Simple Summary
Currently, cytotoxic agents and biological targeted agents are commonly combined for the treatment of advanced or metastatic colorectal cancer. However, questions of ‘which chemotherapy or targeted therapy provides the higher efficacy and lower toxicity’ or ‘whether the addition of targeted therapy to chemotherapy not only increases the treatment effect but also reduces the adverse events’ have been raised. In this study, we firstly calculated the treatment effect on overall survival, which has not been reached in several randomized controlled trials, based on treatment effects on overall response rate and/or progression-free survival. Then we performed the network meta-analysis to compare the efficacy and safety of 12 commonly used regimens. Finally, our analyses showed that FOLFOX+cetuximab and FOLFIRI+bevacizumab have high probabilities of being first-line and second-line treatments in terms of efficacy and safety, respectively.
Abstract
This study aimed to investigate the efficacy and safety of systemic therapies in the treatment of unresectable advanced or metastatic colorectal cancer. Predicted hazard ratios (HRs) and their 95% credible intervals (CrIs) for overall survival (OS) were calculated from the odds ratio (OR) for the overall response rate and/or HR for progression-free survival using multivariate random effects (MVRE) models. We performed a network meta-analysis (NMA) of 49 articles to compare the efficacy and safety of FOLFOX/FOLFIRI±bevacizumab (Bmab)/cetuximab (Cmab)/panitumumab (Pmab), and FOLFOXIRI/CAPEOX±Bmab. The NMA showed significant OS improvement with FOLFOX, FOLFOX+Cmab, and FOLFIRI+Cmab compared with that of FOLFIRI (HR = 0.84, 95% CrI = 0.73–0.98; HR = 0.76, 95% CrI = 0.62–0.94; HR = 0.80, 95% CrI = 0.66–0.96, respectively), as well as with FOLFOX+Cmab and FOLFIRI+Cmab compared with that of FOLFOXIRI (HR = 0.69, 95% CrI = 0.51–0.94 and HR = 0.73, 95% CrI = 0.54–0.97, respectively). The odds of adverse events grade ≥3 were significantly higher for FOLFOX+Cmab vs. FOLFIRI+Bmab (OR = 2.34, 95% CrI = 1.01–4.66). Higher odds of events were observed for FOLFIRI+Pmab in comparison with FOLFIRI (OR = 2.16, 95% CrI = 1.09–3.84) and FOLFIRI+Bmab (OR = 3.14, 95% CrI = 1.51–5.89). FOLFOX+Cmab and FOLFIRI+Bmab showed high probabilities of being first- and second-line treatments in terms of the efficacy and safety, respectively. The findings of the efficacy and safety comparisons may support the selection of appropriate treatments in clinical practice. PROSPERO registration: CRD42020153640.
1. Background
Over the past few decades, colorectal cancer (CRC) has been a global public health issue, with an estimated 1.8 million cases newly diagnosed in 2018 [1]. It is still the second most common cancer in men and the third most common cancer in women. Approximately 25% of CRC patients have metastatic disease at the time of diagnosis, and metastases develop in approximately 20–50% of people who have undergone surgical treatment for the early stage of CRC [2].
In clinical practice, systemic treatments, which commonly combine cytotoxic agents and biological targeted agents to optimize the treatment effects, are proposed for unresectable metastatic CRC (mCRC) [2]. The National Comprehensive Cancer Network (NCCN) guidelines recommend chemotherapy options for people with mCRC, including 5-fluorouracil and folinic acid, in combination with oxaliplatin (FOLFOX), irinotecan (FOLFIRI), oxaliplatin and irinotecan (FOLFOXIRI), and capecitabine in combination with oxaliplatin (CAPEOX) [3,4]. These regimens are introduced and then combined with bevacizumab (Bmab) [5], a monoclonal antibody against vascular endothelial growth factor (VEGF), to increase their activity [3,4]. In patients with mCRC harboring mutations in exons 2, 3, and 4 of the Kirsten rat sarcoma (KRAS) and neuroblastoma rat sarcoma (NRAS) genes, which belong to oncogenes from the rat sarcoma (RAS) gene family, cetuximab (Cmab) and panitumumab (Pmab) are suggested to be combined with the FOLFOX or FOLFIRI regimen [3,4,6,7]. These two anti-epidermal growth factor receptor (EGFR) treatments require confirmation of RAS wild-type before administration.
In clinical practice, questions regarding which chemotherapy or targeted therapy provides higher efficacy and lower toxicity, and whether the addition of targeted therapy to chemotherapy not only increases the treatment effect but also reduces adverse events (AEs), have been raised. A previous network meta-analysis (NMA) of 38 various regimens provided the treatment ranking results to suggest first-line or second-line treatment [8]. However, the data on overall survival (OS), which is known to be the gold standard in clinical trials [9], have not been available for all treatments. Given that findings of various outcomes in the previous NMA may make it difficult to directly answer questions regarding the efficacy and safety, we shifted our interest to calculating the predicted hazard ratio (HR) and 95% credible interval (CrI) for OS based on other surrogate endpoints, including the overall response rate (ORR) and/or progression-free survival (PFS), combined with the observed HR for OS as the efficacy and the odds ratio (OR) for AEs grade ≥3 as safety. Additionally, we performed an NMA of randomized controlled trials (RCTs) to suggest candidate treatments.
2. Methods
2.1. Data Sources
We used data from the previous systematic review and NMA, which included treatment effect sizes from 94 previous RCTs for advanced/metastatic (a/m)CRC [8]. In the current study, RCTs that did not include the following treatments were excluded: FOLFOX/FOLFIRI±Bmab/Cmab/Pmab and CAPEOX/FOLFOXIRI±Bmab [8]. Studies that did not report all ORR, PFS, OS, or AEs grade ≥3 outcomes were also excluded. ORR was defined based on the Response Evaluation Criteria in Solid Tumors guidelines [10] or the World Health Organization recommendations [11]. PFS was defined as the time frame from randomization to objective progression, death, or last tumor assessment without progression before any additional anticancer therapy, whichever occurred first [12]. OS was defined as the time frame from randomization to death or the last date that the patient was known to be alive for those not known to have died, whichever occurred first [12]. As a result, a total of 49 studies from 45 RCTs were included in the final analysis [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61].
2.2. Statistical Analyses
2.2.1. Multivariate Random Effects Model for Surrogate Endpoints
We applied the trivariate random effects model, which was developed and validated by Bujkiewiecz et al., to estimate the joint treatment effects of final outcomes on surrogate endpoints [62]. In general, treatment effects on OS and PFS were presented by calculating HRs, and the treatment effect on ORR was obtained by computing ORs. In the current study, representing log OR on ORR, representing log HR on PFS, and representing log HR on OS are assumed to be correlated and normally distributed [63]:
where are the true treatment effects, are the corresponding variances of treatment effects for individual study and outcome , and are within-study correlations among these estimates. The between-study variability is estimated by modeling in a conditional univariate normal distribution with structured covariance [63]:
where the variances are related to the between-study heterogeneity parameters through the regression coefficients , which are related to both and between-study correlations . Given that HR of OS is positively associated with HR of PFS and negatively associated with OR of ORR, we allocated uniform prior distributions for the between-study correlations, and . Additionally, we assigned half-normal distributions for heterogeneity parameters, , and normal distributions for other parameters, .
