Human Papillomavirus in Sinonasal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Study Selection
2.3. Statistical Analysis
3. Results
3.1. HPV Prevalence
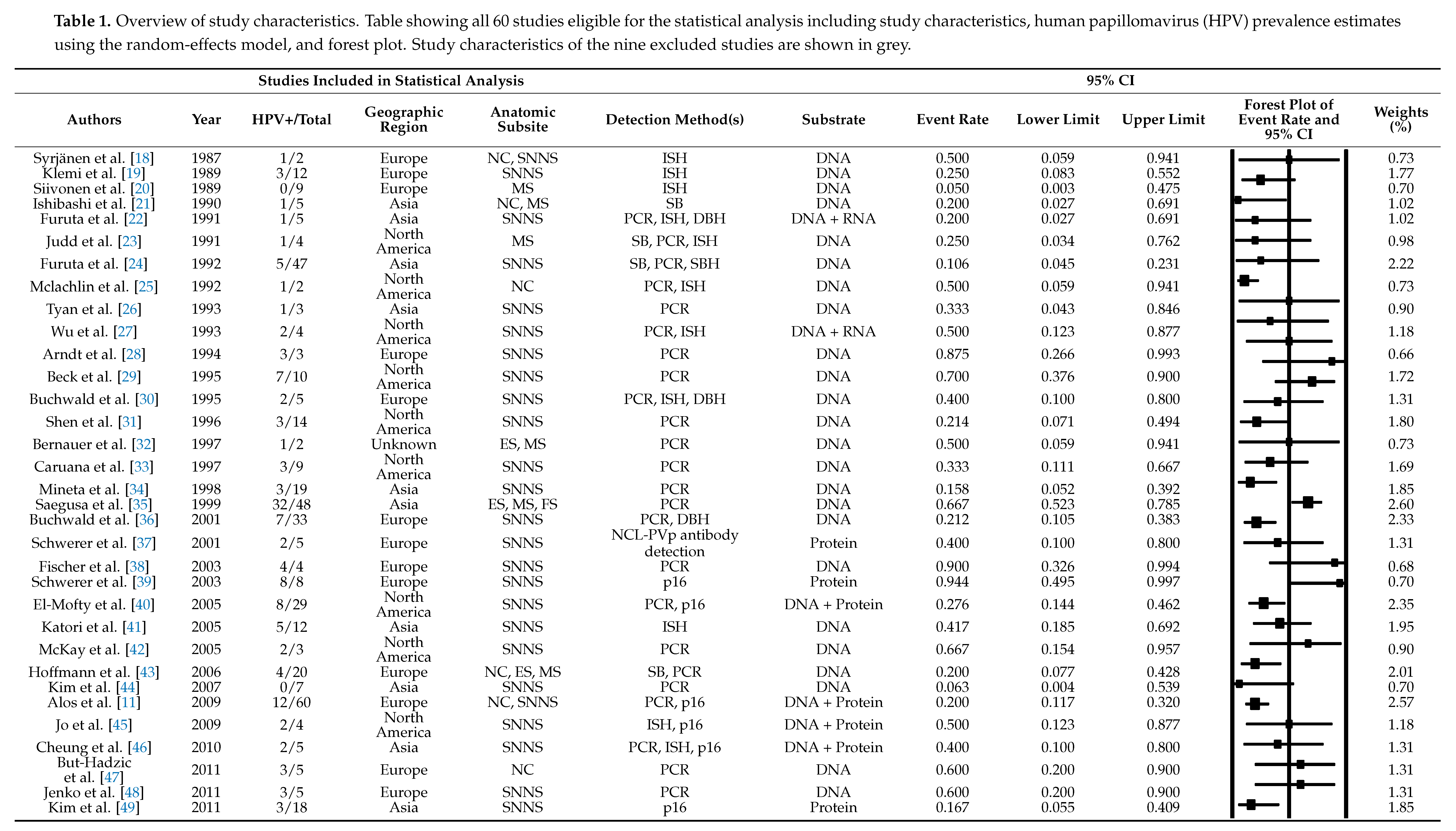
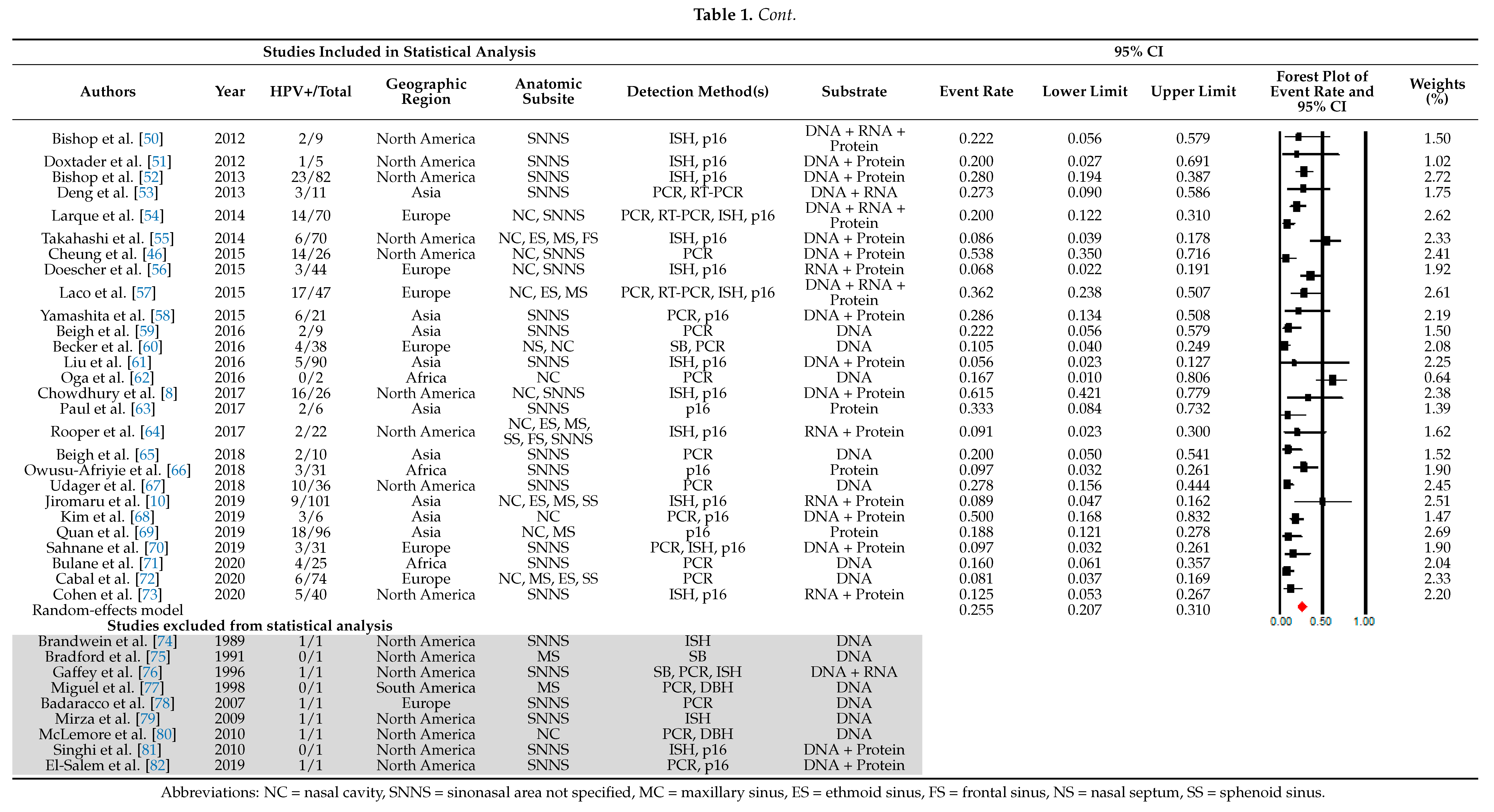
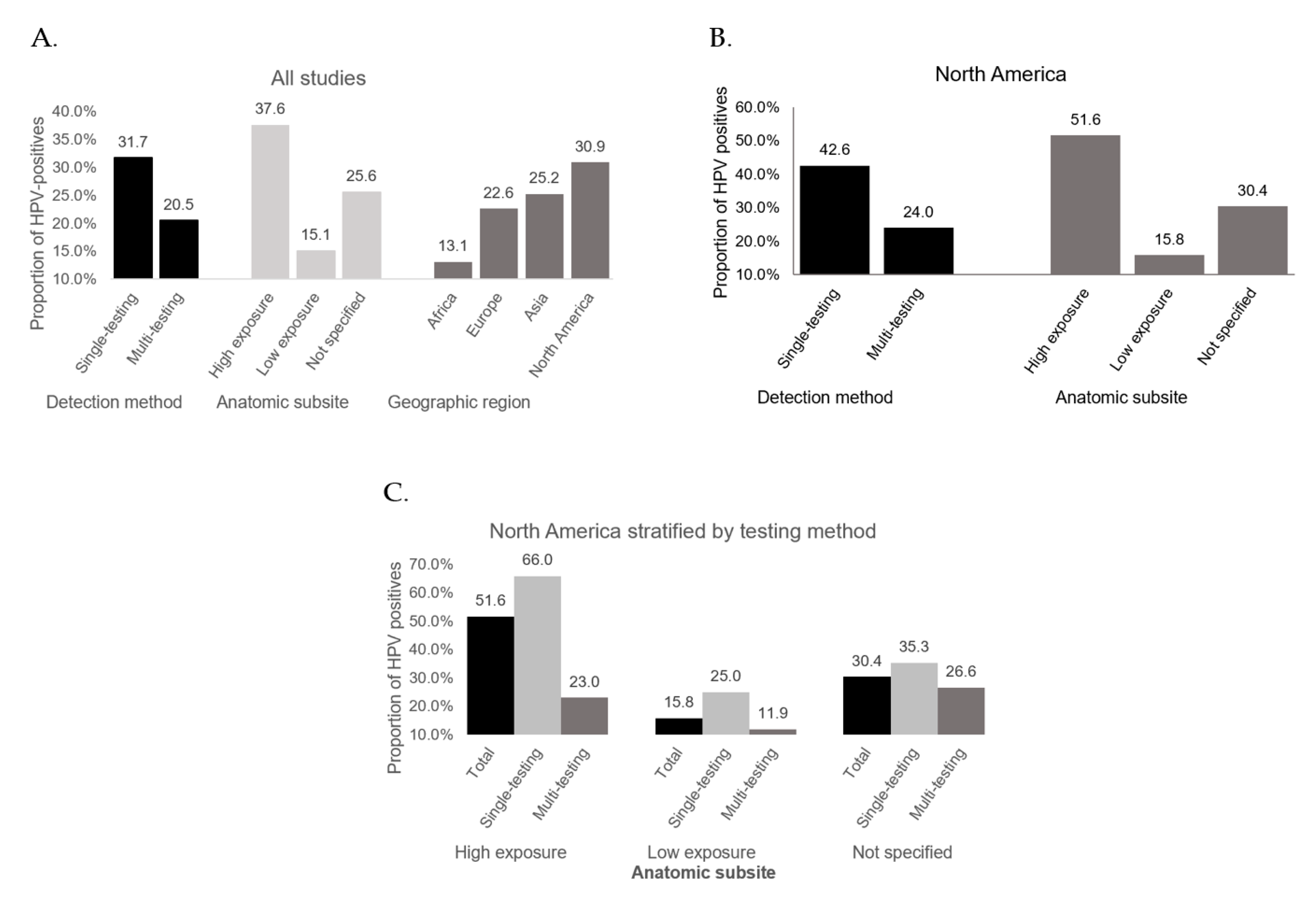
3.2. HPV Detection Method
3.3. Anatomic Subsite
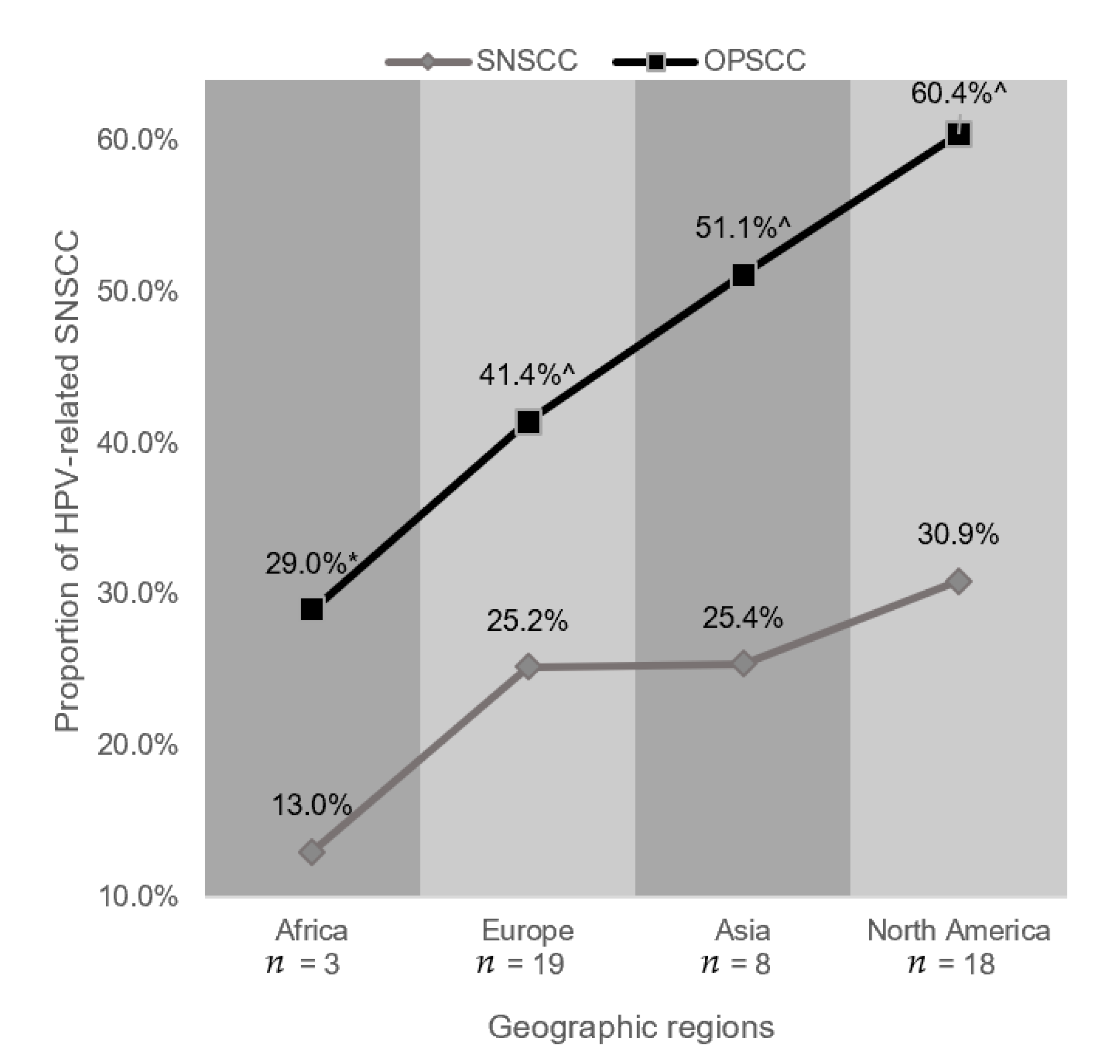
3.4. Geographic Region
3.5. Analysis of Validity, Sensitivity, Data Trends and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gillison, M.L. Human papillomavirus-associated head and neck cancer is a distinct epidemiologic, clinical, and molecular entity. Semin. Oncol. 2004, 31, 744–754. [Google Scholar] [CrossRef]
- Tumban, E. A Current Update on Human Papillomavirus-Associated Head and Neck Cancers. Viruses 2019, 11, 922. [Google Scholar] [CrossRef] [PubMed]
- Ang, K.K.; Harris, J.; Wheeler, R.; Weber, R.; Rosenthal, D.I.; Nguyen-Tân, P.F.; Westra, W.H.; Chung, C.H.; Jordan, R.C.; Lu, C.; et al. Human Papillomavirus and Survival of Patients with Oropharyngeal Cancer. N. Engl. J. Med. 2010, 363, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Faden, D.L.; Ding, F.; Lin, Y.; Zhai, S.; Kuo, F.; Chan, T.A.; Morris, L.G.; Ferris, R.L. APOBEC mutagenesis is tightly linked to the immune landscape and immunotherapy biomarkers in head and neck squamous cell carcinoma. Oral Oncol. 2019, 96, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Faden, D.L.; Thomas, S.; Cantalupo, P.G.; Agrawal, N.; Myers, J.; DeRisi, J. Multi-modality analysis supports APOBEC as a major source of mutations in head and neck squamous cell carcinoma. Oral Oncol. 2017, 74, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Syrjänen, K.J.; Pyrhönen, S.; Syrjänen, S.M. Evidence suggesting human papillomavirus (HPV) etiology for the squamous cell papilloma of the paranasal sinus. Arch. Geschwulstforsch. 1983, 53, 77–82. [Google Scholar]
- Elgart, K.; Faden, D.L. Sinonasal Squamous Cell Carcinoma: Etiology, Pathogenesis, and the Role of Human Papilloma Virus. Curr. Otorhinolaryngol. Rep. 2020, 8, 111–119. [Google Scholar] [CrossRef]
- Chowdhury, N.; Alvi, S.A.; Kimura, K.; Tawfik, O.; Manna, P.; Beahm, D.; Robinson, A.; Kerley, S.; Hoover, L. Outcomes of HPV-related nasal squamous cell carcinoma. Laryngoscope 2017, 127, 1600–1603. [Google Scholar] [CrossRef]
- Kılıç, S.S.; Ma, S.S.K.; Kim, E.S.; Baredes, S.; Mahmoud, O.; Gray, S.T.; Eloy, J.A. Significance of human papillomavirus positivity in sinonasal squamous cell carcinoma. Int. Forum Allergy Rhinol. 2017, 7, 980–989. [Google Scholar] [CrossRef]
- Jiromaru, R.; Yamamoto, H.; Yasumatsu, R.; Hongo, T.; Nozaki, Y.; Hashimoto, K.; Taguchi, K.; Masuda, M.; Nakagawa, T.; Oda, Y. HPV-related Sinonasal Carcinoma: Clinicopathologic Features, Diagnostic Utility of p16 and Rb Immunohistochemistry, and: EGFR: Copy Number Alteration. Am. J. Surg. Pathol. 2019. Publish Ahead of Print. [Google Scholar] [CrossRef]
- Alos, L.L.; Moyano, S.; Nadal, A.; Alobid, I.; Blanch, J.L.; Ayala, E.; Lloveras, B.; Quint, W.; Cardesa, A.; Ordi, J. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer 2009, 115, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.; Higgins, J.; Altman, D. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated July 2019); Higgins, J.P.T., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Cochrane: London, UK, 2019. [Google Scholar]
- Cochran, W.G. The Combination of Estimates from Different Experiments. Biometrics 1954, 10, 101–129. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Duval, S.; Tweedie, R. A Nonparametric "Trim and Fill" Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar]
