Progression-Free Survival Early Assessment Is a Robust Surrogate Endpoint of Overall Survival in Immunotherapy Trials of Hepatocellular Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
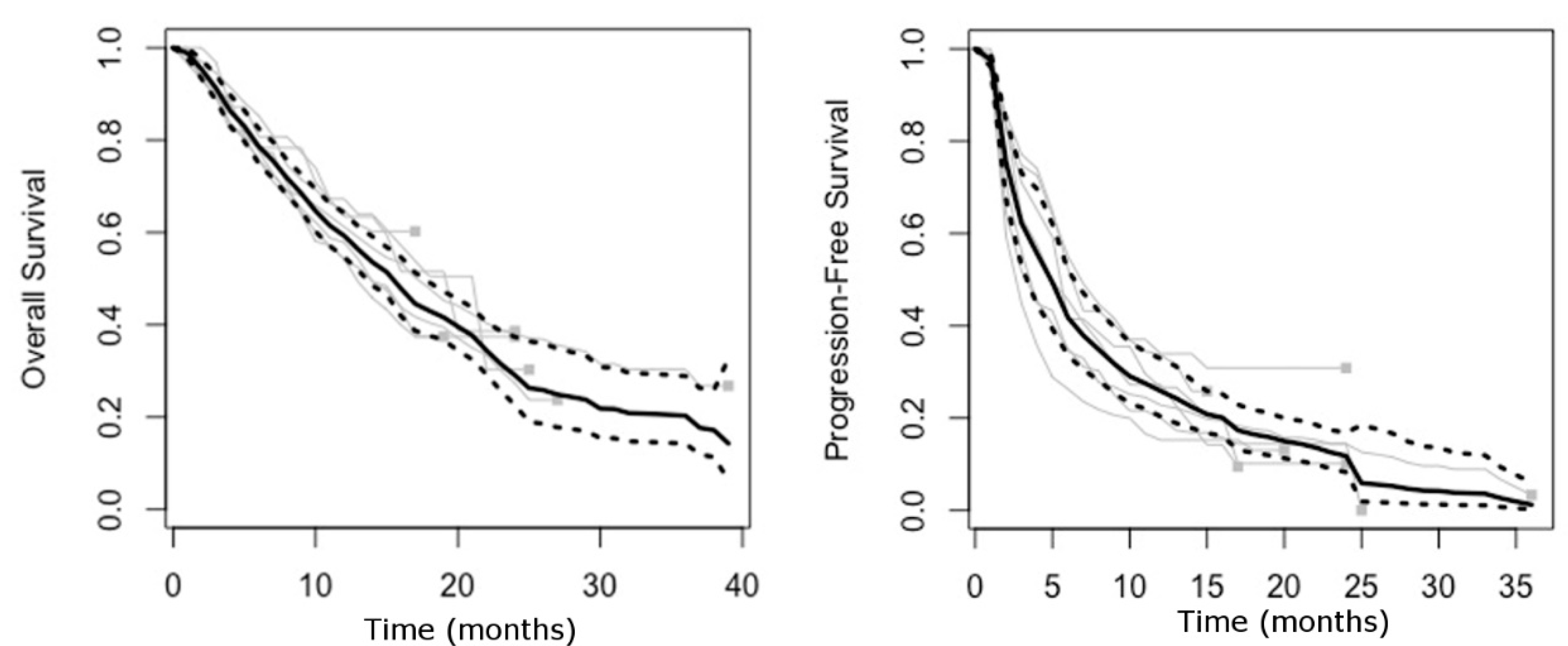
2.1. Trial Selection and Characteristics
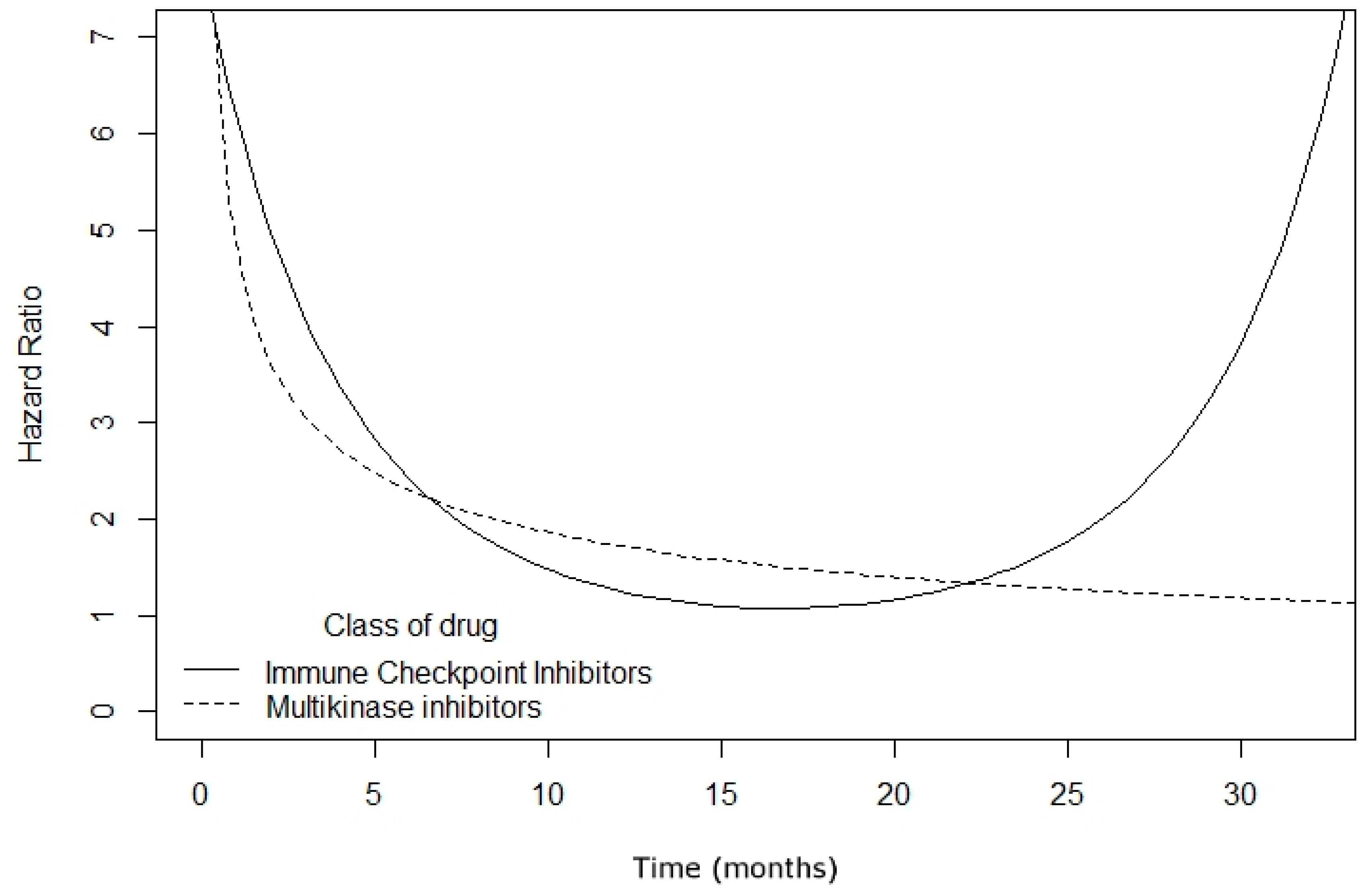
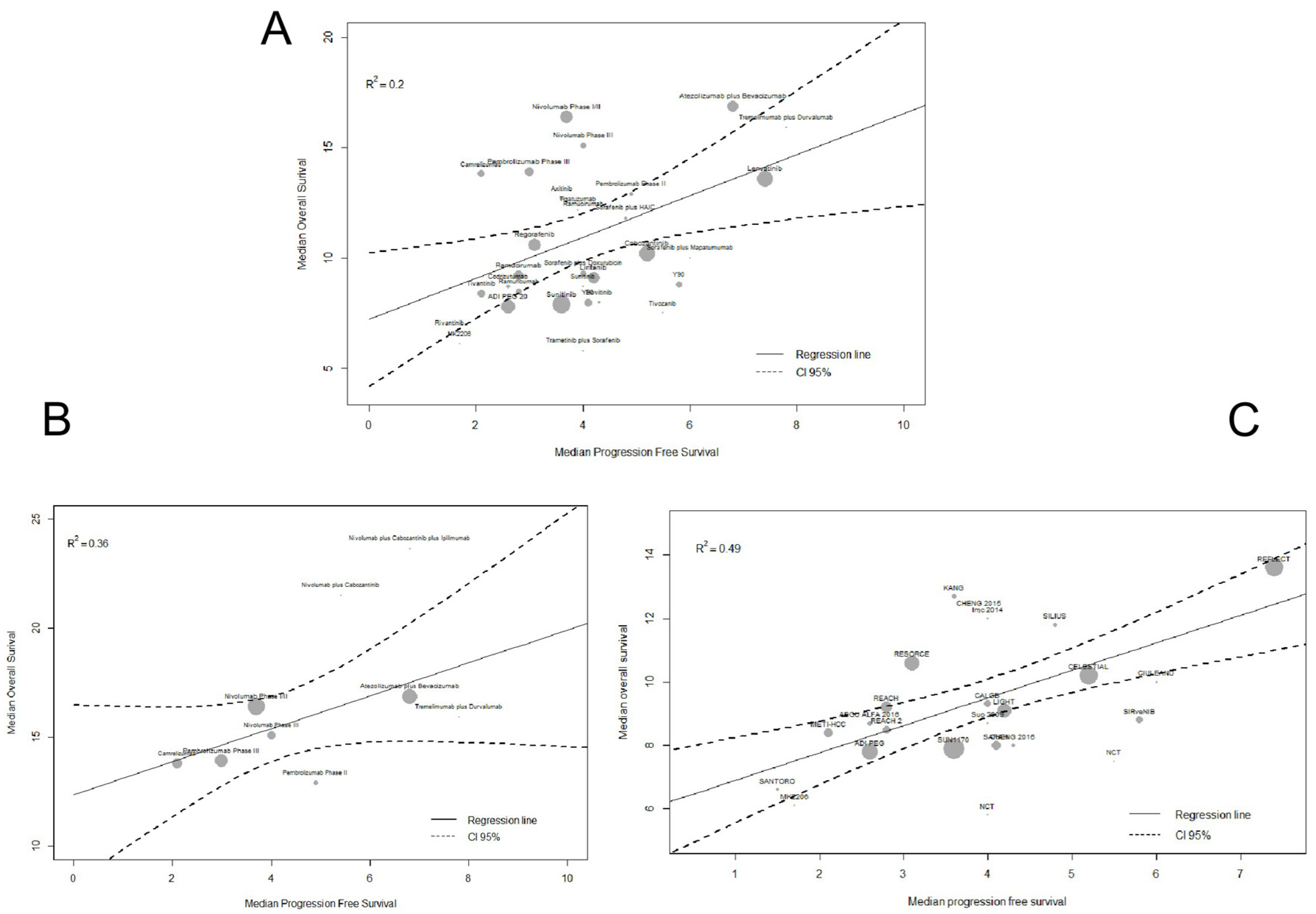
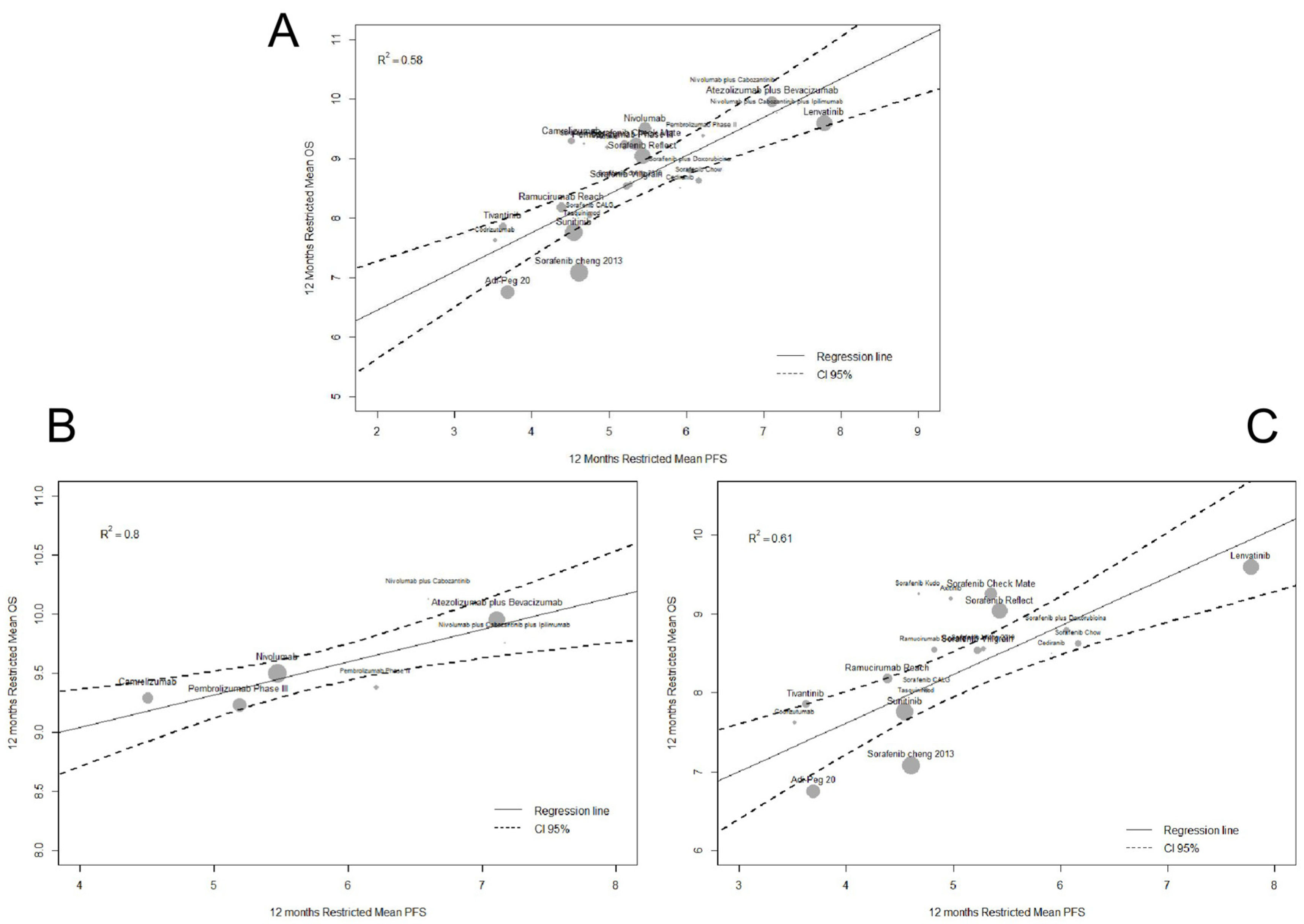
2.2. Surrogacy Metrics
3. Discussion
4. Materials and Methods
4.1. Literature Search and Study Selection
4.2. Trial-Level Data Extraction
4.3. Individual Patient Survival Data Extraction
4.4. Restricted Mean Survival Time (RMST)
4.5. Statistical Analysis
4.5.1. Step 1: Assessing Proportional Hazards Assumption
4.5.2. Step 2: Surrogacy Endpoint Validation
4.6. Subgroup Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bruix, J.; da Fonseca, L.G.; Reig, M. Nat Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Rev. Gastroenterol. Hepatol. 2019, 16, 617–630. [Google Scholar]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Rimassa, L. Finn RS Molecular therapies for HCC: Looking outside the box. J. Hepatol. 2020, 72, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Pazdur, R. Endpoints for assessing drug activity in clinical trials. Oncologist 2008, 13, 19–21. [Google Scholar] [CrossRef]
- Zhao, F. Surrogate end points and their validation in oncology clinical trials. J. Clin. Oncol. 2016, 34, 1436–1437. [Google Scholar] [CrossRef]
- Finn, R.S. Progression-free survival: Starting point or endpoint in advanced HCC trial design? J. Hepatol. 2019, 70, 1062–1064. [Google Scholar] [CrossRef]
- Prasad, V.; Kim, C.; Burotto, M.; Vandross, A. The strength of association between surrogate end points and survival in oncology: A systematic review of trial-level meta-analyses. JAMA Intern. Med. 2015, 175, 1389–1398. [Google Scholar] [CrossRef]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152. [Google Scholar] [CrossRef]
- Llovet, J.M.; Montal, R.; Villanueva, A. Randomized trials and endpoints in advanced HCC: Role of PFS as a surrogate of survival. J. Hepatol. 2019, 70, 1262–1277. [Google Scholar] [CrossRef]
- Mushti, S.L.; Mulkey, F.; Sridhara, R. Evaluation of Overall Response Rate and Progression-Free Survival as Potential Surrogate Endpoints for Overall Survival in Immunotherapy Trials. Clin. Cancer Res. 2018, 24, 2268–2275. [Google Scholar] [CrossRef]
