Acute Radiation Colitis after Preoperative Short-Course Radiotherapy for Rectal Cancer: A Morphological, Immunohistochemical and Genetic Study
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Tumor Regression
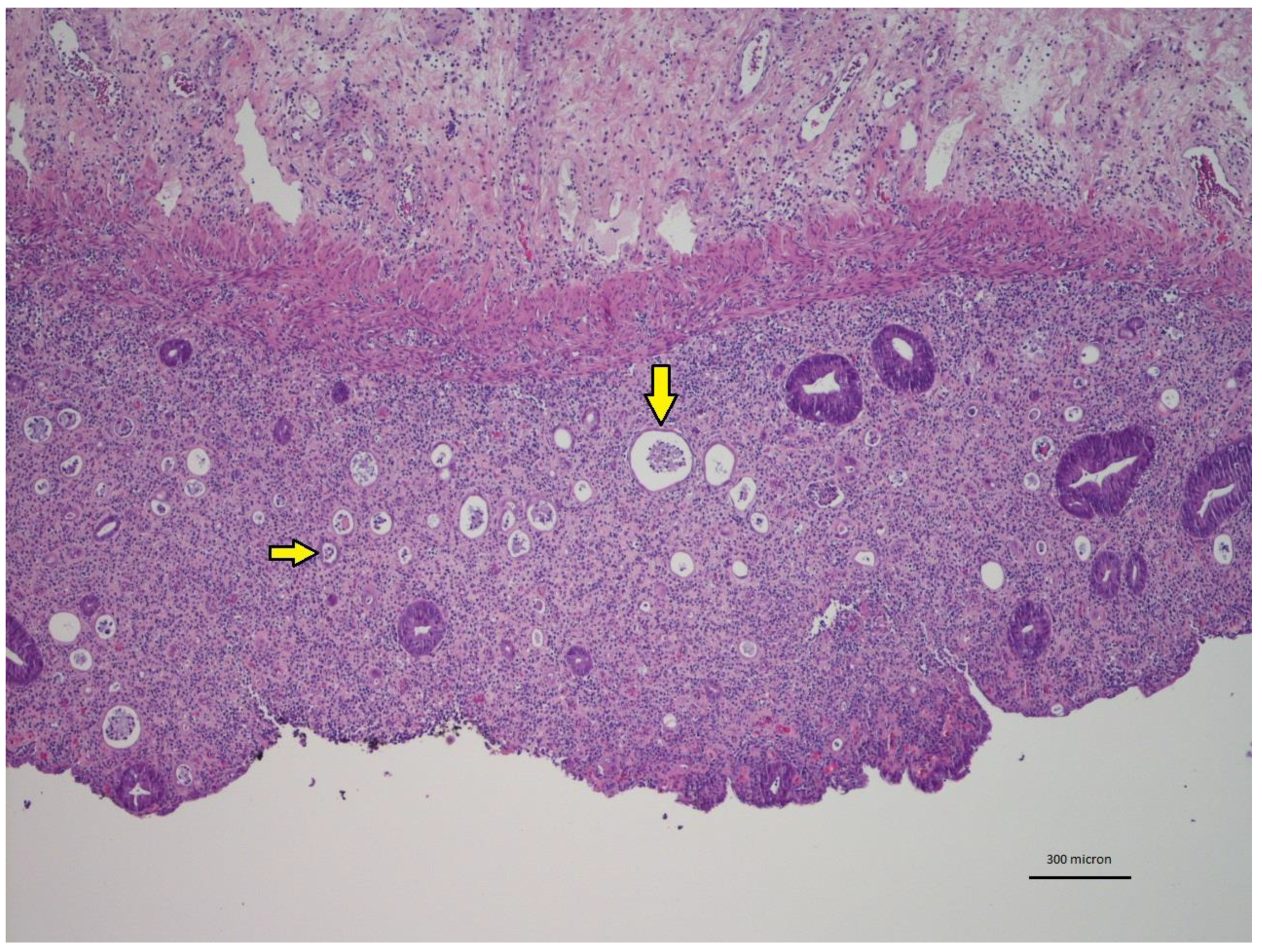
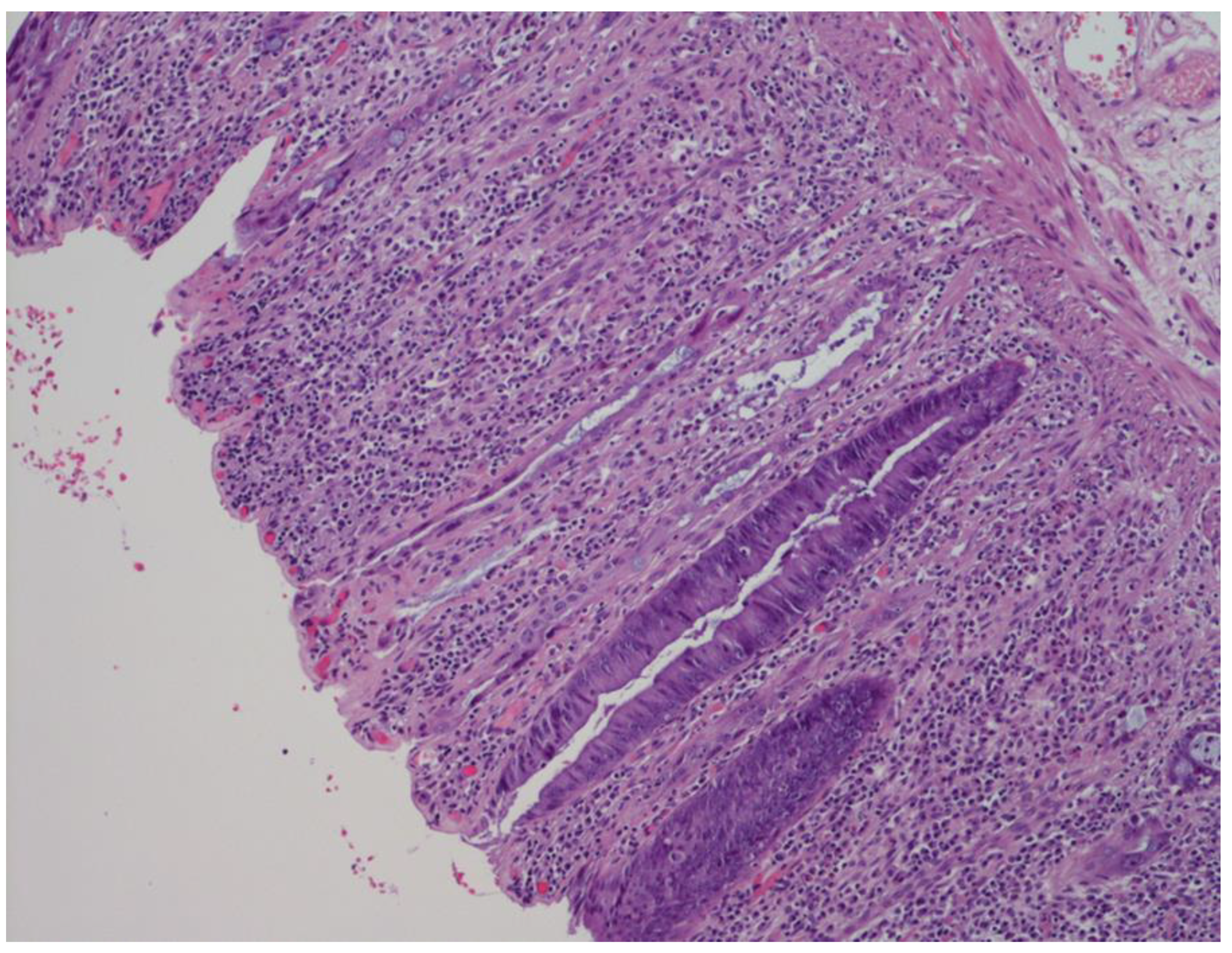
2.2. Radiation-Induced Morphological Features
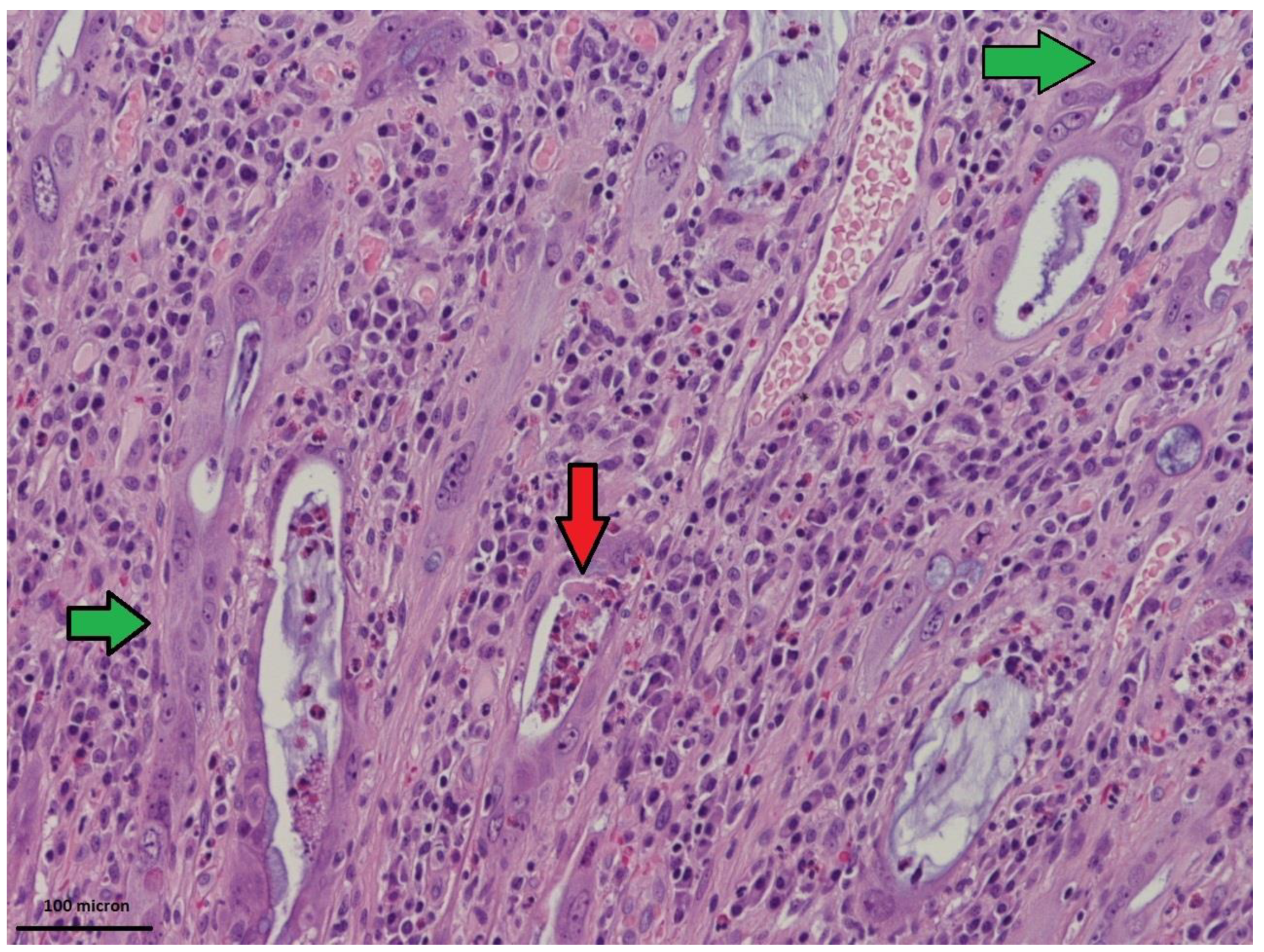
2.3. Inflammatory Component
2.4. “Dysplastic-Like” Features
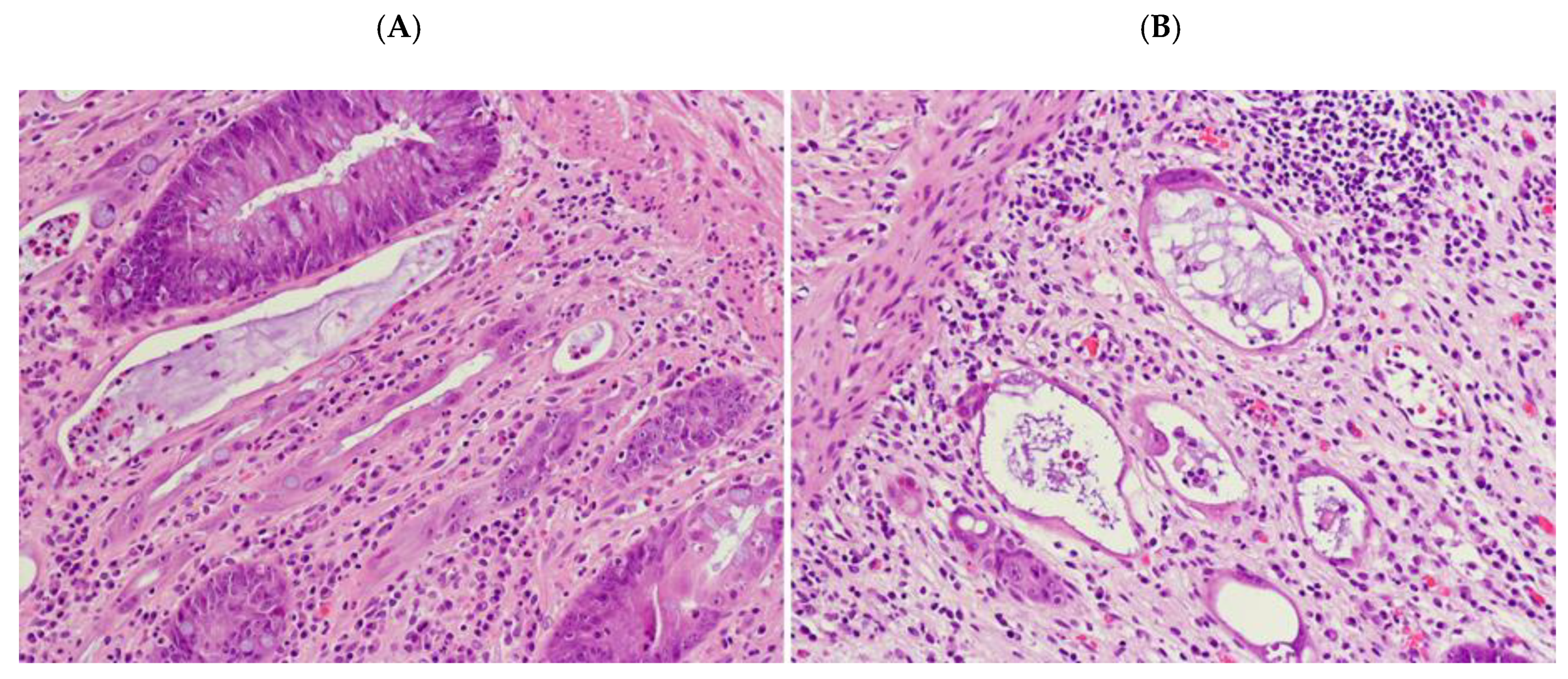
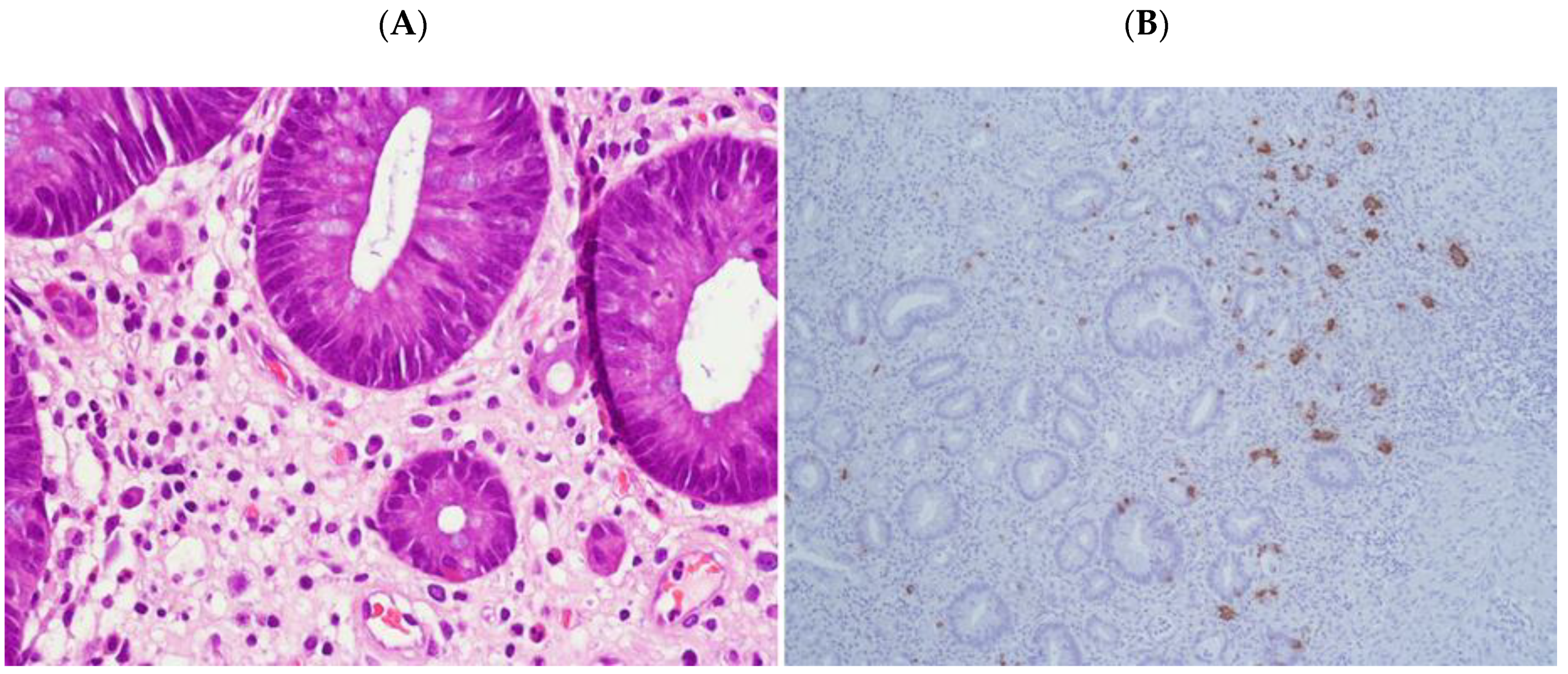
2.5. Endocrine Features
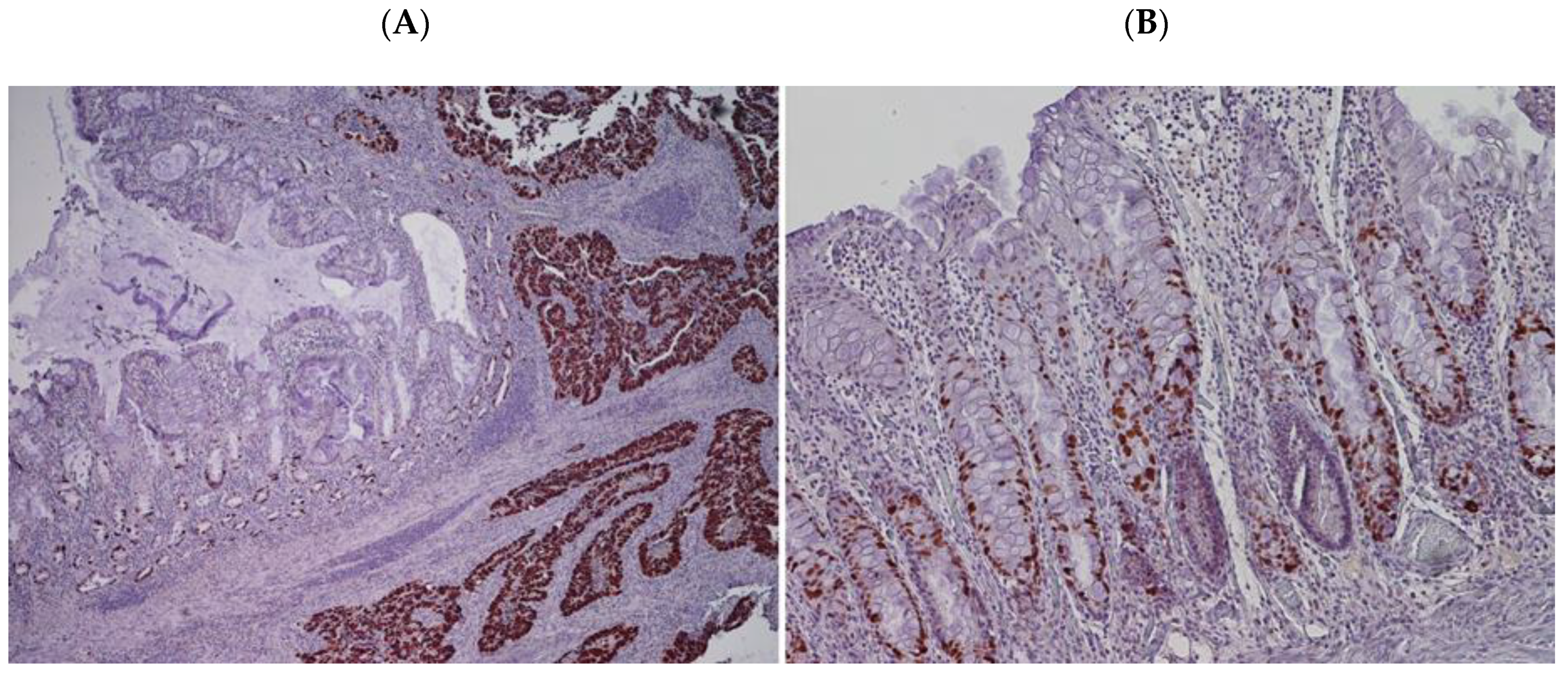
2.6. p53 Immunohistochemical Results
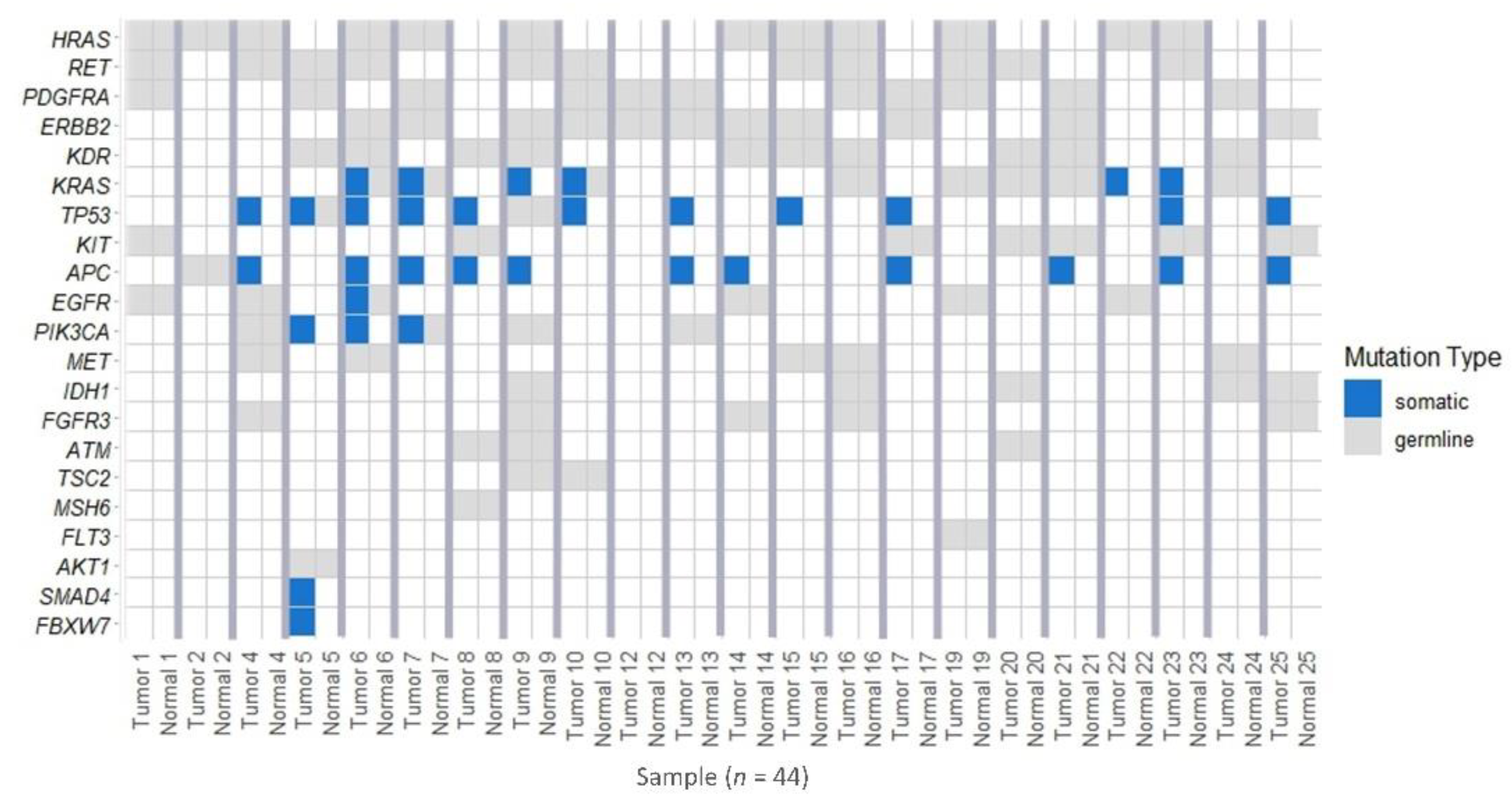
2.7. Genetic Alterations in Tumors and in Tissue Samples with “Dysplastic-Like” Features
2.8. Comparison between P53 Phenotype and TP53 Genotype in Tumor and Mucosa with “Dysplastic-Like” Features
3. Discussion
4. Materials and Methods
4.1. Patients Cohort
4.2. Morphological Examination
4.3. IHC Analysis
4.4. NGS Mutational Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO Classification of Tumours Editorial Board. Digestive System Tumours, 5th ed.; IARC: Lyon, France, 2019. [Google Scholar]
- Hol, J.C.; Van Oostendorp, S.; Tuynman, J.B.; Sietses, C. Long-term oncological results after transanal total mesorectal excision for rectal carcinoma. Tech. Coloproctology 2019, 23, 903–911. [Google Scholar] [CrossRef] [PubMed]
