A Randomized Trial of Physical Activity in Children and Adolescents with Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients Recruitment and Baseline Characteristics
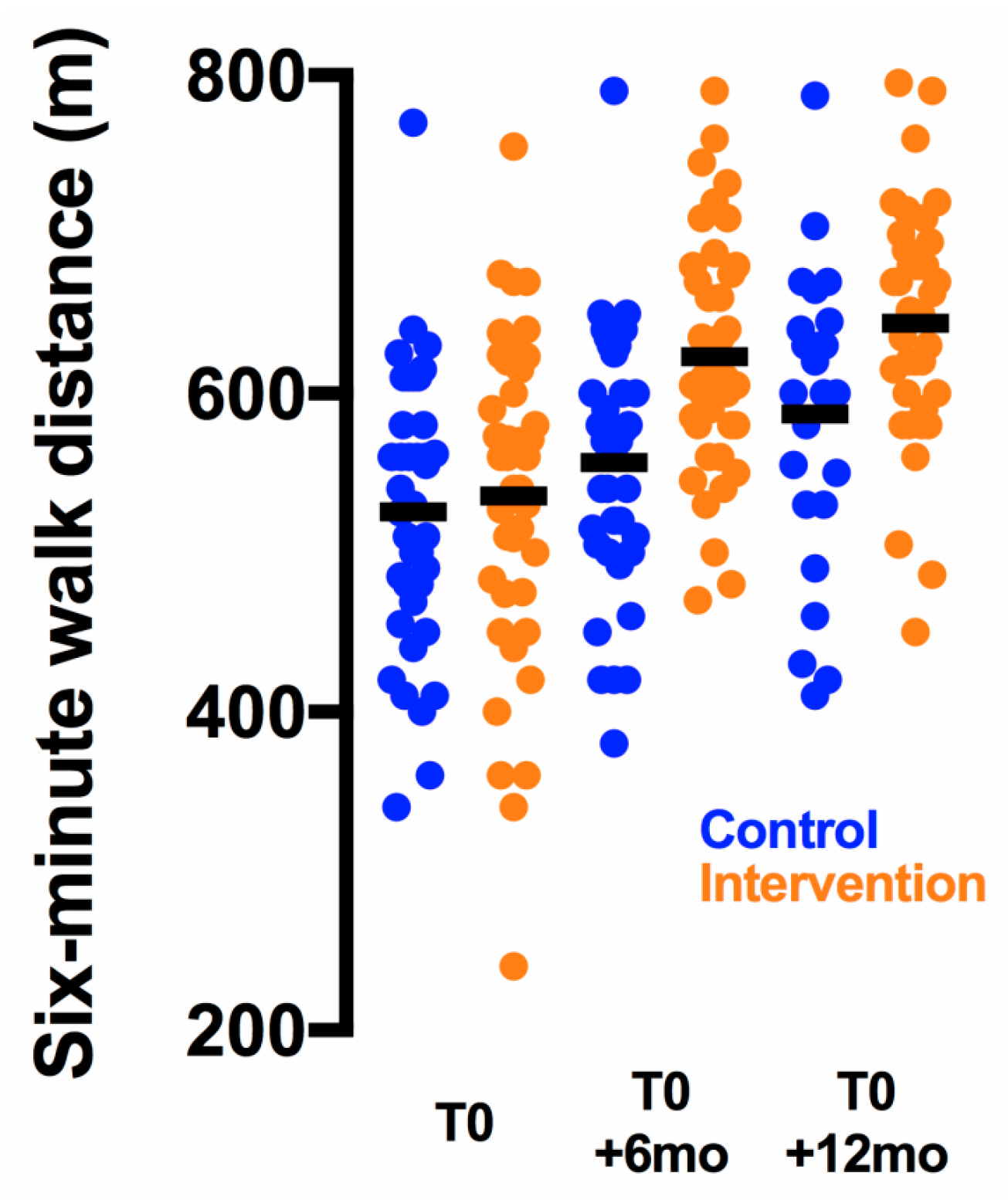
2.2. Physical Endpoints
2.2.1. Change from Baseline to T0 + 6 mo
2.2.2. Change from Baseline to T0 + 12 mo
2.3. Self-Esteem and Quality-of-Life Endpoints
2.3.1. Change from Baseline to T0 + 6 mo
2.3.2. Change from Baseline to T0 + 12 mo
2.4. Adverse Events
3. Discussion
4. Materials and Methods
4.1. Trial Design
4.2. Participants
4.3. Randomization
4.4. Intervention
4.5. Trial Endpoints
4.6. Assessment of Covariates and Adverse Events
4.7. Statistical Analyses and Sample Size Calculation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Götte, M.; Kesting, S.; Winter, C.; Rosenbaum, D.; Boos, J. Comparison of Self-Reported Physical Activity in Children and Adolescents before and during Cancer Treatment: Physical Activity During Cancer Treatment. Pediatric Blood & Cancer 2014, 61, 1023–1028. [Google Scholar] [CrossRef]
- Ness, K.K.; Hudson, M.M.; Ginsberg, J.P.; Nagarajan, R.; Kaste, S.C.; Marina, N.; Whitton, J.; Robison, L.L.; Gurney, J.G. Physical Performance Limitations in the Childhood Cancer Survivor Study Cohort. J. Clin. Oncol. 2009, 27, 2382–2389. [Google Scholar] [CrossRef] [PubMed]
- Strong, W.B.; Malina, R.M.; Blimkie, C.J.R.; Daniels, S.R.; Dishman, R.K.; Gutin, B.; Hergenroeder, A.C.; Must, A.; Nixon, P.A.; Pivarnik, J.M.; et al. Evidence Based Physical Activity for School-Age Youth. J. Pediatr. 2005, 146, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Götte, M.; Taraks, S.; Boos, J. Sports in Pediatric Oncology: The Role(s) of Physical Activity for Children with Cancer. J. Pediatr. Hematol. Oncol. 2014, 36, 85–90. [Google Scholar] [CrossRef]
- Brown, J.C.; Winters-Stone, K.; Lee, A.; Schmitz, K.H. Cancer, Physical Activity, and Exercise. Compr Physiol 2012, 2, 2775–2809. [Google Scholar] [CrossRef]
- Mishra, S.I.; Scherer, R.W.; Snyder, C.; Geigle, P.M.; Berlanstein, D.R.; Topaloglu, O. Exercise Interventions on Health-Related Quality of Life for People with Cancer during Active Treatment. Cochrane Database Syst Rev 2012, 8, CD008465. [Google Scholar] [CrossRef]
- Braam, K.I.; van der Torre, P.; Takken, T.; Veening, M.A.; van Dulmen-den Broeder, E.; Kaspers, G.J.L. Physical Exercise Training Interventions for Children and Young Adults during and after Treatment for Childhood Cancer. Cochrane Database Syst Rev 2016, 3, CD008796. [Google Scholar] [CrossRef]