- Tan, A.; Porcher, R.; Crequit, P.; Ravaud, P.; Dechartres, A. Differences in Treatment Effect Size Between Overall Survival and Progression-Free Survival in Immunotherapy Trials: A Meta-Epidemiologic Study of Trials with Results Posted at ClinicalTrials.gov. J. Clin. Oncol. 2017, 35, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.S.; Ryoo, B.Y.; Merle, P.; Kudo, M.; Bouattour, M.; Lim, H.Y.; Breder, V.; Edeline, J.; Chao, Y.; Ogasawara, S.; et al. Pembrolizumab As Second-Line Therapy in Patients with Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J. Clin. Oncol. 2020, 38, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.d.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Qin, S.; Ren, Z.; Meng, Z.; Chen, Z.; Chai, X.; Xiong, J.; Bai, Y.; Yang, L.; Zhu, H.; Fang, W.; et al. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: A multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 571–580. [Google Scholar] [CrossRef]
- Sangro, B.; Gomez-Martin, C.; de la Mata, M.; Iñarrairaegui, M.; Garralda, E.; Barrera, P.; Riezu-Boj, J.I.; Larrea, E.; Alfaro, C.; Sarobe, P.; et al. A clinical trial of CTLA-4 blockade with tremelimumab in patients with hepatocellular carcinoma and chronic hepatitis C. J. Hepatol. 2013, 59, 81–88. [Google Scholar] [CrossRef]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.; Mathurin, P.; Edeline, J.; Kudo, M.; Han, K.; Harding, J.J.; Merle, P.; et al. Checkmate 459: A Randomized, Multi-Center Phase 3 Study of Nivolumab (Nivo) Vs Sorafenib (Sor) As First-Line (1l) Treatment in Patients (Pts) with Advanced Hepatocellular Carcinoma (Ahcc). Ann. Oncol. 2019, 30 (Suppl. 5), v851–v934. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. NCT01693562. Available online: https://clinicaltrials.gov/ct2/show/NCT01693562 (accessed on 15 May 2020).
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Nivolumab (NIVO) + ipilimumab (IPI) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J. Clin. Oncol. 2019, 37 (Suppl. 15), 4012. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. NCT02821754. Available online: https://clinicaltrials.gov/ct2/show/NCT02821754 (accessed on 15 May 2020).
- Yau, T.; Zagonel, V.; Santoro, A.; Acosta-Rivera, M.; Choo, S.P.; Matilla, A.; He, A.R.; Gracian, A.C.; El-Khoueiry, A.B.; Sangro, B.; et al. Nivolumab (NIVO) + ipilimumab (IPI) + cabozantinib (CABO) combination therapy in patients (pts) with advanced hepatocellular carcinoma (aHCC): Results from CheckMate 040. J. Clin. Oncol. 2020, 38 (Suppl. 4), 478. [Google Scholar] [CrossRef]
- Cheng, A.L.; Kang, Y.K.; Lin, D.Y.; Park, J.W.; Kudo, M.; Qin, S.; Chung, H.C.; Song, X.; Xu, J.; Poggi, G.; et al. Sunitinib versus sorafenib in advanced hepatocellular cancer: Results of a randomized phase III trial. J. Clin. Oncol. 2013, 31, 4067–4075. [Google Scholar] [CrossRef]
- Vilgrain, V.; Pereira, H.; Assenat, E.; Guiu, B.; Ilonca, A.D.; Pageaux, G.P.; Sibert, A.; Bouattour, M.; Lebtahi, R.; Allaham, W.; et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): An open-label randomised controlled phase 3 trial. Lancet Oncol. 2017, 18, 1624–1636. [Google Scholar] [CrossRef]