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: A systematic overview of 8507 patients from 22 randomised trials. Lancet 2001, 358, 1291–1304. [Google Scholar] [CrossRef]
- Ngan, S.Y.; Burmeister, B.; Fisher, R.J.; Solomon, M.J.; Goldstein, D.; Joseph, D.; Ackland, S.; Schache, D.; McClure, B.; McLachlan, S.A.; et al. Randomized Trial of Short-Course Radiotherapy Versus Long-Course Chemoradiation Comparing Rates of Local Recurrence in Patients with T3 Rectal Cancer: Trans-Tasman Radiation Oncology Group Trial. J. Clin. Oncol. 2012, 30, 3827–3833. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Burock, S.; Wernecke, K.D.; Kretzschmar, A.; Dietel, M.; Loy, V.; Koswig, S.; Budach, V.; Schlag, P.M. Preoperative short-course radiotherapy versus combined radiochemotherapy in locally advanced rectal cancer: A multi-centre prospectively randomised study of the Berlin Cancer Society. BMC Cancer 2009, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Stephens, R.J.; Thompson, L.C.; Quirke, P.; Steele, R.; Grieve, R.; Couture, J.; Griffiths, G.O.; Sebag-Montefiore, D. Impact of short-course preoperative radiotherapy for rectal cancer on patients’ quality of life: Data from the Medical Research Council CR07/National Cancer Institute of Canada Clinical Trials Group C016 randomized clinical trial. J. Clin. Oncol. 2010, 28, 4233–4239. [Google Scholar] [CrossRef] [PubMed]