For studies reporting OS and a single treatment effect of ORR or PFS only, the reduced model of bivariate random effects [63] was similarly applied to investigate the association between OS and ORR or between OS and PFS.
The estimated parameters were borrowed from multivariate random effects (MVRE) models, and the predicted log HR for OS was calculated for studies reporting OR for ORR and/or HR for PFS but not HR for OS.
2.2.2. Network Meta-Regression Analysis of Treatment Therapies
In the network meta-analysis, we calculated the pooled HR of OS and the pooled OR of AEs grade ≥3 to compare the pairwise efficacy and safety between mCRC treatments, following a generalized linear model [64,65]:
where the trial-specific effects of treatment in arm 1 of trial i denote , the trial-specific effects of treatment in arm k compared with the treatment in arm 1 in the same trial denote , and and are the treatments in arm k and arm 1 of trial i. The trial-level subgroup indicator is defined as
Furthermore, the consistency assumption between direct and indirect estimates and the between-study heterogeneity were evaluated by conducting the node-splitting statistic and calculating the I2 values [66].
We additionally ranked the treatment based on the surface under the cumulative ranking curve (SUCRA) values [67]. The SUCRA value for treatment is defined as follows:
where is the number of treatments, and is the cumulative probability that treatment ranks th best and is calculated as
where P(i,j) is the probability that treatment i ranks th for a particular outcome of OS and AEs grade ≥3.
The SUCRA value is therefore a representative number of the overall ranking, which ranges from 0 to 1 [67]. A higher SUCRA value indicates a higher probability of the efficacy or safety endpoint [67]. The SUCRA values were standardized and presented in a two-dimensional plot according to the efficacy and safety outcomes. We then applied the k-means clustering method to group treatments showing high efficacy and safety, high efficacy and low safety, high safety and low efficacy, and low efficacy and safety [68].
All the models regarding the Bayesian approach were performed in WinBUGS 1.4.3 (MRC Biostatistics Unit, UK) [69], using 3 chains and 150,000 iterations of the Markov chain Monte Carlo simulation process (including 50,000 burn-in iterations).
The study methodology and progress were registered and approved by the National Institute for Health Research—an international prospective register of systematic reviews (PROSPERO registration number: CRD42020153640).
3. Results
3.1. Association between Surrogacy Endpoint and Correlated Outcome
The characteristics and findings from 49 included studies are summarized in Table 1. Bivariate and trivariate random effect models were carried out for the surrogacy associations between treatment effect sizes. Then, we calculated the predicted HRs (95% CIs) for the OS of 17 and five study populations that reported results for ORR only and both ORR and PFS, respectively (Table 1). The predicted OS was not significantly different for all 22 pairwise treatment comparisons.

Table 1.
Individual study characteristics and treatment effect predictions on correlated outcome.
Table 2 shows the surrogacy parameters of the MVRE models. The 95% CrIs of posterior intercepts containing zero confirmed that no treatment effect on a surrogate endpoint(s) suggested no treatment effect on the outcome. In other words, in studies with no significant differences between the intervention and comparison groups in terms of ORR and/or PFS, there were no differences in OS between the groups either.

Table 2.
Surrogacy parameters of multivariate random effects models.
When the 95% CrI posterior slope did not contain a zero, positive slopes indicated significant positive associations and negative slopes indicated significant negative associations between treatment effects on surrogate endpoints and the outcome. As a result, the treatment effect on OS was observed to be significantly positively associated with the treatment effect on PFS (posterior slope = 0.79, 95% CrI = 0.49–1.09), and the adjusted R-squared value was relatively high (posterior R-squared = 0.54, 95% CrI = 0.25–0.76), with somewhat low variance (posterior variance = 0.02, 95% CrI = 0.01–0.04) in the bivariate model. Although there was a significant negative association between the treatment effects on OS and ORR, the relationship was not strong, with the upper limit of the slope (−0.04) and the lower limit of the R-squared value (0.01) close to zero and somewhat high variance (posterior variance = 0.07, 95% CrI = 0.04–0.13). In the trivariate model, we observed a strong relationship between the treatment effects on OS and both ORR and PFS (posterior slope = 0.76, 95% CrI = 0.46–1.06; posterior R-squared = 0.53, 95% CrI = 0.22–0.76; posterior variance = 0.04, 95% CrI = 0.02–0.07), which was similar to that of the bivariate model of PFS as the surrogate endpoint.
3.2. Network Geometry for the Efficacy and Safety of CRC Treatments
A total of 12 commonly used regimens for a/mCRC were included in the NMA for efficacy (Figure 1A). After including the predicted estimates from MVRE models, there was new evidence of the direct comparison between FOLFOX+Bmab and FOLFOX+Cmab, FOLFOXIRI+Bmab and FOLFOXIRI, and CAPEOX and CAPEOX+Bmab (Figure 1B). The NMA for safety included 11 regimens, except for FOLFOXIRI, because data for comparative AEs grade ≥3 for FOLFOXIRI were not available from RCTs (Figure 1C).
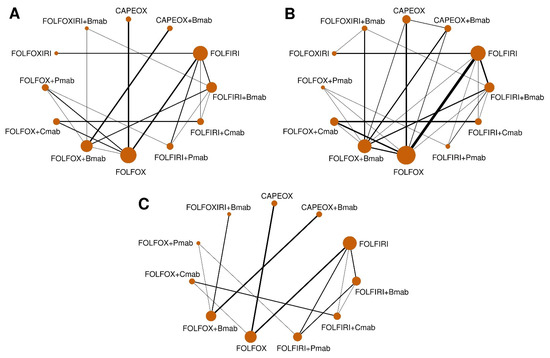
Figure 1.
Network geometry of head-to-head trials. Data are presented as networks for comparison of (A) overall survival (observed values only), (B) overall survival (combining observed and predicted values), and (C) adverse events grade ≥3. The width of each line reflects the number of studies. The sizes of the circles are proportional to the number of study participants. FOLFOX (5-fluorouracil, folinic acid, and oxaliplatin), FOLFIRI (5-fluorouracil, folinic acid, and irinotecan), FOLFOXIRI (5-fluorouracil, folinic acid, oxaliplatin, and irinotecan), CAPEOX (capecitabine and oxaliplatin), Bmab (bevacizumab), Cmab (cetuximab), and Pmab (panitumumab).