- Syrjänen, S.; Happonen, R.-P.; Virolainen, E.; Siivonen, L.; Syrjänen, K. Detection of human papillomavirus (HPV) structural antigens and DNA types in inverted papillomas and squamous cell carcinomas of the nasal cavities and paranasal sinuses. Acta Oto-Laryngol. 1987, 104, 334–341. [Google Scholar] [CrossRef]
- Klemi, P.J.; Joensuu, H.; Siivonen, L.; Virolainen, E.; Syrjänen, S.; Syrjänen, K. Association of DNA Aneuploidy with Human Papillomavirus-Induced Malignant Transformation of Sinonasal Transitional Papillomas. Otolaryngol. Neck Surg. 1989, 100, 563–567. [Google Scholar] [CrossRef]
- Siivonen, L.; Virolainen, E. Transitional Papilloma of the Nasal Cavity and Paranasal Sinuses. ORL 1989, 51, 262–267. [Google Scholar] [CrossRef]
- Ishibashi, T.; Matsushima, S.; Tsunokawa, Y.; Asai, M.; Nomura, Y.; Sugimura, T.; Terada, M. Human Papillomavirus DNA in Squamous Cell Carcinoma of the Upper Aerodigestive Tract. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 294–298. [Google Scholar] [CrossRef]
- Furuta, Y.; Shinohara, T.; Sano, K.; Nagashima, K.; Inoue, K.; Tanaka, K.; Inuyama, Y. Molecular Pathologic Study of Human Papillomavirus Infection in Inverted Papilloma and Squamous Cell Carcinoma of the Nasal Cavities and Paranasal Sinuses. Laryngoscope 1991, 101, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Judd, R.; Zaki, S.R.; Coffield, L.M.; Evatt, B.L. Human papillomavirus type 6 detected by the polymerase chain reaction in invasive sinonasal papillary squamous cell carcinoma. Arch. Pathol. Lab. Med. 1991, 115, 1150–1153. [Google Scholar] [PubMed]
- Furuta, Y.; Takasu, T.; Asai, T.; Shinohara, T.; Sawa, H.; Nagashima, K.; Inuyama, Y. Detection of human papillomavirus DNA in carcinomas of the nasal cavities and paranasal sinuses by polymerase chain reaction. Cancer 1992, 69, 353–357. [Google Scholar] [CrossRef]
- McLachlin, C.M.; A Kandel, R.; Colgan, T.J.; Swanson, D.B.; Witterick, I.J.; Ngan, B.Y. Prevalence of human papillomavirus in sinonasal papillomas: A study using polymerase chain reaction and in situ hybridization. Mod. Pathol. 1992, 5, 406–409. [Google Scholar] [PubMed]
- Tyan, Y.S.; Liu, S.T.; Ong, W.R.; Chen, M.L.; Shu, C.H.; Chang, Y.S. Detection of Epstein-Barr virus and human papillomavirus in head and neck tumors. J. Clin. Microbiol. 1993, 31, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.C.; Trujillo, J.M.; Kashima, H.K.; Mounts, P. Association of human papillomavirus with nasal neoplasia. Lancet 1993, 341, 522–524. [Google Scholar] [CrossRef]
- Arndt, O.; Nottelmann, K.; Brock, J.; Neumann, O.G. Inverted papilloma and its association with human papillomavirus (HPV). A study with polymerase chain reaction (PCR). HNO 1994, 42, 670–676. [Google Scholar]
- Beck, J.C.; McCLATCHEY, K.D.; Lesperance, M.M.; Esclamado, R.M.; Carey, T.E.; Bradford, C.R. Presence of Human papillomavirus types important in progression of inverted papilloma. Otolaryngol. Head Neck Surg. 1995, 113, 558–563. [Google Scholar]
- Buchwald, C.; Franzmann, M.-B.; Jacobsen, G.K.; Lindeberg, H. Human papillomavirus (HPV) in sinonasal papillomas: A study of 78 cases using in situ hybridization and polymerase chain reaction. Laryngoscope 1995, 105, 66–71. [Google Scholar] [CrossRef]
- Shen, J.; E Tate, J.; Crum, C.P.; Goodman, M.L. Prevalence of human papillomaviruses (HPV) in benign and malignant tumors of the upper respiratory tract. Mod. Pathol. 1996, 9, 15–20. [Google Scholar]
- Bernauer, H.S.; Welkoborsky, H.-J.; Tilling, A.; Amedee, R.G.; Mann, W.J. Inverted Papillomas of the Paranasal Sinuses and the Nasal Cavity: DNA Indices and HPV Infection. Am. J. Rhinol. 1997, 11, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Caruana, S.M.; Zwiebel, N.; Cocker, R.; A McCormick, S.; Eberle, R.C.; Lazarus, P. p53 alteration and human papilloma virus infection in paranasal sinus cancer. Cancer 1997, 79, 1320–1328. [Google Scholar] [CrossRef]