- Bujko, K.; Nowacki, M.P.; Nasierowska-Guttmejer, A.; Michalski, W.; Bebenek, M.; Kryj, M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. BJS 2006, 93, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Latkauskas, T.; Pauzas, H.; Kairevičė, L.; Petrauskas, A.; Saladzinskas, Z.; Janciauskiene, R.; Gudaityte, J.; Lizdenis, P.; Svagzdys, S.; Tamelis, A.; et al. Preoperative conventional chemoradiotherapy versus short-course radiotherapy with delayed surgery for rectal cancer: Results of a randomized controlled trial. BMC Cancer 2016, 16, 927. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Liersch, T.; Fietkau, R.; Hohenberger, W.; Beißbarth, T.; Hess, C.; Becker, H.; Ghadimi, M.; Mrak, K.; Merkel, S.; et al. Tumor Regression Grading After Preoperative Chemoradiotherapy for Locally Advanced Rectal Carcinoma Revisited: Updated Results of the CAO/ARO/AIO-94 Trial. J. Clin. Oncol. 2014, 32, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Fokas, E.; Ströbel, P.; Fietkau, R.; Ghadimi, M.; Liersch, T.; Grabenbauer, G.G.; Hartmann, A.; Kaufmann, M.; Sauer, R.; Graeven, U.; et al. Tumor Regression