3.3. Pairwise Treatment Effect of Included Regimens
The comparative treatment effects on OS as efficacy and AEs grade ≥3 as safety are provided in Table 3. On the one hand, NMAs showed significant OS improvements with FOLFOX, FOLFOX+Cmab, and FOLFIRI+Cmab in comparison with FOLFIRI, with HR = 0.84, 95% CrI = 0.73–0.98; HR = 0.76, 95% CrI = 0.62–0.94; HR = 0.80, 95% CrI = 0.66–0.96, respectively. When irinotecan or oxaliplatin in the FOLFOXIRI regimen was replaced with Cmab, the results showed an improvement in OS by 31% (HR = 0.69, 95% CrI = 0.51–0.94) or 27% (HR = 0.73, 95% CrI = 0.54–0.97), respectively. On the other hand, the rates of AEs grade ≥3 were significantly higher among patients treated with FOLFOX+Cmab than among those treated with FOLFIRI+Bmab (OR = 2.34, 95% CrI = 1.01–4.66). Additionally, higher odds of events were observed when adding Pmab into the FOLFIRI regimen (OR = 2.16, 95% CrI = 1.09–3.84 for FOLFIRI+Pmab vs. FOLFIRI) or replacing Bmab with Pmab in the FOLFIRI+Bmab regimen (OR = 3.14, 95% CrI = 1.51–5.89 for FOLFIRI+Pmab vs. FOLFIRI+Bmab).

Table 3.
Comparative efficacy and safety of advanced or metastatic colorectal cancer treatments.
The test for consistency assumption showed that there was no significant difference between direct and indirect evidence (p > 0.05, Table 4). Additionally, substantial heterogeneity was observed for safety outcome (pairwise I2 = 71% and consistent I2 = 76%) but not for efficacy outcome (pairwise I2 = 29% and consistent I2 = 36%).

Table 4.
Assumption checking of consistency and heterogeneity.
3.4. Investigation of Treatment Ranking
Figure 2A,B show the probability of regimens becoming first- and second-line treatments, and other-line treatment probabilities are detailed in Table 5 and Table 6. As a result, while FOLFOX+Cmab had the highest probabilities of being primary (43.3%) and secondary (24.6%) treatments in terms of OS, FOLFIRI+Bmab had the highest probabilities of being both primary and secondary treatments in terms of OS (37.8% and 30.8%, respectively) for AEs grade ≥ 3. Additionally, FOLFOX+Cmab and CAPEOX also had high probabilities of being a secondary treatment regarding OS (24.6%) and primary treatment regarding AEs grade ≥ 3 (37.1%), respectively.
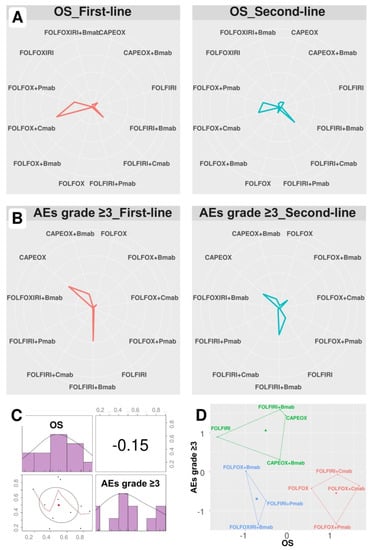
Figure 2.
Treatment ranking probability and SUCRA ranking plots. Data are presented as the probability of first- and second-line treatment ranking according to (A) overall survival and (B) adverse events grade ≥ 3. (C) Correlation and (D) k-means clustering analysis of SUCRA values. FOLFOX (5-fluorouracil, folinic acid, and oxaliplatin), FOLFIRI (5-fluorouracil, folinic acid, and irinotecan), FOLFOXIRI (5-fluorouracil, folinic acid, oxaliplatin, and irinotecan), CAPEOX (capecitabine and oxaliplatin), Bmab (bevacizumab), Cmab (cetuximab), Pmab (panitumumab), OS (overall survival), and AEs (adverse events).

Table 5.
Treatment ranking probability (%) for overall survival.

Table 6.
Treatment ranking probability (%) for adverse events grade ≥3.
As shown in Table 7, FOLFOX+Cmab was the most effective treatment regimen (SUCRA = 0.88), whereas FOLFIRI+Bmab was the safest treatment regimen (SUCRA = 0.87). The SUCRA values of regimens for cumulative ranking probabilities between OS and AEs grade ≥ 3 were slightly correlated (correlation coefficient −0.15, Figure 2C).

Table 7.
SUCRA value for treatment ranking.
K-means clustering analysis identified three clusters based on SUCRA values (Figure 2D): low efficacy and high safety (FOLFIRI±Bmab and CAPEOX±Bmab), low efficacy and safety (FOLFOX/FOLFOXIRI+Bmab and FOLFIRI+Pmab), and high efficacy and low safety (FOLFOX±Pmab and FOLFOX/FOLFIRI Cmab).
4. Discussion
This study applied MVRE models to calculate the predicted treatment effect on the correlated outcome of OS based on treatment effects on surrogated endpoints, including ORR and/or PFS. Both the observed and predicted HRs for OS as the efficacy and ORs for AEs grade ≥ 3 as safety were included in the Bayesian framework of NMA, which is based on both direct and indirect comparisons. Our findings indicated the high probabilities of FOLFOX+Cmab becoming a primary and secondary treatment in terms of efficacy and FOLFIRI+Bmab becoming primary and secondary treatment in terms of safety in the treatment of a/mCRC.
Although OS is the meaningful gold standard in oncology research and practice, surrogate endpoints have also been investigated over the past few decades because of the limitation of obtaining OS. It was reported that 84% of trials used surrogate endpoints during 2005–2012 [70] and 66% oncology indications between 2009 and 2014 [71] were approved by the United States Food and Drug Administration. In mCRC, PFS was mostly evaluated for the prognosis of OS outcome, in addition to response rate and tumor shrinkage criteria [72,73,74,75,76]. Cicero et al. recently found a moderate correlation between OS and PFS in first-line and second-line treatments with FOLFOX+Bmab for mCRC [72]. Similar findings were presented in a systematic review of twenty individual RCTs in the second-line treatment of mCRC, with moderate (0.73) and poor (0.17) correlations of PFS and ORR with OS, respectively [73]. However, the surrogacy relationships, which were performed by Bujkiewicz et al. in the Bayesian framework [62], were determined to have the advantage of considering the uncertainty of measurement errors of treatment effect on surrogate endpoints and allowed us to combine both treatments on ORR and PFS in the calculation of HR for OS [63]. In the present study, we also observed that the association between HR for OS and HR for PFS (R-squared = 0.54, 95% CrI = 0.25–0.76) was not much improved when adding OR for ORR as the second surrogate endpoint by applying the MVRE models (R-squared = 0.53, 95% CrI = 0.22–0.76).
