Clinical Studies Applying Cytokine-Induced Killer Cells for the Treatment of Renal Cell Carcinoma
Simple Summary
Abstract
1. Introduction
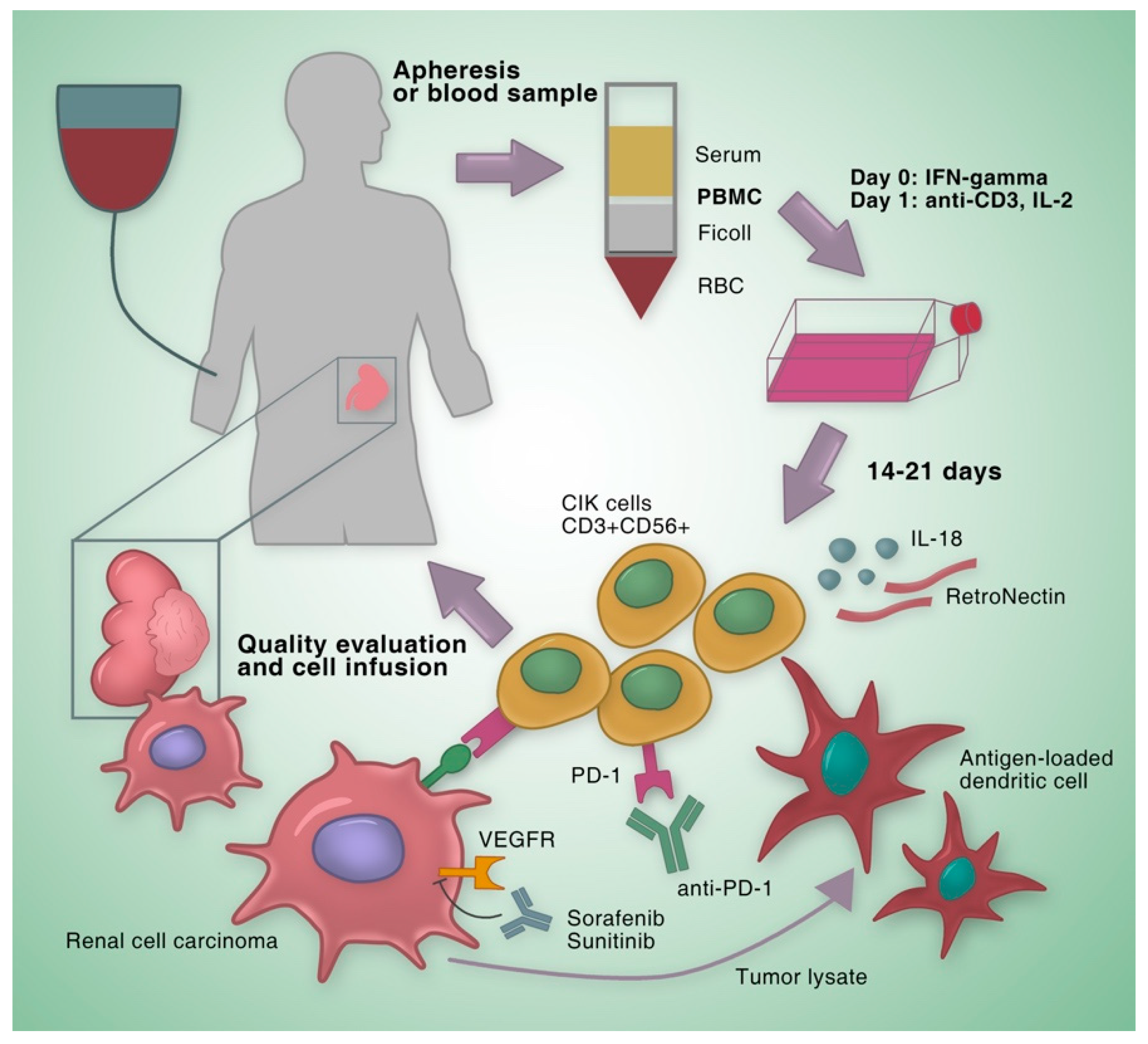
2. Clinical Studies on CIK Cells for the Treatment of RCC
2.1. Clinical Studies on Autologous CIK cells
2.2. Clinical Studies on CIK Cells with Improvement of Culture Methods
2.3. Clinical Studies on CIK Cells Combined with Dendritic Cell Vaccination
2.4. Clinical Studies on CIK Cells Combined with Targeted Agents
2.5. Clinical Studies on CIK Cells Combined with Immune Checkpoint Inhibitors
3. Preclinical Researches to Improve the Anti-Tumoral Activity of CIK Cells
| Study | Method | Conclusion |
|---|---|---|
| Finke et al. [37] | CIK cells transfected with IL-7 gene | Improved proliferation rate and increased TNF-α production of transfected CIK cells; significantly higher cytotoxic activity against the RCC cell line |
| Hillebrand et al. [38] | DCs transduced with adenoviruses carrying human CD40L (Ad-hCD40L) + CIK cells | Co-culture of Ad-hCD40L DCs with CIK cells led to a significant stimulation of tumor-specific CIK cells, with increased proliferation and cytotoxicity |
| Sievers et al. [40] | Telomerase peptide pulsed DCs + CIK cells | Significantly increased cytotoxic activity against RCC cell lines and autologous, telomerase positive primary cell cultures |
| Zhang et al. [41] | An anti-TIGIT functional antibody + CIK cells | CIK cells with TIGIT blocked indicated increased proliferation, higher cytotoxicity against tumor cells expressing CD155 and higher expression of IFN-γ, IL-6, and TNF-α |
| Setiawan et al. [42] | Peptide P60+ CIK cells | CIK cells combined with P60 resulted in a significant decrease in the viability of renal and pancreatic cancer cell lines |
| Dehno et al. [43] | CIK cells treated with nivolumab and ipilimumab | CIK cells treated with nivolumab and ipilimumab had no remarkable effect on the viability of RCC cells; the combination of nivolumab and ipilimumab significantly increased the proliferation and IFN-γ secretion of CIK cells |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Capitanio, U.; Bensalah, K.; Bex, A.; Boorjian, S.A.; Bray, F.; Coleman, J.; Gore, J.L.; Sun, M.; Wood, C.; Russo, P. Epidemiology of renal cell carcinoma. Eur. Urol. 2019, 75, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Abu-Ghanem, Y.; Bensalah, K.; Dabestani, S.; Fernández-Pello, S.; Giles, R.H.; Hofmann, F.; Hora, M.; Kuczyk, M.A. European association of urology guidelines on renal cell carcinoma: The 2019 update. Eur. Urol. 2019, 75, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2016, 387, 894–906. [Google Scholar] [CrossRef]
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef]
- Coppin, C.; Kollmannsberger, C.; Le, L.; Porzsolt, F.; Wilt, T.J. Targeted therapy for advanced renal cell cancer (RCC): A Cochrane systematic review of published randomised trials. Bju Int. 2011, 108, 1556–1563. [Google Scholar] [CrossRef]
- Hudes, G.R. Targeting mTOR in renal cell carcinoma. Cancer 2009, 115, 2313–2320. [Google Scholar] [CrossRef]