- Mineta, H.; Ogino, T.; Amano, H.M.; Ohkawa, Y.; Araki, K.; Takebayashi, S.; Miura, K. Human papilloma virus (HPV) type 16 and 18 detected in head and neck squamous cell carcinoma. Anticancer. Res. 1999, 18, 4765–4768. [Google Scholar]
- Saegusa, M.; Nitta, H.; Hashimura, M.; Okayasu, I. Down-regulation of p27Kip1 expression is correlated with increased cell proliferation but not expression of p21waf1 and p53, and human papillomavirus infection in benign and malignant tumours of sinonasal regions. Histopathology 1999, 35, 55–64. [Google Scholar] [CrossRef]
- Buchwald, C.; Lindeberg, H.; Pedersen, B.L.; Franzmann, M.B. Human Papilloma Virus and p53 Expression in Carcinomas Associated With Sinonasal Papillomas: A Danish Epidemiological Study 1980–1998. Laryngoscope 2001, 111, 1104–1110. [Google Scholar] [CrossRef]
- Schwerer, M.J.; Sailer, A.; Kraft, K.; Baczako, K.; Maier, H. Patterns of p21waf1/cip1 expression in non-papillomatous nasal mucosa, endophytic sinonasal papillomas, and associated carcinomas. J. Clin. Pathol. 2001, 54, 871–876. [Google Scholar] [CrossRef][Green Version]
- Fischer, M.; Von Winterfeld, F. Evaluation and application of a broad-spectrum polymerase chain reaction assay for human papillomaviruses in the screening of squamous cell tumours of the head and neck. Acta Oto-Laryngologica 2003, 123, 752–758. [Google Scholar] [CrossRef]
- Schwerer, M.J.; Sailer, A.; Kraft, K.; Baczako, K.; Maier, H. Expression of retinoblastoma gene product in respiratory epithelium and sinonasal neoplasms: Relationship with p16 and cyclin D1 expression. Histol. Histopathol. 2003, 18, 143–151. [Google Scholar]
- El-Mofty, S.K.; Lu, D.W. Prevalence of High-Risk Human Papillomavirus DNA in Nonkeratinizing (Cylindrical Cell) Carcinoma of the Sinonasal Tract: A Distinct Clinicopathologic and Molecular Disease Entity. Am. J. Surg. Pathol. 2005, 29, 1367–1372. [Google Scholar] [CrossRef]
- Katori, H.; Nozawa, A.; Tsukuda, M. Markers of malignant transformation of sinonasal inverted papilloma. Eur. J. Surg. Oncol. 2005, 31, 905–911. [Google Scholar] [CrossRef]
- McKay, S.P.; Grégoire, L.; Lonardo, F.; Reidy, P.; Mathog, R.H.; Lancaster, W.D. Human Papillomavirus (HPV) Transcripts in Malignant Inverted Papilloma are from Integrated HPV DNA. Laryngoscope 2005, 115, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Klose, N.; Gottschlich, S.; Görögh, T.; Fazel, A.; Lohrey, C.; Rittgen, W.; Ambrosch, P.; Schwarz, E.; Kahn, T. Detection of human papillomavirus DNA in benign and malignant sinonasal neoplasms. Cancer Lett. 2006, 239, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Yoon, J.-K.; Citardi, M.J.; Batra, P.S.; Roh, H.-J. The Prevalence of Human Papilloma virus Infection in Sinonasal Inverted Papilloma Specimens Classified by Histological Grade. Am. J. Rhinol. 2007, 21, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Jo, V.Y.; Mills, S.E.; Stoler, M.H.; Stelow, E.B. Papillary Squamous Cell Carcinoma of the Head and Neck: Frequent Association With Human Papillomavirus Infection and Invasive Carcinoma. Am. J. Surg. Pathol. 2009, 33, 1720–1724. [Google Scholar] [CrossRef]
- Cheung, F.M.; Lau, T.W.; Cheung, L.K.; Li, A.S.; Chow, S.K.; Lo, A.W. Schneiderian Papillomas and Carcinomas: A Retrospective Study with Special Reference to p53 and p16 tumor suppressor gene expression and association with HPV. Ear Nose Throat J. 2010, 89, E5–E12. [Google Scholar] [CrossRef]
- But-Hadzic, J.; Jenko, K.; Poljak, M.; Kocjan, B.J.; Gale, N.; Strojan, P. Sinonasal inverted papilloma associated with squamous cell carcinoma. Radiol. Oncol. 2011, 45, 267. [Google Scholar] [CrossRef]
