Association between Growth Differentiation Factor-15 (GDF-15) Serum Levels, Anorexia and Low Muscle Mass among Cancer Patients
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patient’s Characteristics
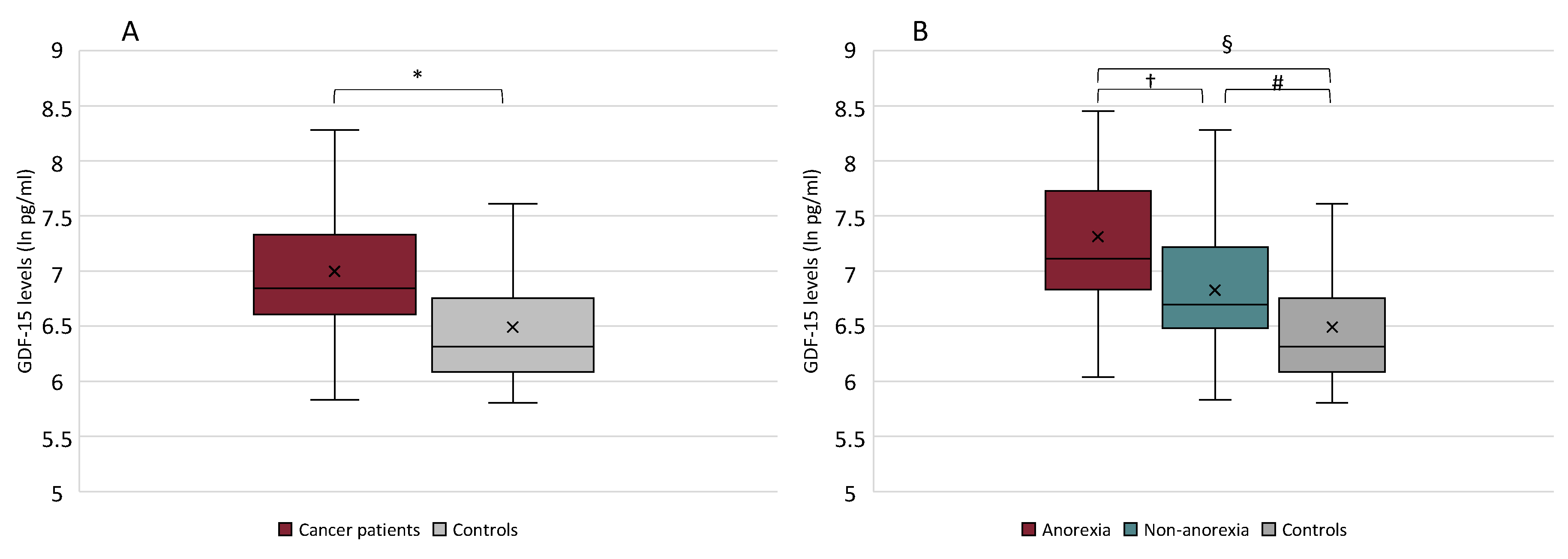
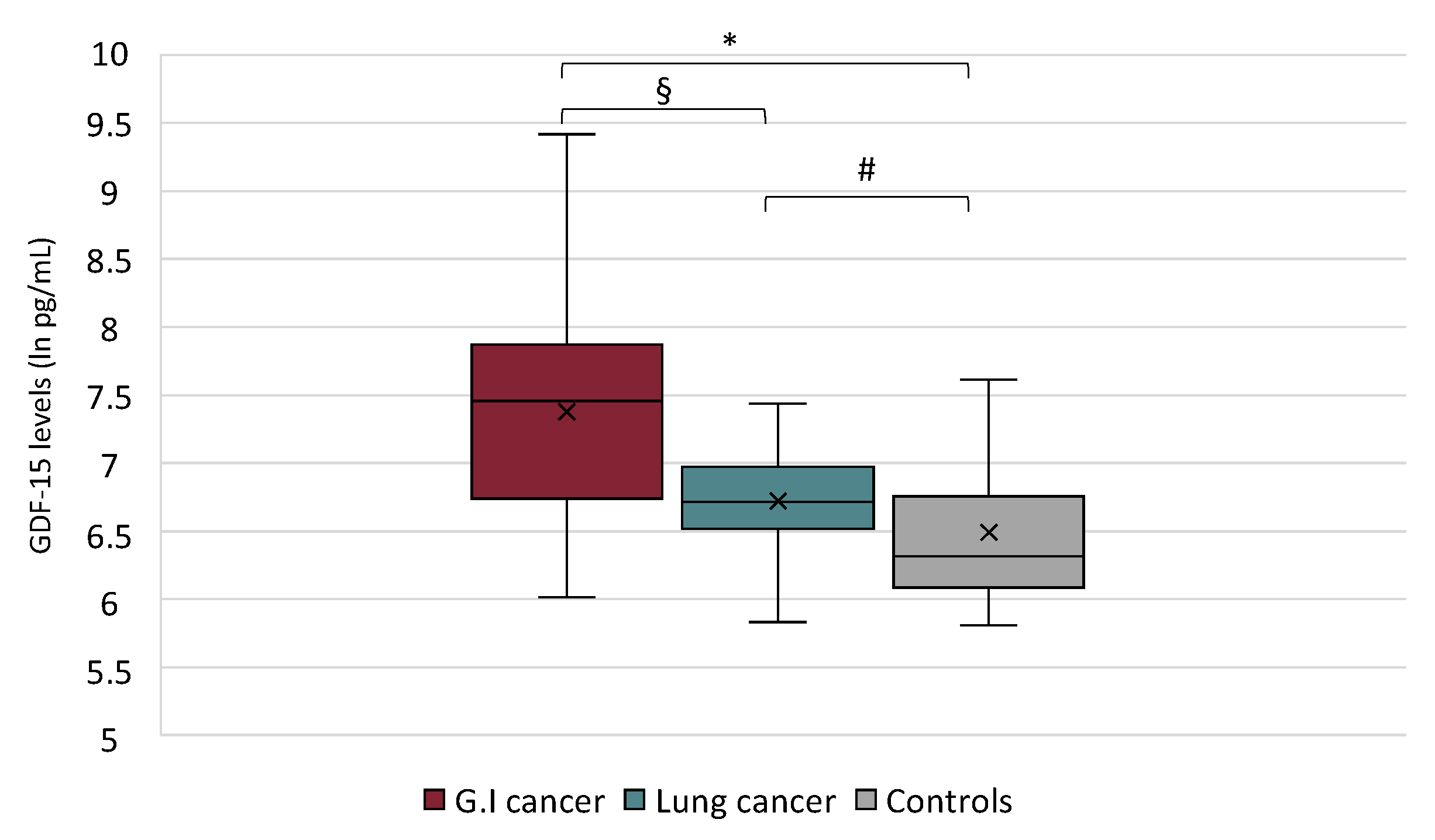
2.2. GDF-15 Serum Levels in Anorexic Cancer Patients, Non-Anorexic Cancer Patients and in Controls
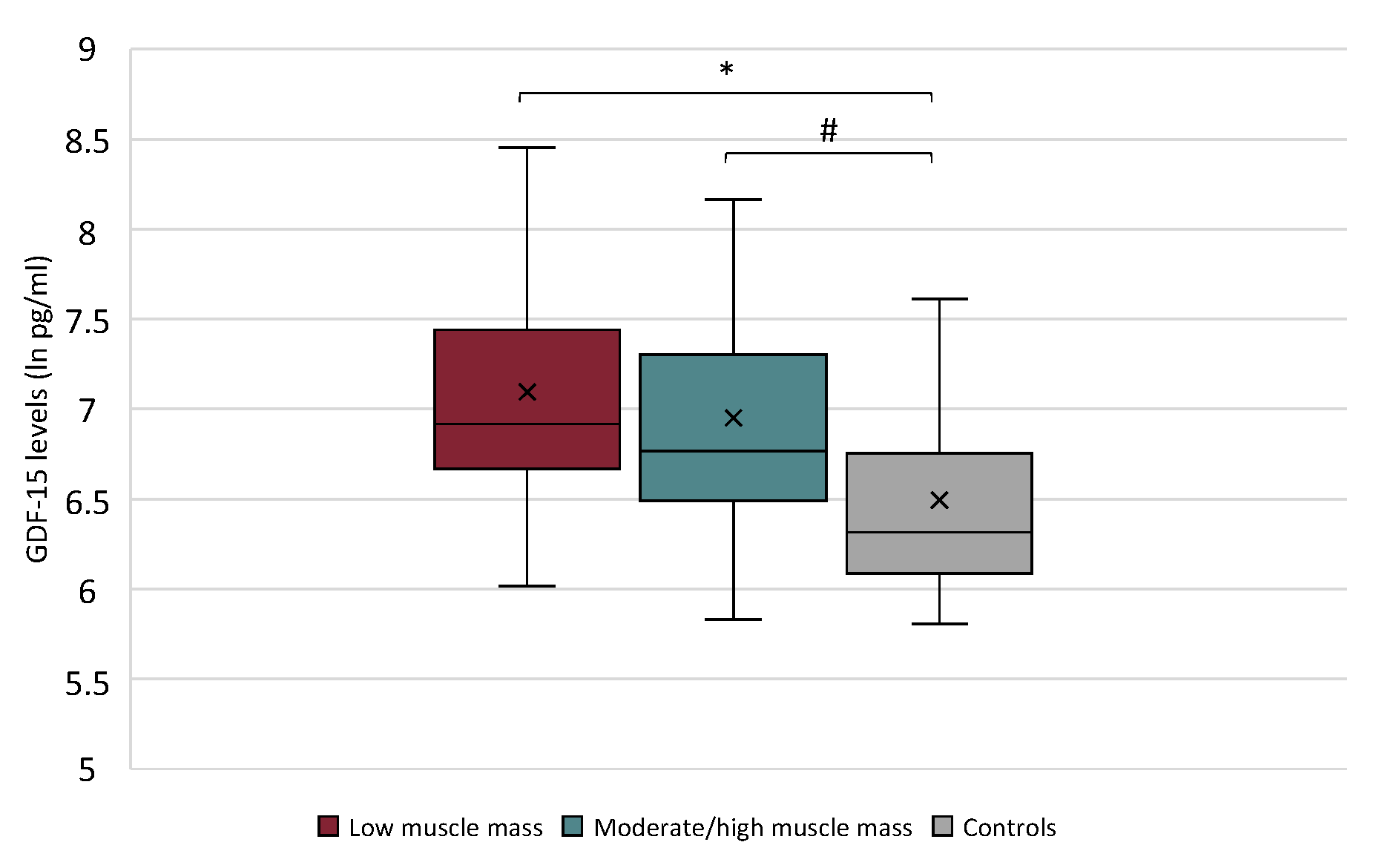
2.3. GDF-15 Serum Levels in Cancer Patients with Low Muscle Mass vs. Moderate/High Muscle Mass and in Controls
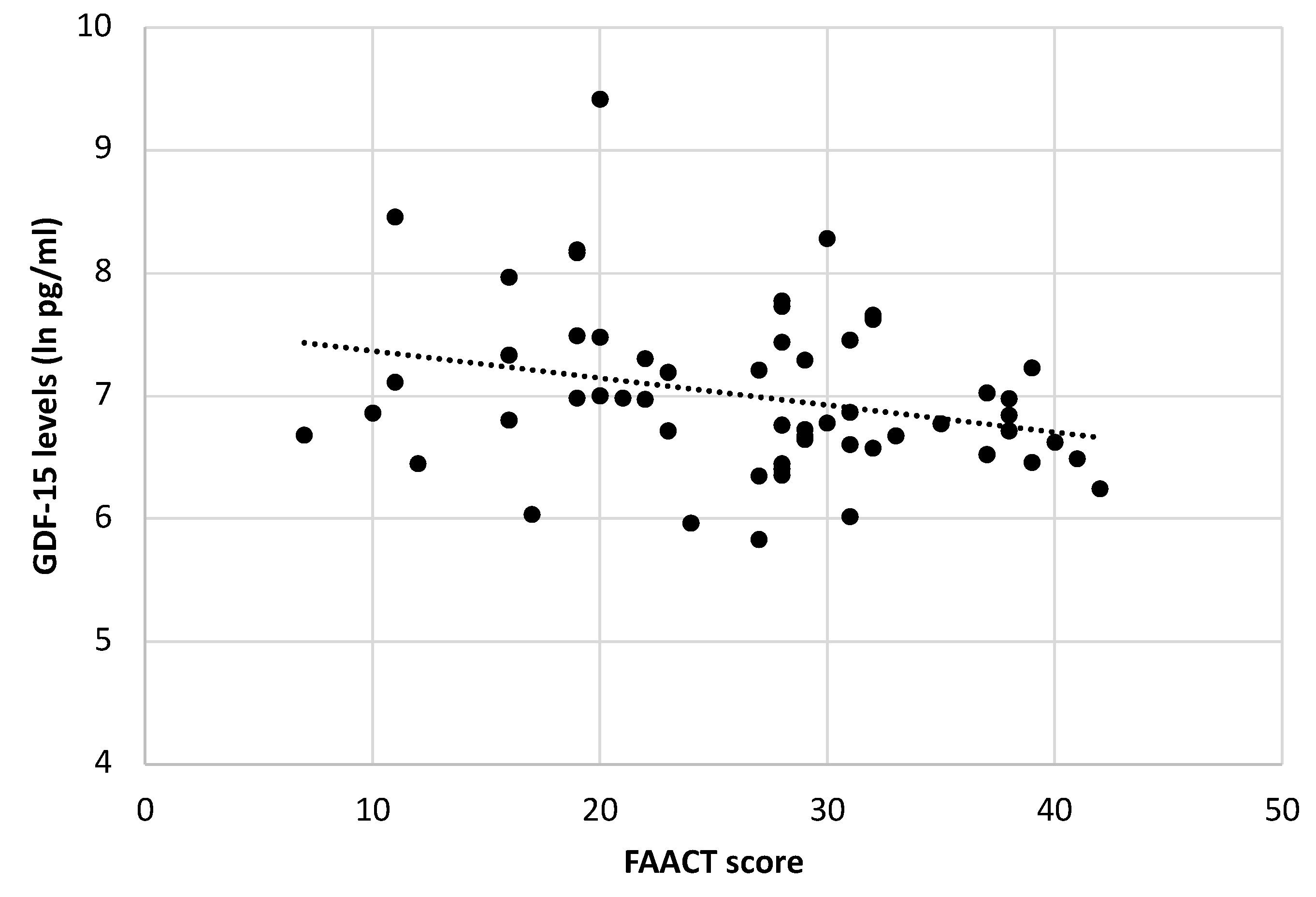
2.4. Association between Body Weight Loss, Anorexia, and GDF-15 Serum Levels
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Nutritional and Clinical Assessment
4.3. Anorexia Assessment
4.4. Evaluation of Muscle Mass by CT Scans
4.5. Serum GDF-15 Levels
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muscaritoli, M.; Molfino, A.; Lucia, S.; Rossi Fanelli, F. Cachexia: A preventable comorbidity of cancer. A T.A.R.G.E.T. approach. Crit. Rev. Oncol. Hematol. 2015, 94, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.; Kordatou, Z.; Barriuso, J.; Lamarca, A.; Weaver, J.M.J.; Cipriano, C.; Papaxoinis, G.; Backen, A.; Mansoor, W. Early recognition of anorexia through patient-generated assessment predicts survival in patients with oesophagogastric cancer. PLoS ONE 2019, 14, e0224540. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Lucia, S.; Farcomeni, A.; Lorusso, V.; Saracino, V.; Barone, C.; Plastino, F.; Gori, S.; Magarotto, R.; Carteni, G.; et al. Prevalence of malnutrition in patients at first medical oncology visit: The PreMiO study. Oncotarget 2017, 8, 79884–79896. [Google Scholar] [CrossRef] [PubMed]