In contrast, several studies have questioned the accuracy of surrogate endpoints in the prediction of treatment outcomes especially in oncology research [77]. A lack of validation due to weak to moderate correlations between ORR or PFS and OS was reported in patients with cancer treated with immune checkpoint inhibitors [77]. Additionally, a cross-sectional study of 51 products (26 products were assessed through conditional marketing authorization, and 25 products were assessed through accelerated assessment), regardless of treatment indications, which were authorized between 2011 and 2018 by the European Medicines Agency found that 46 approvals were based on surrogate endpoints which had not been demonstrated to obviously predict clinical outcomes [78]. In patients with mCRC, although PFS has been examined as a surrogate endpoint of OS in either literature-based (50 RCTs) [79] or individual patient-based (22 RCTs) [80] analysis, further studies of individual patient data at different time points are needed to validate the findings.
The combinations of 5-fluorouracil and folinic acid with oxaliplatin or irinotecan which were first launched in the 1990s showed a significant improvements in the response rate and survival time compared with those of regimens without oxaliplatin or irinotecan [81]. Several RCTs have been conducted to directly compare the activity of FOLFOX and FOLFIRI since then [51,53,58]. While FOLFOX was reported to be associated with an approximately 30% risk of death [51,58], another head-to-head trial [53], as well as our predicted values from RCTs reporting surrogate endpoints [56,59], showed comparative effects. The current NMA supported the superior efficacy of FOLFOX over FOLFIRI, while the safety was still equivalent. However, the choice of oxaliplatin-based or irinotecan-based therapy remains controversial. Physicians might prefer FOLFOX due to the consideration of the significant cost-effectiveness and the lower nausea and vomiting side effects than FOLFIRI, which might not be appropriate for older female patients [82]. In contrast, hand-foot syndrome is more frequent in patients treated with FOLFOX, which might not be preferred in some polar countries. Among European countries, the preference of using FOLFOX- and FOLFIRI-containing regimens in first-line and second-line treatments was also reversed between Germany–Spain and Italy–France [83]. Nevertheless, our NMA showed that the efficacy and safety between FOLFOX- and FOLFIRI-containing regimens (FOLFOX+Bmab vs. FOLFIRI+Bmab, FOLFOX+Cmab vs. FOLFIRI+Cmab, and FOLFOX+Pmab vs. FOLFIRI+Pmab) were not significantly different.
While including anti-VGFR therapy such as Bmab in chemotherapy regimens was introduced to exhibit significant benefits on OS in the ARTIST trial [38], the treatment effect on OS was not reported in other trials [17,25,34,57]. A previous NMA reported consistent findings of comparable OS and AEs grade ≥3 for FOLFOX/FOLFIRI/FOLFOXIRI/CAPEOX plus Bmab vs. chemotherapy alone, although FOLFOX/FOLFIRI plus Bmab resulted in a significantly better disease control rate and PFS than did FOLFOX/FOLFIRI [8]. Wu et al. recently reported a nonsignificant difference in OS between chemotherapy+Bmab and chemotherapy alone in the subset of KRAS (HR = 1.17, 95% CI = 0.93–1.48) and RAS wild-type (HR = 0.88, 95% CI = 0.63–1.23) mCRC patients, despite the small number of individual studies [84].
In this study, we did not observe any significant differences in OS among subjects who received chemotherapy plus Bmab, Cmab, or Pmab. However, pooled analysis for the CRC side suggested Cmab and Pmab for left-sided mCRC treatments and Bmab for right-sided mCRC treatments [84]. Additionally, the effect of anti-EGFR therapies was different according to the presence of KRAS or NRAS mutations [85]. While Cmab and Pmab showed a significant prolongation of OS or PFS among RAS wild-type mCRC patients, the survival outcomes tended to be worse among patients with RAS mutations [85]. The addition of VEGF or EGFR inhibitors into chemotherapy showed similar effects in the first-line treatment of nonmutated RAS mCRC [85]. Cmab- and Pmab-based therapies revealed significant improvements in OS of 25% and 32%, respectively, compared with chemotherapy+Bmab among subjects harboring KRAS wild-type but not RAS wild-type subjects [84].
In the present study, we took the strengths of MVRE models that take into account surrogate endpoints in the final clinical outcome. Despite the consistency of the treatment effects with the previous NMA [8], the treatment effects tended to be close to the null hypothesis in our study because we additionally considered the treatment effect on OS from RCTs that did not report the HR on OS or the HR was not reached. We additionally considered whether the treatment was used for the primary or secondary indication in the meta-regression model to justify the effect of the treatment line.
Despite its strengths, the study has some limitations. Subgroup analyses of cancer side-specific or genotype were not evaluated in the current study. We also combined the treatments based on the components of the regimens, regardless of the schedule (sequentially or continuously, doses, and orders) and drug administration routes (bolus or infusion). Although the dose reduction due to side effects was reported to not have an effect on survival for chemotherapy treatments of colon cancer, we were unable to investigate the impact of these parameters [86]. Furthermore, the types or choices of chemotherapy can depend on the site or a regional preference of the hospital when standard cares show no differences. Finally, publication bias was not assessed because of the small number of head-to-head RCTs for each treatment comparison.
5. Conclusions
In summary, we found a significant relationship between the correlated outcome of the treatment effect on OS and surrogated endpoints. The findings of efficacy and safety comparisons may support the selection of appropriate treatments in clinical practice.
Author Contributions
Conceptualization, J.K. and T.H.; methodology, T.H. and J.K.; validation, J.K.; formal analysis, T.H.; data curation, T.H. and J.K.; writing—original draft preparation, T.H.; writing—review and editing, J.K.; project administration, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the National Cancer Center Korea (Nos. 2010260 and 1910330).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D.; Group, E.G.W. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25 (Suppl. 3), iii1–iii9. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network (NCCN) Guidelines for Patients. Colon Cancer. Available online: https://www.nccn.org/patients/guidelines/colon/files/assets/common/downloads/files/colon.pdf (accessed on 4 July 2019).
- National Comprehensive Cancer Network (NCCN) Guidelines for Patients. Rectal Cancer. Available online: https://www.nccn.org/patients/guidelines/rectal/files/assets/common/downloads/files/rectal.pdf (accessed on 4 July 2019).
- Kim, L.A.; D’Amore, P.A. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am. J. Pathol. 2012, 181, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Downward, J. Targeting RAS signalling pathways in cancer therapy. Nat. Rev. Cancer 2003, 3, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S. The molecular perspective: The ras oncogene. Oncologist 1999, 4, 263–264. [Google Scholar] [CrossRef]
- Hoang, T.; Son, D.K.; Kim, B.C.; Cha, Y.; Kim, J. Efficacy and safety of systemic treatments among colorectal cancer patients: A network meta-analysis of randomized controlled trials. Crit. Rev. Oncol. Hematol. 2020. under review. [Google Scholar]
- Driscoll, J.J.; Rixe, O. Overall survival: Still the gold standard: Why overall survival remains the definitive end point in cancer clinical trials. Cancer J. 2009, 15, 401–405. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- World Health Organization. WHO Handbook for Reporting Results of Cancer Treatment. Available online: https://apps.who.int/iris/handle/10665/37200 (accessed on 12 December 2019).
