Review of Experimental Studies to Improve Radiotherapy Response in Bladder Cancer: Comments and Perspectives
Abstract
Simple Summary
Abstract
1. Introduction
2. Radiotherapy as Part of Bladder Preserving Treatment in Clinics
3. Limitations of Use of RT in MIBC in Clinics
4. MIBC Molecular Subtypes as Biomarkers for CCRT Response
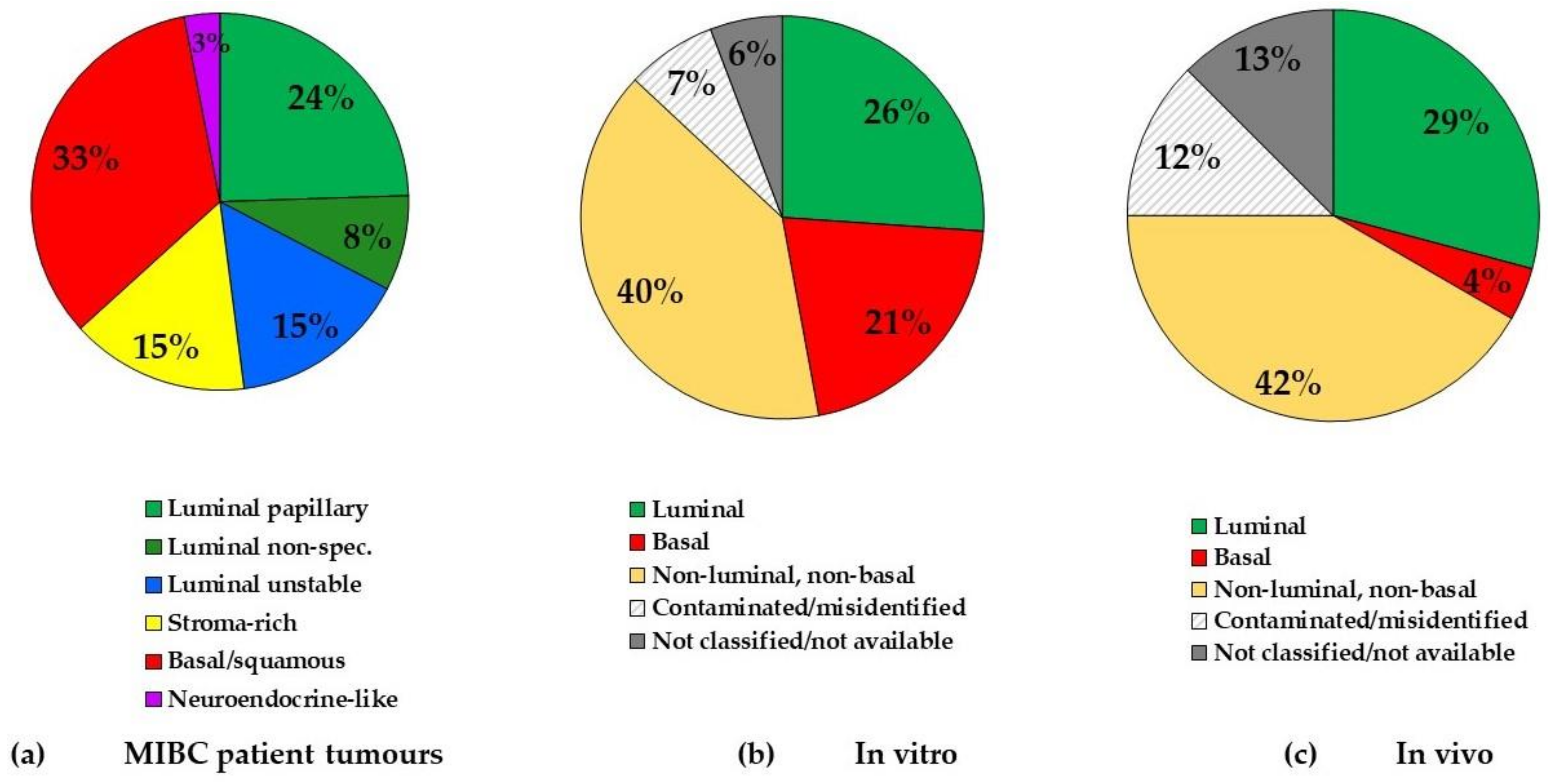
4.1. BCa Tumour Subtypes
4.2. BCa Cell Line Molecular Subtypes
5. BCa Experimental Models in RT Studies
5.1. BCa Cell Lines
5.1.1. Molecular Subtypes
5.1.2. Gender
5.1.3. Intrinsic Radiosensitivity
5.2. BCa Xenografts
5.3. Syngeneic Models
6. Use of CT Agents in Combination with RT in Experimental Studies for BCa Treatment
6.1. Cisplatin
6.2. Gemcitabine
6.3. In Vivo Study Reporting/Design
7. Targeted Agents to Improve RT Response in BCa
7.1. Epidermal Growth Factor Receptor
7.2. Chromatin Modifiers/Epigenetic Regulators
7.3. Radio-Immunotherapy
8. Suggestions to Improve the Design of Future Experimental BCa Studies: New Agents and Relevant Models
8.1. Molecular Subtype Consideration
8.2. New Targeted Agents
8.3. Pelvic Toxisity Assessment
8.4. Use of Orthotopic Mice Models
8.5. Humanised Mouse Models
9. Methods
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shipley, W.U.; Prout, G.R., Jr.; Einstein, A.B.; Coombs, L.J.; Wajsman, Z.; Soloway, M.S.; Englander, L.; Barton, B.A.; Hafermann, M.D. Treatment of Invasive Bladder Cancer by Cisplatin and Radiation in Patients Unsuited for Surgery. JAMA 1987, 258, 931–935. [Google Scholar] [CrossRef]
- Housset, M.; Maulard, C.; Chretien, Y.; Dufour, B.; Delanian, S.; Huart, J.; Colardelle, F.; Brunel, P.; Baillet, F. Combined radiation and chemotherapy for invasive transitional-cell carcinoma of the bladder: A prospective study. J. Clin. Oncol. 1993, 11, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Coppin, C.M.; Gospodarowicz, M.K.; James, K.; Tannock, I.F.; Zee, B.; Carson, J.; Pater, J.; Sullivan, L.D. Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. J. Clin. Oncol. 1996, 14, 2901–2907. [Google Scholar] [CrossRef]
- James, N.D.; Hussain, S.A.; Hall, E.; Jenkins, P.; Tremlett, J.; Rawlings, C.; Crundwell, M.; Sizer, B.; Sreenivasan, T.; Hendron, C.; et al. Radiotherapy with or without Chemotherapy in Muscle-Invasive Bladder Cancer. N. Engl. J. Med. 2012, 366, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.M.; Chung, P.; Kulkarni, G.S.; Sridhar, S.S. Trimodality Therapy for Muscle-Invasive Bladder Cancer: Recent Advances and Unanswered Questions. Curr. Oncol. Rep. 2020, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.; Joseph, N.; Sanderson, B.; Logue, J.; Wylie, J.; Elliott, T.; Lyons, J.; Anandadas, C.; Choudhury, A. Tolerability of Concurrent Chemoradiation Therapy With Gemcitabine (GemX), With and Without Prior Neoadjuvant Chemotherapy, in Muscle Invasive Bladder Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Rojas, A.M.; Bentzen, S.M.; Saunders, M.I. Radiotherapy with concurrent carbogen and nicotinamide in bladder carcinoma. J. Clin. Oncol. 2010, 28, 4912–4918. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; Winter, K.A.; Gunderson, L.L.; Pedersen, J.; Benson, A.B.; Thomas, C.R.; Mayer, R.J.; Haddock, M.G.; Rich, T.A.; Willett, C. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: A randomized controlled trial. JAMA J. Am. Med. Assoc. 2008, 299, 1914–1921. [Google Scholar] [CrossRef]
- Forastiere, A.A.; Zhang, Q.; Weber, R.S.; Maor, M.H.; Goepfert, H.; Pajak, T.F.; Morrison, W.; Glisson, B.; Trotti, A.; Ridge, J.A.; et al. Long-term results of RTOG 91-11: A comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J. Clin. Oncol. 2013, 31, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Budach, V.; Stuschke, M.; Budach, W.; Baumann, M.; Geismar, D.; Grabenbauer, G.; Lammert, I.; Jahnke, K.; Stueben, G.; Herrmann, T.; et al. Hyperfractionated accelerated chemoradiation with concurrent fluorouracil-mitomycin is more effective than dose-escalated hyperfractionated accelerated radiation therapy alone in locally advanced head and neck cancer: Final results of the Radiotherapy Cooperative Clinical Trials Group of the German Cancer Society 95-06 prospective randomized trial. J. Clin. Oncol. 2005, 23, 1125–1135. [Google Scholar] [CrossRef]
- El-Taji, O.M.S.; Alam, S.; Hussain, S.A. Bladder Sparing Approaches for Muscle-Invasive Bladder Cancers. Curr. Treat. Options Oncol. 2016, 17, 15. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W. NCCN Guidelines Updates: Management of Muscle-Invasive Bladder Cancer. J. Natl. Compr. Canc. Netw. 2019, 17, 591–593. [Google Scholar] [PubMed]
- Witjes, J.A.; Bruins, H.M.; Cathomas, R.; Compérat, E.M.; Cowan, N.C.; Gakis, G.; Hernández, V.; Linares Espinós, E.; Lorch, A.; Neuzillet, Y.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur. Urol. 2020, 79, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Pal, S.K.; Chowdhury, S.; Harshman, L.C.; Crabb, S.J.; Wong, Y.N.; Yu, E.Y.; Powles, T.; Moshier, E.L.; Ladoire, S.; et al. Comparative effectiveness of gemcitabine plus cisplatin versus methotrexate, vinblastine, doxorubicin, plus cisplatin as neoadjuvant therapy for muscle-invasive bladder cancer. Cancer 2015, 121, 2586–2593. [Google Scholar] [CrossRef] [PubMed]