- Vuky, J.; Motzer, R.J. Cytokine therapy in renal cell cancer. Urol. Oncol. Semin. Orig. Investig. 2000, 5, 249–257. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Frontera, O.A.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Houot, R.; Schultz, L.M.; Marabelle, A.; Kohrt, H. T-cell–based immunotherapy: Adoptive cell transfer and checkpoint inhibition. Cancer Immunol. Res. 2015, 3, 1115–1122. [Google Scholar] [CrossRef]
- Kim, J.S.; Chung, I.S.; Lim, S.H.; Park, Y.; Park, M.J.; Kim, J.Y.; Kim, Y.G.; Hong, J.T.; Kim, Y.; Han, S.-B. Preclinical and clinical studies on cytokine-induced killer cells for the treatment of renal cell carcinoma. Arch. Pharm. Res. 2014, 37, 559–566. [Google Scholar] [CrossRef]
- Schmidt-Wolf, I.; Finke, S.; Trojaneck, B.; Denkena, A.; Lefterova, P.; Schwella, N.; Heuft, H.; Prange, G.; Korte, M.; Takeya, M. Phase I clinical study applying autologous immunological effector cells transfected with the interleukin-2 gene in patients with metastatic renal cancer, colorectal cancer and lymphoma. Br. J. Cancer 1999, 81, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Wolf, I.; Negrin, R.S.; Kiem, H.-P.; Blume, K.G.; Weissman, I.L. Use of a SCID mouse/human lymphoma model to evaluate cytokine-induced killer cells with potent antitumor cell activity. J. Exp. Med. 1991, 174, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhu, A.; Cai, X.; Jia, Z.; Han, W.; Ma, L.; Zhou, M.; Qian, K.; Cen, L.; Chen, B. Role of NKG2D in cytokine-induced killer cells against multiple myeloma cells. Cancer Biol. 2012, 13, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Diefenbach, A.; Jamieson, A.M.; Liu, S.D.; Shastri, N.; Raulet, D.H. Ligands for the murine NKG2D receptor: Expression by tumor cells and activation of NK cells and macrophages. Nat. Immunol. 2000, 1, 119–126. [Google Scholar] [CrossRef]
- Introna, M. CIK as therapeutic agents against tumors. J. Autoimmun. 2017, 85, 32–44. [Google Scholar] [CrossRef]
- Baker, J.; Verneris, M.R.; Ito, M.; Shizuru, J.A.; Negrin, R.S. Expansion of cytolytic CD8+ natural killer T cells with limited capacity for graft-versus-host disease induction due to interferon γ production. Blood 2001, 97, 2923–2931. [Google Scholar] [CrossRef]
- Verneris, M.R.; Ito, M.; Baker, J.; Arshi, A.; Negrin, R.S.; Shizuru, J.A. Engineering hematopoietic grafts: Purified allogeneic hematopoietic stem cells plus expanded CD8+ NK-T cells in the treatment of lymphoma. Biol. Blood Marrow Transpl. 2001, 7, 532–542. [Google Scholar] [CrossRef]
- Jäkel, C.E.; Hauser, S.; Rogenhofer, S.; Müller, S.C.; Brossart, P.; Schmidt-Wolf, I.G. Clinical studies applying cytokine-induced killer cells for the treatment of renal cell carcinoma. Clin. Dev. Immunol. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Wang, Y.; Lu, X.-C.; Fan, H.; Liu, Y.; Zhang, Y.; Feng, K.-C.; Zhang, W.-Y.; Chen, M.-X. Autologous CIK cell immunotherapy in patients with renal cell carcinoma after radical nephrectomy. Clin. Dev. Immunol. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, L.; Wei, J.; Liu, L.; Yin, Y.; Gu, Y.; Shu, Y. The effects of cytokine-induced killer cells for the treatment of patients with solid tumors: A clinical retrospective study. J. Cancer Res. Clin. Oncol. 2012, 138, 1057–1062. [Google Scholar] [CrossRef]