- Jenko, K.; Kocjan, B.J.; Zidar, N.; Poljak, M.; Strojan, P.; Žargi, M.; Blatnik, O.; Gale, N. In Inverted Papillomas HPV more likely represents incidental colonization than an etiological factor. Virchows Archiv 2011, 459, 529. [Google Scholar] [CrossRef]
- Kim, S.-G.; Lee, O.-Y.; Choi, J.W.; Park, Y.-H.; Kim, Y.M.; Yeo, M.-K.; Kim, J.M.; Rha, K.-S. Pattern of Expression of Cell Cycle–related Proteins in Malignant Transformation of Sinonasal Inverted Papilloma. Am. J. Rhinol. Allergy 2011, 25, 75–81. [Google Scholar] [CrossRef]
- Bishop, J.A.; Ma, X.-J.; Wang, H.; Luo, Y.; Illei, P.B.; Begum, S.; Taube, J.M.; Koch, W.M.; Westra, W.H. Detection of Transcriptionally Active High-risk HPV in Patients With Head and Neck Squamous Cell Carcinoma as Visualized by a Novel E6/E7 mRNA In Situ Hybridization Method. Am. J. Surg. Pathol. 2012, 36, 1874–1882. [Google Scholar] [CrossRef]
- Doxtader, E.E.; Katzenstein, A.-L.A. The relationship between p16 expression and high-risk human papillomavirus infection in squamous cell carcinomas from sites other than uterine cervix: A study of 137 cases. Hum. Pathol. 2012, 43, 327–332. [Google Scholar] [CrossRef]
- Bishop, J.A.; Guo, T.W.; Smith, D.F.; Wang, H.; Ogawa, T.; Pai, S.I.; Westra, W.H. Human Papillomavirus-related Carcinomas of the Sinonasal Tract. Am. J. Surg. Pathol. 2013, 37, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Hasegawa, M.; Kiyuna, A.; Matayoshi, S.; Uehara, T.; Agena, S.; Yamashita, Y.; Ogawa, K.; Maeda, H.; Suzuki, M. Viral load, physical status, andE6/E7mRNA expression of human papillomavirus in head and neck squamous cell carcinoma. Head Neck 2012, 35, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Larque, A.B.; Hakim, S.; Ordi, J.; Nadal, A.; Diaz, A.; Del Pino, M.; Marimon, L.; Alobid, I.; Cardesa, A.; Alos, L. High-risk human papillomavirus is transcriptionally active in a subset of sinonasal squamous cell carcinomas. Mod. Pathol. 2013, 27, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Bell, D.; Agarwal, G.; Roberts, D.; Xie, T.-X.; El-Naggar, A.; Myers, J.N.; Hanna, E.Y. Comprehensive assessment of prognostic markers for sinonasal squamous cell carcinoma. Head Neck 2014, 36, 1094–1102. [Google Scholar] [CrossRef]
- Doescher, J.; Piontek, G.; Wirth, M.; Bettstetter, M.; Schlegel, J.; Haller, B.; Brockhoff, G.; Reiter, R.; Pickhard, A.C. Epstein-Barr virus infection is strictly associated with the metastatic spread of sinonasal squamous-cell carcinomas. Oral Oncol. 2015, 51, 929–934. [Google Scholar] [CrossRef]
- Laco, J.; Sieglová, K.; Vošmiková, H.; Dundr, P.; Němejcová, K.; Michálek, J.; Čelakovský, P.; Chrobok, V.; Mottl, R.; Mottlová, A.; et al. The presence of high-risk human papillomavirus (HPV) E6/E7 mRNA transcripts in a subset of sinonasal carcinomas is evidence of involvement of HPV in its etiopathogenesis. Virchows Archiv 2015, 467, 405–415. [Google Scholar] [CrossRef]
- Yamashita, Y.; Hasegawa, M.; Deng, Z.; Maeda, H.; Kondo, S.; Kyuna, A.; Matayoshi, S.; Agena, S.; Uehara, T.; Kouzaki, H.; et al. Human papillomavirus infection and immunohistochemical expression of cell cycle proteins pRb, p53, and p16INK4a in sinonasal diseases. Infect. Agents Cancer 2015, 10, 23. [Google Scholar] [CrossRef]
- Ambreen, B.; Reyaz, T.A.; Sheikh, J.; Imtiyaz, H.; Summyia, F.; Ruby, R. Histopathological study of lesions of nose and paranasal sinuses and association of Human Papilloma Virus (HPV) with sinonasal papillomas and squamous cell carcinoma. Int. J. Med. Res. Health Sci. 2016, 5, 7–16. [Google Scholar]