- Gohar, S.F.; Comito, M.; Price, J.; Marchese, V. Feasibility and Parent Satisfaction of a Physical Therapy Intervention Program for Children with Acute Lymphoblastic Leukemia in the First 6 Months of Medical Treatment. Pediatr Blood Cancer 2011, 56, 799–804. [Google Scholar] [CrossRef]
- Wurz, A.; Chamorro-Vina, C.; Guilcher, G.M.T.; Schulte, F.; Culos-Reed, S.N. The Feasibility and Benefits of a 12-Week Yoga Intervention for Pediatric Cancer out-Patients. Pediatr Blood Cancer 2014, 61, 1828–1834. [Google Scholar] [CrossRef]
- Beulertz, J.; Prokop, A.; Rustler, V.; Bloch, W.; Felsch, M.; Baumann, F.T. Effects of a 6-Month, Group-Based, Therapeutic Exercise Program for Childhood Cancer Outpatients on Motor Performance, Level of Activity, and Quality of Life. Pediatr Blood Cancer 2016, 63, 127–132. [Google Scholar] [CrossRef]
- Morales, J.S.; Santana-Sosa, E.; Santos-Lozano, A.; Baño-Rodrigo, A.; Valenzuela, P.L.; Rincón-Castanedo, C.; Fernández-Moreno, D.; Vicent, M.G.; Pérez-Somarriba, M.; Madero, L.; et al. Inhospital Exercise Benefits in Childhood Cancer: A Prospective Cohort Study. Scand J. Med. Sci. Sports 2019. [Google Scholar] [CrossRef] [PubMed]
- Moyer-Mileur, L.J.; Ransdell, L.; Bruggers, C.S. Fitness of Children With Standard-Risk Acute Lymphoblastic Leukemia During Maintenance Therapy: Response to a Home-Based Exercise and Nutrition Program. J. Pediatric Hematol. Oncol. 2009, 31, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Fiuza-Luces, C.; Padilla, J.R.; Soares-Miranda, L.; Santana-Sosa, E.; Quiroga, J.V.; Santos-Lozano, A.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Lorenzo-González, R.; Verde, Z.; et al. Exercise Intervention in Pediatric Patients with Solid Tumors: The Physical Activity in Pediatric Cancer Trial. Med. Sci. Sports Exerc. 2017, 49, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Braam, K.I.; van Dijk-Lokkart, E.M.; Kaspers, G.J.L.; Takken, T.; Huisman, J.; Buffart, L.M.; Bierings, M.B.; Merks, J.H.M.; van den Heuvel-Eibrink, M.M.; Veening, M.A.; et al. Effects of a Combined Physical and Psychosocial Training for Children with Cancer: A Randomized Controlled Trial. BMC Cancer 2018, 18, 1289. [Google Scholar] [CrossRef]
- Speyer, E.; Vuillemin, A.; Herbinet, A.; Chastagner, P.; Briançon, S. Effect of Adapted Physical Activity on Health-Related Quality of Life among Hospitalized Children and Adolescents (the ACTIV’HOP Randomized Controlled Trial): Design and Methods. Contemp Clin Trials 2010, 31, 165–171. [Google Scholar] [CrossRef]