- Donat, S.M.; Shabsigh, A.; Savage, C.; Cronin, A.M.; Bochner, B.H.; Dalbagni, G.; Herr, H.W.; Milowsky, M.I. Potential Impact of Postoperative Early Complications on the Timing of Adjuvant Chemotherapy in Patients Undergoing Radical Cystectomy: A High-Volume Tertiary Cancer Center Experience. Eur. Urol. 2009, 55, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; May, M.; Burger, M.; Palisaar, R.-J.; Trinh, Q.-D.; Fritsche, H.-M.; Rink, M.; Chun, F.; Martini, T.; Bolenz, C.; et al. Prediction of 90-day Mortality After Radical Cystectomy for Bladder Cancer in a Prospective European Multicenter Cohort. Eur. Urol. 2014, 66, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.L.; Kassouf, W. Radical cystectomy is the best choice for most patients with muscle-invasive bladder cancer? Opinion: Yes. Int. Braz J. Urol. 2017, 43, 184–187. [Google Scholar] [CrossRef]
- Weldon, T.E.; Kursh, E.; Novak, L.J.; Persky, L. Combination radiotherapy and chemotherapy in murine bladder cancer. Urology 1979, 14, 47–52. [Google Scholar] [CrossRef]
- Ploussard, G.; Daneshmand, S.; Efstathiou, J.A.; Herr, H.W.; James, N.D.; Rödel, C.M.; Shariat, S.F.; Shipley, W.U.; Sternberg, C.N.; Thalmann, G.N.; et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: A systematic review. Eur. Urol. 2014, 66, 120–137. [Google Scholar] [CrossRef]
- Mak, R.H.; Hunt, D.; Shipley, W.U.; Efstathiou, J.A.; Tester, W.J.; Hagan, M.P.; Kaufman, D.S.; Heney, N.M.; Zietman, A.L. Long-term outcomes in patients with muscle-invasive bladder cancer after selective bladder-preserving combined-modality therapy: A pooled analysis of radiation therapy oncology group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J. Clin. Oncol. 2014, 32, 3801–3809. [Google Scholar] [CrossRef]
- Huddart, R.A.; Hall, E.; Hussain, S.A.; Jenkins, P.; Rawlings, C.; Tremlett, J.; Crundwell, M.; Adab, F.A.; Sheehan, D.; Syndikus, I.; et al. Randomized noninferiority trial of reduced high-dose volume versus standard volume radiation therapy for muscle-invasive bladder cancer: Results of the BC2001 Trial (CRUK/01/004). Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.; Masodkar, R.; Kalyani, N.; Mahantshetty, U.; Bakshi, G.; Prakash, G.; Joshi, A.; Prabhash, K.; Ghonge, S.; Shrivastava, S. Clinical outcomes with dose-escalated adaptive radiation therapy for urinary bladder cancer: A prospective study. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 60–66. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, Y.-H.; Zhang, Y.; Qu, W.; Li, J. Radiotherapy in muscle-invasive bladder cancer: The latest research progress and clinical application. Am. J. Cancer Res. 2015, 5, 854–868. [Google Scholar] [PubMed]
- Daro-Faye, M.; Kassouf, W.; Souhami, L.; Marcq, G.; Cury, F.; Niazi, T.; Sargos, P. Combined radiotherapy and immunotherapy in urothelial bladder cancer: Harnessing the full potential of the anti-tumor immune response. World J. Urol. 2020, 1. [Google Scholar] [CrossRef] [PubMed]
- Marks, L.B.; Carroll, P.R.; Dugan, T.C.; Anscher, M.S. The response of the urinary bladder, urethra, and ureter to radiation and chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 1257–1280. [Google Scholar] [CrossRef]
- Viswanathan, A.N.; Yorke, E.D.; Marks, L.B.; Eifel, P.J.; Shipley, W.U. Radiation Dose-Volume Effects of the Urinary Bladder. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, S116. [Google Scholar] [CrossRef]
- Forker, L.J.; Choudhury, A.; Kiltie, A.E. Biomarkers of Tumour Radiosensitivity and Predicting Benefit from Radiotherapy. Clin. Oncol. 2015, 27, 561–569. [Google Scholar] [CrossRef]
- Koga, F.; Takemura, K.; Fukushima, H. Biomarkers for predicting clinical outcomes of chemoradiation-based bladder preservation therapy for muscle-invasive bladder cancer. Int. J. Mol. Sci. 2018, 19, 2777. [Google Scholar] [CrossRef]
- Desai, N.B.; Bagrodia, A. The challenge of matching assays to biology in DNA damage response biomarkers for response to radiotherapy in bladder cancer. Transl. Androl. Urol. 2019, 8, S514–S516. [Google Scholar] [CrossRef]
- Choudhury, A.; Nelson, L.D.; Teo, M.T.W.; Chilka, S.; Bhattarai, S.; Johnston, C.F.; Elliott, F.; Lowery, J.; Taylor, C.F.; Churchman, M.; et al. MRE11 expression is predictive of cause-specific survival following radical radiotherapy for muscle-invasive bladder cancer. Cancer Res. 2010, 70, 7017–7026. [Google Scholar] [CrossRef]
- Kawashima, A.; Nakayama, M.; Kakuta, Y.; Abe, T.; Hatano, K.; Mukai, M.; Nagahara, A.; Nakai, Y.; Oka, D.; Takayama, H.; et al. Excision repair cross-complementing group 1 may predict the efficacy of chemoradiation therapy for muscle-invasive bladder cancer. Clin. Cancer Res. 2011, 17, 2561–2569. [Google Scholar] [CrossRef]