- Chow, P.K.H.; Gandhi, M.; Tan, S.B.; Khin, M.W.; Khasbazar, A.; Ong, J.; Choo, S.P.; Cheow, P.C.; Chotipanich, C.; Lim, K.; et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J. Clin. Oncol. 2018, 36, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Shi, Q.; Knox, J.J.; Kaubisch, A.; Niedzwiecki, D.; Posey, J.; Tan, B.R., Jr.; Kavan, P.; Goel, R.; Lammers, P.E.; et al. Assessment of Treatment With Sorafenib Plus Doxorubicin vs Sorafenib Alone in Patients With Advanced Hepatocellular Carcinoma: Phase 3 CALGB 80802 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1582–1588, Epub ahead of print, Erratum in JAMA Oncol. 10 October 2019. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Ueshima, K.; Yokosuka, O.; Ogasawara, S.; Obi, S.; Izumi, N.; Aikata, H.; Nagano, H.; Hatano, E.; Sasaki, Y.; et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 424–432. [Google Scholar] [CrossRef]
- Hsu, C.; Yang, T.S.; Huo, T.I.; Hsieh, R.K.; Yu, C.W.; Hwang, W.S.; Hsieh, T.Y.; Huang, W.T.; Chao, Y.; Meng, R.; et al. Vandetanib in patients with inoperable hepatocellular carcinoma: A phase II, randomized, double-blind, placebo-controlled study. J. Hepatol. 2012, 56, 1097–1103. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Johnson, P.; Knox, J.J.; Capanu, M.; Davidenko, I.; Lacava, J.; Leung, T.; Gansukh, B.; Saltz, L.B. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: A randomized trial. JAMA 2010, 304, 2154–2160. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Zhu, A.X.; Ancukiewicz, M.; Supko, J.G.; Sahani, D.V.; Blaszkowsky, L.S.; Meyerhardt, J.A.; Abrams, T.A.; McCleary, N.J.; Bhargava, P.; Muzikansky, A.; et al. Efficacy, safety, pharmacokinetics, and biomarkers of cediranib monotherapy in advanced hepatocellular carcinoma: A phase II study. Clin. Cancer Res. 2013, 19, 1557–1566. [Google Scholar] [CrossRef]
- Bruix, J.; Qin, S.; Merle, P.; Granito, A.; Huang, Y.H.; Bodoky, G.; Pracht, M.; Yokosuka, O.; Rosmorduc, O.; Breder, V.; et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 389, 56–66. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Meyer, T.; Cheng, A.L.; El-Khoueiry, A.B.; Rimassa, L.; Ryoo, B.Y.; Cicin, I.; Merle, P.; Chen, Y.H.; Park, J.W.; et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N. Engl. J. Med. 2018, 379, 54–63. [Google Scholar] [CrossRef]
- Zhu, A.X.; Park, J.O.; Ryoo, B.Y.; Yen, C.J.; Poon, R.; Pastorelli, D.; Blanc, J.F.; Chung, H.C.; Baron, A.D.; Pfiffer, T.E.F.; et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015, 16, 859–870. [Google Scholar] [CrossRef]
- Zhu, A.X.; Kang, Y.K.; Yen, C.J.; Finn, R.S.; Galle, P.R.; Llovet, J.M.; Assenat, E.; Brandi, G.; Pracht, M.; Lim, H.Y.; et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased α-fetoprotein concentrations (REACH-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 282–296. [Google Scholar] [CrossRef]
