Recent Progress in the Systemic Treatment of Advanced/Metastatic Cholangiocarcinoma
Simple Summary
Abstract
1. Introduction
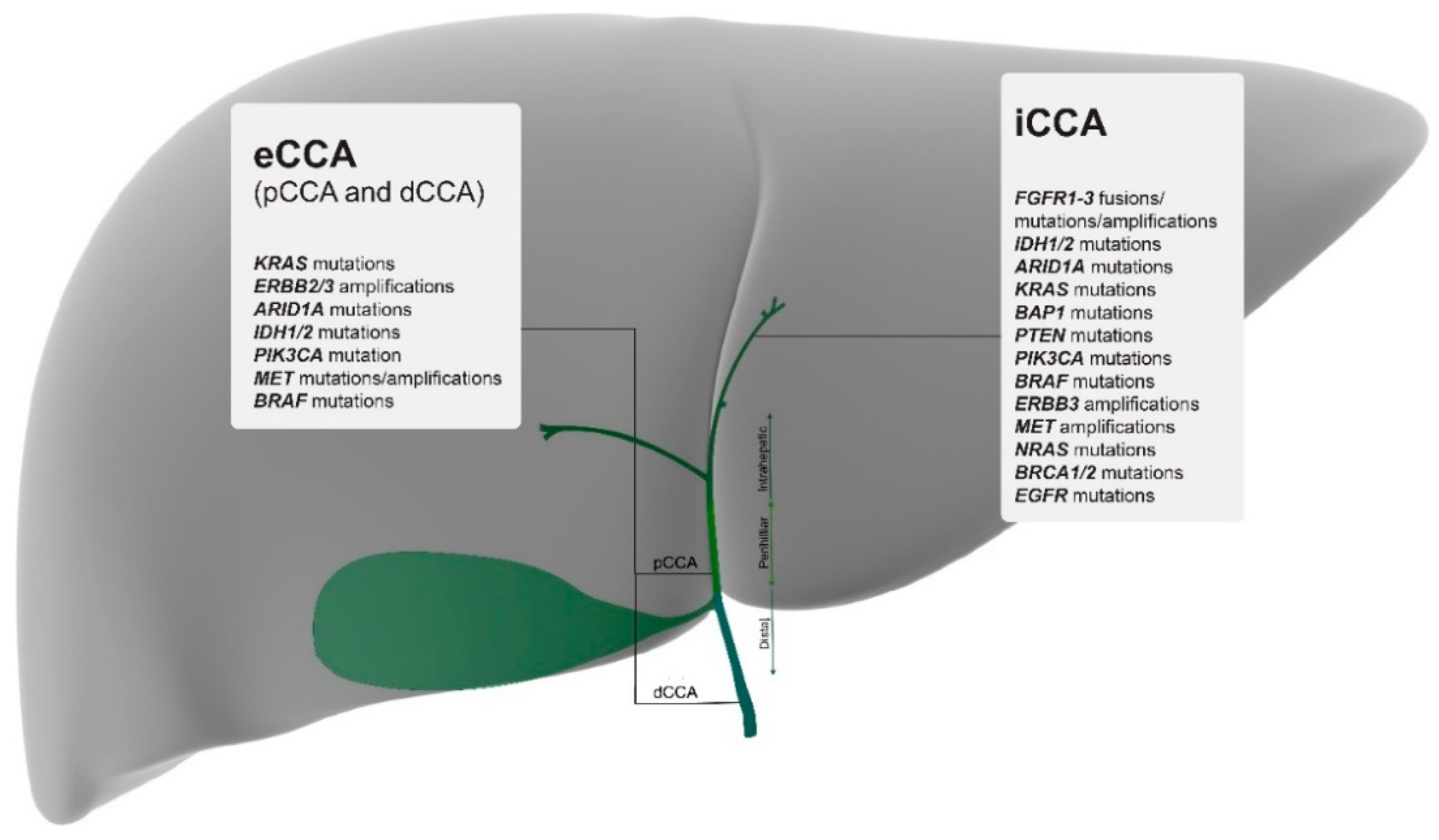
2. Genomic Characterisation by Tumour Site
3. Potential Clinical Applications of Molecular Profiling
3.1. FGFR Inhibition
3.2. IDH1 Inhibition
3.3. BRAF Inhibition
3.4. HER-2 Inhibition
3.5. Anti-Angiogenesis Targeting
3.6. DDR Targeting
3.7. Other Targeted Therapies
4. Immunotherapy Approaches
4.1. Immunology of CCAs
4.2. Checkpoint Inhibitors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Drug (Mechanism of Action) | Target | Phase | Total Number of CCA Patients Enrolled | Outcome in CCA |
|---|---|---|---|---|
| Pemigatinib (FGFR1-3 inhibitor) | FGFR alterations | 2 | 146 | ORR = 36% CR (n = 3) PR (n = 35) DOR > 12 mo (n = 7) [13,14] |
| Infigratinib (FGFR1-3 inhibitor) | FGFR2 fusions | 2 | 61 | ORR = 14.8% PFS = 5.8 mo [15] |
| Futibatenib (FGFR 1-4 inhibitor) | FGFR2 fusions, mutations, amplifications or re-arrangements | 1/2 | 45 | ORR = 25% PR (n = 4) [17,18] |
| Futibatenib (FGFR 1-4 inhibitor) | FGFR alterations + acquired resistance to FGFR inhibitors | N/A | 6 | PR (n = 2) SD (n = 2) [19] |
| Ivosidenib (IDH1 inhbitor) | IDH1 mutations | 3 | 185 | ORR = 2.4% PFS = 32% at 6 mo; 21.9% at 12 mo; OS = 9.7 mo [20] |
| Dabrafenib (BRAFV600E inhibitor) Trametinib (MEK-inhibitor) | Ras-Raf-MEK-ERK pathway BRAFV600E mutation | 2 (basket) | 33 | CCA cohort: ORR = 41% PFS = 7.2 mo OS = 11.3 mo [21] |
| Vemurafenib (BRAFV600E inhibitor) | BRAFV600E mutation | 2 (basket) | 9 | PR (n = 3) SD (n = 4) CCA survival data NA [25] |
| Trastuzumab Pertuzumab (anti-HER2-antibodies) | HER2 (amplification/overexpression) | 2 (basket) | 11 | PR (n = 4) SD (n = 3) PFS 2.8 mo–4.2 mo [28] |
| Regorafenib (tyrosine kinase inhibitor) | Angiogenesis | 2 | 66 | PFS 3.0 mo vs. 1.5 mo, HR 0.49 p: 0.004 [30] |
| Ramucirumab (anti-VEGFR) Pembrolizumab (anti- PD-1) | Angiogenesis Immune checkpoint | 1 | 26 | ORR = 4% PFS = 1.6 mo OS = 6.4 mo [31] |
| Pralsetinib (RET inhibitor) | RET mutation | 1 (basket) | 2 | PR (n = 1) DOR > 7.5 mo CCA survival NA [34] |
| Drug | Target | Phase | Trial Number |
|---|---|---|---|
| Derazantinib | FGFR2 fusion | II | NCT03230318 |
| BGJ398 (infigratinib) | FGFR2 mutation | II | NCT02150967 |
| BGJ398 + Gemcitabine + Cisplatin | FGFR2 fusion/translocation | III | NCT03773302 |
| Oral Infigratinib | FGFR 1-3 fusion or other FGFR alterations | II | NCT04233567 |
| TAS-120 | FGFR2 fusion, mutation, rearrangement or amplification | I/II | NCT02052778 |