- U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER). Guidance for Industry: Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. Available online: https://www.fda.gov/downloads/drugsGuidanceComplianceRegulatoyInformation/Guidance/UCM071590.pdf (accessed on 12 December 2019).
- Uetake, H.; Emi, Y.; Yamanaka, T.; Muro, K.; Oki, E.; Takahashi, T.; Nagasaka, T.; Hatano, E.; Ojima, H.; Manaka, D.; et al. A randomized phase II study of mFOLFOX6 plus bevacizumab versus mFOLFOX6 plus cetuximab for previously untreated, liverlimited metastatic colorectal cancer that is unsuitable for resection (ATOM trial). J. Clin. Oncol. 2018, 36. [Google Scholar] [CrossRef]
- Qin, S.; Guo, W.; Xu, J.M.; Li, Q.; Cheng, Y.; Liu, T.S.; Chen, J.; Chen, W.F.; Li, J. Final overall survival (OS) analysis of first-line (1L) FOLFOX-4 ± cetuximab (cet) in patients (pts) with RAS wild-type (wt) metastatic colorectal cancer (mCRC) in the phase 3 TAILOR trial. J. Clin. Oncol. 2018, 36. [Google Scholar] [CrossRef]
- Maiello, E.; Di Maggio, G.; Cordio, S.S.; Cinieri, S.; Giuliani, F.; Pisconti, S.; Rinaldi, A.; Febbraro, A.; Pia Latiano, T.; Aieta, M.; et al. Bevacizumab (B) + bi-weekly capecitabine (C) and oxaliplatin (O) (XELOX2) or FOLFOX4 in first-line treatment of metastatic colorectal cancer (mCRC): Final results of a multicenter randomized phase II trial of the Gruppo Oncologico dell’Italia Meridionale (GOIM protocol 2802). J. Clin. Oncol. 2018, 36. [Google Scholar] [CrossRef]
- Hurwitz, H.I.; Tan, B.R.; Reeves, J.A.; Xiong, H.; Somer, B.; Lenz, H.J.; Hochster, H.S.; Scappaticci, F.; Palma, J.F.; Price, R.; et al. Phase II randomized trial of sequential or concurrent FOLFOXIRI-bevacizumab versus FOLFOX-bevacizumab for metastatic colorectal cancer (STEAM). Oncologist 2019, 24, 921–932. [Google Scholar] [CrossRef] [PubMed]
- Hou, G.; Song, R.; Yang, J.; Zhang, Y.; Xiao, C.; Wang, C.; Yuan, J.; Chai, T.; Liu, Z. Treatment effect of conversion therapy and its correlation with VEGF expression in unresectable rectal cancer with liver metastasis. Oncol. Lett. 2018, 16, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Vieitez, J.M.; Gil, S.; Salvia, A.S.; Suárez, B.G.; Alfonso, P.G.; De Castro, E.M.; Quintero Aldana, G.A.; Reina, J.J.; Flores, E.G.; et al. Safety analysis of a phase III randomized trial comparing FOLFOX + Bevacizumab vs FOLFOXIRI + Bevacizumab as 1st line treatment in patients with metastatic colorectal cancer (mCRC) with ≥3 circulating tumor cells (CTCs) (VISNU-1 TTD TRIAL). J. Clin. Oncol. 2018, 36. [Google Scholar] [CrossRef]
- Schmoll, H.J.; Meinert, F.M.; Cygon, F.; Garlipp, B.; Junghanss, C.; Leithäuser, M.; Vogel, A.; Schaefers, M.; Kaiser, U.; Hoeffkes, H.G.; et al. “CHARTA”: FOLFOX/bevacizumab vs. FOLFOXIRI/bevacizumab in advanced colorectal cancer-Final results, prognostic, and potentially predictive factors from the randomized phase II trial of the AIO. J. Clin. Oncol. 2017, 35, 3533. [Google Scholar] [CrossRef]
- Carrato, A.; Abad, A.; Massuti, B.; Gravalos, C.; Escudero, P.; Longo-Munoz, F.; Manzano, J.L.; Gomez, A.; Safont, M.J.; Gallego, J.; et al. First-line panitumumab plus FOLFOX4 or FOLFIRI in colorectal cancer with multiple or unresectable liver metastases: A randomised, phase II trial (PLANET-TTD). Eur. J. Cancer 2017, 81, 191–202. [Google Scholar] [CrossRef]
- Yamazaki, K.; Nagase, M.; Tamagawa, H.; Ueda, S.; Tamura, T.; Murata, K.; Eguchi Nakajima, T.; Baba, E.; Tsuda, M.; Moriwaki, T.; et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first-line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann. Oncol. 2016, 27, 1539–1546. [Google Scholar] [CrossRef]
- Shitara, K.; Yonesaka, K.; Denda, T.; Yamazaki, K.; Moriwaki, T.; Tsuda, M.; Takano, T.; Okuda, H.; Nishina, T.; Sakai, K.; et al. Randomized study of FOLFIRI plus either panitumumab or bevacizumab for wild-type KRAS colorectal cancer-WJOG 6210G. Cancer Sci. 2016, 107, 1843–1850. [Google Scholar] [CrossRef]
- Ciardiello, F.; Normanno, N.; Martinelli, E.; Troiani, T.; Pisconti, S.; Cardone, C.; Nappi, A.; Bordonaro, A.R.; Rachiglio, M.; Lambiase, M.; et al. Cetuximab continuation after first progression in metastatic colorectal cancer (CAPRI-GOIM): A randomized phase II trial of FOLFOX plus cetuximab versus FOLFOX. Ann. Oncol. 2016, 27, 1055–1061. [Google Scholar] [CrossRef]
- Tournigand, C.; Chibaudel, B.; Samson, B.; Scheithauer, W.; Lledo, G.; Artru, P.; Viret, F.; Ramee, J.F.; Tubiana-Mathieu, N.; Dauba, J.; et al. Improving safety in first-line metastatic colorectal cancer (MCRC) therapy with bevacizumab: Modified FOLFOX7 versus XELOX2-Results of the induction phase of the GERCOR DREAM randomized phase III study. J. Clin. Oncol. 2015, 33. [Google Scholar] [CrossRef]
- Passardi, A.; Nanni, O.; Tassinari, D.; Turci, D.; Cavanna, L.; Fontana, A.; Ruscelli, S.; Mucciarini, C.; Lorusso, V.; Ragazzini, A.; et al. Effectiveness of bevacizumab added to standard chemotherapy in metastatic colorectal cancer: Final results for first-line treatment from the ITACa randomized clinical trial. Ann. Oncol. 2015, 26, 1201–1207. [Google Scholar] [CrossRef]
- Hecht, J.R.; Cohn, A.; Dakhil, S.; Saleh, M.; Piperdi, B.; Cline-Burkhardt, M.; Tian, Y.; Go, W.Y. SPIRITT: A randomized, multicenter, phase II study of panitumumab with FOLFIRI and bevacizumab with FOLFIRI as second-line treatment in patients with unresectable wild type KRAS metastatic colorectal cancer. Clin. Colorectal Cancer 2015, 14, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Gruenberger, T.; Bridgewater, J.; Chau, I.; Garcia Alfonso, P.; Rivoire, M.; Mudan, S.; Lasserre, S.; Hermann, F.; Waterkamp, D.; Adam, R. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: The OLIVIA multinational randomised phase II trial. Ann. Oncol. 2015, 26, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Loupakis, F.; Antoniotti, C.; Lupi, C.; Sensi, E.; Lonardi, S.; Mezi, S.; Tomasello, G.; Ronzoni, M.; Zaniboni, A.; et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015, 16, 1306–1315. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Rivera, F.; Karthaus, M.; Fasola, G.; Canon, J.L.; Hecht, J.R.; Yu, H.; Oliner, K.S.; Go, W.Y. PEAK: A randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J. Clin. Oncol. 2014, 32, 2240–2247. [Google Scholar] [CrossRef]
