Flavokawain B and Doxorubicin Work Synergistically to Impede the Propagation of Gastric Cancer Cells via ROS-Mediated Apoptosis and Autophagy Pathways
Simple Summary
Abstract
1. Introduction
2. Results
2.1. Combination Effects of FKB and Doxorubicin on AGS Cells
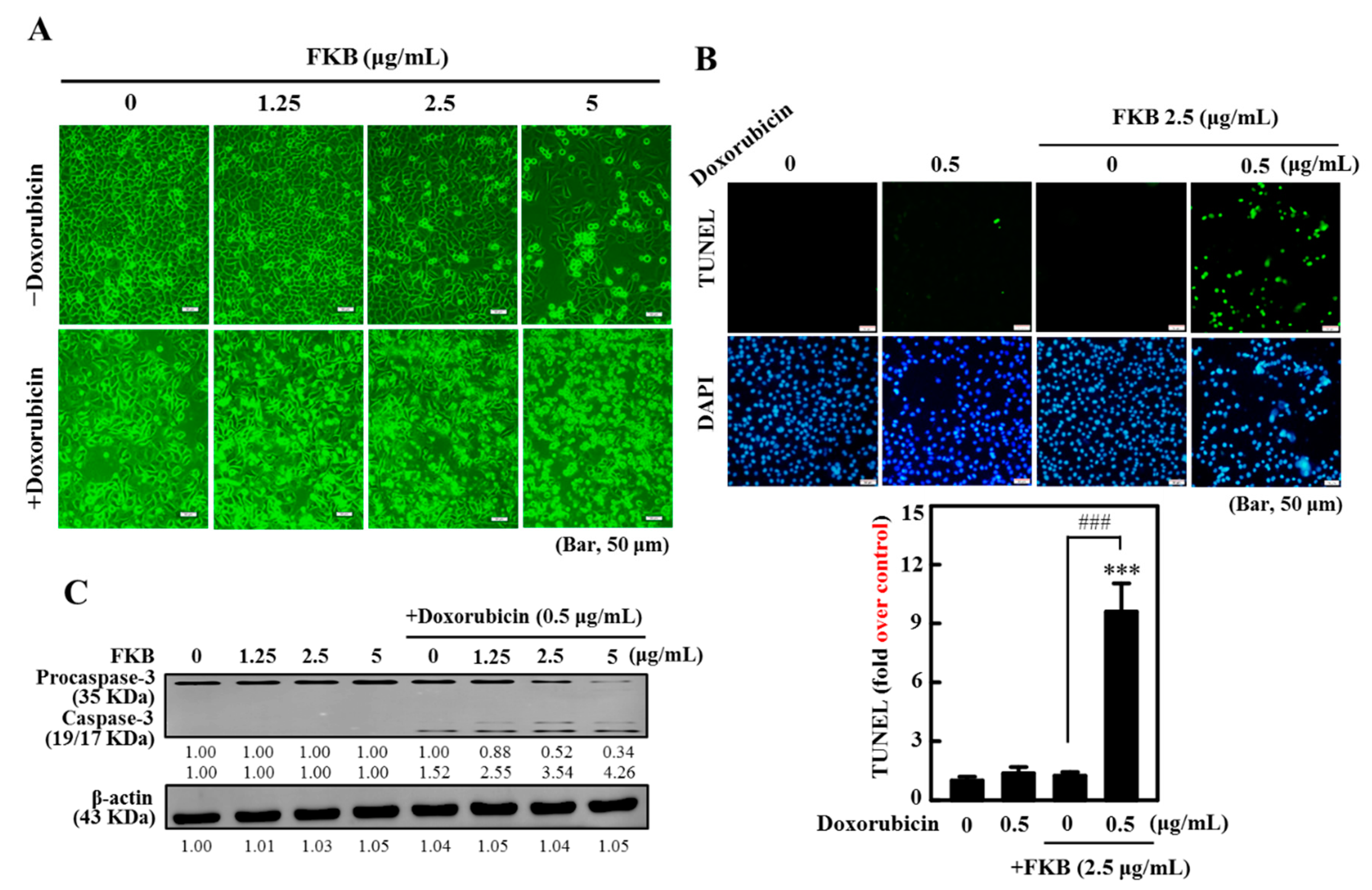
2.2. Doxorubicin-Induced Apoptosis Is Potentiated by FKB Treatment in AGS Cells
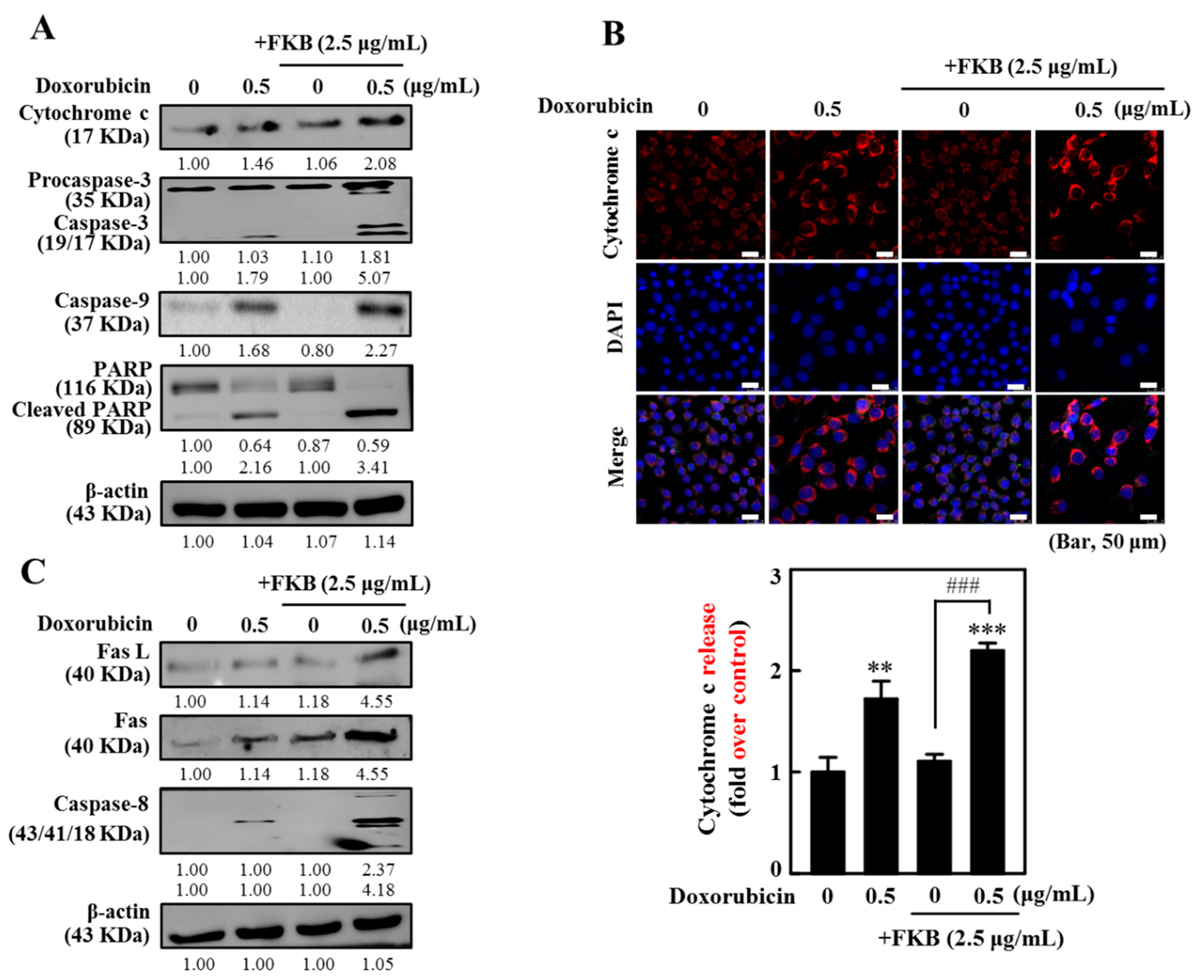
2.3. FKB Enhanced Doxorubicin-Mediated Mitochondrial Cascades in AGS Cells
2.4. FKB Enhanced Doxorubicin-Mediated FasL/Fas Expression and Caspase-8 Activation in AGS Cells
2.5. Caspase Inhibitor Attenuates the Induction of Apoptosis by FKB+Doxorubicin in AGS Cells
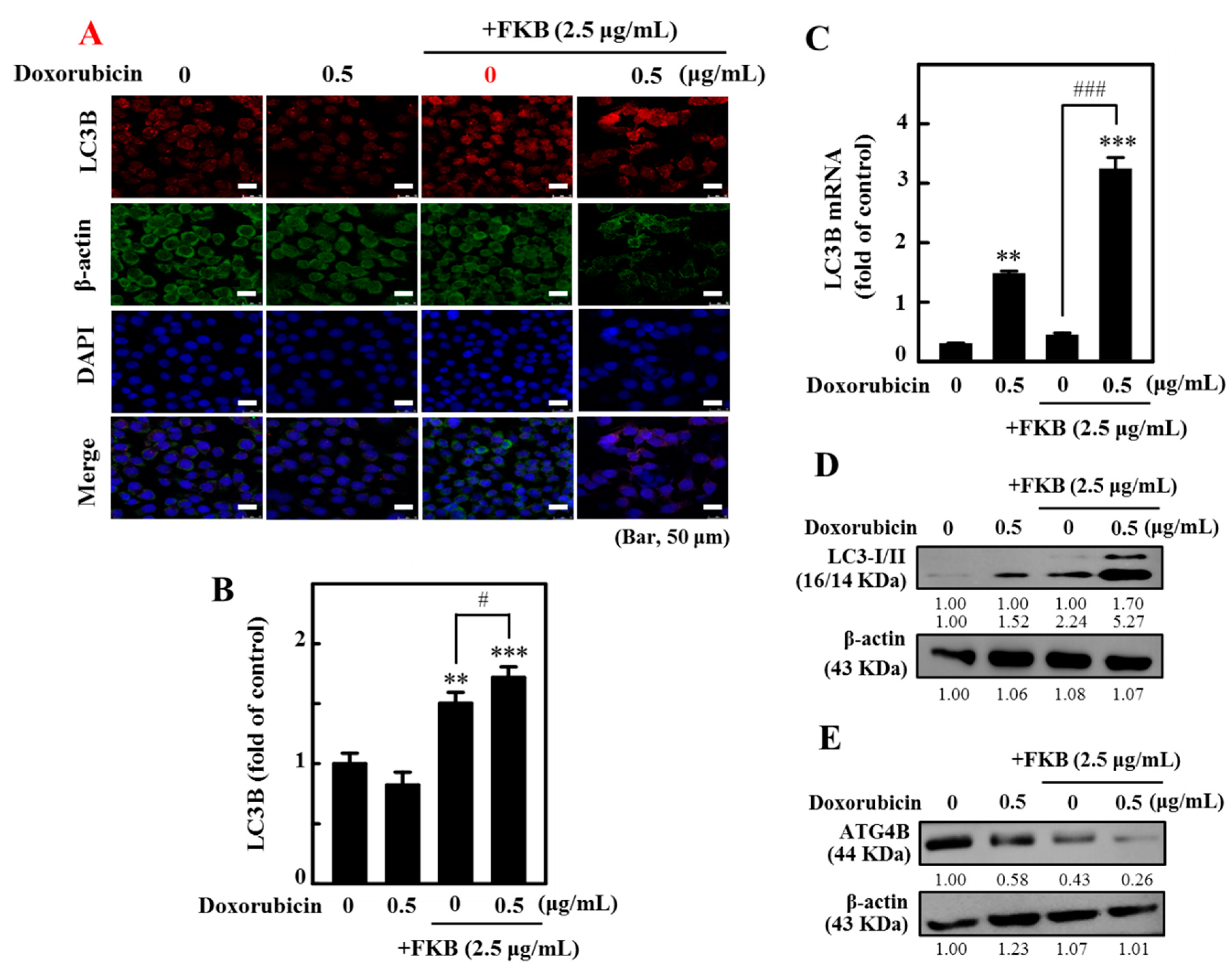
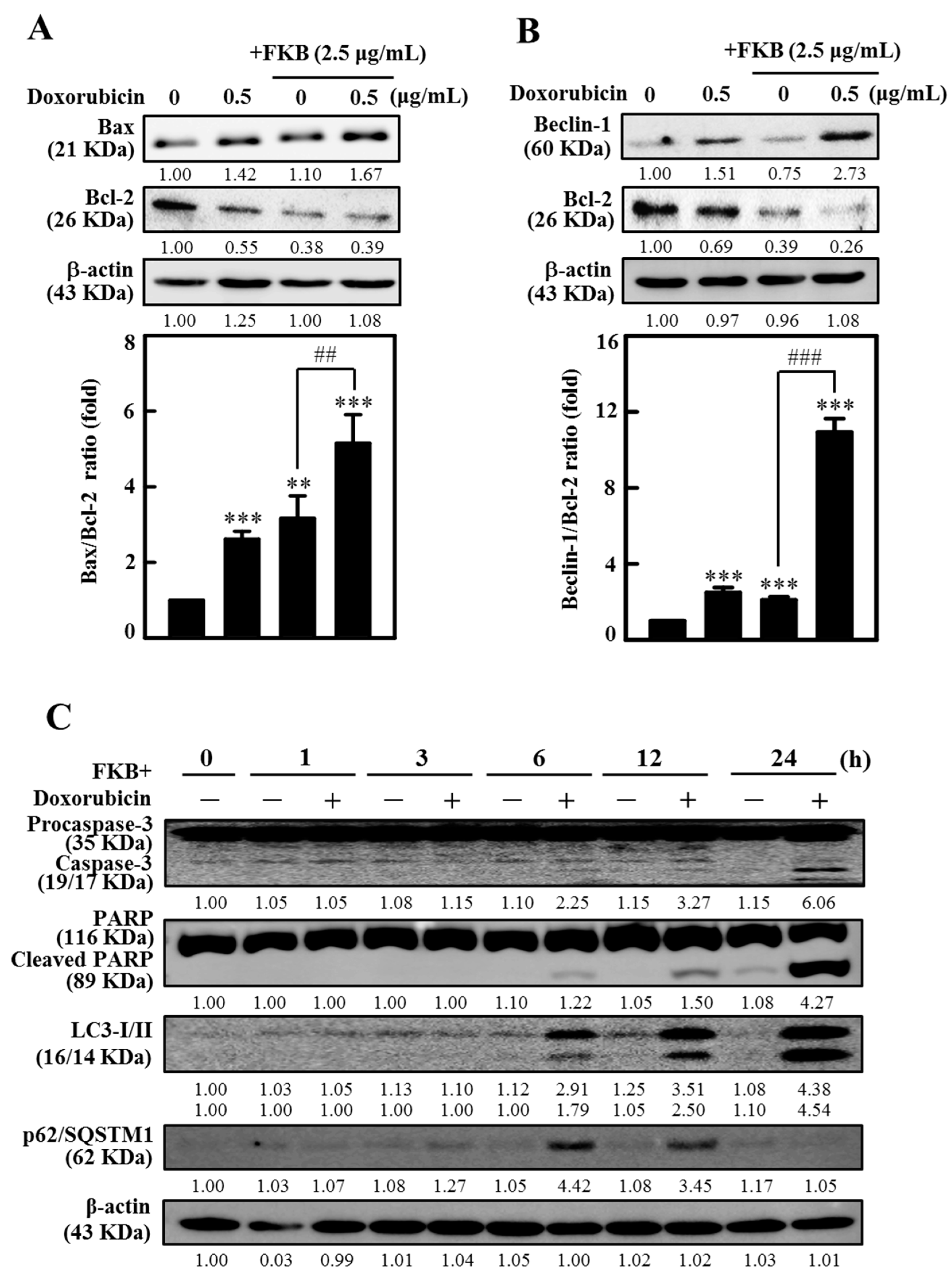
2.6. FKB+Doxorubicin Is Able to Activate Autophagy in AGS Cells from the Increased LC3 Accumulation
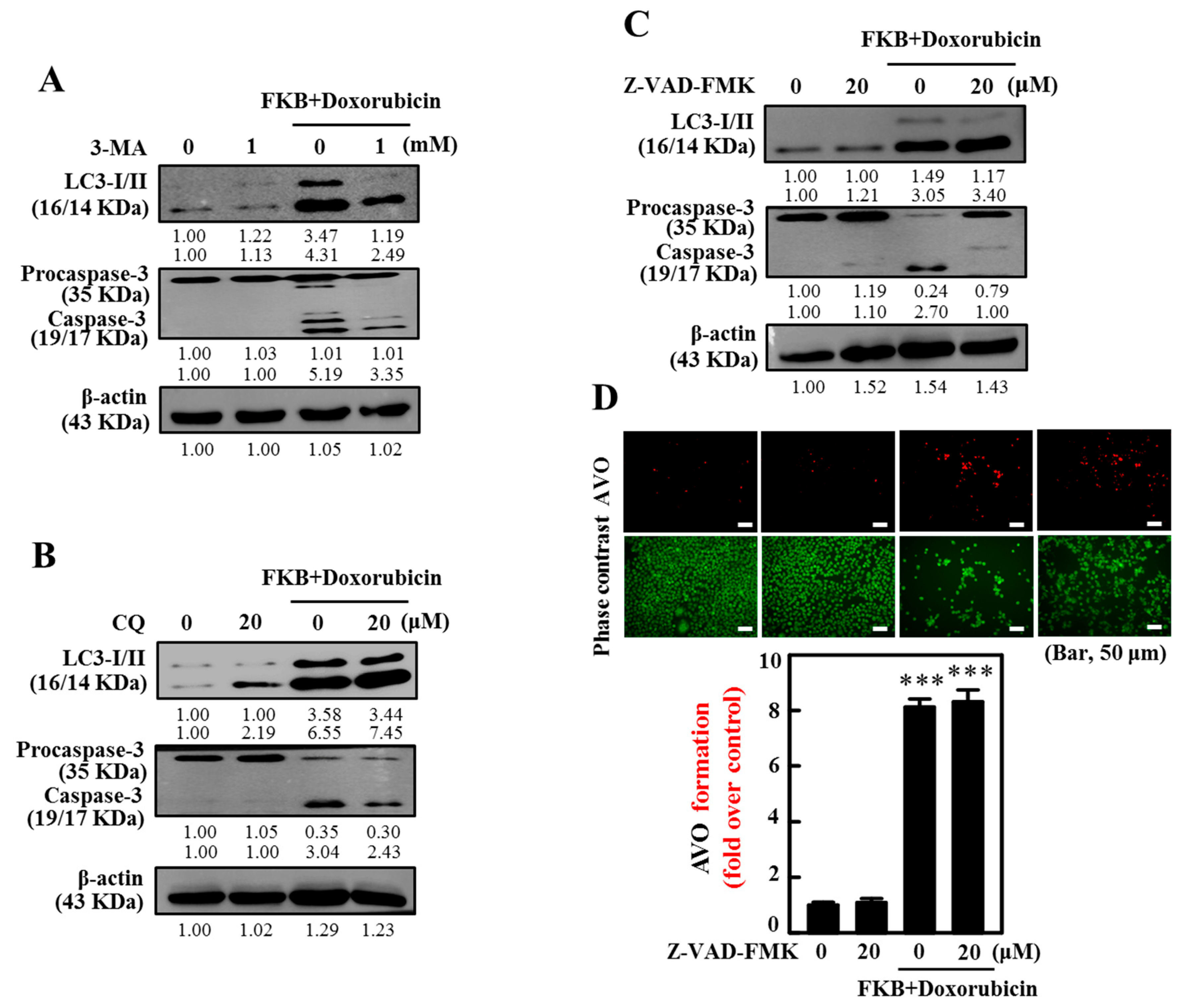
2.7. AVOs in AGS Cells Are Enhanced by FKB+Doxorubicin Treatment
2.8. FKB+Doxorubicin-Treated AGS Cells Indicate the Activation of Autophagy Signaling Cascade as a Death Mechanism
2.9. FKB Enhanced Doxorubicin-Modulated Expression of Bcl-2 Family Proteins in AGS Cells
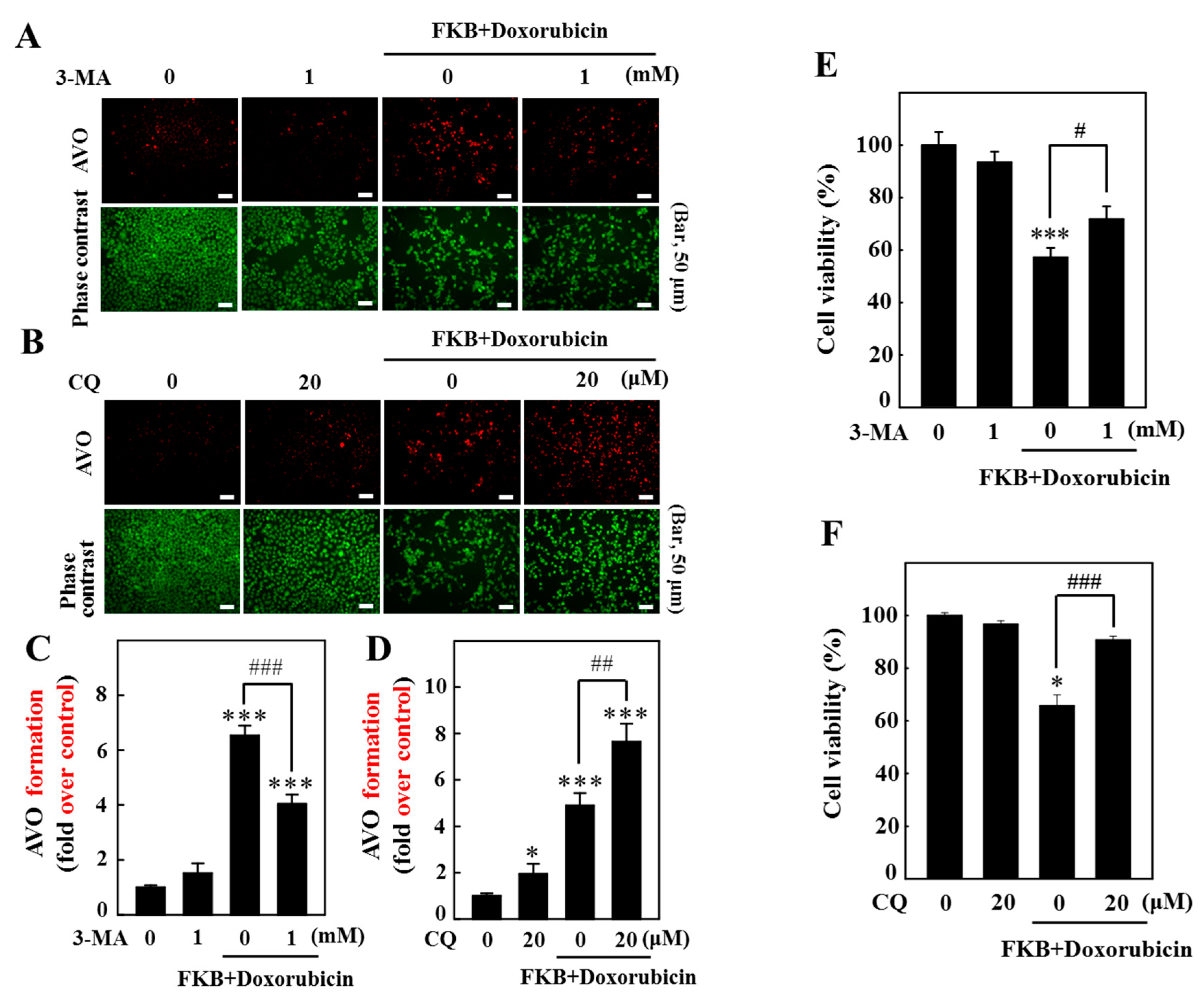
2.10. Inhibition of Autophagy Suppressed FKB+Doxorubicin-Induced Apoptosis
2.11. Inhibition of Apoptosis Suppressed FKB+Doxorubicin-Induced Autophagy
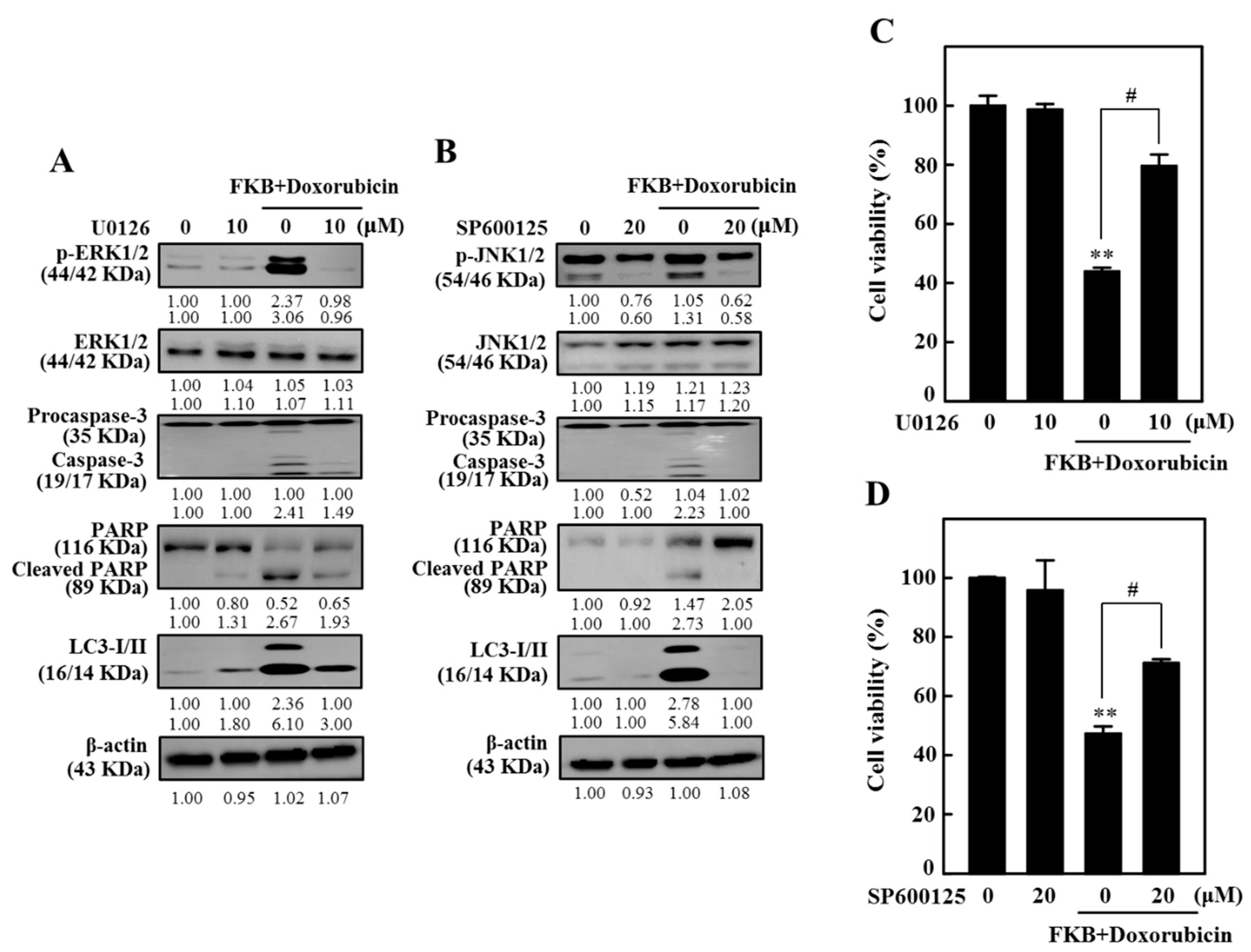
2.12. FKB+Doxorubicin Induced Apoptosis and Autophagy in AGS Cells Through JNK and ERK Signaling Pathways
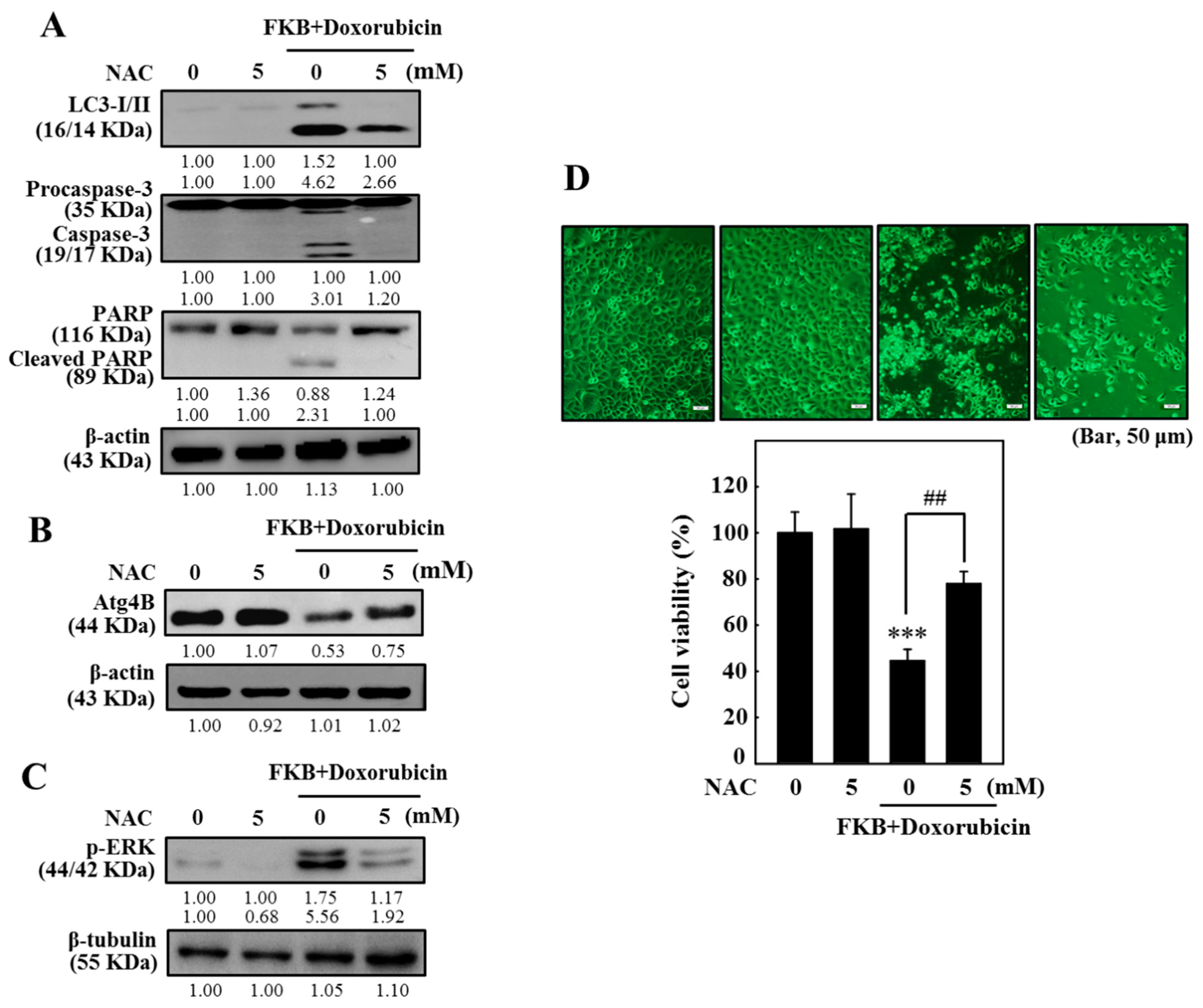
2.13. FKB+Doxorubicin Induces ROS-Mediated Apoptotic and Autophagic Cascades in AGS Cells
2.14. FKB+Doxorubicin Inhibits ROS-Related ATG4B and Enhances ERK Pathway in AGS Cells
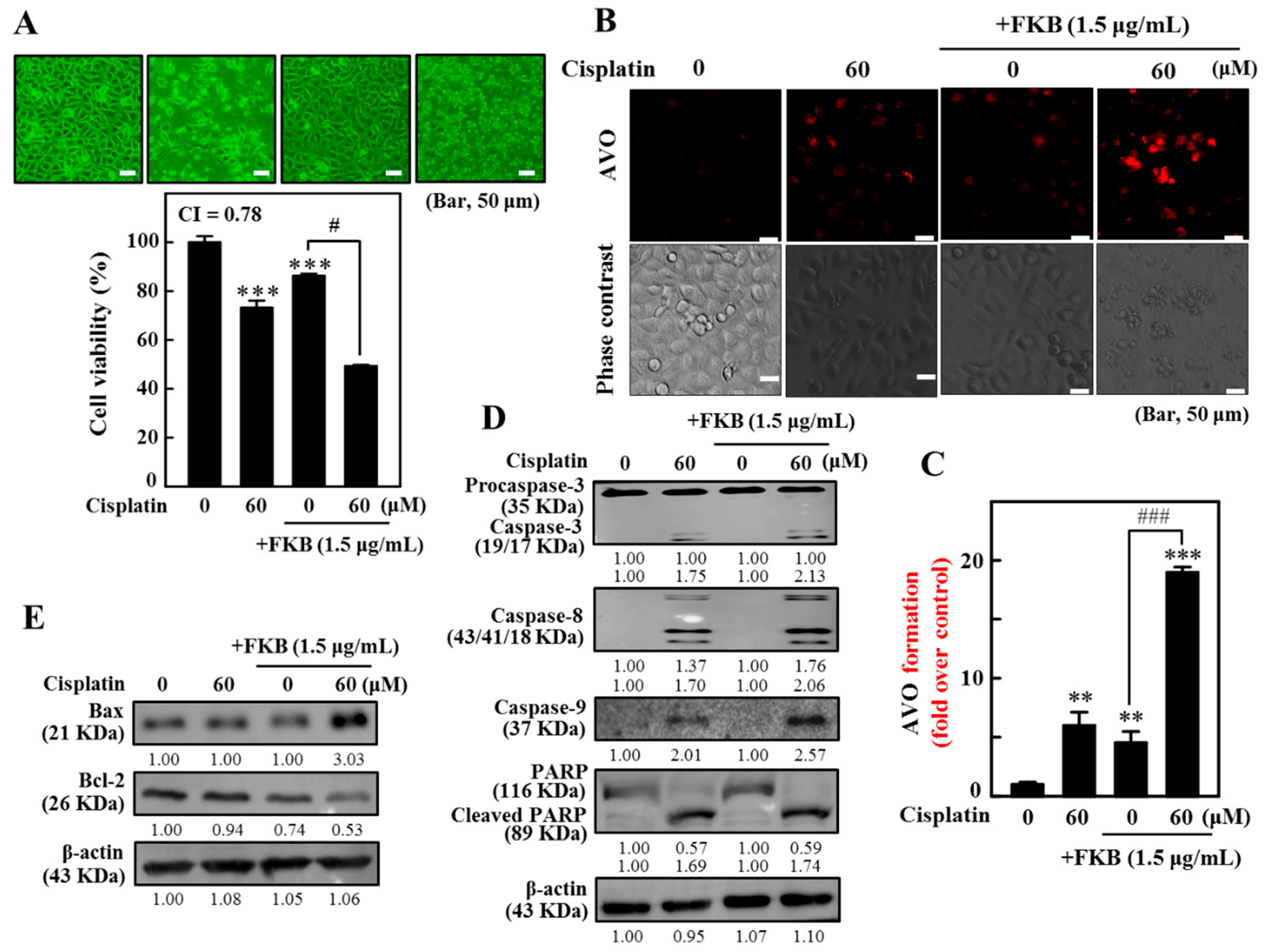
2.15. Combination of Cisplatin and FKB-Induced Cell Death, Apoptosis, and Autophagy in AGS Cells
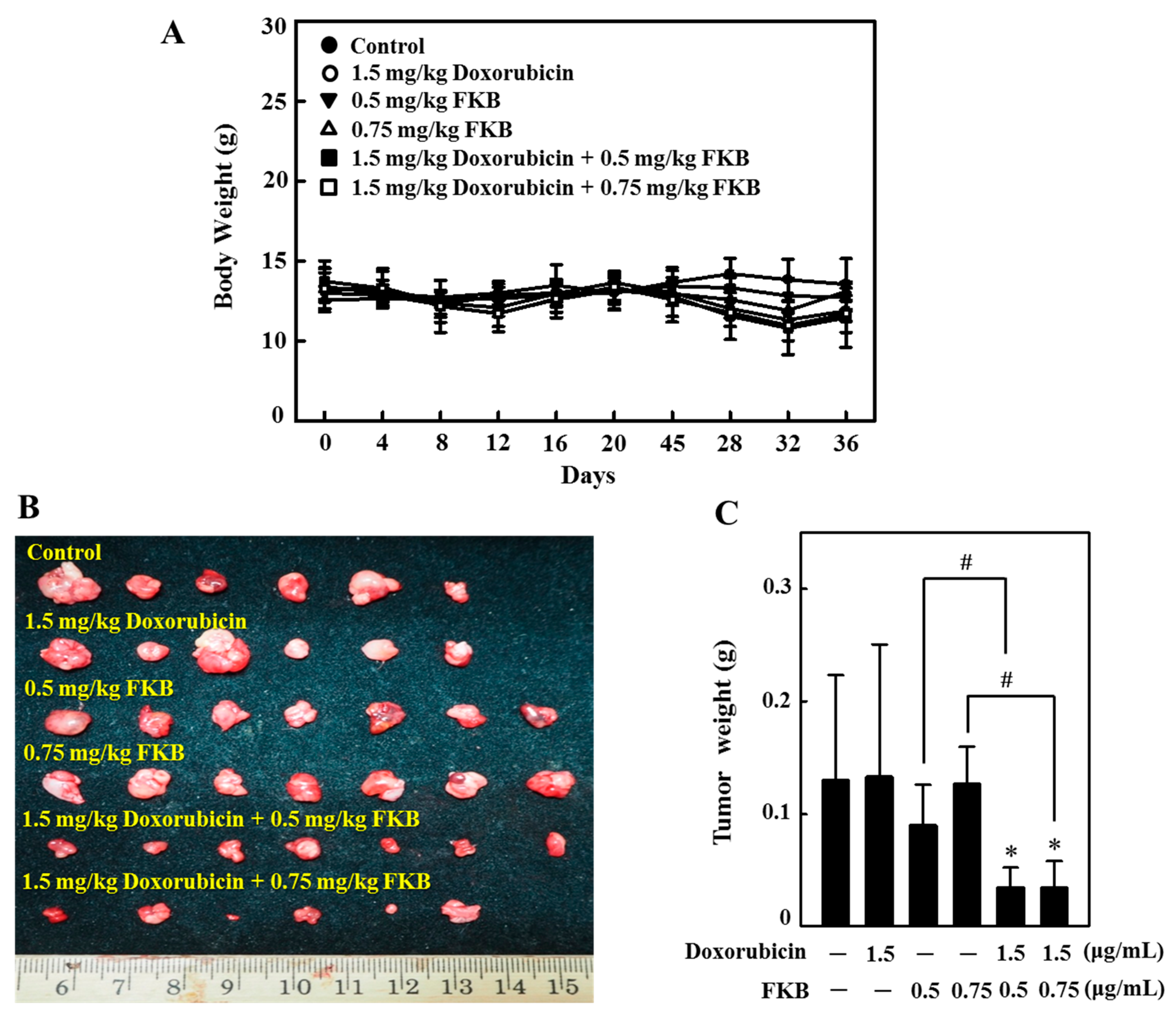