| INCB062079 | FGF/FGFR alterations | I | NCT03144661 |
| FT 2102 Nivolumab Gemcitabin+Cisplatin | IDH1 mutation | IB/II | NCT03684811 |
| Olaparib | IDH1/2 mutation | II | NCT03212274 |
| AG-120 | IDH1 mutation | III | NCT02989857 |
| Olaparib and ceralasertib | IDH1/2-mutation | II | NCT03878095 |
| IDH305 | IDH mutations | I | NCT02381886 |
| ABM-1310 | BRAF mutation | I | NCT04190628 |
| Lenvatinib plus Pembrolizumab | VEGFR, FGFR, PDGFRα, RET, KITPD-1 checkpoint | II | NCT03797326 |
| Niraparib | BAP1 and other DNA Damage Response (DDR) Pathway alterations | II | NCT03207347 |
| Rucaparib plus Nivolumab | DNA Damage Response (DDR) Pathway alterations PD-1 checkpoint | II | NCT03639935 |
| GEMOX Cetuximab Trastuzumab Gefitinib Lapatinib Everolimus Sorafenib Crizotinib | Mutations or abnormal activation of HER-2 receptor tyrosine kinase signalling pathway | II | NCT02836847 |
| Pevonedistat Paclitaxel+Carboplatin | NEDD8-activating enzyme | II | NCT04175912 |
| ABC294640 (Opaganib) | Sphingosine-kinase2 | I/II | NCT03377179 |
| Aldesleukin Autologous CD8+ T Cell Therapy Pembrolizumab Cyclophosphamide | Targeted therapy CD8+ T Cell therapy | I | NCT02757391 |
| A166 | HER-2 Antigen or Amplified HER-2 Gene | I/II | NCT03602079 |
| Nedisertib, Avelumab and hypofractionated radiation | DNA-dependent protein kinase | I/II | NCT04068194 |
| Atezolizumab Cobimetinib | Immune checkpoint inhibitor MEK | II | NCT03201458 |
| Nivolumab Ipilimumab | CTLA-4 PD-1 checkpoints | II | NCT02834013 |
| Guadecitabine Durvalumab | DNA methyltransferase and immune checkpoint | I | NCT03257761 |
References
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B., III; D’Angelica, M.I.; Abbott, D.E.; Abrams, T.A.; Anaya, D.A.; Anders, R.; Are, C.; Borad, M.; Brown, D.; Chahal, P.; et al. NCCN Guidelines v. 4.2020 Hepatobiliary Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf (accessed on 17 July 2020).
- Rizzo, A.; Ricci, A.D.; Tober, N.; Nigro, M.C.; Mosca, M.; Palloni, A.; Abbati, F.; Frega, G.; De Lorenzo, S.; Tavolari, S.; et al. Second-line Treatment in Advanced Biliary Tract Cancer: Today and Tomorrow. Anticancer Res. 2020, 40, 3013–3030. [Google Scholar] [CrossRef] [PubMed]
- Demols, A.; Borbath, I.; Eynde, M.V.D.; Houbiers, G.; Peeters, M.; Marechal, R.; Delaunoit, T.; Goemine, J.-C.; Laurent, S.; Holbrechts, S.; et al. Regorafenib after failure of gemcitabine and platinum-based chemotherapy for locally advanced/metastatic biliary tumors: A randomized, double-blind, phase 2 trial—REACHIN. Ann. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Eckel, F.; Schmid, R.M. Chemotherapy and Targeted Therapy in Advanced Biliary Tract Carcinoma: A Pooled Analysis of Clinical Trials. Chemotherapy 2014, 60, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Bertuccio, P.; Malvezzi, M.; Carioli, G.; Hashim, D.; Boffetta, P.; El-Serag, H.B.; La Vecchia, C.; Negri, E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J. Hepatol. 2019, 71, 104–114. [Google Scholar] [CrossRef]
- Nakamura, H.; Arai, Y.; Totoki, Y.; Shirota, T.; ElZawahry, A.; Kato, M.; Hama, N.; Hosoda, F.; Urushidate, T.; Ohashi, S.; et al. Genomic spectra of biliary tract cancer. Nat. Genet. 2015, 47, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Lowery, M.A.; Ptashkin, R.N.; Jordan, E.J.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef] [PubMed]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Ni Huang, M.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef]
- Ong, C.K.; Subimerb, C.; Pairojkul, C.; Wongkham, S.; Cutcutache, I.; Yu, W.; McPherson, J.R.; Allen, G.E.; Ng, C.C.Y.; Wong, B.H.; et al. Exome sequencing of liver fluke–associated cholangiocarcinoma. Nat. Genet. 2012, 44, 690–693. [Google Scholar] [CrossRef]
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Pemigatinib: First Approval. Drugs 2020, 80, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Sahai, V.; Hollebecque, A.; Vaccaro, G.; Melisi, D.; Al-Rajabi, R.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: A multicentre, open-label, phase 2 study. Lancet Oncol. 2020, 21, 671–684. [Google Scholar] [CrossRef]
- A Gilbert, J. BGJ398 for FGFR-altered advanced cholangiocarcinoma. Lancet Oncol. 2018, 19, e16. [Google Scholar] [CrossRef]
- Goyal, L.; Saha, S.K.; Liu, L.Y.; Siravegna, G.; Leshchiner, I.; Ahronian, L.G.; Lennerz, J.K.; Vu, P.; Deshpande, V.; Kambadakone, A.; et al. Polyclonal secondary FGFR2 mutations drive acquired resistance to FGFR inhibition in patients with FGFR2 fusion-positive cholangiocarcinoma. Cancer Discov. 2016, 7, 252–263. [Google Scholar] [CrossRef]
- Tran, B.; Meric-Bernstam, F.; Arkenau, H.-T.; Bahleda, R.; Kelley, R.; Hierro, C.; Ahn, D.; Zhu, A.; Javle, M.; Winkler, R.; et al. Efficacy of TAS-120, an irreversible fibroblast growth factor receptor inhibitor (FGFRi), in patients with cholangiocarcinoma and FGFR pathway alterations previously treated with chemotherapy and other FGFRi’s. Ann. Oncol. 2018, 29, ix49–ix50. [Google Scholar] [CrossRef]
- Bahleda, R.; Meric-Bernstam, F.; Goyal, L.; Tran, B.; He, Y.; Yamamiya, I.; Benhadji, K.A.; Matos, I.; Arkenau, H.T. Phase 1, First-in-Human Study of Futibatinib, a Highly Selective, Irreversible FGFR1–4 Inhibitor in Patients with Advanced Solid Tumors. Ann. Oncol. 2020, in press. [Google Scholar] [CrossRef]