- Becker, C.; Kayser, G.; Pfeiffer, J. Squamous cell cancer of the nasal cavity: New insights and implications for diagnosis and treatment. Head Neck 2016, 38, E2112–E2117. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.; Zhang, Y.; Xing, L.; Liu, H.G. Human papillomavirus-related squamous cell carcinomas of the oropharynx and sinonasal tract in 156 Chinese patients. Int. J. Clin. Exp. Pathol. 2016, 9, 1839–1848. [Google Scholar]
- Oga, E.A.; Schumaker, L.M.; Alabi, B.S.; Obaseki, D.; Umana, A.; Bassey, I.-A.; Ebughe, G.; Oluwole, O.; Akeredolu, T.; Adebamowo, S.N.; et al. Paucity of HPV-Related Head and Neck Cancers (HNC) in Nigeria. PLoS ONE 2016, 11, e0152828. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.; Ray, S.; Sengupta, M.; Sengupta, A. Spectrum of Sinonasal Lesions with Expression of p16 and Ki-67 in Premalignant Lesions and Squamous Cell Carcinomas. J. Clin. Diagn. Res. 2017, 11, EC05–EC08. [Google Scholar] [CrossRef]
- Rooper, L.M.; Bishop, J.A.; Westra, W.H. Transcriptionally Active High-Risk Human Papillomavirus is Not a Common Etiologic Agent in the Malignant Transformation of Inverted Schneiderian Papillomas. Head Neck Pathol. 2017, 11, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Beigh, A.; Rashi, R.; Junaid, S.; Khuroo, M.S.; Farook, S. Human Papilloma Virus (HPV) in Sinonasal Papillomas and Squamous Cell Carcinomas: A PCR-based Study of 60 cases. Gulf J. Oncol. 2018, 1, 37–42. [Google Scholar]
- Owusu-Afriyie, O.; Owiredu, W.K.B.A.; Owusu-Danquah, K.; Larsen-Reindorf, R.; Donkor, P.; Acheampong, E.; Quayson, S.E. Expression of immunohistochemical markers in non-oropharyngeal head and neck squamous cell carcinoma in Ghana. PLoS ONE 2018, 13, e0202790. [Google Scholar]
- Udager, A.M.; McHugh, J.; Goudsmit, C.; Weigelin, H.; Lim, M.; Elenitoba-Johnson, K.; Betz, B.; Carey, T.; Brown, N. Human papillomavirus (HPV) and somatic EGFR mutations are essential, mutually exclusive oncogenic mechanisms for inverted sinonasal papillomas and associated sinonasal squamous cell carcinomas. Ann. Oncol. 2018, 29, 466–471. [Google Scholar] [CrossRef]
- Kim, T.; Jung, S.-H.; Kim, S.-K.; Kwon, H.J. P16 expression and its association with PD-L1 expression and FOXP3-positive tumor infiltrating lymphocytes in head and neck squamous cell carcinoma. Mol. Cell. Toxicol. 2019, 15, 137–143. [Google Scholar] [CrossRef]
- Quan, H.; Yan, L.; Wang, S.; Wang, S.Z. Clinical relevance and significance of programmed death-ligand 1 expression, tumor-infiltrating lymphocytes, and p16 status in sinonasal squamous cell carcinoma. Cancer Manag. Res. 2019, 11, 4335–4345. [Google Scholar] [CrossRef]
- Sahnane, N.; Ottini, G.; Turri-Zanoni, M.; Furlan, D.; Battaglia, P.; Karligkiotis, A.; Albeni, C.; Cerutti, R.; Mura, E.; Chiaravalli, A.M.; et al. Comprehensive analysis of HPV infection, EGFR exon 20 mutations and LINE1 hypomethylation as risk factors for malignant transformation of sinonasal-inverted papilloma to squamous cell carcinoma. Int. J. Cancer 2019, 144, 1313–1320. [Google Scholar] [CrossRef]
- Bulane, A.; Goedhals, D.; Seedat, R.Y.; Goedhals, J.; Burt, F. Human papillomavirus DNA in head and neck squamous cell carcinomas in the Free State, South Africa. J. Med Virol. 2020, 92, 227–233. [Google Scholar] [CrossRef]
- Cabal, V.; Menendez, M.; Vivanco, B.; Potes-Ares, S.; Riobello, C.; Suarez-Fernandez, L.; Garcia-Marin, R.; Blanco-Lorenzo, V.; Lopez, F.; Alvarez-Marcos, C.; et al. EGFR mutation and HPV infection in sinonasal inverted papilloma and squamous cell carcinoma. Rhinology 2020, 58, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Bs, C.C.; Menaker, S.; Martinez-Duarte, E.; Gomez, C.; Lo, K.; Kerr, D.; Franzmann, E.; Leibowitz, J.; Sargi, Z. P16 and human papillomavirus in sinonasal squamous cell carcinoma. Head Neck 2020, 42, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Brandwein, M.; Steinberg, B.; Thung, S.; Biller, H.; Dilorenzo, T.; Galli, R. Human papillomavirus 6/11 and 16/18 in Schneiderian inverted papillomas. In situ hybridization with human papillomavirus RNA probes. Cancer 1989, 63, 1708–1713. [Google Scholar] [CrossRef]