2.16. In Vivo Tumor Growth Inhibition by FKB+Doxorubicin Treatment in Nude Mice
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Drug Treatment
4.3. Cell Culture
4.4. MTT Assay
4.5. Drug Interaction Effects
4.6. TUNEL Assay for Apoptotic DNA Fragmentation
4.7. Western Blotting
4.8. LC3 Immunofluorescence
4.9. RNA Extraction and RT-PCR
4.10. Acridine Orange Staining
4.11. ROS Generation Assay
4.12. Animal Study
4.13. Tumor Cell Inoculation
4.14. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huang, C.C.; Lien, H.H.; Sung, Y.C.; Liu, H.T.; Chie, W.C. Quality of life of patients with gastric cancer in Taiwan: Validation and clinical application of the Taiwan Chinese version of the EORTC QLQ-C30 and EORTC QLQ-STO22. Psychooncology 2007, 16, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, K.; Ding, J.; Xu, H.; Zhu, L.; Zhang, K.; Li, X.; Sun, W. Harmine induces apoptosis and inhibits tumor cell proliferation, migration and invasion through down-regulation of cyclooxygenase-2 expression in gastric cancer. Phytomedicine 2014, 21, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, E.; Shotorbani, S.S.; Baradaran, B. Herbal medicine as inducers of apoptosis in cancer treatment. Adv. Pharm. Bull. 2014, 4, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, V.; Tsai, M.J.; Weng, C.F. Antroquinonol targets FAK-signaling pathway suppressed cell migration, invasion, and tumor growth of C6 glioma. PLoS ONE 2015, 10, e0141285. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Thiyagarajan, V.; Ou, T.T.; Yang, H.L. CoQ0-induced mitochondrial PTP opening triggers apoptosis via ROS-mediated VDAC1 upregulation in HL-60 leukemia cells and suppresses tumor growth in athymic nude mice/xenografted nude mice. Arch. Toxicol. 2018, 92, 301–322. [Google Scholar] [CrossRef]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef]
- Morselli, E.; Galluzzi, L.; Kepp, O.; Vicencio, J.M.; Criollo, A.; Maiuri, M.C.; Kroemer, G. Anti- and pro-tumor functions of autophagy. Biochim. Biophys. Acta 2009, 1793, 1524–1532. [Google Scholar] [CrossRef]