- Peeters, M.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; André, T.; Chan, E.; Lordick, F.; Punt, C.J.A.; et al. Final results from a randomized phase 3 study of FOLFIRI ± panitumumab for second-line treatment of metastatic colorectal cancer. Ann. Oncol. 2014, 25, 107–116. [Google Scholar] [CrossRef]
- Heinemann, V.; von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.E.; Heintges, T.; Lerchenmuller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Folprecht, G.; Gruenberger, T.; Bechstein, W.; Raab, H.R.; Weitz, J.; Lordick, F.; Hartmann, J.T.; Stoehlmacher-Williams, J.; Lang, H.; Trarbach, T.; et al. Survival of patients with initially unresectable colorectal liver metastases treated with FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary concept (CELIM study). Ann. Oncol. 2014, 25, 1018–1025. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Siena, S.; Cassidy, J.; Tabernero, J.; Burkes, R.; Barugel, M.; Humblet, Y.; Bodoky, G.; Cunningham, D.; Jassem, J.; et al. Final results from PRIME: Randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann. Oncol. 2014, 25, 1346–1355. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, S.; Ma, D.; Hu, L. A multi-center randomized phase II clinical study of bevacizumab plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second-line treatment for Chinese patients with metastatic colorectal cancer. Med. Oncol. 2014, 32, 1–5. [Google Scholar] [CrossRef]
- Personeni, N.; Rimassa, L.; Verusio, C.; Barni, S.; Destro, A.; Raschioni, C.; Armenia, S.; Floriani, I.; Gerardi, C.; Monteforte, M.; et al. Prognostic factors in KRAS wild-type (wt) metastatic colorectal cancer (mCRC) patients (pts) treated with biweekly cetuximab (C) plus irinotecan, fluorouracil, and leucovorin (FOLFIRI): A phase II study. J. Clin. Oncol. 2013, 31, e14611. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Kohne, C.H.; Lang, I.; Folprecht, G.; Nowacki, M.P.; Cascinu, S.; Shchepotin, I.; Maurel, J.; Cunningham, D.; Tejpar, S.; et al. Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: Updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J. Clin. Oncol. 2011, 29, 2011–2019. [Google Scholar] [CrossRef] [PubMed]
- Masi, G.; Vasile, E.; Loupakis, F.; Cupini, S.; Fornaro, L.; Baldi, G.; Salvatore, L.; Cremolini, C.; Stasi, I.; Brunetti, I.; et al. Randomized trial of two induction chemotherapy regimens in metastatic colorectal cancer: An updated analysis. J. Natl. Cancer Inst. 2011, 103, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.Z.; Xu, J.M.; Luo, R.C.; Feng, F.Y.; Wang, L.W.; Shen, L.; Yu, S.Y.; Ba, Y.; Liang, J.; Wang, D.; et al. Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: A randomized phase III ARTIST trial. Chin. J. Cancer 2011, 30, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Ducreux, M.; Bennouna, J.; Hebbar, M.; Ychou, M.; Lledo, G.; Conroy, T.; Adenis, A.; Faroux, R.; Rebischung, C.; Bergougnoux, L.; et al. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/leucovorin plus oxaliplatin (FOLFOX-6) as first-line treatment for metastatic colorectal cancer. Int. J. Cancer 2011, 128, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.; Clarke, S.; Diaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Rittweger, K.; Gilberg, F.; Saltz, L. XELOX vs FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results. Br. J. Cancer 2011, 105, 58–64. [Google Scholar] [CrossRef]
- Vamvakas, L.; Athanasiadis, A.; Karampeazis, A.; Kakolyris, S.; Polyzos, A.; Kouroussis, C.; Ziras, N.; Kalbakis, K.; Georgoulias, V.; Souglakos, J. Clinical outcome of elderly patients with metastatic colorectal cancer treated with FOLFOXIRI versus FOLFIRI: Subgroup analysis of a randomized phase III trial from the Hellenic Oncology Research Group (HORG). Crit. Rev. Oncol. Hematol. 2010, 76, 61–70. [Google Scholar] [CrossRef]
- Peeters, M.; Price, T.J.; Cervantes, A.; Sobrero, A.F.; Ducreux, M.; Hotko, Y.; Andre, T.; Chan, E.; Lordick, F.; Punt, C.J.; et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J. Clin. Oncol. 2010, 28, 4706–4713. [Google Scholar] [CrossRef]
- Ocvirk, J.; Brodowicz, T.; Wrba, F.; Ciuleanu, T.E.; Kurteva, G.; Beslija, S.; Koza, I.; Papai, Z.; Messinger, D.; Yilmaz, U.; et al. Cetuximab plus FOLFOX6 or FOLFIRI in metastatic colorectal cancer: CECOG trial. World J. Gastroenterol. 2010, 16, 3133–3143. [Google Scholar] [CrossRef]
- Folprecht, G.; Gruenberger, T.; Bechstein, W.O.; Raab, H.R.; Lordick, F.; Hartmann, J.T.; Lang, H.; Frilling, A.; Stoehlmacher, J.; Weitz, J.; et al. Tumour response and secondary resectability of colorectal liver metastases following neoadjuvant chemotherapy with cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol. 2010, 11, 38–47. [Google Scholar] [CrossRef]
- Bokemeyer, C.; Bondarenko, I.; Makhson, A.; Hartmann, J.T.; Aparicio, J.; de Braud, F.; Donea, S.; Ludwig, H.; Schuch, G.; Stroh, C.; et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J. Clin. Oncol. 2009, 27, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.L.; Cox, J.V.; Butts, C.; Navarro, M.; Bang, Y.J.; Goel, R.; Gollins, S.; Siu, L.L.; Laguerre, S.; Cunningham, D. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: A randomized phase III noninferiority study. Ann. Oncol. 2008, 19, 1720–1726. [Google Scholar] [CrossRef] [PubMed]