- Rimassa, L.; Assenat, E.; Peck-Radosavljevic, M.; Pracht, M.; Zagonel, V.; Mathurin, P.; Rota Caremoli, E.; Porta, C.; Daniele, B.; Bolondi, L.; et al. Tivantinib for second-line treatment of MET-high, advanced hepatocellular carcinoma (METIV-HCC): A final analysis of a phase 3, randomised, placebo-controlled study. Lancet Oncol. 2018, 19, 682–693. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Qin, S.; Ryoo, B.Y.; Lu, S.N.; Yen, C.J.; Feng, Y.H.; Lim, H.Y.; Izzo, F.; Colombo, M.; Sarker, D.; et al. Phase III randomized study of second line ADI-PEG 20 plus best supportive care versus placebo plus best supportive care in patients with advanced hepatocellular carcinoma. Ann. Oncol. 2018, 29, 1402–1408. [Google Scholar] [CrossRef]
- Kang, Y.K.; Yau, T.; Park, J.W.; Lim, H.Y.; Lee, T.Y.; Obi, S.; Chan, S.L.; Qin, S.; Kim, R.D.; Casey, M.; et al. Randomized phase II study of axitinib versus placebo plus best supportive care in second-line treatment of advanced hepatocellular carcinoma. Ann. Oncol. 2015, 26, 2457–2463. [Google Scholar] [CrossRef]
- Escudier, B.; Faivre, S.; Van Cutsem, E.; Germann, N.; Pouget, J.C.; Plummer, R.; Vergote, I.; Thistlethwaite, F.; Bjarnason, G.A.; Jones, R.; et al. A Phase II Multicentre, Open-Label, Proof-of-Concept Study of Tasquinimod in Hepatocellular, Ovarian, Renal Cell, and Gastric Cancers. Target Oncol. 2017, 12, 655–661. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Puig, O.; Daniele, B.; Kudo, M.; Merle, P.; Park, J.W.; Ross, P.; Peron, J.M.; Ebert, O.; Chan, S.; et al. Randomized phase II placebo controlled study of codrituzumab in previously treated patients with advanced hepatocellular carcinoma. J. Hepatol. 2016, 65, 289–295. [Google Scholar] [CrossRef]
- Cabibbo, G.; Celsa, C.; Enea, M.; Battaglia, S.; Rizzo, G.E.M.; Grimaudo, S.; Matranga, D.; Attanasio, M.; Bruzzi, P.; Craxì, A.; et al. Optimizing Sequential Systemic Therapies for Advanced Hepatocellular Carcinoma: A Decision Analysis. Cancers 2020, 12, 2132. [Google Scholar] [CrossRef]
- Mulkey, F.; Theoret, M.R.; Keegan, P.; Pazdur, R.; Sridhara, R. Comparison of iRECIST versus RECIST V.1.1 in patients treated with an anti-PD-1 or PD-L1 antibody: Pooled FDA analysis. J. ImmunoTher. Cancer 2020, 8, e000146. [Google Scholar] [CrossRef]
- Ferrara, R.; Pilotto, S.; Caccese, M.; Grizzi, G.; Sperduti, I.; Giannarelli, D.; Milella, M.; Besse, B.; Tortora, G.; Bria, E. Do immune checkpoint inhibitors need new studies methodology? J. Thorac. Dis. 2018, 10 (Suppl. 13), S1564–S1580. [Google Scholar] [CrossRef]