- Bradford, C.R.; Zacks, S.E.; Androphy, E.J.; Gregoire, L.; Lancaster, W.D.; Carey, T.E. Human Papillomavirus DNA Sequences in Cell Lines Derived from Head and Neck Squamous Cell Carcinomas. Otolaryngol. Neck Surg. 1991, 104, 303–310. [Google Scholar] [CrossRef]
- Gaffey, M.J.; Frierson, J.H.F.; Weiss, L.M.; Barber, M.C.M.; Baber, B.G.B.; Stoler, M.H. Human Papillomavirus and Epstein-Barr Virus in Sinonasal Schneiderian Papillomas:An In Situ Hybridization and Polymerase Chain Reaction Study. Am. J. Clin. Pathol. 1996, 106, 475–482. [Google Scholar] [CrossRef]
- Miguel, R.E.V.; Villa, L.L.; Cordeiro, A.C.; Prado, J.C.M.; Sobrinho, J.S.P.; Kowalski, L.P. Low prevalence of human papillomavirus in a geographic region with a high incidence of head and neck cancer. Am. J. Surg. 1998, 176, 428–429. [Google Scholar] [CrossRef]
- Badaracco, G.; Rizzo, C.; Mafera, B.; Pichi, B.; Giannarelli, D.; Rahimi, S.; Vigili, M.G.; Venuti, A. Molecular analyses and prognostic relevance of HPV in head and neck tumours. Oncol. Rep. 2007, 17, 931–939. [Google Scholar] [CrossRef]
- Mirza, N.; Montone, K.; Sato, Y.; Kroger, H.; Kennedy, D.W. Identification of p53 and Human Papilloma Virus in Schneiderian Papillomas. Laryngoscope 1998, 108, 497–501. [Google Scholar] [CrossRef]
- McLemore, M.S.; Haigentz, M., Jr.; Smith, R.V.; Nuovo, G.J.; Alos, L.; Cardesa, A.; Brandwein-Gensler, M. Head and Neck Squamous Cell Carcinomas in HIV-Positive Patients: A Preliminary Investigation of Viral Associations. Head Neck Pathol. 2010, 4, 97–105. [Google Scholar] [CrossRef][Green Version]
- Singhi, A.D.; Westra, W.H. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer 2010, 116, 2166–2173. [Google Scholar] [CrossRef]
- El-Salem, F.; Mansour, M.; Gitman, M.; Miles, B.; Posner, M.; Bakst, R.; Genden, E.; Westra, W. Real-time PCR HPV genotyping in fine needle aspirations of metastatic head and neck squamous cell carcinoma: Exposing the limitations of conventional p16 immunostaining. Oral Oncol. 2019, 90, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Ba, J.R.O.; Lieberman, S.M.; Tam, M.M.; Liu, C.Z.; Oliver, J.R.; Hu, K.S.; Morris, L.G.T.; Givi, B. Human papillomavirus and survival of patients with sinonasal squamous cell carcinoma. Cancer 2020, 126, 1413–1423. [Google Scholar]
- Jalouli, J.; Ibrahim, S.O.; Sapkota, D.; Jalouli, M.M.; Vasstrand, E.N.; Hirsch, J.-M.; Larsson, P.-A. Presence of human papilloma virus, herpes simplex virus and Epstein-Barr virus DNA in oral biopsies from Sudanese patients with regard to toombak use. J. Oral Pathol. Med. 2010, 39, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Jalouli, J.; Jalouli, M.M.; Sapkota, D.; O Ibrahim, S.; Larsson, P.-A.; Sand, L. Human papilloma virus, herpes simplex virus and epstein barr virus in oral squamous cell carcinoma from eight different countries. Anticancer. Res. 2012, 32, 571–580. [Google Scholar]
- Ndiaye, C.; Mena, M.; Alemany, L.; Arbyn, M.; Castellsagué, X.; Laporte, L.; Bosch, F.; De Sanjosé, S.; Trottier, H. HPV DNA, E6/E7 mRNA, and p16INK4a detection in head and neck cancers: A systematic review and meta-analysis. Lancet Oncol. 2014, 15, 1319–1331. [Google Scholar] [CrossRef]