- Laurberg, J.R.; Brems-Eskildsen, A.S.; Nordentoft, I.; Fristrup, N.; Schepeler, T.; Ulhøi, B.P.; Agerbæk, M.; Hartmann, A.; Bertz, S.; Wittlinger, M.; et al. Expression of TIP60 (tat-interactive protein) and MRE11 (meiotic recombination 11 homolog) predict treatment-specific outcome of localised invasive bladder cancer. BJU Int. 2012, 110, E1228–E1236. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.K.; Karaszi, K.; Valentine, H.; Strauss, V.Y.; Choudhury, A.; McGill, S.; Wen, K.; Brown, M.D.; Ramani, V.; Bhattarai, S.; et al. MRE11 as a Predictive Biomarker of Outcome After Radiation Therapy in Bladder Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Efstathiou, J.A.; Spiegel, D.Y.; Shipley, W.U.; Heney, N.M.; Kaufman, D.S.; Niemierko, A.; Coen, J.J.; Skowronski, R.Y.; Paly, J.J.; McGovern, F.J.; et al. Long-term outcomes of selective bladder preservation by combined-modality therapy for invasive bladder cancer: The MGH experience. Eur. Urol. 2012, 61, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Mitin, T.; George, A.; Zietman, A.L.; Heney, N.M.; Kaufman, D.S.; Uzzo, R.G.; Dreicer, R.; Wallace, H.J.; Souhami, L.; Dobelbower, M.C.; et al. Long-term outcomes among patients who achieve complete or near-complete responses after the induction phase of bladder-preserving combined-modality therapy for muscle-invasive bladder cancer: A pooled analysis of NRG Oncology/RTOG 9906 and 0233. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 67–74. [Google Scholar] [CrossRef]
- Kamoun, A.; de Reyniès, A.; Allory, Y.; Sjödahl, G.; Robertson, A.G.; Seiler, R.; Hoadley, K.A.; Groeneveld, C.S.; Al-Ahmadie, H.; Choi, W.; et al. A Consensus Molecular Classification of Muscle-invasive Bladder Cancer[Formula presented]. Eur. Urol. 2020, 77, 420–433. [Google Scholar] [CrossRef]
- Choi, W.; Porten, S.; Kim, S.; Willis, D.; Plimack, E.R.; Hoffman-Censits, J.; Roth, B.; Cheng, T.; Tran, M.; Lee, I.L.; et al. Identification of Distinct Basal and Luminal Subtypes of Muscle-Invasive Bladder Cancer with Different Sensitivities to Frontline Chemotherapy. Cancer Cell 2014, 25, 152–165. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; Van Der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Seiler, R.; Ashab, H.A.D.; Erho, N.; van Rhijn, B.W.G.; Winters, B.; Douglas, J.; Van Kessel, K.E.; Fransen van de Putte, E.E.; Sommerlad, M.; Wang, N.Q.; et al. Impact of Molecular Subtypes in Muscle-invasive Bladder Cancer on Predicting Response and Survival after Neoadjuvant Chemotherapy [Figure presented]. Eur. Urol. 2017, 72, 544–554. [Google Scholar] [CrossRef]
- Mariathasan, S.; Turley, S.J.; Nickles, D.; Castiglioni, A.; Yuen, K.; Wang, Y.; Kadel, E.E.; Koeppen, H.; Astarita, J.L.; Cubas, R.; et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554, 544–548. [Google Scholar] [CrossRef]
- Rebouissou, S.; Bernard-Pierrot, I.; De Reyniès, A.; Lepage, M.L.; Krucker, C.; Chapeaublanc, E.; Hérault, A.; Kamoun, A.; Caillault, A.; Letouzé, E.; et al. EGFR as a potential therapeutic target for a subset of muscle-invasive bladder cancers presenting a basal-like phenotype. Sci. Transl. Med. 2014, 6, 244ra91. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, T.M.; Keyomarsi, K. Role of cell cycle in mediating sensitivity to radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 928–942. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Overgaard, J. The impact of hypoxia and its modification of the outcome of radiotherapy. J. Radiat. Res. 2016, 57 Suppl 1, i90–i98. [Google Scholar] [CrossRef]
- Efstathiou, J.A.; Gibb, E.; Miyamoto, D.T.; Wu, C.L.; Drumm, M.R.; Lehrer, J.; Ashab, H.A.D.M.; Erho, N.G.; du Plessis, M.; Ong, K.; et al. Subtyping Muscle-Invasive Bladder Cancer to Assess Clinical Response to Trimodality Therapy. Int. J. Radiat. Oncol. 2017, 99, S118. [Google Scholar] [CrossRef][Green Version]
- Choudhury, A.; Yang, L.; Irlam, J.J.; Williamson, A.; Denley, H.; Hoskin, P.; West, C. A hypoxia transcriptomic signature to predict benefit from hypoxia-modifying treatment for muscle-invasive bladder cancer patients. J. Clin. Oncol. 2017, 35, 301. [Google Scholar] [CrossRef]
- Shi, M.J.; Meng, X.Y.; Fontugne, J.; Chen, C.L.; Radvanyi, F.; Bernard-Pierrot, I. Identification of new driver and passenger mutations within APOBEC-induced hotspot mutations in bladder cancer. Genome Med. 2020, 12. [Google Scholar] [CrossRef]
- Earl, J.; Rico, D.; Carrillo-de-Santa-Pau, E.; Rodríguez-Santiago, B.; Méndez-Pertuz, M.; Auer, H.; Gómez, G.; Grossman, H.B.; Pisano, D.G.; Schulz, W.A.; et al. The UBC-40 Urothelial Bladder Cancer cell line index: A genomic resource for functional studies. BMC Genomics 2015, 16, 403. [Google Scholar] [CrossRef]
- Zuiverloon, T.C.M.; De Jong, F.C.; Costello, J.C.; Theodorescu, D. Systematic Review: Characteristics and Preclinical Uses of Bladder Cancer Cell Lines. Bladder Cancer 2018, 4, 169–183. [Google Scholar] [CrossRef]
- Ruan, J.L.; Hsu, J.W.; Browning, R.J.; Stride, E.; Yildiz, Y.O.; Vojnovic, B.; Kiltie, A.E. Mouse Models of Muscle-invasive Bladder Cancer: Key Considerations for Clinical Translation Based on Molecular Subtypes. Eur. Urol. Oncol. 2019, 2, 239–247. [Google Scholar] [CrossRef]
- Cellosaurus—SIB Swiss Institute of Bioinformatics|Expasy. Available online: https://www.expasy.org/resources/cellosaurus (accessed on 19 November 2020).