Grading After Preoperative Chemoradiotherapy as a Prognostic Factor and Individual-Level Surrogate for Disease-Free Survival in Rectal Cancer. J. Natl. Cancer Inst. 2017, 109, 109. [Google Scholar] [CrossRef] [PubMed]
- Salmo, E.; El-Dhuwaib, Y.; Haboubi, N.Y. Histological grading of tumour regression and radiation colitis in locally advanced rectal cancer following neoadjuvant therapy: A critical appraisal. Color. Dis. 2011, 13, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Leupin, N.; Curschmann, J.; Kranzbühler, H.; Maurer, C.A.; Laissue, J.A.; Mazzucchelli, L. Acute Radiation Colitis in Patients Treated with Short-Term Preoperative Radiotherapy for Rectal Cancer. Am. J. Surg. Pathol. 2002, 26, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Hall, P.A.; Lane, D.P. P53 in tumor pathology: Can we trust immunohistochemistry? Revisited! J. Pathol. 1994, 172, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yemelyanova, A.; Vang, R.; Kshirsagar, M.; Lu, D.; A Marks, M.; Shih, I.-M.; Kurman, R.J. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: An immunohistochemical and nucleotide sequencing analysis. Mod. Pathol. 2011, 24, 1248–1253. [Google Scholar] [CrossRef]