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Imbimbo, G.; Emiliani, A.; Ramaccini, C.; Lahaye, E.; Takagi, K.; Fetissov, S.O. Plasma enterobacterial ClpB levels and ClpB- and α-MSH-reactive immunoglobulins in lung cancer patients with and without anorexia. Nutrition 2020, 78, 110952. [Google Scholar] [CrossRef]
- Laviano, A.; Molfino, A.; Seelaender, M.; Frascaria, T.; Bertini, G.; Ramaccini, C.; Bollea, M.R.; Citro, G.; Rossi Fanelli, F. Carnitine administration reduces cytokine levels, improves food intake, and ameliorates body composition in tumor-bearing rats. Cancer Investig. 2011, 29, 696–700. [Google Scholar] [CrossRef]
- Laviano, A.; Meguid, M.M.; Guijarro, A.; Muscaritoli, M.; Cascino, A.; Preziosa, I.; Molfino, A.; Rossi Fanelli, F. Antimyopathic effects of carnitine and nicotine. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 442–448. [Google Scholar] [CrossRef]
- Peixoto da Silva, S.; Santos, J.M.O.; Costa, E.; Silva, M.P.; Gil da Costa, R.M.; Medeiros, R. Cancer cachexia and its pathophysiology: Links with sarcopenia, anorexia and asthenia. J. Cachexia Sarcopenia Muscle 2020, 11, 619–635. [Google Scholar] [CrossRef]
- Yang, L.; Chang, C.C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjær, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J.; et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef]