- Goyal, L.; Shi, L.; Liu, L.Y.; De La Cruz, F.F.; Lennerz, J.K.; Raghavan, S.; Leschiner, I.; Elagina, L.; Siravegna, G.; Ng, R.W.; et al. TAS-120 Overcomes Resistance to ATP-Competitive FGFR Inhibitors in Patients with FGFR2 Fusion–Positive Intrahepatic Cholangiocarcinoma. Cancer Discov. 2019, 9, 1064–1079. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Wainberg, Z.A.; Lassen, U.N.; Elez, E.; Italiano, A.; Curigliano, G.; De Braud, F.G.; Prager, G.; Greil, R.; Stein, A.; Fasolo, A.; et al. Efficacy and safety of dabrafenib (D) and trametinib (T) in patients (pts) with BRAF V600E–mutated biliary tract cancer (BTC): A cohort of the ROAR basket trial. J. Clin. Oncol. 2019, 37, 187. [Google Scholar] [CrossRef]
- Bunyatov, T.; Zhao, A.; Kovalenko, J.; Gurmikov, B.; Vishnevsky, V. Personalised approach in combined treatment of cholangiocarcinoma: A case report of healing from cholangiocellular carcinoma at stage IV. J. Gastrointest. Oncol. 2019, 10, 815–820. [Google Scholar] [CrossRef]
- Judit, K.; Árokszállási, A.; András, C.; Balogh, I.; Béres, E.; Déri, J.; Peták, I.; Jánváry, L.; Horváth, Z. Combined dabrafenib and trametinib treatment in a case of chemotherapy-refractory extrahepatic BRAF V600E mutant cholangiocarcinoma: Dramatic clinical and radiological response with a confusing synchronic new liver lesion. J. Gastrointest. Oncol. 2017, 8, E32–E38. [Google Scholar] [CrossRef]
- Loaiza-Bonilla, A.; Clayton, E.; Furth, E.; O’Hara, M.H.; Morrissette, J. Dramatic response to dabrafenib and trametinib combination in a BRAF V600E-mutated cholangiocarcinoma: Implementation of a molecular tumour board and next-generation sequencing for personalized medicine. Ecancermedicalscience 2014, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Puzanov, I.; Blay, J.-Y.; Chau, I.; Lockhart, A.C.; Raje, N.S.; Wolf, J.; Baselga, J.; Meric-Bernstam, F.; Roszik, J.; et al. Pan-Cancer Efficacy of Vemurafenib in BRAFV600-Mutant Non-Melanoma Cancers. Cancer Discov. 2020, 10, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Peck, J.; Wei, L.; Zalupski, M.; O’Neil, B.; Calero, M.V.; Bekaii-Saab, T. HER2/neu May Not Be an Interesting Target in Biliary Cancers: Results of an Early Phase II Study with Lapatinib. Oncology 2012, 82, 175–179. [Google Scholar] [CrossRef] [PubMed]