- Liu, J.; Sui, J.; Zhang, Z.; Ren, X.; Luan, L.; Yang, Q.; Gu, S.; Wank, R.; Laumbacher, B.; Song, X. Inhibition of pleural metastasis of collecting duct carcinoma of the kidney by modified cytokine-induced killer cells: A case report and review of the literature. Oncol. Lett. 2010, 1, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, Y.; Zhao, L.; Xu, L.; Zhang, Y.; Mai, L.; Gao, Q. Efficacy of RetroNectin-activated cytokine-induced killer cell therapy in metastatic brain tumor patients. Oncol. Res. Treat. 2015, 38, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Kimizuku, F.; Taguchi, Y.; Ohdate, Y.; Kawase, Y.; Shimojo, T.; Hashino, K.; Kato, I.; Sekiguchi, K.; Titani, K. Production and characterization of functional domains of human fibronectin expressed in Escherichia coli. J. Biochem. 1991, 110, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Liu, Y.; Wang, L.; Zhao, L.; Yang, T.; He, C.; Song, Y.; Gao, Q. Association of myeloid-derived suppressor cells and efficacy of cytokine-induced killer cell immunotherapy in metastatic renal cell carcinoma patients. J. Immunother. 2014, 37, 43–50. [Google Scholar] [CrossRef]
- Santos, P.M.; Butterfield, L.H. Dendritic cell–based cancer vaccines. J. Immunol. 2018, 200, 443–449. [Google Scholar] [CrossRef]
- Zhan, H.; Xin, G.; Pu, X.; Li, W.; Li, Z.; Zhou, X.; Qiu, J. A randomized controlled trial of postoperative tumor lysate-pulsed dendritic cells and cytokine-induced killer cells immunotherapy in patients with localized and locally advanced renal cell carcinoma. Chin. Med. J. 2012, 125, 3771–3777. [Google Scholar]
- Cui, Y.; Yang, X.; Zhu, W.; Li, J.; Wu, X.; Pang, Y. Immune response, clinical outcome and safety of dendritic cell vaccine in combination with cytokine-induced killer cell therapy in cancer patients. Oncol. Lett. 2013, 6, 537–541. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, B.; Gao, H.; Ding, G.; Wu, Q.; Zhang, J.; Liao, L.; Chen, H. Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer. BMC Cancer 2014, 14, 251. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Z.; Li, H.; Huang, J.; Yang, S.; Xie, T.; Huang, L.; Yue, D.; Xu, L.; Wang, L. Cytokine induced killer cell-based immunotherapies in patients with different stages of renal cell carcinoma. Cancer Lett. 2015, 362, 192–198. [Google Scholar] [CrossRef]
- Zheng, K.; Tan, J.-M.; Wu, W.-Z.; Qiu, Y.-M.; Zhang, H.; Xu, T.-Z.; Sun, X.-H.; Zhuo, W.-L.; Wang, D.; Zhang, J.-P. Adjuvant dendritic cells vaccine combined with cytokine-induced-killer cell therapy after renal cell carcinoma surgery. J. BUON 2015, 20, 505–513. [Google Scholar]
- Li, C.; Zhu, D.; Zhao, Y.; Guo, Q.; Sun, W.; Li, L.; Gao, D.; Zhao, P. Dendritic Cells Therapy with Cytokine-Induced Killer Cells and Activated Cytotoxic T Cells Attenuated Th2 Bias Immune Response. Immunol. Investig. 2019, 49, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, H.; Zhao, L.; Song, Y.; Gao, Q. Combination of sorafenib and cytokine-induced killer cells in metastatic renal cell carcinoma: A potential regimen. Immunotherapy 2017, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.-X.; Mei, G.-H.; Zhao, F.-L.; Li, B.-T.; Tang, Y.-Y.; Zhang, B.; Xu, X.-J.; Chen, L.-J. Retrospective analysis on the efficacy of sunitinib/sorafenib in combination with dendritic cells-cytokine-induced killer in metastasis renal cell carcinoma after radical nephrectomy. J. Cancer Res. 2018, 14, 427. [Google Scholar]
