- Debele, T.A.; Yu, L.Y.; Yang, C.S.; Shen, Y.A.; Lo, C.L. pH- and GSH-Sensitive Hyaluronic Acid-MP Conjugate Micelles for Intracellular Delivery of Doxorubicin to Colon Cancer Cells and Cancer Stem Cells. Biomacromolecules 2018, 19, 3725–3737. [Google Scholar] [CrossRef] [PubMed]
- Mero, A.; Campisi, M. Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.-C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Debele, T.; Peng, S.; Tsai, H.-C. Drug Carrier for Photodynamic Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [PubMed]
- Ponka, P.; Lok, C.N. The transferrin receptor: Role in health and disease. Int. J. Biochem. Cell Biol. 1999, 31, 1111–1137. [Google Scholar] [CrossRef]
- Qian, Z.M.; Li, H.; Sun, H.; Ho, K. Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharm. Rev. 2002, 54, 561–587. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Yamamoto, M.; Saitoh, Y. The significance of CD44 in human pancreatic cancer: I. High expression of CD44 in human pancreatic adenocarcinoma. Pancreas 1994, 9, 748–752. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Wu, H.C.; Wu, S.R.; Shan, Y.S.; Su, W.P. Combination Delivery of Alpha-Tocopheryl Succinate and Curcumin Using a GSH-Sensitive Micelle (PAH-SS-PLGA) to Treat Pancreatic Cancer. Pharmaceutics 2020, 12, 778. [Google Scholar] [CrossRef]
- Hu, K.; Zhou, H.; Liu, Y.; Liu, Z.; Liu, J.; Tang, J.; Li, J.; Zhang, J.; Sheng, W.; Zhao, Y.; et al. Hyaluronic acid functional amphipathic and redox-responsive polymer particles for the co-delivery of doxorubicin and cyclopamine to eradicate breast cancer cells and cancer stem cells. Nanoscale 2015, 7, 8607–8618. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ehrich, M.; Fuhrman, K.; Zhang, C. In vitro performance of lipid-PLGA hybrid nanoparticles as an antigen delivery system: Lipid composition matters. Nanoscale Res. Lett. 2014, 9, 1–10. [Google Scholar] [CrossRef]
- Lele, B.S.; Leroux, J.C. Synthesis and Micellar Characterization of Novel Amphiphilic A−B−A Triblock Copolymers of N-(2-Hydroxypropyl)methacrylamide or N-Vinyl-2-pyrrolidone with Poly(ε-caprolactone). Macromolecules 2002, 35, 6714–6723. [Google Scholar] [CrossRef]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. A pH-sensitive micelle composed of heparin, phospholipids, and histidine as the carrier of photosensitizers: Application to enhance photodynamic therapy of cancer. Int. J. Biol. Macromol. 2017, 98, 125–138. [Google Scholar] [CrossRef]
- Stingl, L.; Stühmer, T.; Chatterjee, M.; Jensen, M.R.; Flentje, M.; Djuzenova, C.S. Novel HSP90 inhibitors, NVP-AUY922 and NVP-BEP800, radiosensitise tumour cells through cell-cycle impairment, increased DNA damage and repair protraction. Br. J. Cancer 2010, 102, 1578–1591. [Google Scholar] [CrossRef] [PubMed]
- Rong, B.; Yang, S. Molecular mechanism and targeted therapy of Hsp90 involved in lung cancer: New discoveries and developments (Review). Int. J. Oncol. 2018, 52, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Bagatell, R.; Paine-Murrieta, G.D.; Taylor, C.W.; Pulcini, E.J.; Akinaga, S.; Benjamin, I.J.; Whitesell, L. Induction of a heat shock factor 1-dependent stress response alters the cytotoxic activity of hsp90-binding agents. Clin. Cancer Res. 2000, 6, 3312–3318. [Google Scholar]