- Hernán, M.A. The hazards of hazard ratios [published correction appears in Epidemiology. 2011 Jan;22(1):134]. Epidemiology 2010, 21, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Royston, P.; Parmar, M.K. Restricted mean survival time: An alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med. Res. Methodol. 2013, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Pak, K.; Uno, H.; Kim, D.H.; Tian, L.; Kane, R.C.; Takeuchi, M.; Fu, H.; Claggett, B.; Wei, L.J. Interpretability of Cancer Clinical Trial Results Using Restricted Mean Survival Time as an Alternative to the Hazard Ratio. JAMA Oncol. 2017, 3, 1692–1696. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Allison, J.P. Immune checkpoint targeting in cancer therapy: Toward combination strategies with curative potential. Cell 2015, 161, 205–214. [Google Scholar] [CrossRef]
- Cabibbo, G.; Petta, S.; Barbara, M.; Attardo, S.; Bucci, L.; Farinati, F.; Giannini, E.G.; Negrini, G.; Ciccarese, F.; Rapaccini, G.L.; et al. Hepatic decompensation is the major driver of death in HCV-infected cirrhotic patients with successfully treated early hepatocellular carcinoma. J. Hepatol. 2017, 67, 65–71. [Google Scholar] [CrossRef]
- Mitchell, M.; Muftakhidinov, B.; Winchen, T. Engauge Digitizer Software. Available online: http://markummitchell.github.io/engauge-digitizer (accessed on 22 April 2020).
- Guyot, P.; Ades, A.E.; Ouwens, M.J.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef]
- Combescure, C.; Foucher, Y.; Jackson, D. Meta-analysis of single-arm survival studies: A distribution-free approach for estimating summary survival curves with random effects. Stat. Med. 2014, 33, 2521–2537. [Google Scholar] [CrossRef]
- Earle, C.C.; Pham, B.; Wells, G.A. An assessment of methods to combine published survival curves. Med. Decis. Making 2000, 20, 104–111. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Uno H: Vignette for survRM2 Package: Comparing Two Survival Curves Using the Restricted Mean Survival Time. Available online: https://cran.r-project.org/web/packages/survRM2/vignettes/survRM2-vignette3-1.pdf (accessed on 15 May 2020).
- Klein, J.P.; Moeschberger, M.L. Survival Analysis: Techniques for Censored and Truncated Data, 2nd ed.; Springer: New York, NY, USA, 2003; Volume 9, pp. 302–308. [Google Scholar]
- Sterne, J.A.; Jüni, P.; Schulz, K.F.; Altman, D.G.; Bartlett, C.; Egger, M. Statistical methods for assessing the influence of study characteristics on treatment effects in ‘meta-epidemiological’ research. Stat. Med. 2002, 21, 1513–1524. [Google Scholar] [CrossRef]
| Trial | Line of Treatment | Arms | Overall Survival | Progression-Free Survival | Objective Response Rate (%) | Time to First Radiological Assessment | Duration of Follow-Up (Months) | Reference | |||||||