- Chen, C.-L.; Pan, Q.-Z.; Weng, D.-S.; Xie, C.-M.; Zhao, J.-J.; Chen, M.-S.; Peng, R.-Q.; Li, D.-D.; Wang, Y.; Tang, Y. Safety and activity of PD-1 blockade-activated DC-CIK cells in patients with advanced solid tumors. Oncoimmunology 2018, 7, e1417721. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Till, B.; Sun, M.; Li, X.; Gao, Q. Combination of cytokine-induced killer cells and programmed cell death-1 blockade works synergistically to enhance therapeutic efficacy in metastatic renal cell carcinoma and non-small cell lung cancer. Front. Immunol. 2018, 9, 1513. [Google Scholar] [CrossRef]
- Zoll, B.; Lefterova, P.; Csipai, M.; Finke, S.; Trojaneck, B.; Ebert, O.; Micka, B.; Roigk, K.; Fehlinger, M.; Schmidt-Wolf, G.D. Generation of cytokine-induced killer cells using exogenous interleukin-2,-7 or-12. Cancer Immunol. Immunother. 1998, 47, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Finke, S.; Trojaneck, B.; Lefterova, P.; Csipai, M.; Wagner, E.; Kircheis, R.; Neubauer, A.; Huhn, D.; Wittig, B.; Schmidt-Wolf, I. Increase of proliferation rate and enhancement of antitumor cytotoxicity of expanded human CD3+ CD56+ immunologic effector cells by receptor-mediated transfection with the interleukin-7 gene. Gene Ther. 1998, 5, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Hillebrand, R.M.; Vogt, A.; Strassburg, C.P.; Gonzalez-Carmona, M.A.; Schmidt-Wolf, I.G. Immune Check Point CD40–CD40L Activates Dendritic and Effector Cells Against Human Renal Carcinoma Cells. Anticancer Res. 2019, 39, 4643–4652. [Google Scholar] [CrossRef] [PubMed]
- French, R.R.; Chan, H.C.; Tutt, A.L.; Glennie, M.J. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat. Med. 1999, 5, 548–553. [Google Scholar] [CrossRef]
- Sievers, E.; Albers, P.; Schmidt-Wolf, I.G.; MÄRTEN, A. Telomerase pulsed dendritic cells for immunotherapy for renal cell carcinoma. J. Urol. 2004, 171, 114–119. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, W.; Li, H.; Chen, Y.; Tian, H.; Li, L.; Zhang, L.; Gao, C.; Zheng, J. Immunoreceptor TIGIT inhibits the cytotoxicity of human cytokine-induced killer cells by interacting with CD155. Cancer Immunol. Immunother. 2016, 65, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, M.F.; Rudan, O.; Vogt, A.; Gonzalez-Carmona, M.A.; Langhans, B.; Schmidt-Wolf, R.; Garofano, F.; Strassburg, C.P.; Lasarte, J.J.; Casares, N. FOXP3 Inhibitory Peptide P60 Increases Efficacy of Cytokine-induced Killer Cells Against Renal and Pancreatic Cancer Cells. Anticancer Res. 2019, 39, 5369–5374. [Google Scholar] [CrossRef] [PubMed]