- Andersen, L.B.; Harro, M.; Sardinha, L.B.; Froberg, K.; Ekelund, U.; Brage, S.; Anderssen, S.A. Physical Activity and Clustered Cardiovascular Risk in Children: A Cross-Sectional Study (The European Youth Heart Study). Lancet 2006, 368, 299–304. [Google Scholar] [CrossRef]
- Winter, C.; Müller, C.; Brandes, M.; Brinkmann, A.; Hoffmann, C.; Hardes, J.; Gosheger, G.; Boos, J.; Rosenbaum, D. Level of Activity in Children Undergoing Cancer Treatment. Pediatr Blood Cancer 2009, 53, 438–443. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. New Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef]
- Geiger, R.; Strasak, A.; Treml, B.; Gasser, K.; Kleinsasser, A.; Fischer, V.; Geiger, H.; Loeckinger, A.; Stein, J.I. Six-Minute Walk Test in Children and Adolescents. J. Pediatrics 2007, 150, 395–399. [Google Scholar] [CrossRef]
- Nixon, P.A.; Joswiak, M.L.; Fricker, F.J. A Six-Minute Walk Test for Assessing Exercise Tolerance in Severely Ill Children. J. Pediatr. 1996, 129, 362–366. [Google Scholar] [CrossRef]
- Li, A.M.; Yin, J.; Au, J.T.; So, H.K.; Tsang, T.; Wong, E.; Fok, T.F.; Ng, P.C. Standard Reference for the Six-Minute-Walk Test in Healthy Children Aged 7 to 16 Years. Am. J. Respir Crit Care Med. 2007, 176, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Hooke, M.C.; Garwick, A.W.; Neglia, J.P. Assessment of Physical Performance Using the 6-Minute Walk Test in Children Receiving Treatment for Cancer. Cancer Nurs 2013, 36, E9–E16. [Google Scholar] [CrossRef] [PubMed]
- Ekeland, E.; Heian, F.; Hagen, K.B.; Abbott, J.; Nordheim, L. Exercise to Improve Self-Esteem in Children and Young People. Cochrane Database Syst Rev 2004, 1, CD003683. [Google Scholar] [CrossRef] [PubMed]
- Maïano, C.; Morin, A.J.S.; Ninot, G.; Monthuy-Blanc, J.; Stephan, Y.; Florent, J.-F.; Vallée, P. A Short and Very Short Form of the Physical Self-Inventory for Adolescents: Development and Factor Validity. Psychol. Sport Exerc. 2008, 9, 830–847. [Google Scholar] [CrossRef]
- McTiernan, A.; Friedenreich, C.M.; Katzmarzyk, P.T.; Powell, K.E.; Macko, R.; Buchner, D.; Pescatello, L.S.; Bloodgood, B.; Tennant, B.; Vaux-Bjerke, A.; et al. Physical Activity in Cancer Prevention and Survival: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical Activity, Sedentary Behaviour, Diet, and Cancer: An Update and Emerging New Evidence. Lancet Oncol. 2017, 18, e457–e471. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories ATS Statement: Guidelines for the Six-Minute Walk Test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [CrossRef]