- Hochster, H.S.; Hart, L.L.; Ramanathan, R.K.; Childs, B.H.; Hainsworth, J.D.; Cohn, A.L.; Wong, L.; Fehrenbacher, L.; Abubakr, Y.; Saif, M.W.; et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: Results of the TREE Study. J. Clin. Oncol. 2008, 26, 3523–3529. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, J.; Clarke, S.; Diaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.S.; Rivera, F.; et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J. Clin. Oncol. 2008, 26, 2006–2012. [Google Scholar] [CrossRef] [PubMed]
- Porschen, R.; Arkenau, H.T.; Kubicka, S.; Greil, R.; Seufferlein, T.; Freier, W.; Kretzschmar, A.; Graeven, U.; Grothey, A.; Hinke, A.; et al. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: A final report of the AIO Colorectal Study Group. J. Clin. Oncol. 2007, 25, 4217–4223. [Google Scholar] [CrossRef]
- Falcone, A.; Ricci, S.; Brunetti, I.; Pfanner, E.; Allegrini, G.; Barbara, C.; Crino, L.; Benedetti, G.; Evangelista, W.; Fanchini, L.; et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: The Gruppo Oncologico Nord Ovest. J. Clin. Oncol. 2007, 25, 1670–1676. [Google Scholar] [CrossRef]
- Goldberg, R.M.; Sargent, D.J.; Morton, R.F.; Fuchs, C.S.; Ramanathan, R.K.; Williamson, S.K.; Findlay, B.P.; Pitot, H.C.; Alberts, S. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: A North American Intergroup Trial. J. Clin. Oncol. 2006, 24, 3347–3353. [Google Scholar] [CrossRef]
- Polikoff, J.; Mitchell, E.P.; Badarinath, S.; Graham, C.D.; Jennis, A.; Chen, T.T.; Gustafson, T.N.; Langer, C. Erbitux (cetuximab) plus FOLFOX for colorectal cancer (EXPLORE): Preliminary efficacy analysis of a randomized phase III trial. Annu. Meet. ASCO 2005, 23, 264. [Google Scholar]
- Kalofonos, H.P.; Aravantinos, G.; Kosmidis, P.; Papakostas, P.; Economopoulos, T.; Dimopoulos, M.; Skarlos, D.; Bamias, A.; Pectasides, D.; Chalkidou, S.; et al. Irinotecan or oxaliplatin combined with leucovorin and 5-fluorouracil as first-line treatment in advanced colorectal cancer: A multicenter, randomized, phase II study. Ann. Oncol. 2005, 16, 869–877. [Google Scholar] [CrossRef]
- Comella, P.; Massidda, B.; Filippelli, G.; Palmeri, S.; Natale, D.; Farris, A.; De Vita, F.; Buzzi, F.; Tafuto, S.; Maiorino, L.; et al. Oxaliplatin plus high-dose folinic acid and 5-fluorouracil i.v. bolus (OXAFAFU) versus irinotecan plus high-dose folinic acid and 5-fluorouracil i.v. bolus (IRIFAFU) in patients with metastatic colorectal carcinoma: A Southern Italy Cooperative Oncology Group phase III trial. Ann. Oncol. 2005, 16, 878–886. [Google Scholar] [CrossRef]
- Colucci, G.; Gebbia, V.; Paoletti, G.; Giuliani, F.; Caruso, M.; Gebbia, N.; Carteni, G.; Agostara, B.; Pezzella, G.; Manzione, L.; et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: A multicenter study of the Gruppo Oncologico Dell’Italia Meridionale. J. Clin. Oncol. 2005, 23, 4866–4875. [Google Scholar] [CrossRef] [PubMed]
- Tournigand, C.; Andre, T.; Achille, E.; Lledo, G.; Flesh, M.; Mery-Mignard, D.; Quinaux, E.; Couteau, C.; Buyse, M.; Ganem, G.; et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J. Clin. Oncol. 2004, 22, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Hurwitz, H.; Fehrenbacher, L.; Novotny, W.; Cartwright, T.; Hainsworth, J.; Heim, W.; Berlin, J.; Baron, A.; Griffing, S.; Holmgren, E.; et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 2004, 350, 2335–2342. [Google Scholar] [CrossRef]
- Goldberg, R.M.; Sargent, D.J.; Morton, R.F.; Fuchs, C.S.; Ramanathan, R.K.; Williamson, S.K.; Findlay, B.P.; Pitot, H.C.; Alberts, S.R. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J. Clin. Oncol. 2004, 22, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Rougier, P.; Lepille, D.; Bennouna, J.; Marre, A.; Ducreux, M.; Mignot, L.; Hua, A.; Méry-Mignard, D. Antitumor activity of three second-line treatment combinations in patients with metastatic colorectal cancer after optimal 5-FU regimen failure: A randomised, multicentre phase II study. Ann. Oncol. 2002, 13, 1558–1567. [Google Scholar] [CrossRef]
- Nct. Study of Bevacizumab + mFOLFOX6 Versus Bevacizumab + FOLFIRI with Biomarker Stratification in Participants with Previously Untreated Metastatic Colorectal Cancer (mCRC). Available online: https://clinicaltrials.gov/show/nct01374425 (accessed on 22 December 2019).
- Nct. Study Evaluating the Safety and Efficacy of FOLFIRI Plus Cetuximab or FOLFOX Plus Cetuximab as First-Line Therapy in Subjects with KRAS Wild-Type Metastatic Colorectal Cancer (APEC-Study) (APEC). Available online: https://clinicaltrials.gov/show/nct00778830 (accessed on 22 December 2019).
- Bujkiewicz, S.; Thompson, J.R.; Sutton, A.J.; Cooper, N.J.; Harrison, M.J.; Symmons, D.P.; Abrams, K.R. Multivariate meta-analysis of mixed outcomes: A Bayesian approach. Stat. Med. 2013, 32, 3926–3943. [Google Scholar] [CrossRef]
- Elia, E.G.; Stadler, N.; Ciani, O.; Taylor, R.S.; Bujkiewicz, S. Combining tumour response and progression free survival as surrogate endpoints for overall survival in advanced colorectal cancer. arXiv 2018, arXiv:1809.02935. [Google Scholar] [CrossRef]
- Dias, S.; Sutton, A.J.; Ades, A.E.; Welton, N.J. Evidence synthesis for decision making 2: A generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med. Decis. Mak. 2013, 33, 607–617. [Google Scholar] [CrossRef]
- Dias, S.; Sutton, A.J.; Welton, N.J.; Ades, A.E. Evidence synthesis for decision making 3: Heterogeneity—Subgroups, meta-regression, bias, and bias-adjustment. Med. Decis. Mak. 2013, 33, 618–640. [Google Scholar] [CrossRef]
- van Valkenhoef, G.; Kuiper, J. Gemtc: Network Meta-Analysis Using Bayesian Methods. Available online: https://cran.r-project.org/web/packages/gemtc/gemtc.pdf (accessed on 17 August 2019).