- Kudryavtsev, V.A.; Khokhlova, A.V.; Mosina, V.A.; Selivanova, E.I.; Kabakov, A.E. Induction of Hsp70 in tumor cells treated with inhibitors of the Hsp90 activity: A predictive marker and promising target for radiosensitization. PLoS ONE 2017, 12, e0173640. [Google Scholar] [CrossRef]
- Anckar, J.; Sistonen, L. Regulation of HSF1 function in the heat stress response: Implications in aging and disease. Annu. Rev. Biochem. 2011, 80, 1089–1115. [Google Scholar] [CrossRef]
- Solárová, Z.; Mojžiš, J.; Solár, P. Hsp90 inhibitor as a sensitizer of cancer cells to different therapies (review). Int. J. Oncol. 2015, 46, 907–926. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Xie, Z.; Sun, G.; Yang, P.; Li, J.; Yang, H.; Xiao, S.; Liu, Y.; Qiu, H.; Qin, L.; et al. Reversing drug resistance of cisplatin by hsp90 inhibitors in human ovarian cancer cells. Int. J. Clin. Exp. Med. 2015, 8, 6687–6701. [Google Scholar]
- Tatokoro, M.; Koga, F.; Yoshida, S.; Kawakami, S.; Fujii, Y.; Neckers, L.; Kihara, K. Potential role of Hsp90 inhibitors in overcoming cisplatin resistance of bladder cancer-initiating cells. Int. J. Cancer 2012, 131, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Chun, J.; Li, D.; Boese, A.C.; Li, J.; Kang, J.; Umano, A.; Jiang, Y.; Song, L.; Magliocca, K.R.; et al. Hsp90B enhances MAST1-mediated cisplatin resistance by protecting MAST1 from proteosomal degradation. J. Clin. Invest. 2019, 129, 4110–4123. [Google Scholar] [CrossRef] [PubMed]









| Cell Type | IC50 Value (nM) | ||
|---|---|---|---|
| Free AUY922 | NP-AUY922 | TF-NP-AUY922 | |
| P5 | ~37 | ~17 | ~17 |
| P5-TMZ-R | ~39 | ~17 | ~12 |
| U87 | ~16 | ~14 | ~10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debele, T.A.; Wu, P.-C.; Wei, Y.-F.; Chuang, J.-Y.; Chang, K.-Y.; Tsai, J.-H.; Su, W.-P. Transferrin Modified GSH Sensitive Hyaluronic Acid Derivative Micelle to Deliver HSP90 Inhibitors to Enhance the Therapeutic Efficacy of Brain Cancers. Cancers 2021, 13, 2375. https://doi.org/10.3390/cancers13102375
Debele TA, Wu P-C, Wei Y-F, Chuang J-Y, Chang K-Y, Tsai J-H, Su W-P. Transferrin Modified GSH Sensitive Hyaluronic Acid Derivative Micelle to Deliver HSP90 Inhibitors to Enhance the Therapeutic Efficacy of Brain Cancers. Cancers. 2021; 13(10):2375. https://doi.org/10.3390/cancers13102375
Chicago/Turabian StyleDebele, Tilahun Ayane, Ping-Ching Wu, Yu-Feng Wei, Jian-Ying Chuang, Kwang-Yu Chang, Jui-Hung Tsai, and Wen-Pin Su. 2021. "Transferrin Modified GSH Sensitive Hyaluronic Acid Derivative Micelle to Deliver HSP90 Inhibitors to Enhance the Therapeutic Efficacy of Brain Cancers" Cancers 13, no. 10: 2375. https://doi.org/10.3390/cancers13102375
APA StyleDebele, T. A., Wu, P.-C., Wei, Y.-F., Chuang, J.-Y., Chang, K.-Y., Tsai, J.-H., & Su, W.-P. (2021). Transferrin Modified GSH Sensitive Hyaluronic Acid Derivative Micelle to Deliver HSP90 Inhibitors to Enhance the Therapeutic Efficacy of Brain Cancers. Cancers, 13(10), 2375. https://doi.org/10.3390/cancers13102375