- Kondo, Y.; Kanzawa, T.; Sawaya, R.; Kondo, S. The role of autophagy in cancer development and response to therapy. Nat. Rev. Cancer 2005, 5, 726–734. [Google Scholar] [CrossRef]
- Gozuacik, D.; Kimchi, A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene 2004, 23, 2891–2906. [Google Scholar] [CrossRef]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Ly, J.D.; Grubb, D.R.; Lawen, A. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 2003, 8, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Kim, J.H.; Chi, G.Y.; Kim, G.Y.; Chang, Y.C.; Moon, S.K.; Nam, S.W.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Induction of apoptosis and autophagy by sodium selenite in A549 human lung carcinoma cells through generation of reactive oxygen species. Toxicol. Lett. 2012, 212, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Denard, B.; Lee, C.; Ye, J. Doxorubicin blocks proliferation of cancer cells through proteolytic activation of CREB3L1. Elife 2012, 1, e00090. [Google Scholar] [CrossRef] [PubMed]
- Denard, B.; Pavia-Jimenez, A.; Chen, W.; Williams, N.S.; Naina, H.; Collins, R.; Brugarolas, J.; Ye, J. Identification of CREB3L1 as a biomarker predicting doxorubicin treatment outcome. PLoS ONE 2015, 10, e0129233. [Google Scholar] [CrossRef]
- Kaye, S.; Merry, S. Tumour cell resistance to anthracyclines—A review. Cancer Chemother. Pharm. 1985, 14, 96–103. [Google Scholar] [CrossRef]
- Weiss, R.B. The anthracyclines: Will we ever find a better doxorubicin? Semin Oncol. 1992, 19, 670–686. [Google Scholar]
- Fong, M.Y.; Jin, S.; Rane, M.; Singh, R.K.; Gupta, R.; Kakar, S.S. Withaferin A synergizes the therapeutic effect of doxorubicin through ROS-mediated autophagy in ovarian cancer. PLoS ONE 2012, 7, e42265. [Google Scholar] [CrossRef]