- El-Khoueiry, A.B.; Rankin, C.; Siegel, A.B.; Iqbal, S.; Gong, I.-Y.; Micetich, K.C.; Kayaleh, O.R.; Lenz, H.-J.; Blanke, C.D. S0941: A phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br. J. Cancer 2014, 110, 882–887. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Hurwitz, H.; Raghav, K.P.S.; McWilliams, R.R.; Fakih, M.; VanderWalde, A.; Swanton, C.; Kurzrock, R.; Burris, H.; Sweeney, C.; et al. Pertuzumab plus trastuzumab for HER2-amplified metastatic colorectal cancer (MyPathway): An updated report from a multicentre, open-label, phase 2a, multiple basket study. Lancet Oncol. 2019, 20, 518–530. [Google Scholar] [CrossRef]
- Yamashita-Kashima, Y.; Yoshimura, Y.; Fujimura, T.; Shu, S.; Yanagisawa, M.; Yorozu, K.; Furugaki, K.; Higuchi, R.; Shoda, J.; Harada, N. Molecular targeting of HER2-overexpressing biliary tract cancer cells with trastuzumab emtansine, an antibody–cytotoxic drug conjugate. Cancer Chemother. Pharmacol. 2019, 83, 659–671. [Google Scholar] [CrossRef]
- Arkenau, H.-T.; Martin-Liberal, J.; Calvo, E.; Penel, N.; Krebs, M.G.; Herbst, R.S.; Walgren, R.A.; Widau, R.C.; Mi, G.; Jin, J.; et al. Ramucirumab Plus Pembrolizumab in Patients with Previously Treated Advanced or Metastatic Biliary Tract Cancer: Nonrandomized, Open-Label, Phase I Trial (JVDF). Oncology 2018, 23, 1407. [Google Scholar] [CrossRef]
- Golan, T.; Raitses-Gurevich, M.; Kelley, R.K.; Bocobo, A.G.; Borgida, A.; Shroff, R.T.; Holter, S.; Gallinger, S.; Ahn, D.H.; Aderka, D.; et al. Overall Survival and Clinical Characteristics of BRCA-Associated Cholangiocarcinoma: A Multicenter Retrospective Study. Oncology 2017, 22, 804–810. [Google Scholar] [CrossRef]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef]
- Subbiah, V.; Hu, M.I.-N.; Gainor, J.F.; Mansfield, A.S.; Alonso, G.; Taylor, M.H.; Zhu, V.W.; Lopez, P.G.; Amatu, A.; Doebele, R.C.; et al. Clinical activity of the RET inhibitor pralsetinib (BLU-667) in patients with RET fusion+ solid tumors. J. Clin. Oncol. 2020, 38, 109. [Google Scholar] [CrossRef]
- Simile, M.M.; Bagella, P.; Vidili, G.; Spanu, A.; Manetti, R.; Seddaiu, M.A.; Babudieri, S.; Maddeddu, G.; Serra, P.A.; Altana, M.; et al. Targeted Therapies in Cholangiocarcinoma: Emerging Evidence from Clinical Trials. Medicina 2019, 55, 42. [Google Scholar] [CrossRef] [PubMed]
- Loeuillard, E.; Conboy, C.B.; Gores, G.J.; Rizvi, S. Immunobiology of cholangiocarcinoma. JHEP Rep. 2019, 1, 297–311. [Google Scholar] [CrossRef]
- Goeppert, B.; Frauenschuh, L.; Zucknick, M.; Stenzinger, A.; Andrulis, M.; Klauschen, F.; Joehrens, K.; Warth, A.; Renner, M.; Mehrabi, A.; et al. Prognostic impact of tumour-infiltrating immune cells on biliary tract cancer. Br. J. Cancer 2013, 109, 2665–2674. [Google Scholar] [CrossRef] [PubMed]