- Lerner, L.; Hayes, T.G.; Tao, N.; Krieger, B.; Feng, B.; Wu, Z.; Nicoletti, R.; Chiu, M.I.; Gyuris, J.; Garcia, J.M. Plasma growth differentiation factor 15 is associated with weight loss and mortality in cancer patients. J. Cachexia Sarcopenia Muscle 2015, 6, 317–324. [Google Scholar] [CrossRef]
- Johnen, H.; Lin, S.; Kuffner, T.; Brown, D.A.; Tsai, V.W.; Bauskin, A.R.; Wu, L.; Pankhurst, G.; Jiang, L.; Junankar, S.; et al. Tumor-induced anorexia and weight loss are mediated by the TGF-beta superfamily cytokine MIC-1. Nat. Med. 2007, 13, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Borner, T.; Shaulson, E.D.; Ghidewon, M.Y.; Barnett, A.B.; Horn, C.C.; Doyle, R.P.; Grill, H.J.; Hayes, M.R.; De Jonghe, B.C. GDF15 Induces Anorexia through Nausea and Emesis. Cell Metab. 2020, 31, 351–362.e5. [Google Scholar] [CrossRef]
- Turcott, J.G.M.; Oñate-Ocaña, L.F.; Soca-Chafre, G.; Ramírez-Tirado, L.A.; Flores-Estrada, D.; Zatarain-Barrón, Z.L.; Arrieta, O. FAACT-Anorexia Cachexia Scale: Cutoff Value for Anorexia Diagnosis in Advanced Non-Small Cell Lung Cancer Patients. Nutr. Cancer 2019, 71, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Laviano, A.; Meguid, M.M.; Inui, A.; Muscaritoli, M.; Rossi Fanelli, F. Therapy insight: Cancer anorexia-cachexia syndrome-when all you can eat is yourself. Nat. Clin. Pract. Oncol. 2005, 2, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Molfino, A.; Iannace, A.; Colaiacomo, M.C.; Farcomeni, A.; Emiliani, A.; Gualdi, G.; Laviano, A.; Rossi Fanelli, F. Cancer anorexia: Hypothalamic activity and its association with inflammation and appetite-regulating peptides in lung cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 40–47. [Google Scholar] [CrossRef]