- Daly, C.H.; Neupane, B.; Beyene, J.; Thabane, L.; Straus, S.E.; Hamid, J.S. Empirical evaluation of SUCRA-based treatment ranks in network meta-analysis: Quantifying robustness using Cohen’s kappa. BMJ Open 2019, 9, e024625. [Google Scholar] [CrossRef]
- Kassambara, A.; Fabian, M. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. Available online: http://www.sthda.com/english/rpkgs/factoextra (accessed on 22 December 2019).
- van de Schoot, R.; Kaplan, D.; Denissen, J.; Asendorpf, J.B.; Neyer, F.J.; van Aken, M.A.G. A gentle introduction to bayesian analysis: Applications to developmental research. Child. Dev. 2014, 85, 842–860. [Google Scholar] [CrossRef] [PubMed]
- Haslam, A.; Hey, S.P.; Gill, J.; Prasad, V. A systematic review of trial-level meta-analyses measuring the strength of association between surrogate end-points and overall survival in oncology. Eur. J. Cancer 2019, 106, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Kemp, R.; Prasad, V. Surrogate endpoints in oncology: When are they acceptable for regulatory and clinical decisions, and are they currently overused? BMC Med. 2017, 15, 134. [Google Scholar] [CrossRef]
- Cicero, G.; De Luca, R.; Dieli, F. Progression-free survival as a surrogate endpoint of overall survival in patients with metastatic colorectal cancer. Onco. Targets Ther. 2018, 11, 3059–3063. [Google Scholar] [CrossRef] [PubMed]
- Cremolini, C.; Antoniotti, C.; Pietrantonio, F.; Berenato, R.; Tampellini, M.; Baratelli, C.; Salvatore, L.; Marmorino, F.; Borelli, B.; Nichetti, F.; et al. Surrogate Endpoints in Second-Line Trials of Targeted Agents in Metastatic Colorectal Cancer: A Literature-Based Systematic Review and Meta-Analysis. Cancer Res. Treat. 2017, 49, 834–845. [Google Scholar] [CrossRef]
- Montagnani, F.; Di Leonardo, G.; Pino, M.S.; Martella, F.; Perboni, S.; Ribecco, A.; Fioretto, L. Progression-free Survival as a Surrogate End-point in Advanced Colorectal Cancer Treated with Antiangiogenic Therapies. Anticancer Res. 2016, 36, 4259–4265. [Google Scholar]
- Sidhu, R.; Rong, A.; Dahlberg, S. Evaluation of progression-free survival as a surrogate endpoint for survival in chemotherapy and targeted agent metastatic colorectal cancer trials. Clin. Cancer Res. 2013, 19, 969–976. [Google Scholar] [CrossRef]
- Tang, P.A.; Bentzen, S.M.; Chen, E.X.; Siu, L.L. Surrogate end points for median overall survival in metastatic colorectal cancer: Literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J. Clin. Oncol. 2007, 25, 4562–4568. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, M.; Costanzo, A.; Rampulla, V.; Varricchio, A.; Tomasello, G. Surrogate endpoints in immunotherapy trials for solid tumors. Ann. Transl. Med. 2019, 7, 154. [Google Scholar] [CrossRef]
- Schuster Bruce, C.; Brhlikova, P.; Heath, J.; McGettigan, P. The use of validated and nonvalidated surrogate endpoints in two European Medicines Agency expedited approval pathways: A cross-sectional study of products authorised 2011–2018. PLoS Med. 2019, 16, e1002873. [Google Scholar] [CrossRef]
- Giessen, C.; Laubender, R.P.; Ankerst, D.P.; Stintzing, S.; Modest, D.P.; Mansmann, U.; Heinemann, V. Progression-free survival as a surrogate endpoint for median overall survival in metastatic colorectal cancer: Literature-based analysis from 50 randomized first-line trials. Clin. Cancer Res. 2013, 19, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; de Gramont, A.; Grothey, A.; Zalcberg, J.; Chibaudel, B.; Schmoll, H.J.; Seymour, M.T.; Adams, R.; Saltz, L.; Goldberg, R.M.; et al. Individual patient data analysis of progression-free survival versus overall survival as a first-line end point for metastatic colorectal cancer in modern randomized trials: Findings from the analysis and research in cancers of the digestive system database. J. Clin. Oncol. 2015, 33, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Mohelnikova-Duchonova, B.; Melichar, B.; Soucek, P. FOLFOX/FOLFIRI pharmacogenetics: The call for a personalized approach in colorectal cancer therapy. World J. Gastroenterol. 2014, 20, 10316–10330. [Google Scholar] [CrossRef] [PubMed]
- Neugut, A.I.; Lin, A.; Raab, G.T.; Hillyer, G.C.; Keller, D.; O’Neil, D.S.; Accordino, M.K.; Kiran, R.P.; Wright, J.; Hershman, D.L. FOLFOX and FOLFIRI Use in Stage IV Colon Cancer: Analysis of SEER-Medicare Data. Clin. Colorectal Cancer 2019, 18, 133–140. [Google Scholar] [CrossRef]
- Zhao, Z.; Pelletier, E.; Barber, B.; Bhosle, M.; Wang, S.; Gao, S.; Klingman, D. Patterns of treatment with chemotherapy and monoclonal antibodies for metastatic colorectal cancer in Western Europe. Curr. Med. Res. Opin. 2012, 28, 221–229. [Google Scholar] [CrossRef]
- Wu, C.C.; Wang, J.H.; Lin, P.C.; Liang, C.A.; Huang, C.Y.; Lien, H.C.; Chen, C.Y.; Chou, K.J.; Su, Y.C. Tumor sidedness and efficacy of first-line therapy in patients with RAS/BRAF wild-type metastatic colorectal cancer: A network meta-analysis. Crit. Rev. Oncol. Hematol. 2019, 145, 102823. [Google Scholar] [CrossRef]
- Bupathi, M.; Ahn, D.H.; Bekaii-Saab, T. Spotlight on bevacizumab in metastatic colorectal cancer: Patient selection and perspectives. Gastrointest Cancer 2016, 6, 21–30. [Google Scholar] [CrossRef]
- Munker, S.; Gerken, M.; Fest, P.; Ott, C.; Schnoy, E.; Fichtner-Feigl, S.; Wiggermann, P.; Vogelhuber, M.; Herr, W.; Stroszczynski, C.; et al. Chemotherapy for metastatic colon cancer: No effect on survival when the dose is reduced due to side effects. BMC Cancer 2018, 18, 455. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).