- Zhang, Y. Understanding the gender disparity in bladder cancer risk: The impact of sex hormones and liver on bladder susceptibility to carcinogens. J. Environ. Sci. Heal. Part C Environ. Carcinog. Ecotoxicol. Rev. 2013, 31, 287–304. [Google Scholar] [CrossRef]
- Dobruch, J.; Daneshmand, S.; Fisch, M.; Lotan, Y.; Noon, A.P.; Resnick, M.J.; Shariat, S.F.; Zlotta, A.R.; Boorjian, S.A. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur. Urol. 2016, 69, 300–310. [Google Scholar] [CrossRef] [PubMed]
- Ide, H.; Inoue, S.; Mizushima, T.; Jiang, G.; Chuang, K.H.; Oya, M.; Miyamoto, H. Androgen receptor signaling reduces radiosensitivity in bladder cancer. Mol. Cancer Ther. 2018, 17, 1566–1574. [Google Scholar] [CrossRef] [PubMed]
- Yard, B.D.; Adams, D.J.; Chie, E.K.; Tamayo, P.; Battaglia, J.S.; Gopal, P.; Rogacki, K.; Pearson, B.E.; Phillips, J.; Raymond, D.P.; et al. A genetic basis for the variation in the vulnerability of cancer to DNA damage. Nat. Commun. 2016, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Groselj, B.; Ruan, J.L.; Scott, H.; Gorrill, J.; Nicholson, J.; Kelly, J.; Anbalagan, S.; Thompson, J.; Stratford, M.R.L.; Jevons, S.J.; et al. Radiosensitization in vivo by histone deacetylase inhibition with no increase in early normal tissue radiation toxicity. Mol. Cancer Ther. 2018, 17, 381–392. [Google Scholar] [CrossRef]
- Williams, K.J.; Albertella, M.R.; Fitzpatrick, B.; Loadman, P.M.; Shnyder, S.D.; Chinje, E.C.; Telfer, B.A.; Dunk, C.R.; Harris, P.A.; Stratford, I.J. In vivo activation of the hypoxia-targeted cytotoxin AQ4N in human tumor xenografts. Mol. Cancer Ther. 2009, 8, 3266–3275. [Google Scholar] [CrossRef]
- Paillas, S.; Then, C.K.; Kilgas, S.; Ruan, J.L.; Thompson, J.; Elliott, A.; Smart, S.; Kiltie, A.E. The Histone Deacetylase Inhibitor Romidepsin Spares Normal Tissues While Acting as an Effective Radiosensitizer in Bladder Tumors in Vivo. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 212–221. [Google Scholar] [CrossRef]
- Then, C.K.; Paillas, S.; Wang, X.; Hampson, A.; Kiltie, A.E. Association of Bacteroides acidifaciens relative abundance with high-fibre diet-associated radiosensitisation. BMC Biol. 2020, 18. [Google Scholar] [CrossRef]
- Schaffer, M.; Schaffer, P.M.; Corti, L.; Sotti, G.; Hofstetter, A.; Jori, G.; Dühmke, E. Photofrin II as an Efficient Radiosensitizing Agent in an Experimental Tumor. Oncol. Res. Treat. 2001, 24, 482–485. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Xiao, J.; Luo, W.; Wang, B.H.; Chen, J.M. Caffeine suppresses apoptosis of bladder cancer RT4 cells in response to ionizing radiation by inhibiting ataxia telangiectasia mutated-Chk2-p53 axis. Chin. Med. J. 2015, 128, 2938–2945. [Google Scholar] [CrossRef]
- Jiang, H.; Hu, X.; Zhang, H.; Li, W. Down-regulation of LncRNA TUG1 enhances radiosensitivity in bladder cancer via suppressing HMGB1 expression. Radiat. Oncol. 2017, 12. [Google Scholar] [CrossRef]
- Yagisawa, T.; Okumi, M.; Omoto, K.; Sawada, Y.; Morikawa, S.; Tanabe, K. Novel approach for bladder cancer treatment using sulfoquinovosylacylpropanediol as a radiosensitizer. Int. J. Urol. 2016, 23, 270–272. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhao, M.J.; Song, Y.F.; Niu, H.T.; Tian, Y.X.; Yang, X.G.; Xie, K.; Jing, Y.H.; Wang, D.G. Adenovirus-mediated downregulation of the ubiquitin ligase RNF8 sensitizes bladder cancer to radiotherapy. Oncotarget 2016, 7, 8956–8967. [Google Scholar] [CrossRef]
- Wang, F.; Tang, J.; Li, P.; Si, S.; Yu, H.; Yang, X.; Tao, J.; Lv, Q.; Gu, M.; Yang, H.; et al. Chloroquine Enhances the Radiosensitivity of Bladder Cancer Cells by Inhibiting Autophagy and Activating Apoptosis. Cell. Physiol. Biochem. 2018, 45, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhang, Q.; Yuan, A.; Wang, B.; Zhang, F.; Ding, Y.; Cao, W.; Chen, W.; Guo, H. Synergy of tumor microenvironment remodeling and autophagy inhibition to sensitize radiation for bladder cancer treatment. Theranostics 2020, 10, 7683–7696. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Cerniglia, G.J.; Mick, R.; Ahmed, M.S.; Bakanauskas, V.J.; Muschel, R.J.; McKenna, W.G. Radiation sensitization of human cancer cells in vivo by inhibiting the activity of PI3K using LY294002. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 846–853. [Google Scholar] [CrossRef]
- Cohen-Jonathan, E.; Muschel, R.J.; McKenna, W.G.; Evans, S.M.; Cerniglia, G.; Mick, R.; Kusewitt, D.; Sebti, S.M.; Hamilton, A.D.; Oliff, A.; et al. Farnesyltransferase inhibitors potentiate the antitumor effect of radiation on a human tumor xenograft expressing activated HRAS. Radiat. Res. 2000, 154, 125–132. [Google Scholar] [CrossRef]
- Shrivastava, S.; Mansure, J.J.; Almajed, W.; Cury, F.; Ferbeyre, G.; Popovic, M.; Seuntjens, J.; Kassouf, W. The Role of HMGB1 in Radioresistance of Bladder Cancer. Mol. Cancer Ther. 2016, 15, 471–479. [Google Scholar] [CrossRef]
- Yoshida, S.; Koga, F.; Tatokoro, M.; Kawakami, S.; Fujii, Y.; Kumagai, J.; Neckers, L.; Kihara, K. Low-dose Hsp90 inhibitors tumor-selectively sensitize bladder cancer cells to chemoradiotherapy. Cell Cycle 2011, 10, 4291–4299. [Google Scholar] [CrossRef]
- Colquhoun, A.J.; Mchugh, L.A.; Tulchinsky, E.; Kriajevska, M.; Mellon, J.K. Combination Treatment with Ionising Radiation and Gefitinib (“Iressa”, ZD1839), an Epidermal Growth Factor Receptor (EGFR) Inhibitor, Significantly Inhibits Bladder Cancer Cell Growth in vitro and in vivo. J. Radiat. Res 2007, 48, 351–360. [Google Scholar] [CrossRef][Green Version]