- Colomer, A.; Erill, N.; Verdú, M.; Roman, R.; Vidal, A.; Cordon-Cardo, C.; Puig, X. Lack of p53 Nuclear Immunostaining Is Not Indicative of Absence of TP53 Gene Mutations in Colorectal Adenocarcinomas. Appl. Immunohistochem. Mol. Morphol. 2003, 11, 130–137. [Google Scholar] [CrossRef]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int. J. Color. Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, E.A.; Oshima, K.; Voltaggio, L. Survival Guide to gastrointestinal mucosal biopsies. Pathology Survival Guide First Series-Vol. 1, 1st ed.; Innovative Pathology Press: Arlington, VA, USA, 2017. [Google Scholar]
- Brien, T.P.; Farraye, F.A.; Odze, R.D. Gastric Dysplasia-Like Epithelial Atypia Associated with Chemoradiotherapy for Esophageal Cancer: A Clinicopathologic and Immunohistochemical Study of 15 Cases. Mod. Pathol. 2001, 14, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.; Duong, H. The molecular characteristics of colorectal cancer: Implications for diagnosis and therapy. Oncol. Lett. 2018, 16, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Stewart, C.J.; Hillery, S. Mucosal endocrine cell micronests and single endocrine cells following neo-adjuvant therapy for adenocarcinoma of the distal oesophagus and oesophagogastric junction. J. Clin. Pathol. 2007, 60, 1284–1289. [Google Scholar] [CrossRef] [PubMed]