| 1st Quartile (Months) | Median (Months) | 3rd Quartile (Months) | HR (95% CI) | 1st Quartile (Months) | Median (Months) | 3rd Quartile (Months) | HR (95% CI) | ||||||||
| Immune-checkpoint Inhibitors Alone Or In Combination | KEYNOTE-240, 2019 (phase III, full text) | Second-line | Pembrolizumab (n = 278) | 6.15 | 13.9 | 24 | 0.781 (0.611–0.998) | 1.46 | 3.0 | 8.45 | 0.718 0.570–0.904) | 18.3 | 6 weeks | 28 | [12] |
| Placebo (n = 135) | - | 10.6 | - | - | 2.8 | - | 4.4 | ||||||||
| KEYNOTE-224, 2018 (phase II, full text) | Second-line | Pembrolizumab (n = 104) | 7.25 | 12.9 | 16.1 | - | 2.12 | 4.9 | 12.5 | - | 17.3 | 9 weeks | 19 | [13] | |
| CheckMate 040, 2017 (phase I/II, full text) | Second-line | Nivolumab (n = 182) | - | 15.1 | - | - | - | 4.0 | - | - | 14.3 | - | 57 | [14] | |
| Qin et al., 2020 (phase II, full text) | Second-line | Camrelizumab (n = 217) | 6.45 | 13.8 | 16.9 | - | 1.88 | 2.1 | 6.1 | - | 14.7 | 8 weeks | 22 | [15] | |
| Sangro et al., 2013 (phase II, full text)° | Both first- and second-line | Tremelimumab (n = 21) | 6 | 8.2 | 21.6 | - | - | NA | - | 17.6 | 12 weeks | 25 | [16] | ||
| CheckMate 459, 2019 (phase III abstract) | First-line | Nivolumab (n = 371) | 6.35 | 16.4 | 36.3 | 0.85 (0.72–1.02) | 1.98 | 3.7 | 9.90 | 0.93 (0.79–1.10) | 15.4 | - | 39 | [17] | |
| Sorafenib (n = 372) | 6.0 | 14.7 | 27.3 | 1.95 | 3.8 | 7.65 | 7.0 | 37 | |||||||
| NCT01693562, 2017 (phase I/II, abstract) | Second-line | Durvalumab (n = 39) | - | 13.2 | - | - | - | NA | - | - | 10.3 | - | - | [18] | |
| CheckMate 040, 2019 (phase I/II, abstract) | Second-line | Nivolumab plus Ipilimumab * Arm A: n = 50 | - | Arm A: 23 | - | - | - | NA | - | - | Arm A: 32 | - | |||
| Arm B: n = 49 | - | Arm B: 12 | - | - | - | ArmB:30.6 | 37 | [19] | |||||||
| Arm C: n = 49 | - | Arm C: 13 | - | - | - | Arm C: 30.6 | |||||||||
| NCT02821754, 2019 (phase I/II, abstract) | Second-line | Tremelimumab plus Durvalumab (n = 10) | - | 15.9 | - | - | - | 7.8 | - | - | 20 | - | - | [20] | |
| Immune-checkpoint inhibitors in combination with multikinase inhibitors | CheckMate 040, 2020 (phase I/II, abstract) | Both first- (n = 12) and second-line (n = 19) | Nivolumab plus Ipilimumab plus Cabozantinib (n = 35) | 9 | 24 | NR | - | 3.4 | 6.8 | NR | - | 31 | - | 24 | [21] |
| Both first- (n = 12) and second-line (n = 23) | Nivolumab plus Cabozantinib (n = 36) | 8.7 | 21.5 | NR | - | 2.73 | 5.4 | 12.5 | - | 14 | |||||
| IMbrave 150, 2019 (phase III, published) | First-line | Atezolizumab plus Bevacizumab (n = 336) | 7.87 | NR | NR | 0.58(0.42–0.79) | 2.9 | 6.8 | 13.5 | 0.59 (0.47–0.76) | 26.5 | 6 weeks | 17 | [2] | |
| Sorafenib (n = 165) | 3.5 | 13.2 | - | 1.17 | 4.3 | 7.3 | 11.9 | ||||||||
| Class of Drug | Trial Arm | 6-Month Restricted Mean Survival Time | 12-Month Restricted Mean Survival Time | ||