- Zhang, Z.; Shirakawa, T.; Hinata, N.; Matsumoto, A.; Fujisawa, M.; Okada, H.; Kamidono, S.; Matsuo, M.; Gotoh, A. Combination with CD/5-FC gene therapy enhances killing of human bladder-cancer cells by radiation. J. Gene Med. 2003, 5, 860–867. [Google Scholar] [CrossRef]
- Wu, C.-T.; Chen, W.C.; Chang, Y.H.; Lin, W.Y.; Chen, M.F. The role of PD-L1 in the radiation response and clinical outcome for bladder cancer. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; Shinde-Jadhav, S.; Mansure, J.J.; Alvarez, F.; Connell, T.; Seuntjens, J.; Piccirillo, C.A.; Kassouf, W. The immune mediated role of extracellular HMGB1 in a heterotopic model of bladder cancer radioresistance. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Prack Mc Cormick, B.; Langle, Y.; Belgorosky, D.; Vanzulli, S.; Balarino, N.; Sandes, E.; Eiján, A.M. Flavonoid silybin improves the response to radiotherapy in invasive bladder cancer. J. Cell. Biochem. 2018, 119, 5402–5412. [Google Scholar] [CrossRef] [PubMed]
- Prack Mc Cormick, B.; Belgorosky, D.; Langle, Y.; Balarino, N.; Sandes, E.; Eiján, A.M. Bacillus Calmette-Guerin improves local and systemic response to radiotherapy in invasive bladder cancer. Nitric Oxide Biol. Chem. 2017, 64, 22–30. [Google Scholar] [CrossRef]
- Mu, Y.; Sun, D. Lapatinib, a dual inhibitor of epidermal growth factor receptor (EGFR) and HER-2, enhances radiosensitivity in mouse bladder tumor line-2 (MBT-2) cells in vitro and in vivo. Med. Sci. Monit. 2018, 24, 5811–5819. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Yeh, C.H.; Tzen, K.Y.; Ho, P.Y.; Tuan, T.F.; Pu, Y.S.; Cheng, A.L.; Cheng, J.C.H. Targeting epidermal growth factor receptor/human epidermal growth factor receptor 2 signalling pathway by a dual receptor tyrosine kinase inhibitor afatinib for radiosensitisation in murine bladder carcinoma. Eur. J. Cancer 2013, 49, 1458–1466. [Google Scholar] [CrossRef]
- Flaig, T.W.; Spiess, P.E.; Agarwal, N.; Bangs, R.; Boorjian, S.A.; Buyyounouski, M.K.; Chang, S.; Downs, T.M.; Efstathiou, J.A.; Friedlander, T.; et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 329–354. [Google Scholar] [CrossRef]
- Kyriazis, A.P.; Kyriazis, A.A.; Yagoda, A. Time dependence of the radiation-modifying effect of cis-diamminedichloroplatinum II (cisplatin, DDP) on human urothelial cancer grown in nude mice. Cancer Investig. 1986, 4, 217–222. [Google Scholar] [CrossRef]
- Bedford, P.; Shellard, S.A.; Walker, M.C.; Whelan, R.D.H.; Masters, J.R.W.; Hill, B.T. Differential expression of collateral sensitivity or resistance to cisplatin in human bladder carcinoma cell lines pre-exposedin vitro to either X-irradiation or cisplatin. Int. J. Cancer 1987, 40, 681–686. [Google Scholar] [CrossRef]
- Hoskin, P.J.; Sibtain, A.; Daley, F.M.; Wilson, G.D. GLUT1 and CAIX as intrinsic markers of hypoxia in bladder cancer: Relationship with vascularity and proliferation as predictors of outcome of ARCON. Br. J. Cancer 2003, 89, 1290–1297. [Google Scholar] [CrossRef]
- Caffo, O.; Thompson, C.; De Santis, M.; Kragelj, B.; Hamstra, D.A.; Azria, D.; Fellin, G.; Pappagallo, G.L.; Galligioni, E.; Choudhury, A. Concurrent gemcitabine and radiotherapy for the treatment of muscle-invasive bladder cancer: A pooled individual data analysis of eight phase I–II trials. Radiother. Oncol. 2016, 121, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Fechner, G.; Perabo, F.G.E.; Schmidt, D.H.; Haase, L.; Ludwig, E.; Schueller, H.; Blatter, J.; Müller, S.C.; Albers, P. Preclinical evaluation of a radiosensitizing effect of gemcitabine in p53 mutant and p53 wild type bladder cancer cells. Urology 2003, 61, 468–473. [Google Scholar] [CrossRef]
- Pauwels, B.; Korst, A.E.C.; Pattyn, G.G.O.; Lambrechts, H.A.J.; Van Bockstaele, D.R.; Vermeulen, K.; Lenjou, M.; De Pooter, C.M.J.; Vermorken, J.B.; Lardon, F. Cell cycle effect of gemcitabine and its role in the radiosensitizing mechanism in vitro. Int. J. Radiat. Oncol. Biol. Phys. 2003, 57, 1075–1083. [Google Scholar] [CrossRef]
- Sangar, V.K.; Cowan, R.; Margison, G.P.; Hendry, J.H.; Clarke, N.W. An evaluation of gemcitabines differential radiosensitising effect in related bladder cancer cell lines. Br. J. Cancer 2004, 90, 542–548. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Capes-Davis, A.; Theodosopoulos, G.; Atkin, I.; Drexler, H.G.; Kohara, A.; MacLeod, R.A.F.; Masters, J.R.; Nakamura, Y.; Reid, Y.A.; Reddel, R.R.; et al. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int. J. Cancer 2010, 127, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.; Zhao, H.; Jalali, F.; Al Rashid, S.; Ran, J.; Supiot, S.; Kiltie, A.E.; Bristow, R.G. Targeting homologous recombination using imatinib results in enhanced tumor cell chemosensitivity and radiosensitivity. Mol. Cancer Ther. 2009, 8, 203–213. [Google Scholar] [CrossRef]
- Kerr, M.; Scott, H.E.; Groselj, B.; Stratford, M.R.L.; Karaszi, K.; Sharma, N.L.; Kiltie, A.E. Deoxycytidine kinase expression underpins response to gemcitabine in bladder cancer. Clin. Cancer Res. 2014, 20, 5435–5445. [Google Scholar] [CrossRef]
- Paget, V.; Ben Kacem, M.; Dos Santos, M.; Benadjaoud, M.A.; Soysouvanh, F.; Buard, V.; Georges, T.; Vaurijoux, A.; Gruel, G.; François, A.; et al. Multiparametric radiobiological assays show that variation of X-ray energy strongly impacts relative biological effectiveness: Comparison between 220 kV and 4 MV. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef]