- Molfino, A.; Amabile, M.I.; Muscaritoli, M. Nutrition support for treating cancer-associated weight loss: An update. Curr. Opin. Support. Palliat. Care 2018, 12, 434–438. [Google Scholar] [CrossRef]
- Molfino, A.; Laviano, A.; Rossi Fanelli, F. Contribution of anorexia to tissue wasting in cachexia. Curr. Opin. Support. Palliat. Care 2010, 4, 249–253. [Google Scholar] [CrossRef]
- Hsu, J.Y.; Crawley, S.; Chen, M.; Ayupova, D.A.; Lindhout, D.A.; Higbee, J.; Kutach, A.; Joo, W.; Gao, Z.; Fu, D.; et al. Non-homeostatic body weight regulation through a brainstem-restricted receptor for GDF15. Nature 2017, 550, 255–259. [Google Scholar] [CrossRef]
- Suriben, R.; Chen, M.; Higbee, J.; Oeffinger, J.; Ventura, R.; Li, B.; Mondal, K.; Gao, Z.; Ayupova, D.; Taskar, P.; et al. Antibody-mediated inhibition of GDF15-GFRAL activity reverses cancer cachexia in mice. Nat. Med. 2020, 26, 1264–1270. [Google Scholar] [CrossRef]
- Skipworth, R.J.M.; Deans, D.A.; Tan, B.H.; Sangster, K.; Paterson-Brown, S.; Brown, D.A.; Hunter, M.; Breit, S.N.; Ross, J.A.; Fearon, K.C. Plasma MIC-1 correlates with systemic inflammation but is not an independent determinant of nutritional status or survival in oesophago-gastric cancer. Br. J. Cancer 2010, 102, 665–672. [Google Scholar] [CrossRef]
- Borner, T.; Wald, H.S.; Ghidewon, M.Y.; Zhang, B.; Wu, Z.; De Jonghe, B.C.; Breen, D.; Grill, H.J. GDF15 Induces an Aversive Visceral Malaise State that Drives Anorexia and Weight Loss. Cell Rep. 2020, 31, 107543. [Google Scholar] [CrossRef] [PubMed]