- Yan, J.; Dou, X.; Zhou, J.; Xiong, Y.; Mo, L.; Li, L.; Lei, Y. Tubeimoside-I sensitizes colorectal cancer cells to chemotherapy by inducing ROS-mediated impaired autophagolysosomes accumulation. J. Exp. Clin. Cancer Res. 2019, 38, 353. [Google Scholar] [CrossRef]
- Abu, N.; Ho, W.Y.; Yeap, S.K.; Akhtar, M.N.; Abdullah, M.P.; Omar, A.R.; Alitheen, N.B. The flavokawains: Uprising medicinal chalcones. Cancer Cell Int. 2013, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Zi, X.; Simoneau, A.R. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res. 2005, 65, 3479–3486. [Google Scholar] [CrossRef] [PubMed]
- Abu, N.; Mohamed, N.E.; Yeap, S.K.; Lim, K.L.; Akhtar, M.N.; Zulfadli, A.J.; Kee, B.B.; Abdullah, M.P.; Omar, A.R.; Alitheen, N.B. In vivo antitumor and antimetastatic effects of flavokawain B in 4T1 breast cancer cell-challenged mice. Drug Des. Dev. Ther. 2015, 9, 1401–1417. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Lee, M.S.; Wu, C.R.; Cho, H.J.; Lin, K.Y.; Lai, G.H.; Wang, S.Y.; Kuo, Y.H.; Kumar, K.J.; Yang, H.L. The chalcone flavokawain B induces G2/M cell-cycle arrest and apoptosis in human oral carcinoma HSC-3 cells through the intracellular ROS generation and downregulation of the Akt/p38 MAPK signaling pathway. J. Agric. Food Chem. 2012, 60, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Huang, Y.C.; Thiyagarajan, V.; Mathew, D.C.; Lin, K.Y.; Chen, S.C.; Liu, J.Y.; Hsu, L.S.; Li, M.L.; Yang, H.L. Anticancer activities of chalcone flavokawain B from Alpinia pricei Hayata in human lung adenocarcinoma (A549) cells via induction of reactive oxygen species-mediated apoptotic and autophagic cell death. J. Cell Physiol. 2019, 234, 17514–17526. [Google Scholar] [CrossRef] [PubMed]
- Orlikova, B.; Tasdemir, D.; Golais, F.; Dicato, M.; Diederich, M. Dietary chalcones with chemopreventive and chemotherapeutic potential. Genes Nutr. 2011, 6, 125–147. [Google Scholar] [CrossRef]