- Ben Kacem, M.; Benadjaoud, M.A.; Dos Santos, M.; Soysouvanh, F.; Buard, V.; Tarlet, G.; Le Guen, B.; François, A.; Guipaud, O.; Milliat, F.; et al. Variation of 4 MV X-ray dose rate strongly impacts biological response both in vitro and in vivo. Sci. Rep. 2020, 10, 7021. [Google Scholar] [CrossRef]
- Contessa, J.N.; Hampton, J.; Lammering, G.; Mikkelsen, R.B.; Dent, P.; Valerie, K.; Schmidt-Ullrich, R.K. Ionizing radiation activates Erb-B receptor dependent Akt and p70 S6 kinase signaling in carcinoma cells. Oncogene 2002, 21, 4032–4041. [Google Scholar] [CrossRef]
- Schmidt-Ullrich, R.K.; Contessa, J.N.; Lammering, G.; Amorino, G.; Lin, P.S. ERBB receptor tyrosine kinases and cellular radiation responses. Oncogene 2003, 22, 5855–5865. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chaux, A.; Cohen, J.S.; Schultz, L.; Albadine, R.; Jadallah, S.; Murphy, K.M.; Sharma, R.; Schoenberg, M.P.; Netto, G.J. High epidermal growth factor receptor immunohistochemical expression in urothelial carcinoma of the bladder is not associated with EGFR mutations in exons 19 and 21: A study using formalin-fixed, paraffin-embedded archival tissues. Hum. Pathol. 2012, 43, 1590–1595. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Escrig, J.L.; Kelly, J.D.; Neal, D.E.; King, S.M.; Davies, B.R. Evaluation of the therapeutic potential of the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib in preclinical models of bladder cancer. Clin. Cancer Res. 2004, 10, 4874–4884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Giannopoulou, A.F.; Velentzas, A.D.; Konstantakou, E.G.; Avgeris, M.; Katarachia, S.A.; Papandreou, N.C.; Kalavros, N.I.; Mpakou, V.E.; Iconomidou, V.; Anastasiadou, E.; et al. Revisiting histone deacetylases in human tumorigenesis: The paradigm of urothelial bladder cancer. Int. J. Mol. Sci. 2019, 20, 1291. [Google Scholar] [CrossRef] [PubMed]
- Grivas, P.; Mortazavi, A.; Picus, J.; Hahn, N.M.; Milowsky, M.I.; Hart, L.L.; Alva, A.; Bellmunt, J.; Pal, S.K.; Bambury, R.M.; et al. Mocetinostat for patients with previously treated, locally advanced/metastatic urothelial carcinoma and inactivating alterations of acetyltransferase genes. Cancer 2019, 125, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-C.; Ho, P.-Y.; Tzen, K.-Y.; Tuan, T.-F.; Liu, W.-L.; Cheng, A.-L.; Pu, Y.-S.; Cheng, J.C.-H. Synergistic Blockade of EGFR and HER2 by New-Generation EGFR Tyrosine Kinase Inhibitor Enhances Radiation Effect in Bladder Cancer Cells. Mol. Cancer Ther. 2015, 14, 810–820. [Google Scholar] [CrossRef]
- Inoue, T.; Terada, N.; Kobayashi, T.; Ogawa, O. Patient-derived xenografts as in vivo models for research in urological malignancies. Nat. Rev. Urol. 2017, 14, 267–283. [Google Scholar] [CrossRef]
- Hedegaard, J.; Lamy, P.; Nordentoft, I.; Algaba, F.; Høyer, S.; Ulhøi, B.P.; Vang, S.; Reinert, T.; Hermann, G.G.; Mogensen, K.; et al. Comprehensive Transcriptional Analysis of Early-Stage Urothelial Carcinoma. Cancer Cell 2016, 30, 27–42. [Google Scholar] [CrossRef]
- Robertson, A.G.; Kim, J.; Al-Ahmadie, H.; Bellmunt, J.; Guo, G.; Cherniack, A.D.; Hinoue, T.; Laird, P.W.; Hoadley, K.A.; Akbani, R.; et al. Comprehensive Molecular Characterization of Muscle-Invasive Bladder Cancer. Cell 2017, 171, 540–556.e25. [Google Scholar] [CrossRef]
- Mahe, M.; Dufour, F.; Neyret-Kahn, H.; Moreno-Vega, A.; Beraud, C.; Shi, M.; Hamaidi, I.; Sanchez-Quiles, V.; Krucker, C.; Dorland-Galliot, M.; et al. An FGFR 3/ MYC positive feedback loop provides new opportunities for targeted therapies in bladder cancers. EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.M.; SenthilKumar, G.; Hu, R.; Goldstein, S.; Ong, I.M.; Miller, M.C.; Brennan, S.R.; Kaushik, S.; Abel, L.; Nickel, K.P.; et al. Fibroblast Growth Factor Receptors as Targets for Radiosensitization in Head and Neck Squamous Cell Carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Dufour, F.; Silina, L.; Neyret-Kahn, H.; Moreno-Vega, A.; Krucker, C.; Karboul, N.; Dorland-Galliot, M.; Maillé, P.; Chapeaublanc, E.; Allory, Y.; et al. TYRO3 as a molecular target for growth inhibition and apoptosis induction in bladder cancer. Br. J. Cancer 2019, 120. [Google Scholar] [CrossRef] [PubMed]
- Tormoen, G.W.; Crittenden, M.R.; Gough, M.J. The TAM family as a therapeutic target in combination with radiation therapy. Emerg. Top. Life Sci. 2017, 1, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, T.A.; Rafat, M.; Castellini, L.; Shehade, H.; Kariolis, M.S.; Hui, A.B.Y.; Stehr, H.; Von Eyben, R.; Jiang, D.; Ellies, L.G.; et al. Reprogramming the immunological microenvironment through radiation and targeting Axl. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Grapin, M.; Richard, C.; Limagne, E.; Boidot, R.; Morgand, V.; Bertaut, A.; Derangere, V.; Laurent, P.A.; Thibaudin, M.; Fumet, J.D.; et al. Optimized fractionated radiotherapy with anti-PD-L1 and anti-TIGIT: A promising new combination. J. Immunother. Cancer 2019, 7. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef]
- Formenti, S.C.; Rudqvist, N.P.; Golden, E.; Cooper, B.; Wennerberg, E.; Lhuillier, C.; Vanpouille-Box, C.; Friedman, K.; Ferrari de Andrade, L.; Wucherpfennig, K.W.; et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat. Med. 2018, 24, 1845–1851. [Google Scholar] [CrossRef]
- Choi, W.; Czerniak, B.; Ochoa, A.; Su, X.; Siefker-Radtke, A.; Dinney, C.; McConkey, D.J. Intrinsic basal and luminal subtypes of muscle-invasive bladder cancer. Nat. Rev. Urol. 2014, 11, 400–410. [Google Scholar] [CrossRef]
- J Southgate; K A Hutton; D F Thomas; L K Trejdosiewicz Normal Human Urothelial Cells in Vitro: Proliferation and Induction of Stratification - PubMed. Lab. Investig. 1994, 71, 583–594.