- Muscaritoli, M.; Anker, S.D.; Argilés, J.; Aversa, Z.; Bauer, J.M.; Biolo, G.; Boirie, Y.; Bosaeus, I.; Cederholm, T.; Costelli, P.; et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010, 29, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Blauwhoff-Buskermolen, S.; Ruijgrok, C.; Ostelo, R.W.; de Vet, H.C.W.; Verheul, H.M.W.; de van der Schueren, M.A.E.; Langius, J.A.E. The assessment of anorexia in patients with cancer: Cut-off values for the FAACT-A/CS and the VAS for appetite. Support. Care Cancer 2016, 24, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Arezzo di Trifiletti, A.; Misino, P.; Giannantoni, P.; Giannantoni, B.; Cascino, A.; Fazi, L.; Rossi Fanelli, F.; Laviano, A. Comparison of the performance of four different tools in diagnosing disease-associated anorexia and their relationship with nutritional, functional and clinical outcome measures in hospitalized patients. Clin. Nutr. 2013, 32, 527–532. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.; Kavanagh, R.G.; Carey, B.W.; Maher, M.M.; O’Connor, O.J.; Andrews, E.J. The impact of sarcopenia and myosteatosis on postoperative outcomes in patients with inflammatory bowel disease. Eur. Radiol. Exp. 2018, 2, 37. [Google Scholar] [CrossRef]
- Van Dijk, D.P.; Bakens, M.J.; Coolsen, M.M.; Rensen, S.S.; van Dam, R.M.; Bours, M.J.; Weijenberg, M.P.; Dejong, C.H.; Olde Damink, S.W. Low skeletal muscle radiation attenuation and visceral adiposity are associated with overall survival and surgical site infections in patients with pancreatic cancer. J. Cachexia Sarcopenia Muscle 2017, 8, 317–326. [Google Scholar] [CrossRef]