- Adam, C.; Arruda, V.; Ravazzolo, M.; Adams, R.; Tuxworth, W. EUROFIT: European Test of Physical Fitness.; Council of Europe, Committee for Development of Sport: Rome, Italy, 1988. [Google Scholar]
- Bubanj, S.; Stankovi, R.; Boji, I. Reliability of Myotest Tested by a Countermovement Jump. Acta Kinesiologica 2010, 2, 46–48. [Google Scholar]
- McQuade, K.J.; Turner, J.A.; Buchner, D.M. Physical Fitness and Chronic Low Back Pain. An Analysis of the Relationships among Fitness, Functional Limitations, and Depression. Clin. Orthop. Relat. Res. 1988, 198–204. [Google Scholar]
- Auquier, P.; Clement, A.; Sapin, C.; El Khammar, M.; San Marco, J.-L.; Simeoni, M.-C. Development, Validation of HRQL Measurement in Children: VSP-Ae. Qual. Life Res. 2001, 10, 268. [Google Scholar]
- Simeoni, M.C.; Sapin, C.; Antoniotti, S.; Auquier, P. Health-Related Quality of Life Reported by French Adolescents: A Predictive Approach of Health Status? J. Adolesc Health 2001, 28, 288–294. [Google Scholar] [CrossRef]
- Sapin, C.; Simeoni, M.-C.; El Khammar, M.; Antoniotti, S.; Auquier, P. Reliability and Validity of the VSP-A, a Health-Related Quality of Life Instrument for Ill and Healthy Adolescents. J. Adolesc Health 2005, 36, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, MI, USA, 1988. [Google Scholar]
- Sawilowsky, S. Very Large and Huge Effect Sizes. J. Mod. Appl. Stat. Methods 2009, 8, 597–599. [Google Scholar] [CrossRef]

| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. Characteristics | Intervention n = 41 | Control n = 39 |
|---|---|---|
| Age (years) | 11.4 ± 0.6 | 11.2 ± 0.6 |
| Age group‡ | ||
| Children | 25 (61%) | 25 (64%) |
| Adolescents | 16 (39%) | 14 (36%) |
| Sex | ||
| Male | 23 (56%) | 23 (59%) |
| Female | 18 (44%) | 16 (41%) |
| Family | ||
| Parents in couple | 31 (76%) | 28 (72%) |
| Single-parent family | 10 (24%) | 11 (28%) |
| Number of siblings § | ||
| 1–2 | 27 (66%) | 27 (69%) |
| >2 | 14 (34%) | 12 (31%) |
| Distance from home to the treating center (km) | 64 ± 11 | 57 ± 7 |
| Physical activity before diagnosis | ||
| Regular physical activity | 38 (93%) | 35 (90%) |
| Sport in a club | 25 (61%) | 21 (54%) |
| Disease | ||
| Localized | 38 (93%) | 35 (90%) |
| Metastatic | 3 (7%) | 4 (10%) |
| Primary disease | 34 (83%) | 37 (95%) |
| Relapse | 7 (17%) | 2 (5%) |
| Leukemia treated with HSCT | 5 (12%) | 3 (8%) |
| Brain or bone tumor | 12 (29%) | 12 (31%) |
| Other tumor | 24 (59%) | 24 (62%) |
| Leukemia | 16 (39%) | 15 (38%) |
| Lymphoma | 8 (20%) | 8 (21%) |