- Shia, J.; Tickoo, S.K.; Guillem, J.G.; Qin, J.; Nissan, A.; Hoos, A.; Stojadinovic, A.; Ruo, L.; Wong, W.D.; Paty, P.B.; et al. Increased endocrine cells in treated rectal adenocarcinomas: A possible reflection of endocrine differentiation in tumor cells induced by chemotherapy and radiotherapy. Am. J. Surg. Pathol. 2002, 26, 863–872. [Google Scholar] [CrossRef] [PubMed]

| Clinicopathological Features | PSRT | % | PLRT | % | p Value |
|---|---|---|---|---|---|
| 45 | 47.4% | 50 | 52.6% | ||
| Age | 75.2 years | 62.4 years | <0.001 | ||
| (46–90) | (38–79) | ||||
| Sex | 0.410 | ||||
| Female | 18 | 18.9% | 25 | 26.3% | |
| Male | 27 | 28.4% | 25 | 26.3% | |
| Tumor regression system by Dworak et al. [16] | <0.001 | ||||
| Grade 0 | 31 | 32.6% | 12 | ||
| Grade 1 | 14 | 14.7% | 15 | 15.8% | |
| Grade 2 | 0 | 0.0% | 20 | 21.1% | |
| Grade 3 | 0 | 0.0% | 2 | 2.1% | |
| Grade 4 | 0 | 0.0% | 1 | 1.1% | |
| Inflammation | <0.001 | ||||
| absent | 4 | 4.2% | 35 | ||
| mild | 4 | 4.2% | 13 | 13.7% | |
| moderate | 23 | 24.2% | 1 | 1.1% | |
| severe | 14 | 14.7% | 1 | 1.1% | |
| Architectural crypt distortion | <0.001 | ||||
| absent | 4 | 4.2% | 47 | ||
| mild | 4 | 4.2% | 2 | 2.1% | |
| moderate | 19 | 20.0% | 1 | 1.1% | |
| severe | 18 | 18.9% | 0 | 0.0% | |
| Nuclear and cytoplasmic atypia of glandular epithelium | <0.001 | ||||
| absent | 4 | 4.2% | 49 | ||