- Varley, C.L.; Stahlschmidt, J.; Lee, W.-C.; Holder, J.; Diggle, C.; Selby, P.J.; Trejdosiewicz, L.K.; Southgate, J. Role of PPARgamma and EGFR signalling in the urothelial terminal differentiation programme. J. Cell Sci. 2004, 117, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Cross, W.R.; Eardley, I.; Leese, H.J.; Southgate, J. A biomimetic tissue from cultured normal human urothelial cells: Analysis of physiological function. Am. J. Physiol. Ren. Physiol. 2005, 289. [Google Scholar] [CrossRef] [PubMed]
- Jäger, W.; Moskalev, I.; Janssen, C.; Hayashi, T.; Awrey, S.; Gust, K.M.; So, A.I.; Zhang, K.; Fazli, L.; Li, E.; et al. Ultrasound-Guided Intramural Inoculation of Orthotopic Bladder Cancer Xenografts: A Novel High-Precision Approach. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Zhang, Z.T.; Pak, J.; Shapiro, E.; Sun, T.T.; Wu, X.R. Urothelium-specific expression of an oncogene in transgenic mice induced the formation of carcinoma in situ and invasive transitional cell carcinoma. Cancer Res. 1999, 59, 3512–3517. [Google Scholar] [PubMed]
- Vasoncelos-Nobrega, C.; Colaco, A.; Lopes, C.; Oliveira, P.A. BBN as an Urothelial Carcinogen. In Vivo (Brooklyn). 2012, 26, 727–739. [Google Scholar]
- De La Rochere, P.; Guil-Luna, S.; Decaudin, D.; Azar, G.; Sidhu, S.S.; Piaggio, E. Humanized Mice for the Study of Immuno-Oncology. Trends Immunol. 2018, 39, 748–763. [Google Scholar] [CrossRef]
- Shultz, L.D.; Ishikawa, F.; Greiner, D.L. Humanized mice in translational biomedical research. Nat. Rev. Immunol. 2007, 7, 118–130. [Google Scholar] [CrossRef]
- Borcoman, E.; De La Rochere, P.; Richer, W.; Vacher, S.; Chemlali, W.; Krucker, C.; Sirab, N.; Radvanyi, F.; Allory, Y.; Pignot, G.; et al. Inhibition of PI3K pathway increases immune infiltrate in muscle-invasive bladder cancer. Oncoimmunology 2019, 8, e1581556. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]

| Cell Line | Cellosaurus Accession No. [50] | Molecular Subtype [46] | |
|---|---|---|---|
| Human | RT112 | CVCL_1670 | luminal |
| SW780 | CVCL_1728 | ||
| UMUC5 | CVCL_2750 | ||
| UMUC9 | CVCL_2753 | ||
| RT4 1 | CVCL_0036 | ||
| 5637 | CVCL_0126 | basal | |
| 647V | CVCL_1049 | ||
| HT1197 | CVCL_1291 | ||
| HT1376 | CVCL_1292 | ||
| KU19-19 | CVCL_1344 | ||
| UMUC6 | CVCL_2751 | ||
| VMCUB1 | CVCL_1786 | ||
| 253J B-V | CVCL_7937 | non-luminal, non-basal | |
| 639-V | CVCL_1048 | ||
| J82 | CVCL_0359 | ||
| KK47 | CVCL_8253 | ||
| T24 | CVCL_0554 | ||
| TCC-SUP | CVCL_1738 | ||
| UMUC3 | CVCL_1783 | ||
| CAL29 | CVCL_1808 | n/c | |
| NTUB1 | CVCL_RW29 | n/a | |
| OBR | n/a | ||
| SW-800 | CVCL_A684 | ||
| UCRU-BL13 | CVCL_M873 | ||
| UCRU-BL17 | CVCL_M007 | ||
| UCRU-BL28 | CVCL_4904 | ||
| Mouse | MB49 | CVCL_7076 | basal (mouse) 2 |
| MB49-I | CVCL_VL62 | ||
| MBT2 | CVCL_4660 |
| Cell Line | AUC 1 [54] | Molecular Subtype [46] |
|---|---|---|
| HT1376 | 5.228 | basal |
| HT1197 | 4.449 | basal |
| VMCUB1 | 4.412 | basal |
| KMBC2 | 4.126 | luminal |
| TCCSUP | 3.539 | non-luminal/non-basal |
| KU1919 | 3.503 | basal |
| 647V | 3.374 | basal |
| BC3C | 3.362 | basal |
| UMUC1 | 3.346 | luminal |
| SW1710 | 3.309 | n/c |
| UMUC3 | 3.231 | luminal |
| J82 | 3.198 | non-luminal/non-basal |
| RT112 | 3.038 | luminal |
| RT4 2 | 2.935 | luminal |
| UBLC1 | 2.914 | n/c |
| JMSU1 | 2.792 | non-luminal/non-basal |
| 5637 | 2.473 | basal |
| T24 | 2.366 | non-luminal/non-basal |
| SCABER | 1.883 | basal |
| Subtype [46] | Cell Line | IR Regimen | Radiosensitising Agent | Class | Nude Mice Genetic Background (Gender) | Initial Tumour Size (mm3) 1 | Study Follow-Up (Days) 2 | Ref. |
|---|---|---|---|---|---|---|---|---|
| luminal | RT112 | 4 × 5 Gy | Panobinostat (vs. gemcitabine) | HDAC inhibitor | (Unknown strain) (F) | 100 | 10–60 | [55] |
| 2 × 5 Gy | AQ4N (banoxantrone) (vs. cisplatin) | DNA intercalator and Topoisomerase II inhibitor | CBA (F) | 240–280 | 10–60 | [56] | ||
| 1 × 6 Gy | Romidepsin | HDAC inhibitor | CD1 (F) | 50 | 25 | [57] | ||
| 1 × 6 Gy | Low-/soluble high-/insoluble high- and mixed high-fibre diets | Diet | CD1 (F) | 50 | 42 | [58] | ||
| RT4 | 1 × 5 or1 × 15 Gy | Photofrin II | Photosensitiser | (Unknown strain) (F) | 2.6–3.0 | 15 | [59] | |
| 1 × 2 Gy | Caffeine | DNA Damage Response inhibitor | BALB/c (M) | 30–75 | 0 3 | [60] | ||
| SW780 | 2 × 5 Gy | siTUG1 | siRNA | (Unknown strain) (M) | 100 | 21 | [61] | |
| basal | 5637 | 2 × 2 Gy | Sulfoquinovosylacylpropanediol | Synthetic sulfoglycolipid | BALB/c Slc (M) | 100–300 | 33 | [62] |
| Non luminal/non basal | 1 × n/a Gy | shRNF8 | shRNA | BALB/c (M) | 100–150 | 30 | [63] | |
| 1 × 6 Gy | Chloroquine | Other | BALB/c (F) | ∼200 | 25 | [64] | ||
| 1 × 6 Gy | Nanoparticles (chloroquine conjugated) | Nanoparticles | (Unknown strain) (n/a) | 150 | 16 | [65] | ||
| 1 × 6 Gy | LY294002 | TKI | Ncr-nu/n (F) | 300–400 | 40 | [66] | ||
| 1 × 6 Gy | FTI-276 or L744832 | Farnesyltransferase inhibitors | Ncr-nu/n (n/a) | 58 | 80 | [67] | ||
| UMUC3 | 2 × 3 Gy | shHMGB1 | shRNA | (Unknown strain) (F) | n/a | 21 | [68] | |
| 1 × 12 Gy | 17-AAG or 17-DMAG/Trastuzumab/LY294002 | Hsp90 inhibitors/ monoclonal antibody/TKI | BALB/c (M) | 1000 | 12 | [69] | ||
| 2 × 2 Gy | Flutamide/shAR | Antiandrogen/shRNA | NOD-SCID (M) | 30 | 12 | [53] | ||
| J82 | 1 × 5 Gy | Gefitinib (“Iressa”, ZD1839) | TKI | BALB/c (n/a) | 100 | n/a | [70] | |
| n/a | KK47 | 1 × 4 Gy | Ad-RSV-CD+5-FC | A recombinant adenovirus vector | BALB/c (n/a) | n/a | n/a | [71] |