| Brain tumor | 7 (17%) | 5 (13%) |
| Bone tumor | 5 (12%) | 7 (18%) |
| Other solid tumor | 5 (12%) | 4 (10%) |
| Anticancer treatment received | ||
| Chemotherapy | 39 (95%) | 38 (97%) |
| HSCT | 6 (15%) | 5 (13%) |
| Radiotherapy | 11 (27%) | 10 (26%) |
| Surgery | 15 (37%) | 14 (36%) |
| Treatment intensity at intervention (T0) | ||
| High intensity | 26 (63%) | 23 (59%) |
| Maintenance/Metronomic | 8 (20%) | 11 (28%) |
| No anticancer treatment | 7 (17%) | 5 (13%) |
| Time from diagnosis to intervention (T0) * (months) | 11.8 ± 2.0 | 9.3 ± 1.2 |
| Intervention n = 40 | Control n = 38 | Group Effect on Change | ||||||
|---|---|---|---|---|---|---|---|---|
| Endpoints | Baseline | T0 + 6 mo | Change from Baseline | Baseline | T0 + 6 mo | Change from Baseline | p-Value | Effect Size |
| Six-minute walk distance (primary endpoint) (m) | 540 ± 16 | 623 ± 12 | 83 ± 12 | 525 ± 14 | 557 ± 13 | 32 ± 6 | <0.001 | 1.3 |
| Heart rate (min−1) | ||||||||
| Resting | 93 ± 2 | 89 ± 1 | −4 ± 2 | 94 ± 1 | 91 ± 1 | −3 ± 1 | 0.4 | −0.2 |
| End of effort | 174 ± 2 | 173 ± 2 | −1 ± 2 | 173 ± 2 | 173 ± 2 | 0 ± 1 | 0.7 | −0.1 |
| Recovery 1′ | 135 ± 3 | 137 ± 2 | 3 ± 3 | 140 ± 2 | 136 ± 2 | −4 ± 2 | 0.09 | 0.7 |
| Recovery 2′ | 119 ± 2 | 119 ± 2 | 0 ± 2 | 119 ± 1 | 121 ± 1 | 2 ± 1 | 0.3 | −0.3 |
| Recovery 3′ | 111 ± 2 | 107 ± 2 | −5 ± 2 | 112 ± 2 | 108 ± 1 | −4 ± 1 | 0.6 | −0.1 |
| Body mass index (kg.m−2) | 18.4 ± 0.6 | 18.8 ± 0.6 | 0.4 ± 0.2 | 16.8 ± 0.4 | 17.2 ± 0.4 | 0.4 ± 0.2 | 1.0 | 0.0 |
| Fat mass (kg) | 9.2 ± 1.0 | 9.6 ± 1.2 | 0.5 ± 0.3 | 6.7 ± 0.6 | 7.3 ± 0.7 | 0.6 ± 0.2 | 0.7 | −0.1 |
| Lean mass (kg) | 31.6 ± 2.2 | 32.7 ± 2.2 | 1.1 ± 0.3 | 28.2 ± 1.6 | 28.6 ± 1.6 | 0.5 ± 0.3 | 0.1 | 0.3 |
| Flexibility (sit-and-reach test) (cm) | −8.7 ± 1.4 | −6.2 ± 1.3 | 2.6 ± 0.7 | −8.8 ± 1.1 | −8.1 ± 1.1 | 0.7 ± 0.3 | 0.02 | 1.0 |
| Balance (flamingo balance test) (A.U.) | 7.8 ± 1.4 | 5.8 ± 1.0 | −2.0 ± 0.5 | 6.7 ± 1.1 | 5.9 ± 1.0 | −0.8 ± 0.2 | 0.048 | −0.9 |
| Upper limb strength (medicine-ball launch) (m) | 3.0 ± 0.2 | 3.3 ± 0.2 | 0.3 ± 0.0 | 2.8 ± 0.1 | 2.9 ± 0.1 | 0.1 ± 0.0 | <0.001 | 1.9 |
| Lower limb strength | ||||||||
| Myotest® (cm) | 15.1 ± 1.1 | 17.5 ± 1.2 | 2.4 ± 0.5 | 13.5 ± 0.7 | 14.1 ± 0.8 | 0.6 ± 0.3 | 0.04 | 1.0 |
| Chair test (sec) | 45.4 ± 5.3 | 69.7 ± 7.0 | 24.3 ± 4.4 | 35.1 ± 3.9 | 41.1 ± 3.5 | 6.0 ± 2.0 | <0.001 | 1.6 |
| Trunk muscle endurance (bridge trunk muscle endurance test) (sec) | 45.4 ± 4.5 | 69.3 ± 5.7 | 23.9 ± 3.4 | 49.8 ± 8.3 | 50.3 ± 3.4 | 0.6 ± 6.3 | 0.001 | 0.6 |