- Chang, C.T.; Hseu, Y.C.; Thiyagarajan, V.; Lin, K.Y.; Way, T.D.; Korivi, M.; Liao, J.W.; Yang, H.L. Chalcone flavokawain B induces autophagic-cell death via reactive oxygen species-mediated signaling pathways in human gastric carcinoma and suppresses tumor growth in nude mice. Arch. Toxicol. 2017, 91, 3341–3364. [Google Scholar] [CrossRef]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef]
- Chen, J.; Chen, B.; Zou, Z.; Li, W.; Zhang, Y.; Xie, J.; Liu, C. Costunolide enhances doxorubicin-induced apoptosis in prostate cancer cells via activated mitogen-activated protein kinases and generation of reactive oxygen species. Oncotarget 2017, 8, 107701–107715. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Liu, X.; Kim, C.N.; Pohl, J.; Wang, X. Purification and characterization of an interleukin-1beta-converting enzyme family protease that activates cysteine protease P32 (CPP32). J. Biol. Chem. 1996, 271, 13371–13376. [Google Scholar] [CrossRef] [PubMed]
- Eskes, R.; Desagher, S.; Antonsson, B.; Martinou, J.C. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol. Cell Biol. 2000, 20, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; McDonnell, J.M.; Korsmeyer, S.J. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999, 13, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- Hasima, N.; Ozpolat, B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis. 2014, 5, e1509. [Google Scholar] [CrossRef] [PubMed]
- Thiyagarajan, V.; Sivalingam, K.S.; Viswanadha, V.P.; Weng, C.F. 16-hydroxy-cleroda-3,13-dien-16,15-olide induced glioma cell autophagy via ROS generation and activation of p38 MAPK and ERK-1/2. Environ. Toxicol. Pharm. 2016, 45, 202–211. [Google Scholar] [CrossRef]
- Arafa, M.A.; Rabah, D.M. With increasing trends of prostate cancer in the Saudi Arabia and Arab World: Should we start screening programs? World J. Clin. Oncol. 2017, 8, 447–449. [Google Scholar] [CrossRef]
- Xiong, S.; Mu, T.; Wang, G.; Jiang, X. Mitochondria-mediated apoptosis in mammals. Protein Cell 2014, 5, 737–749. [Google Scholar] [CrossRef]