| Cell Line | IR Regimen | Radiosensitising Agent | Class | Mouse Background (Gender) | Initial Tumour Size (mm3) 1 | Study Follow-Up (days) 2 | Ref. |
|---|---|---|---|---|---|---|---|
| MB49 | 1 × 12 Gy | PD-L1 blocking antibody | Immunotherapy | C57BL/6 (F) | 500 | 27 | [72] |
| MB49 | 2 × 5 Gy | Glycyrrhizin | HMGB1 inhibitor | C57BL/6 (M) | Once palpable | 7 | [73] |
| MB49,MB49-I | 6 × 3 Gy | Silybin (Sb) | Flavonoid | C57BL/6J (n/a) | 50 | 30 | [74] |
| MB49-I | 6 × 3 Gy | Bacillus Calmette-Guérin (BCG) | Immunotherapy | C57BL/6J (n/a) | 50 | 21 | [75] |
| MBT-2 | 1 × 15 Gy | Lapatinib | TKI | C3H/HeN (F) | 162 | 21 | [76] |
| MBT-2 | 1 × 15 Gy | Afatinib | TKI | C3H/HeN (F) | 162 | 21 | [77] |
| MBT-2 | 5 × 4 Gy | Cisplatin, doxorubicin hydrochloride (adriamycin), cyclophosphamide | CT | CsH/Hej (n/a) | 6 | 60 | [18] |
| Yoshida et al., 2011 [69] | Williams et al., 2009 [56] | Kyriazis et al., 1986 [79] | Weldon et al., 1979 [18] | |
|---|---|---|---|---|
| Cell lines | UMUC3 (non-luminal, non-basal) | RT112 (luminal) | SW-800 (not classified) | MBT-2 |
| Source/ Dose rate (Gy/min) | X-rays (225 V)/ 0.83 Gy/min | X-rays (230 kV)/ 2 Gy/min | X-rays (250 kV)/ 1.23 Gy/min | X-rays (250 kV)/ n/a |
| IR dose and fractionation | 5 × 2 Gy | 5 × 2 Gy | 1 × 10 Gy | 5 × 4 Gy |
| Cisplatin dose | 3 mg/kg (administered once) | 2 mg/kg (administered once) | 5 mg/kg once on each specified day before or after radiation | 3 mg/kg once a week (3 weeks) |
| Treatment arms | Hsp90 inhibitors (17-AAG or 17-DMAG) Trastuzumab, LY294002 | AQ4N (banoxantrone) | - | Doxorubicin hydrochloride (Adriamycin), cyclophosphamide |
| Normal tissue toxicity | Yes (NHU in vitro) | - | - | - |
| Groselj et al., 2018 [55] | Kerr et al., 2014 [88] | |
|---|---|---|
| Cell lines | - | CALgem |
| Source/ Dose rate (Gy/min) | X-rays (220 kV)/ n/a | gamma (137Cs)/ 1.7 Gy/min |
| IR regimen in vivo | 1× 10, 12, or 14 Gy (acute toxicity) 5 × 5 Gy (late toxicity) | 5 × 2 Gy |
| Gemcitabine dose | single 100 mg/kg injection | single 100 mg/kg injection |
| Normal tissue toxicity | yes (intestinal) | - |
| Class | Name | Target | Cell Line Subtype (According to [46]) | Year | Ref. |
|---|---|---|---|---|---|
| TKI | Gefitinib (ZD1839) | EGFR | J82, non-luminal, non-basal | 2007 | [70] |
| Afatinib, Erlotinib | EGFR/HER2, EGFR | NTUB1, class n/a | 2015 | [98] | |
| PI3K | LY294002 | PI3 kinase | T24, non-luminal, non-basal | 2003 | [66] |
| Epigenetic modifiers | Panobinostat | HDAC (histone deacetylase) | RT112, luminal | 2018 | [55] |
| Romidepsin | HDAC (histone deacetylase) | RT112, luminal | 2020 | [57] | |
| Heat shock protein inhibitors | 17-AAG or 17-DMAG | Hsp90 | UMUC3 non-luminal, non-basal | 2011 | [69] |
| Farnesyltransferase inhibitors | FTI-276 and L744832 | Farnesyltransferase | T24, non-luminal, non-basal | 2000 | [67] |
| Hypoxia | AQ4N | Hypoxia | RT112, luminal | 2009 | [56] |
| Angiogenesis | SQAP | Angiogenesis | 5637, basal | 2016 | [62] |
| Other | Chloroquine | Autophagy | T24, non-luminal, non-basal | 2018 | [64] |
| HSA-MnO2-CQ nanoparticles | Autophagy | T24, non-luminal, non-basal | 2020 | [65] | |
| Ad-RSV-CD+5-FC | - | KK47, non-luminal, non-basal | 2003 | [71] | |
| shRNF8 | DNA Damage Response | T24, non-luminal, non-basal | 2016 | [63] | |
| Caffeine | DNA Damage Response | RT4, luminal | 2015 | [60] | |
| siTUG1 | HMGB1 | SW780, luminal | 2017 | [61] | |
| shHMGB1 | HMGB1 | UMUC3 non-luminal, non-basal | 2016 | [68] | |
| Flutamide/shAR | AR | UMUC3 non-luminal, non-basal | 2018 | [53] | |
| Photofrin II | Angiogenesis | RT4, luminal | 2001 | [59] | |
| Low-fibre, soluble high-fibre, insoluble high-fibre, and mixed soluble/insoluble high-fibre diets | Metabolism | RT112, luminal | 2020 | [58] |
| Class | Name | Target | Cell line (Subtype According to [46]) | Ref. |
|---|---|---|---|---|
| TKI | Afatinib | EGFR/HER2 | MBT-2, mice cell line (basal) | [77] |
| Lapatinib | PDGF-R | MBT-2, mice cell line (basal) | [76] | |
| HMGB1 inhibitor | Glycyrrhizin | HMGB1 | MB49, mice cell line (basal) | [73] |
| Flavonoid | Silybin | MB49, MB49-I, mice cell lines (basal) | [74] | |
| Immune checkpoint inhibitor | Anti-PD-L1 antibody | PD-L1 | MB49, mice cell line (basal) | [72] |
| Non-specific immune stimulator | Bacillus Calmette-Guérin (BCG) | Immune system | MB49-I, mice cell line (basal) | [75] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silina, L.; Maksut, F.; Bernard-Pierrot, I.; Radvanyi, F.; Créhange, G.; Mégnin-Chanet, F.; Verrelle, P. Review of Experimental Studies to Improve Radiotherapy Response in Bladder Cancer: Comments and Perspectives. Cancers 2021, 13, 87. https://doi.org/10.3390/cancers13010087
Silina L, Maksut F, Bernard-Pierrot I, Radvanyi F, Créhange G, Mégnin-Chanet F, Verrelle P. Review of Experimental Studies to Improve Radiotherapy Response in Bladder Cancer: Comments and Perspectives. Cancers. 2021; 13(1):87. https://doi.org/10.3390/cancers13010087
Chicago/Turabian StyleSilina, Linda, Fatlinda Maksut, Isabelle Bernard-Pierrot, François Radvanyi, Gilles Créhange, Frédérique Mégnin-Chanet, and Pierre Verrelle. 2021. "Review of Experimental Studies to Improve Radiotherapy Response in Bladder Cancer: Comments and Perspectives" Cancers 13, no. 1: 87. https://doi.org/10.3390/cancers13010087
APA StyleSilina, L., Maksut, F., Bernard-Pierrot, I., Radvanyi, F., Créhange, G., Mégnin-Chanet, F., & Verrelle, P. (2021). Review of Experimental Studies to Improve Radiotherapy Response in Bladder Cancer: Comments and Perspectives. Cancers, 13(1), 87. https://doi.org/10.3390/cancers13010087