- Amin, M.B.; Edge, S.B. American Joint Committee on C: AJCC Cancer Staging Manual; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Faden, D.L.; Arron, S.T.; Heaton, C.M.; DeRisi, J.L.; South, A.P.; Wang, S.J. Targeted next-generation sequencing of TP53 in oral tongue carcinoma from non-smokers. J. Otolaryngol. Head Neck Surg. 2016, 45, 47. [Google Scholar] [CrossRef]
- Li, R.; Faden, D.L.; Fakhry, C.; Langelier, C.; Jiao, Y.; Wang, Y.; Wilkerson, M.D.; Pedamallu, C.S.; Old, M.; Lang, J.; et al. Clinical, genomic, and metagenomic characterization of oral tongue squamous cell carcinoma in patients who do not smoke. Head Neck 2014, 37, 1642–1649. [Google Scholar] [CrossRef]
- Pickering, C.R.; Zhang, J.; Neskey, D.M.; Zhao, M.; Jasser, S.A.; Wang, J.; Ward, A.; Tsai, C.J.; Alves, M.V.O.; Zhou, J.H.; et al. Squamous Cell Carcinoma of the Oral Tongue in Young Non-Smokers Is Genomically Similar to Tumors in Older Smokers. Clin. Cancer Res. 2014, 20, 3842–3848. [Google Scholar] [CrossRef]
- TCGA. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef]
- Turner, J.H.; Reh, D.D. Incidence and survival in patients with sinonasal cancer: A historical analysis of population-based data. Head Neck 2012, 34, 877–885. [Google Scholar] [CrossRef]
- Arnold, A.; Ziglinas, P.; Ochs, K.; Alter, N.; Geretschläger, A.; Lädrach, K.; Zbären, P.; Caversaccio, M. Therapy options and long-term results of sinonasal malignancies. Oral Oncol. 2012, 48, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, S.; Khan, M.N.; Patel, N.R.; Bs, S.Y.; Baredes, S.; Eloy, J.A. Epidemiology of sinonasal squamous cell carcinoma: A comprehensive analysis of 4994 patients. Laryngoscope 2014, 124, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Youlden, D.R.; Cramb, S.; Peters, S.; Porceddu, S.V.; Moller, H.; Fritschi, L.; Baade, P.D. International comparisons of the incidence and mortality of sinonasal cancer. Cancer Epidemiol. 2013, 37, 770–779. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Li, Y.; Kuan, E.C.; Tajudeen, B.A.; Batra, P.S. Prognostic Factors in Paranasal Sinus Squamous Cell Carcinoma and Adenocarcinoma: A SEER Database Analysis. J. Neurol. Surg. Part B Skull Base 2019, 80, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, B.S.; Stegman, L.D.; Zelefsky, M.J.; Rosenzweig, K.E.; Wolden, S.L.; Patel, S.G.; Shah, J.P.; Kraus, D.H.; Lee, N. Treatment of nasal cavity and paranasal sinus cancer with modern radiotherapy techniques in the postoperative setting—the MSKCC experience. Int. J. Radiat. Oncol. 2007, 67, 691–702. [Google Scholar] [CrossRef]
- Syrjänen, K.J.; Pyrhönen, S.; Syrjänen, S.M.; A Lamberg, M. Immunohistochemical demonstration of human papilloma virus (HPV) antigens in oral squamous cell lesions. Br. J. Oral Surg. 1983, 21, 147–153. [Google Scholar] [CrossRef]
- Chung, C.H.; Guthrie, V.B.; Masica, D.L.; Tokheim, C.; Kang, H.; Richmon, J.; Agrawal, N.; Fakhry, C.; Quon, H.; Subramaniam, R.M.; et al. Genomic alterations in head and neck squamous cell carcinoma determined by cancer gene-targeted sequencing. Ann. Oncol. 2015, 26, 1216–1223. [Google Scholar] [CrossRef]
- Randén-Brady, R.; Carpén, T.; Jouhi, L.; Syrjänen, S.; Haglund, C.; Tarkkanen, J.; Remes, S.; Mäkitie, A.; Mattila, P.S.; Silén, S.; et al. In situ hybridization for high-risk HPV E6/E7 mRNA is a superior method for detecting transcriptionally active HPV in oropharyngeal cancer. Hum. Pathol. 2019, 90, 97–105. [Google Scholar] [CrossRef]
- Gao, G.; Chernock, R.D.; Gay, H.A.; Thorstad, W.L.; Zhang, T.R.; Wang, H.; Ma, X.-J.; Luo, Y.; Lewis, J.S., Jr.; Wang, X. A novel RT-PCR method for quantification of human papillomavirus transcripts in archived tissues and its application in oropharyngeal cancer prognosis. Int. J. Cancer 2012, 132, 882–890. [Google Scholar] [CrossRef]