- Kaufmann, T.; Strasser, A.; Jost, P.J. Fas death receptor signalling: Roles of Bid and XIAP. Cell Death Differ. 2012, 19, 42–50. [Google Scholar] [CrossRef]
- Kantari, C.; Walczak, H. Caspase-8 and bid: Caught in the act between death receptors and mitochondria. Biochim. Biophys. Acta 2011, 1813, 558–563. [Google Scholar] [CrossRef]
- Jiang, P.; Mizushima, N. LC3- and p62-based biochemical methods for the analysis of autophagy progression in mammalian cells. Methods 2015, 75, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Karin, M.; Diaz-Meco, M.T. p62 in cancer: Signaling adaptor beyond autophagy. Cell 2016, 167, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Puissant, A.; Robert, G.; Fenouille, N.; Luciano, F.; Cassuto, J.P.; Raynaud, S.; Auberger, P. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010, 70, 1042–1052. [Google Scholar] [CrossRef] [PubMed]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 2004, 36, 2503–2518. [Google Scholar] [CrossRef] [PubMed]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef]
- Czabotar, P.E.; Lessene, G.; Strasser, A.; Adams, J.M. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 2014, 15, 49–63. [Google Scholar] [CrossRef]
- Bazhin, A.V.; Philippov, P.P.; Karakhanova, S. Reactive oxygen species in cancer biology and anticancer therapy. Oxid. Med. Cell Longev. 2016, 2016, 4197815. [Google Scholar] [CrossRef]
- Thiyagarajan, V.; Lin, S.X.; Lee, C.H.; Weng, C.F. A focal adhesion kinase inhibitor 16-hydroxy-cleroda-3,13-dien-16,15-olide incorporated into enteric-coated nanoparticles for controlled anti-glioma drug delivery. Colloids Surf. B Biointerfaces 2016, 141, 120–131. [Google Scholar] [CrossRef]
- Zhuge, J.; Cederbaum, A.I. Serum deprivation-induced HepG2 cell death is potentiated by CYP2E1. Free. Radic. Biol. Med. 2006, 40, 63–74. [Google Scholar] [CrossRef]
- Hale, A.N.; Ledbetter, D.J.; Gawriluk, T.R.; Rucker, E.B. Autophagy: Regulation and role in development. Autophagy 2013, 9, 951–972. [Google Scholar] [CrossRef]