| mild | 3 | 3.2% | 1 | 1.1% | |
| 20 | 21.1% | 0 | 0.0% | ||
| 18 | 18.9% | 0 | 0.0% | ||
| Apoptotic bodies | <0.001 | ||||
| 5 | 5.3% | 49 | |||
| 40 | 42.1% | 1 | 1.1% | ||
| Endocrine Features | PSRT | PLRT | p Value | ||
|---|---|---|---|---|---|
| N° | % | N° | % | ||
| 25 | 50.0% | 25 | 50.0% | ||
| Endocrine differentiation in radiation-damaged mucosa | <0.001 | ||||
| absent | 1 | 2.0% | 20 | 40.0% | |
| isolated endocrine cells | 5 | 10.0% | 5 | 10.0% | |
| endocrine cells micronests | 19 | 38.0% | 0 | 0.0% | |
| Endocrine differentiation in tumor | 0.490 | ||||
| absent | 23 | 46.0% | 25 | 50.0% | |
| isolated endocrine cells | 1 | 2.0% | 0 | 0.0% | |
| endocrine cells micronests | 1 | 2.0% | 0 | 0.0% | |
| p53 IHC | TP53 NGS | Total of Cases | |
|---|---|---|---|
| MUT | WT | ||
| Negative-pattern | 2 | 3 | 5 |
| Positive-pattern | 9 | 6 | 15 |
| Reactive-pattern | 0 | 2 | 2 |
| Total of cases | 11 | 11 | 22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zanelli, M.; Ciarrocchi, A.; De Petris, G.; Zizzo, M.; Costantini, M.; Bisagni, A.; Torricelli, F.; Nicoli, D.; Ramundo, D.; Ricci, S.; et al. Acute Radiation Colitis after Preoperative Short-Course Radiotherapy for Rectal Cancer: A Morphological, Immunohistochemical and Genetic Study. Cancers 2020, 12, 2571. https://doi.org/10.3390/cancers12092571