- Mody, K.; Starr, J.; Saul, M.; Poorman, K.; Weinberg, B.A.; Salem, M.E.; VanderWalde, A.; Shields, A.F. Patterns and genomic correlates of PD-L1 expression in patients with biliary tract cancers. J. Gastrointest. Oncol. 2019, 10, 1099–1109. [Google Scholar] [CrossRef]
- Lu, J.C.; Zeng, H.Y.; Sun, Q.M.; Meng, Q.N.; Huang, X.Y.; Zhang, P.F.; Yang, X.; Peng, R.; Gao, C.; Wei, C.Y.; et al. Distinct PD-L1/PD1 profiles and clinical implications in intrahepatic cholangiocarcinoma patients with different risk factors. Theranostics 2019, 9, 4678. [Google Scholar] [CrossRef]
- Marabelle, A.; Le, D.T.; Ascierto, P.A.; Di Giacomo, A.M.; De Jesus-Acosta, A.; Delord, J.-P.; Geva, R.; Gottfried, M.; Penel, N.; Hansen, A.R.; et al. Efficacy of Pembrolizumab in Patients with Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J. Clin. Oncol. 2019, 38, 1–10. [Google Scholar] [CrossRef]
- Azad, N.S.; Gray, R.J.; Overman, M.J.; Schoenfeld, J.D.; Mitchell, E.P.; Zwiebel, J.A.; Sharon, E.; Streicher, H.; Li, S.; McShane, L.M.; et al. Nivolumab Is Effective in Mismatch Repair-Deficient Noncolorectal Cancers: Results From Arm Z1D-A Subprotocol of the NCI-MATCH (EAY131) Study. J. Clin. Oncol. 2019, 38, 214–222. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Yarchoan, M.; Cope, L.; Anders, R.A.; Noonan, A.; Goff, L.W.; Goyal, L.; Lacy, J.; Li, D.; Patel, A.; He, A.R.; et al. Abstract CT043: A multicenter randomized phase 2 trial of atezolizumab as monotherapy or in combination with cobimetinib in biliary tract cancers (BTCs): A NCI Experimental Therapeutics Clinical Trials Network (ETCTN) study. AACR 2020, 80. [Google Scholar] [CrossRef]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fostea, R.M.; Fontana, E.; Torga, G.; Arkenau, H.-T. Recent Progress in the Systemic Treatment of Advanced/Metastatic Cholangiocarcinoma. Cancers 2020, 12, 2599. https://doi.org/10.3390/cancers12092599
Fostea RM, Fontana E, Torga G, Arkenau H-T. Recent Progress in the Systemic Treatment of Advanced/Metastatic Cholangiocarcinoma. Cancers. 2020; 12(9):2599. https://doi.org/10.3390/cancers12092599
Chicago/Turabian StyleFostea, Raluca Maria, Elisa Fontana, Gonzalo Torga, and Hendrik-Tobias Arkenau. 2020. "Recent Progress in the Systemic Treatment of Advanced/Metastatic Cholangiocarcinoma" Cancers 12, no. 9: 2599. https://doi.org/10.3390/cancers12092599
APA StyleFostea, R. M., Fontana, E., Torga, G., & Arkenau, H.-T. (2020). Recent Progress in the Systemic Treatment of Advanced/Metastatic Cholangiocarcinoma. Cancers, 12(9), 2599. https://doi.org/10.3390/cancers12092599