| Abdominal muscle endurance (sit-up score) (A.U.) | 9.7 ± 0.8 | 14.7 ± 0.7 | 4.9 ± 0.6 | 9.8 ± 0.7 | 11.4 ± 0.6 | 1.7 ± 0.2 | <0.001 | 2.7 |
| Intervention n = 37 | Control n = 25 | Group Effect on Change | ||||||
|---|---|---|---|---|---|---|---|---|
| Endpoints | Baseline | T0 + 12 mo | Change from Baseline | Baseline | T0 + 12 mo | Change from Baseline | p-Value | Effect Size |
| Six-minute walk distance (m) | 535 ± 17 | 644 ± 13 | 109 ± 15 | 528 ± 17 | 587 ± 19 | 59 ± 9 | 0.007 | 1.1 |
| Heart rate (min−1) | ||||||||
| Resting | 92 ± 2 | 88 ± 1 | −4 ± 2 | 94 ± 2 | 91 ± 1 | −3 ± 2 | 0.8 | −0.1 |
| End of effort | 173 ± 2 | 173 ± 2 | 0 ± 3 | 172 ± 3 | 175 ± 2 | 3 ± 2 | 0.3 | −0.3 |
| Recovery 1′ | 134 ± 4 | 135 ± 2 | 2 ± 4 | 138 ± 3 | 135 ± 2 | −3 ± 2 | 0.4 | 0.4 |
| Recovery 2′ | 118 ± 2 | 117 ± 2 | −1 ± 3 | 119 ± 1 | 120 ± 2 | 1 ± 2 | 0.6 | −0.2 |
| Recovery 3′ | 111 ± 2 | 106 ± 2 | −5 ± 2 | 112 ± 2 | 108 ± 2 | −4 ± 3 | 0.8 | −0.1 |
| Body mass index (kg.m−2) | 18.4 ± 0.6 | 19.1 ± 0.6 | 0.7 ± 0.2 | 16.9 ± 0.5 | 17.9 ± 0.5 | 1.0 ± 0.3 | 0.3 | −0.2 |
| Fat mass (kg) | 9.0 ± 1.0 | 9.7 ± 1.0 | 0.7 ± 0.4 | 7.3 ± 0.9 | 9.0 ± 1.5 | 1.7 ± 1.0 | 0.3 | −0.2 |
| Lean mass (kg) | 30.5 ± 2.2 | 32.9 ± 2.1 | 2.4 ± 0.3 | 27.4 ± 2.0 | 29.6 ± 2.0 | 2.2 ± 0.4 | 0.7 | 0.1 |
| Flexibility (sit-and-reach test) (cm) | −9.3 ± 1.5 | −6.0 ± 1.4 | 3.3 ± 0.8 | −9.0 ± 1.4 | −7.8 ± 1.4 | 1.2 ± 0.5 | 0.02 | 0.9 |
| Balance (flamingo balance test) (A.U.) | 7.9 ± 1.5 | 4.7 ± 0.9 | −3.3 ± 0.8 | 5.7 ± 1.2 | 4.6 ± 1.1 | −1.1 ± 0.4 | 0.01 | −1.1 |
| Upper limb strength (medicine-ball launch) (m) | 3.0 ± 0.2 | 3.4 ± 0.2 | 0.5 ± 0.1 | 2.8 ± 0.1 | 3.1 ± 0.1 | 0.3 ± 0.0 | 0.009 | 1.0 |
| Lower limb strength | ||||||||
| Myotest® (cm) | 14.7 ± 1.1 | 17.8 ± 1.3 | 3.2 ± 0.6 | 13.9 ± 0.8 | 15.5 ± 1.0 | 1.6 ± 0.6 | 0.08 | 0.6 |
| Chair test (sec) | 44.0 ± 5.5 | 73.7 ± 8.7 | 29.7 ± 7.3 | 29.9 ± 2.9 | 52.0 ± 4.7 | 22.1 ± 4.6 | 0.5 | 0.4 |
| Trunk muscle endurance (bridge trunk muscle endurance test) (sec) | 45.1 ± 4.9 | 74.3 ± 5.7 | 29.2 ± 3.6 | 43.8 ± 4.7 | 67.6 ± 4.4 | 23.7 ± 3.3 | 0.3 | 0.3 |
| Abdominal muscle endurance (sit-up score) (A.U.) | 9.5 ± 0.8 | 15.3 ± 0.7 | 5.8 ± 0.5 | 10.0 ± 0.8 | 14.3 ± 0.7 | 4.3 ± 0.5 | 0.04 | 0.6 |
| Intervention n = 37 | Control n = 33 | Group Effect on Change | ||||||
|---|---|---|---|---|---|---|---|---|
| Endpoints | Baseline | T0 + 6 mo | Change from Baseline | Baseline | T0 + 6 mo | Change from Baseline | p-Value | Effect Size |
| Self-esteem (PSI-VSF scale) (A.U.) | ||||||||