Zanelli M, Ciarrocchi A, De Petris G, Zizzo M, Costantini M, Bisagni A, Torricelli F, Nicoli D, Ramundo D, Ricci S, et al. Acute Radiation Colitis after Preoperative Short-Course Radiotherapy for Rectal Cancer: A Morphological, Immunohistochemical and Genetic Study. Cancers. 2020; 12(9):2571. https://doi.org/10.3390/cancers12092571
Chicago/Turabian StyleZanelli, Magda, Alessia Ciarrocchi, Giovanni De Petris, Maurizio Zizzo, Massimo Costantini, Alessandra Bisagni, Federica Torricelli, Davide Nicoli, Dafne Ramundo, Stefano Ricci, and et al. 2020. "Acute Radiation Colitis after Preoperative Short-Course Radiotherapy for Rectal Cancer: A Morphological, Immunohistochemical and Genetic Study" Cancers 12, no. 9: 2571. https://doi.org/10.3390/cancers12092571
APA StyleZanelli, M., Ciarrocchi, A., De Petris, G., Zizzo, M., Costantini, M., Bisagni, A., Torricelli, F., Nicoli, D., Ramundo, D., Ricci, S., Palicelli, A., Sanguedolce, F., Ascani, S., Castro Ruiz, C., Annessi, V., Zamponi, R., Bortesi, M., Martino, V., Marchetti, M., & De Marco, L. (2020). Acute Radiation Colitis after Preoperative Short-Course Radiotherapy for Rectal Cancer: A Morphological, Immunohistochemical and Genetic Study. Cancers, 12(9), 2571. https://doi.org/10.3390/cancers12092571