- Mills, A.M.; Dirks, D.C.; Poulter, M.D.; Mills, S.E.; Stoler, M.H. HR-HPV E6/E7 mRNA In Situ Hybridization: Validation Against PCR, DNA In Situ Hybridization, and p16 Immunohistochemistry in 102 Samples of Cervical, Vulvar, Anal, and Head and Neck Neoplasia. Am. J. Surg. Pathol. 2017, 41, 607–615. [Google Scholar] [CrossRef]
- Syrjänen, K.; Syrjänen, S. Detection of human papillomavirus in sinonasal carcinoma: Systematic review and meta-analysis. Hum. Pathol. 2013, 44, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Dutta, R.; Ba, P.M.D.; Svider, P.F.; Liu, J.K.; Baredes, S.; Eloy, J.A. Sinonasal malignancies: A population-based analysis of site-specific incidence and survival. Laryngoscope 2015, 125, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Robin, T.P.; Jones, B.L.; Ba, O.M.G.; Phan, A.; Abbott, D.; McDermott, J.D.; Goddard, J.A.; Raben, D.; Lanning, R.M.; Karam, S.D. A comprehensive comparative analysis of treatment modalities for sinonasal malignancies. Cancer 2017, 123, 3040–3049. [Google Scholar] [CrossRef] [PubMed]
- Mehrad, M.; Stelow, E.B.; Bishop, J.A.; Wang, X.; Haynes, W.; Oliver, D.; Chernock, R.D.; Lewis, J.S. Transcriptionally Active HPV and Targetable EGFR Mutations in Sinonasal Inverted Papilloma: An Association Between Low-risk HPV, Condylomatous Morphology, and Cancer Risk? Am. J. Surg Pathol. 2020, 44, 340–346. [Google Scholar] [CrossRef]
- Thompson, L.D.R. HPV-Related Multiphenotypic Sinonasal Carcinoma. Ear Nose Throat J. 2020, 99, 94–95. [Google Scholar] [CrossRef]
- Bishop, J.A.; Andreasen, S.; Hang, J.F.; Bullock, M.J.; Chen, T.Y.; Franchi, A.; Garcia, J.J.; Gnepp, D.R.; Gomez-Fernandez, C.R.; Ihrler, S.; et al. HPV-related Multiphenotypic Sinonasal Carcinoma: An Expanded Series of 49 Cases of the Tumor Formerly Known as HPV-related Carcinoma With Adenoid Cystic Carcinoma-like Features. Am. J. Surg. Pathol. 2017, 41, 1690–1701. [Google Scholar] [CrossRef]
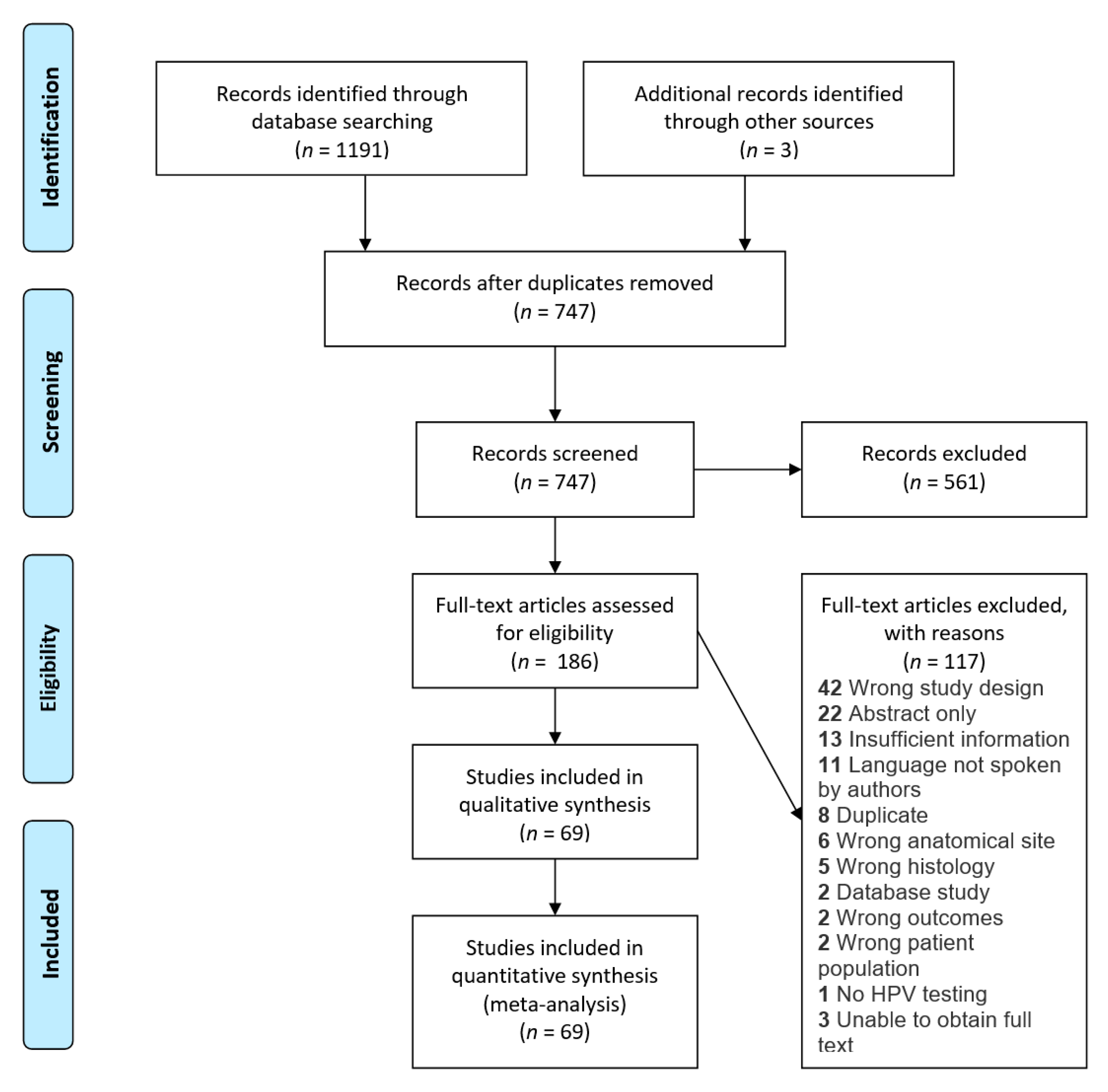
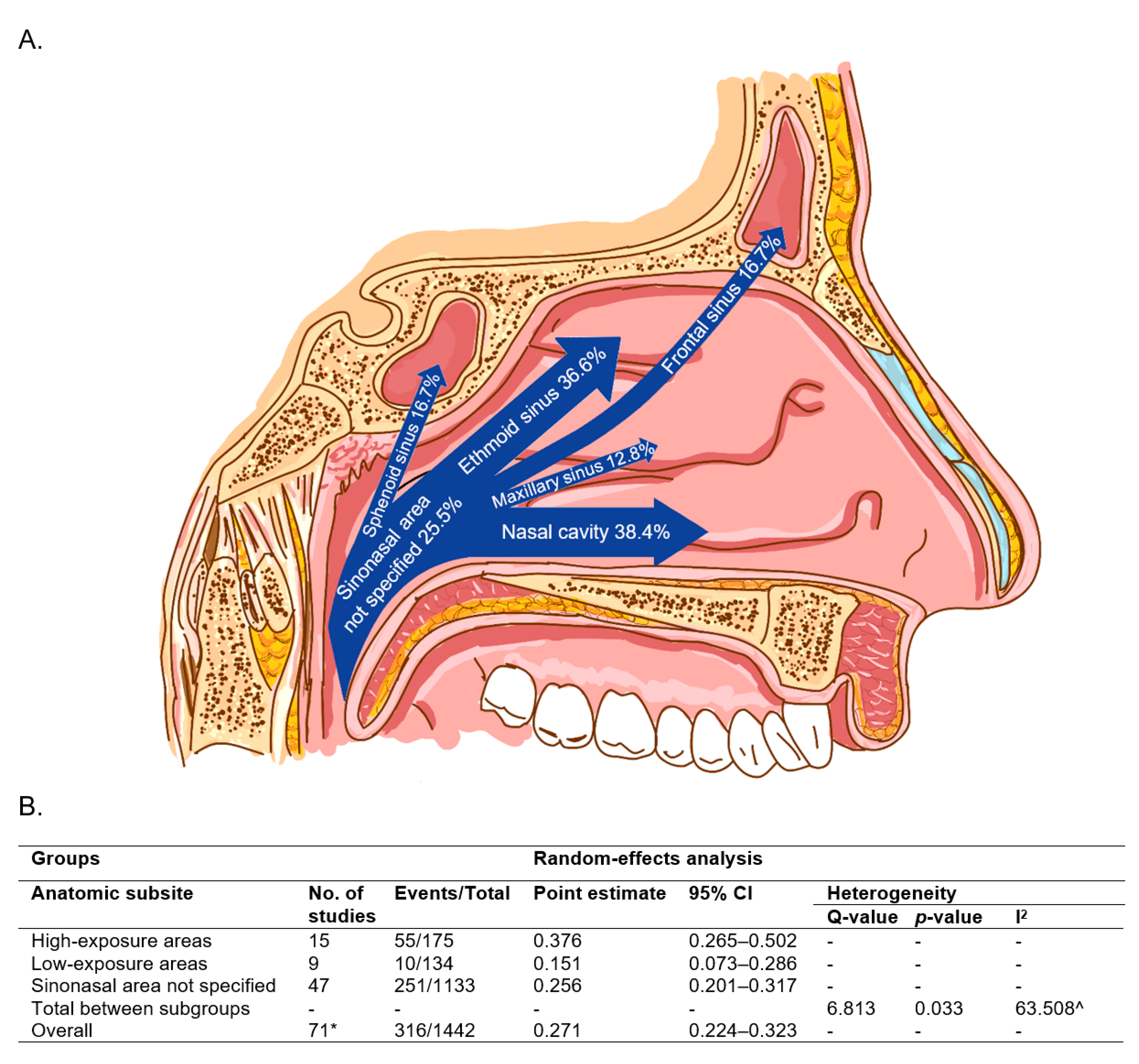
| Groups | Random-Effects Analysis | Heterogeneity | ||||||
|---|---|---|---|---|---|---|---|---|
| Detection Method | No. of Studies | Events/Total | Point Estimate | 95% CI | Q-Value | p-Value | I2 | |
| A | Single testing | 35 | 152/583 | 0.317 | 0.236–0.411 | - | - | - |
| Multi-testing | 20 | 142/727 | 0.205 | 0.145–0.282 | - | - | - | |
| Total between study | - | - | - | - | 3.878 | 0.049 | - | |
| B | Single testing | 35 | 152/583 | 0.317 | 0.236–0.411 | - | - | - |
| Multi-testing without RNA | 12 | 101/455 | 0.233 | 0.150–0.343 | - | - | - | |
| Multi-testing with RNA | 8 | 41/272 | 0.165 | 0.088–0.287 | - | - | - | |
| Total between study | - | - | - | - | 4.595 | 0.101 | - | |
| Overall | 55 | 294/1310 | 0.261 | 0.208–0.322 | - | - | 70.452 ^ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang Sing Pang, K.J.W.; Mur, T.; Collins, L.; Rao, S.R.; Faden, D.L. Human Papillomavirus in Sinonasal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 45. https://doi.org/10.3390/cancers13010045
Chang Sing Pang KJW, Mur T, Collins L, Rao SR, Faden DL. Human Papillomavirus in Sinonasal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers. 2021; 13(1):45. https://doi.org/10.3390/cancers13010045
Chicago/Turabian StyleChang Sing Pang, Kim J. W., Taha Mur, Louise Collins, Sowmya R. Rao, and Daniel L. Faden. 2021. "Human Papillomavirus in Sinonasal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis" Cancers 13, no. 1: 45. https://doi.org/10.3390/cancers13010045
APA StyleChang Sing Pang, K. J. W., Mur, T., Collins, L., Rao, S. R., & Faden, D. L. (2021). Human Papillomavirus in Sinonasal Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Cancers, 13(1), 45. https://doi.org/10.3390/cancers13010045