| Global self-concept | 4.4 ± 0.2 | 5.0 ± 0.1 | 0.5 ± 0.2 | 4.6 ± 0.1 | 4.8 ± 0.1 | 0.1 ± 0.1 | 0.04 | 0.6 |
| Physical self-worth | 4.4 ± 0.2 | 4.6 ± 0.1 | 0.2 ± 0.1 | 4.1 ± 0.2 | 4.3 ± 0.1 | 0.2 ± 0.1 | 1.0 | 0.0 |
| Physical strength | 4.0 ± 0.1 | 4.2 ± 0.1 | 0.2 ± 0.1 | 4.0 ± 0.2 | 4.0 ± 0.1 | 0.0 ± 0.1 | 0.2 | 0.3 |
| Physical attractiveness | 3.9 ± 0.2 | 4.2 ± 0.2 | 0.2 ± 0.1 | 3.8 ± 0.2 | 4.0 ± 0.2 | 0.2 ± 0.1 | 0.8 | 0.1 |
| Physical condition | 3.8 ± 0.2 | 4.1 ± 0.2 | 0.3 ± 0.1 | 3.8 ± 0.2 | 4.1 ± 0.1 | 0.3 ± 0.1 | 0.9 | 0.0 |
| Sport competence | 4.1 ± 0.2 | 4.5 ± 0.1 | 0.4 ± 0.1 | 4.3 ± 0.2 | 4.3 ± 0.1 | 0.0 ± 0.1 | 0.08 | 0.5 |
| Parent-reported quality of life (VSP-A parents scale) (A.U.) | ||||||||
| Relationship with parents | 80 ± 2 | 80 ± 2 | 0 ± 2 | 84 ± 2 | 83 ± 2 | 0 ± 2 | 1.0 | 0.0 |
| Body image | 69 ± 4 | 76 ± 3 | 7 ± 2 | 77 ± 3 | 79 ± 3 | 2 ± 2 | 0.2 | 0.4 |
| Vitality | 67 ± 2 | 74 ± 2 | 7 ± 2 | 68 ± 2 | 71 ± 2 | 3 ± 2 | 0.13 | 0.5 |
| Relationship with friends | 63 ± 3 | 67 ± 3 | 5 ± 3 | 59 ± 4 | 64 ± 3 | 5 ± 4 | 0.8 | 0.0 |
| Leisure activities | 37 ± 4 | 55 ± 3 | 18 ± 3 | 41 ± 4 | 47 ± 3 | 6 ± 2 | 0.003 | 0.9 |
| Psychological well-being | 68 ± 3 | 74 ± 3 | 6 ± 2 | 74 ± 3 | 75 ± 3 | 2 ± 1 | 0.17 | 0.5 |
| Physical well-being | 52 ± 3 | 61 ± 2 | 9 ± 2 | 52 ± 3 | 58 ± 3 | 6 ± 2 | 0.4 | 0.2 |
| School performance | 76 ± 3 | 78 ± 3 | 2 ± 2 | 79 ± 3 | 80 ± 3 | 2 ± 2 | 1.0 | 0.0 |
| Relationship with teachers | 84 ± 2 | 86 ± 2 | 2 ± 2 | 86 ± 2 | 89 ± 2 | 3 ± 1 | 0.7 | -0.1 |
| Relationship with medical staff | 82 ± 2 | 86 ± 2 | 3 ± 2 | 87 ± 3 | 87 ± 3 | 0 ± 4 | 0.6 | 0.1 |
| Summary score | 68 ± 2 | 74 ± 1 | 6 ± 1 | 71 ± 2 | 73 ± 2 | 3 ± 1 | 0.04 | 0.8 |
| Intervention n = 33 | Control n = 23 | Group Effect on Change | ||||||
|---|---|---|---|---|---|---|---|---|
| Endpoints | Baseline | T0 + 12 mo | Change from Baseline | Baseline | T0 + 12 mo | Change from Baseline | p-Value | Effect Size |
| Self-esteem (PSI-VSF scale) (A.U.) | ||||||||
| Global self-concept | 4.4 ±0.2 | 5.1 ± 0.1 | 0.7 ± 0.2 | 4.7 ± 0.2 | 5.1 ± 0.1 | 0.4 ± 0.2 | 0.4 | 0.3 |
| Physical self-worth | 4.3 ± 0.2 | 4.7 ± 0.2 | 0.4 ± 0.2 | 4.2 ± 0.2 | 4.8 ± 0.1 | 0.5 ± 0.2 | 0.3 | −0.3 |
| Physical strength | 4.0 ± 0.2 | 4.3 ± 0.1 | 0.3 ± 0.2 | 4.0 ± 0.2 | 4.2 ± 0.1 | 0.2 ± 0.2 | 0.8 | 0.1 |
| Physical attractiveness | 3.9 ± 0.2 | 4.3 ± 0.2 | 0.4 ± 0.2 | 4.0 ± 0.3 | 4.3 ± 0.2 | 0.3 ± 0.3 | 0.9 | 0.0 |
| Physical condition | 3.8 ± 0.2 | 4.4 ± 0.2 | 0.6 ± 0.2 | 3.8 ± 0.2 | 4.3 ± 0.1 | 0.5 ± 0.2 | 0.6 | 0.2 |