|---|---|---|---|---|---|
| Progression-Free Survival (Months) (95% Confidence Interval) | Overall Survival (Months) (95% Confidence Interval) | Progression-Free Survival (Months) (95% Confidence Interval) | Overall Survival (Months) (95% Confidence Interval) | ||
| Immune-checkpoint inhibitors | IMBrave-150 (Atezolizumab plus Bevacizumab arm) [2] | 4.67 (4.46–4.86) | 5.62 (5.50–5.73) | 7.11 (6.65–7.58) | 9.95 (9.60–10.31) |
| CheckMate 459 (Nivolumab arm) [17] | 3.85 (3.35–4.04) | 5.42 (5.28–5.56) | 5.47 (5.03–5.9) | 9.50 (9.12–9.87) | |
| Keynote-240 (Pembrolizumab arm) [12] | 3.65 (3.40–3.89) | 5.37 (5.22–5.52) | 5.19 (4.68–5.70) | 9.23 (8.79–9.66) | |
| Keynote-224 (Pembrolizumab arm) [13] | 4.23 (3.87–4.59) | 5.44 (5.19–5.69) | 6.21 (5.41–7.02) | 9.38 (8.70–10.05) | |
| Qin et al., 2020 (Camrelizumab arm) [15] | 3.32 (3.07–3.57) | 5.35 (5.16–5.23) | 4.51 (3.99–5.04) | 9.29 (8.79–9.79) | |
| CheckMate 040 (Nivolumab plus Cabozantinib plus Ipilimumab arm) [21] | 4.72 (4.13–5.32) | 5.37 (4.84–5.89) | 7.17 (5.77–8.57) | 9.76 (8.38–11.41) | |
| CheckMate 040 (Nivolumab plus Cabozantinib arm) [21] | 4.44 (3.84–5.03) | 5.62 (5.31–5.93) | 6.6 (5.2–8) | 10.13 (9.02–11.24) | |
| Pooled immune-checkpoint inhibitors | 3.99 (3.88–4.09) | 5.45 (5.38–5.52) | 5.79 (5.56–6.02) | 9.53 (9.34–9.72) | |
| Multikinase inhibitors | SUN1170 (Sorafenib arm) [22] | 3.52 (3.35–3.69) | 4.81 (4.56–5.07) | 4.61 (4.28–7.93) | 7.08 (6.44–7.71) |
| SUN1170 (Sunitinib arm) [22] | 3.68 (3.53–3.84) | 5.00 (4.87–5.13) | 4.54 (4.26–4.82) | 7.76 (7.42–8.09) | |
| SARAH (Sorafenib arm) [23] | 3.91 (3.65–4.18) | 5.21 (5.01–5.4) | 5.22 (4.71–5.72) | 8.54 (8.02–9.06) | |
| SIRVENIB (Sorafenib arm) [24] | 4.48 (4.25–4.72) | 5.28 (5.06–5.49) | 6.16 (5.59–6.72) | 8.63 (8.05–9.21) | |
| CALGB80802 (Sorafenib arm) [25] | 3.68 (3.39–3.97) | 5.00 (4.70–5.24) | 4.75 (4.20–5.30) | 8.03 (7.44–8.63) | |
| SILIUS (Sorafenib arm) [26] | 3.71 (3.35–4.07) | 5.5 (5.39–5.59) | 4.68 (4.00–5.36) | 9.25 (8.93–9.56) | |
| Hsu et al., 2012 (Vandetanib arms) [27] | 2.19 (1.4–2.97) | 4.86 (4.17–5.56) | NA | NA | |
| Abou-Alfa et al., 2010 (Sorafenib plus Doxorubicin arm) [28] | 4.38 (3.79–4.98) | 5.15 (4.66–5.63) | 6.05 (4.85–7.25) | 8.8 (7.6–10.00) | |
| REFLECT (Sorafenib arm) [29] | 3.96 (3.77–4.14) | 5.43 (5.32–5.54) | 5.43 (5.05–5.81) | 9.04 (8.71–9.36) | |
| REFLECT (Lenvatinib arm) [29] | 4.85 (4.67–5.00) | 5.54 (5.43–5.64) | 7.78 (7.38–8.16) | 9.29 (9.6–9.91) | |
| IMBrave-150 (Sorafenib arm) [2] | 3.96 (3.64–4.28) | 5.25 (5.13–5.37) | 5.28 (4.65–5.91) | 8.56 (8.23–8.89) | |
| CheckMate 459 (Sorafenib arm) [17] | 4.02 (3.83–4.21) | 5.40 (5.27–5.54) | 5.35 (4.96–5.74) | 9.25 (8.87–9.63) | |