- Qiao, S.; Dennis, M.; Song, X.; Vadysirisack, D.D.; Salunke, D.; Nash, Z.; Yang, Z.; Liesa, M.; Yoshioka, J.; Matsuzawa, S.; et al. A REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stress-induced autophagy and sustain exercise capacity. Nat. Commun. 2015, 6, 7014. [Google Scholar] [CrossRef] [PubMed]
- Rothe, K.; Lin, H.; Lin, K.B.; Leung, A.; Wang, H.M.; Malekesmaeili, M.; Brinkman, R.R.; Forrest, D.L.; Gorski, S.M.; Jiang, X. The core autophagy protein ATG4B is a potential biomarker and therapeutic target in CML stem/progenitor cells. Blood 2014, 123, 3622–3634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Miao, L.; Lv, C.; Sun, H.; Wei, S.; Wang, B.; Huang, C.; Jiao, B. Wentilactone B induces G2/M phase arrest and apoptosis via the Ras/Raf/MAPK signaling pathway in human hepatoma SMMC-7721 cells. Cell Death Dis. 2013, 4, e657. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.T.; Korivi, M.; Huang, H.C.; Thiyagarajan, V.; Lin, K.Y.; Huang, P.J.; Liu, J.Y.; Hseu, Y.C.; Yang, H.L. Inhibition of ROS production, autophagy or apoptosis signaling reversed the anticancer properties of Antrodia salmonea in triple-negative breast cancer (MDA-MB-231) cells. Food Chem. Toxicol. 2017, 103, 1–17. [Google Scholar] [CrossRef] [PubMed]


| Treatment | (μg/mL) | Cell Number (%) | Predicted Value # | Combination Index (CI) ## |
|---|---|---|---|---|
| FKB | 1.25 | 91.2 ± 2.0 ** | — | — |
| 2.5 | 86.9 ± 4.2 ** | — | — | |
| 5 | 64.4 ± 1.2 *** | — | — | |
| Doxorubicin | 0.5 | 77.1 ± 3.7 *** | — | — |
| FKB + doxorubicin | 1.25 + 0.5 | 66.4 ± 0.4 *** | 70.3 | 0.64 |
| 2.5 + 0.5 | 41.1 ± 2.6 *** | 66.9 | 0.28 | |
| 5 + 0.5 | 6.8 ± 0.8 *** | 49.6 | 0.07 |
| Treatment | (μg/mL) | Cell Number (%) | Predicted Value # | Combination Index (CI) ## |
|---|---|---|---|---|
| FKB | 1.25 | 98.9 ± 3.9 | — | — |
| 2.5 | 96.3 ± 5.8 | — | — | |
| 5 | 76.8 ± 6.4 ** | — | — | |