- Sabel, M.S.; Lee, J.; Cai, S.; Englesbe, M.J.; Holcombe, S.; Wang, S. Sarcopenia as a prognostic factor among patients with stage III melanoma. Ann. Surg. Oncol. 2011, 18, 3579–3585. [Google Scholar] [CrossRef]
- McGrath, E.R.; Himali, J.J.; Levy, D.; Conner, S.C.; DeCarli, C.; Pase, M.P.; Ninomiya, T.; Ohara, T.; Courchesne, P.; Satizabal, C.L.; et al. Growth Differentiation Factor 15 and NT-proBNP as Blood-Based Markers of Vascular Brain Injury and Dementia. J. Am. Heart Assoc. 2020, 9, e014659. [Google Scholar] [CrossRef]
| Cancer Patients (N = 59) | Controls (N = 30) | * p-Value | ||
|---|---|---|---|---|
| Parameter | Lung (N = 34) | Gastrointestinal (N = 25) | ||
| Male (%) | 27 (79%) | 12 (48%) | 13 (43%) | 0.0394 |
| Age (y) | 67.97 ± 12.03 | 71.64 ± 11.22 | 58.53 ± 11.85 | <0.0001 |
| Body mass index, kg/m2 | 23.83 ± 3.91 | 27.04 ± 3.22 | 26.51 ± 4.46 | 0.158 |
| Current weight, kg | 68.43 ± 12.96 | 76.48 ± 12.58 | 74.5 ± 13.01 | 0.371 |
| Usual weight, kg | 73.82 ± 13.26 | 81.22 ± 13.34 | 73.54 ± 11.93 | 0.312 |
| Hemoglobin, g/dl | 13.0 (12.2; 13.4) | 12.1 (9.3; 13.0) | 13.7 (12.2; 15.5) | 0.0108 |
| C-reactive protein, mg/dl | 5.85 (2.87; 9.40) | 1.42 (0.28; 3.23) | 0.29 (0.17; 0.62) | <0.0001 |
| Albumin, g/dl | 3.74 (3.35; 4.05) | 3.20 (3.00; 3.50) | 4 (3.76; 4.1) | 0.0003 |
| Presence of anorexia, n (%) | 8 (24%) | 13 (52%) | / | |
| Percent of body weight loss | 7.72 (5.31; 12.92) | 4.23 (3.53; 7.83) | 0 (0; 0) | <0.0001 |
| L3-SMI, cm2/m2 | M, 47.60 ± 8.78 F, 39.97 ± 5.78 | M, 44.28 ± 8.73 F, 38.77 ± 7.93 | / | |
| Type of cancer | ||||
| Gastric, n (%) | / | 7 (12%) | ||