| Zhu et al., 2013 (Cediranib arm) [30] | 4.29 (3.39–5.19) | 5.15 (4.30–6.00) | 5.92 (4.12–7.72) | 8.50 (6.51–10.50) | |
| RESORCE (Regorafenib arm) [31] | 3.71 (3.51–3.91) | 5.22 (5.08–5.36) | 4.89 (4.49–5.29) | 8.63 (8.23–9.03) | |
| CELESTIAL (Cabozantinib arm) [32] | 4.19 (4.01–4.36) | 5.30 (5.16–5.44) | 5.29 (5.66–6.03) | 8.76 (8.39–9.12) | |
| REACH (Ramucirumab arm) [33] | 3.31 (2.00–3.61) | 5.17 (4.97–5.37) | 4.39 (3.84–4.94) | 8.18 (7.64–8.72) | |
| REACH-2 (Ramucirumab arm) [34] | 3.54 (3.30–3.79) | 5.26 (5.09–5.42) | 4.82 (4.35–5.29) | 8.55 (8.09–9.01) | |
| METIV-HCC (Tivantinib arm) [35] | 3.08 (2.62–3.15) | 4.99 (4.79–5.19) | 3.62 (3.25–3.99) | 7.86 (7.34–8.38) | |
| ADI-PEG20 (ADI-PEG20 arm) [36] | 3.19 (3.01–3.37) | 4.47 (4.29–4.65) | 3.69 (3.40–3.98) | 6.75 (6.34–7.16) | |
| Kang et al., 2015 (Axitinib arm) [37] | 3.75 (3.41–4.09) | 5.37 (4.84–5.89) | 4.98 (4.32–5.62) | 9.19 (8.56–9.82) | |
| Escudier et al., 2017 (Tasquinimod arm) [38] | 3.73 (3.20–4.27) | 5.30 (4.88–5.72) | 4.65 (3.69–5.61) | 7.89 (6.89–8.89) | |
| Abou-Alfa et al., 2016 (Codrituzumab arm) [39] | 3.02 (2.68–3.36) | 4.97 (4.68–5.26) | 3.52 (2.97–4.07) | 7.62 (6.96–8.29) | |
| Pooled multikinase inhibitors | 3.86 (3.80–3.90) | 5.26 (5.22–5.30) | 5.19 (5.08–5.30) | 8.63 (8.53–8.73) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabibbo, G.; Celsa, C.; Enea, M.; Battaglia, S.; Rizzo, G.E.M.; Busacca, A.; Matranga, D.; Attanasio, M.; Reig, M.; Craxì, A.; et al. Progression-Free Survival Early Assessment Is a Robust Surrogate Endpoint of Overall Survival in Immunotherapy Trials of Hepatocellular Carcinoma. Cancers 2021, 13, 90. https://doi.org/10.3390/cancers13010090
Cabibbo G, Celsa C, Enea M, Battaglia S, Rizzo GEM, Busacca A, Matranga D, Attanasio M, Reig M, Craxì A, et al. Progression-Free Survival Early Assessment Is a Robust Surrogate Endpoint of Overall Survival in Immunotherapy Trials of Hepatocellular Carcinoma. Cancers. 2021; 13(1):90. https://doi.org/10.3390/cancers13010090
Chicago/Turabian StyleCabibbo, Giuseppe, Ciro Celsa, Marco Enea, Salvatore Battaglia, Giacomo Emanuele Maria Rizzo, Anita Busacca, Domenica Matranga, Massimo Attanasio, Maria Reig, Antonio Craxì, and et al. 2021. "Progression-Free Survival Early Assessment Is a Robust Surrogate Endpoint of Overall Survival in Immunotherapy Trials of Hepatocellular Carcinoma" Cancers 13, no. 1: 90. https://doi.org/10.3390/cancers13010090
APA StyleCabibbo, G., Celsa, C., Enea, M., Battaglia, S., Rizzo, G. E. M., Busacca, A., Matranga, D., Attanasio, M., Reig, M., Craxì, A., & Cammà, C. (2021). Progression-Free Survival Early Assessment Is a Robust Surrogate Endpoint of Overall Survival in Immunotherapy Trials of Hepatocellular Carcinoma. Cancers, 13(1), 90. https://doi.org/10.3390/cancers13010090