| Doxorubicin | 0.5 | 81.0 ± 1.1 *** | — | — |
| FKB + doxorubicin | 1.25 + 0.5 | 61.4 ± 3.8 *** | 80.1 | 0.53 |
| 2.5 + 0.5 | 57.2 ± 1.6 *** | 78.0 | 0.62 | |
| 5 + 0.5 | 43.6 ± 3.1 *** | 62.2 | 0.67 |
| Treatment | (μg/mL) | Cell Number (%) | Predicted Value # | Combination Index (CI) ## |
|---|---|---|---|---|
| FKB | 1.25 | 98.1 ± 7.0 | — | — |
| 2.5 | 94.9 ± 4.7 | — | — | |
| 5 | 78.7 ± 3.7 ** | — | — | |
| Doxorubicin | 0.5 | 87.7 ± 3.5 * | — | — |
| FKB + doxorubicin | 1.25 + 0.5 | 65.0 ± 4.1 *** | 86.0 | 0.57 |
| 2.5 + 0.5 | 63.4 ± 2.2 *** | 83.1 | 0.70 | |
| 5 + 0.5 | 41.4 ± 3.7 *** | 68.9 | 0.59 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hseu, Y.-C.; Lin, R.-W.; Shen, Y.-C.; Lin, K.-Y.; Liao, J.-W.; Thiyagarajan, V.; Yang, H.-L. Flavokawain B and Doxorubicin Work Synergistically to Impede the Propagation of Gastric Cancer Cells via ROS-Mediated Apoptosis and Autophagy Pathways. Cancers 2020, 12, 2475. https://doi.org/10.3390/cancers12092475
Hseu Y-C, Lin R-W, Shen Y-C, Lin K-Y, Liao J-W, Thiyagarajan V, Yang H-L. Flavokawain B and Doxorubicin Work Synergistically to Impede the Propagation of Gastric Cancer Cells via ROS-Mediated Apoptosis and Autophagy Pathways. Cancers. 2020; 12(9):2475. https://doi.org/10.3390/cancers12092475
Chicago/Turabian StyleHseu, You-Cheng, Ruei-Wan Lin, Yi-Chun Shen, Kai-Yuan Lin, Jiunn-Wang Liao, Varadharajan Thiyagarajan, and Hsin-Ling Yang. 2020. "Flavokawain B and Doxorubicin Work Synergistically to Impede the Propagation of Gastric Cancer Cells via ROS-Mediated Apoptosis and Autophagy Pathways" Cancers 12, no. 9: 2475. https://doi.org/10.3390/cancers12092475
APA StyleHseu, Y.-C., Lin, R.-W., Shen, Y.-C., Lin, K.-Y., Liao, J.-W., Thiyagarajan, V., & Yang, H.-L. (2020). Flavokawain B and Doxorubicin Work Synergistically to Impede the Propagation of Gastric Cancer Cells via ROS-Mediated Apoptosis and Autophagy Pathways. Cancers, 12(9), 2475. https://doi.org/10.3390/cancers12092475