| Pancreas, n (%) | / | 9 (15%) | ||
| Colorectal, n (%) | / | 9 (15%) | ||
| Stage | ||||
| I–II | 3 | 15 | ||
| III–IV | 31 | 10 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molfino, A.; Amabile, M.I.; Imbimbo, G.; Rizzo, V.; Pediconi, F.; Catalano, C.; Emiliani, A.; Belli, R.; Ramaccini, C.; Parisi, C.; et al. Association between Growth Differentiation Factor-15 (GDF-15) Serum Levels, Anorexia and Low Muscle Mass among Cancer Patients. Cancers 2021, 13, 99. https://doi.org/10.3390/cancers13010099
Molfino A, Amabile MI, Imbimbo G, Rizzo V, Pediconi F, Catalano C, Emiliani A, Belli R, Ramaccini C, Parisi C, et al. Association between Growth Differentiation Factor-15 (GDF-15) Serum Levels, Anorexia and Low Muscle Mass among Cancer Patients. Cancers. 2021; 13(1):99. https://doi.org/10.3390/cancers13010099
Chicago/Turabian StyleMolfino, Alessio, Maria Ida Amabile, Giovanni Imbimbo, Veronica Rizzo, Federica Pediconi, Carlo Catalano, Alessandra Emiliani, Roberta Belli, Cesarina Ramaccini, Claudia Parisi, and et al. 2021. "Association between Growth Differentiation Factor-15 (GDF-15) Serum Levels, Anorexia and Low Muscle Mass among Cancer Patients" Cancers 13, no. 1: 99. https://doi.org/10.3390/cancers13010099
APA StyleMolfino, A., Amabile, M. I., Imbimbo, G., Rizzo, V., Pediconi, F., Catalano, C., Emiliani, A., Belli, R., Ramaccini, C., Parisi, C., Nigri, G., & Muscaritoli, M. (2021). Association between Growth Differentiation Factor-15 (GDF-15) Serum Levels, Anorexia and Low Muscle Mass among Cancer Patients. Cancers, 13(1), 99. https://doi.org/10.3390/cancers13010099