| Sport competence | 4.1 ± 0.2 | 4.7 ± 0.2 | 0.6 ± 0.2 | 4.2 ± 0.2 | 4.5 ± 0.2 | 0.3 ± 0.2 | 0.3 | 0.3 |
| Parent-reported quality of life (VSP-A parents scale) (A.U.) | ||||||||
| Relationship with parents | 79 ± 2 | 83 ± 2 | 4 ± 2 | 84 ± 2 | 83 ± 2 | −1 ± 2 | 0.10 | 0.5 |
| Body image | 71 ± 4 | 83 ± 3 | 13 ± 3 | 79 ± 3 | 88 ± 2 | 10 ± 3 | 0.6 | 0.2 |
| Vitality | 66 ± 2 | 81 ± 2 | 14 ± 2 | 70 ± 3 | 78 ± 2 | 8 ± 3 | 0.04 | 0.5 |
| Relationship with friends | 63 ± 3 | 73 ± 2 | 10 ± 4 | 62 ± 4 | 72 ± 3 | 9 ± 3 | 0.6 | 0.1 |
| Leisure activities | 38 ± 4 | 68 ± 3 | 30 ± 3 | 42 ± 5 | 61 ± 3 | 19 ± 4 | 0.03 | 0.6 |
| Psychological well-being | 69 ± 3 | 81 ± 2 | 13 ± 3 | 74 ± 3 | 80 ± 2 | 6 ± 2 | 0.12 | 0.6 |
| Physical well-being | 50 ± 3 | 68 ± 2 | 18 ± 3 | 56 ± 4 | 62 ± 2 | 7 ± 3 | 0.009 | 0.8 |
| School performance | 77 ± 3 | 78 ± 3 | 2 ± 3 | 81 ± 3 | 80 ± 3 | −1 ± 3 | 0.6 | 0.1 |
| Relationship with teachers | 84 ± 3 | 86 ± 3 | 2 ± 3 | 84 ± 3 | 88 ± 3 | 4 ± 2 | 0.5 | −0.2 |
| Relationship with medical staff | 83 ± 3 | 89 ± 3 | 5 ± 3 | 89 ± 4 | 88 ± 3 | −1 ± 4 | 0.3 | 0.3 |
| Summary score | 68 ± 2 | 80 ± 1 | 12 ± 1 | 73 ± 2 | 79 ± 2 | 5 ± 2 | 0.01 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saultier, P.; Vallet, C.; Sotteau, F.; Hamidou, Z.; Gentet, J.-C.; Barlogis, V.; Curtillet, C.; Verschuur, A.; Revon-Riviere, G.; Galambrun, C.; et al. A Randomized Trial of Physical Activity in Children and Adolescents with Cancer. Cancers 2021, 13, 121. https://doi.org/10.3390/cancers13010121
Saultier P, Vallet C, Sotteau F, Hamidou Z, Gentet J-C, Barlogis V, Curtillet C, Verschuur A, Revon-Riviere G, Galambrun C, et al. A Randomized Trial of Physical Activity in Children and Adolescents with Cancer. Cancers. 2021; 13(1):121. https://doi.org/10.3390/cancers13010121
Chicago/Turabian StyleSaultier, Paul, Clothilde Vallet, Frédéric Sotteau, Zeinab Hamidou, Jean-Claude Gentet, Vincent Barlogis, Catherine Curtillet, Arnauld Verschuur, Gabriel Revon-Riviere, Claire Galambrun, and et al. 2021. "A Randomized Trial of Physical Activity in Children and Adolescents with Cancer" Cancers 13, no. 1: 121. https://doi.org/10.3390/cancers13010121
APA StyleSaultier, P., Vallet, C., Sotteau, F., Hamidou, Z., Gentet, J.-C., Barlogis, V., Curtillet, C., Verschuur, A., Revon-Riviere, G., Galambrun, C., Chambost, H., Auquier, P., Michel, G., & André, N. (2021). A Randomized Trial of Physical Activity in Children and Adolescents with Cancer. Cancers, 13(1), 121. https://doi.org/10.3390/cancers13010121