- Dehno, M.N.; Li, Y.; Weiher, H.; Schmidt-Wolf, I.G. Increase in Efficacy of Checkpoint Inhibition by Cytokine-Induced-Killer Cells as a Combination Immunotherapy for Renal Cancer. Int. J. Mol. Sci. 2020, 21, 3078. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Harden, K.; Gonzalez, L.C.; Francesco, M.; Chiang, E.; Irving, B.; Tom, I.; Ivelja, S.; Refino, C.J.; Clark, H. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 2009, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Stanietsky, N.; Simic, H.; Arapovic, J.; Toporik, A.; Levy, O.; Novik, A.; Levine, Z.; Beiman, M.; Dassa, L.; Achdout, H. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 17858–17863. [Google Scholar] [CrossRef]
- Casares, N.; Rudilla, F.; Arribillaga, L.; Llopiz, D.; Riezu-Boj, J.I.; Lozano, T.; López-Sagaseta, J.; Guembe, L.; Sarobe, P.; Prieto, J. A peptide inhibitor of FOXP3 impairs regulatory T cell activity and improves vaccine efficacy in mice. J. Immunol. 2010, 185, 5150–5159. [Google Scholar] [CrossRef]
- Zhang, Y.; Schmidt-Wolf, I.G. Ten-year update of the international registry on cytokine-induced killer cells in cancer immunotherapy. J. Cell. Physiol. 2020. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, L.; Zhang, Y.; Wang, Y.; Lu, X.; Shi, F.; Liu, Y.; Chen, M.; Feng, K.; Zhang, W. Analysis of adverse events following the treatment of autologous cytokine-induced killer cells for adoptive immunotherapy in malignant tumour sufferers. Expert Opin. Biol. 2015, 15, 481–493. [Google Scholar] [CrossRef]
- Introna, M.; Lussana, F.; Algarotti, A.; Gotti, E.; Valgardsdottir, R.; Micò, C.; Grassi, A.; Pavoni, C.; Ferrari, M.L.; Delaini, F. Phase II study of sequential infusion of donor lymphocyte infusion and cytokine-induced killer cells for patients relapsed after allogeneic hematopoietic stem cell transplantation. Biol. Blood Marrow Transpl. 2017, 23, 2070–2078. [Google Scholar] [CrossRef]
- Gibney, G.; Aziz, S.; Camp, R.; Conrad, P.; Schwartz, B.; Chen, C.; Kelly, W.; Kluger, H. c-Met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann. Oncol. 2013, 24, 343–349. [Google Scholar] [CrossRef]
- Balan, M.; Teran, E.M.Y.; Waaga-Gasser, A.M.; Gasser, M.; Choueiri, T.K.; Freeman, G.; Pal, S. Novel roles of c-Met in the survival of renal cancer cells through the regulation of HO-1 and PD-L1 expression. J. Biol. Chem. 2015, 290, 8110–8120. [Google Scholar] [CrossRef] [PubMed]
- Hontscha, C.; Borck, Y.; Zhou, H.; Messmer, D.; Schmidt-Wolf, I. Clinical trials on CIK cells: First report of the international registry on CIK cells (IRCC). J. Cancer Res. Clin. Oncol. 2011, 137, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Schmeel, L.C.; Schmeel, F.C.; Coch, C.; Schmidt-Wolf, I.G. Cytokine-induced killer (CIK) cells in cancer immunotherapy: Report of the international registry on CIK cells (IRCC). J. Cancer Res. Clin. Oncol. 2015, 141, 839–849. [Google Scholar] [CrossRef] [PubMed]

| Study | Number of Patients | Therapeutic Approach | Clinical Response | Adverse Event | Conclusion |
|---|---|---|---|---|---|
| Liu et al. [21] | 1 (1 RCC) | Modified auto-CIKs | Symptomatic improvement (relieved cough, dyspnea, chest distress and thoracalgia) and reduction of pleural fluid | Fever (~38 °C) but recovered 2 days later | The patient achieved partial success |
| Zhan et al. [26] | 137 (137 RCC) | Group 1: tumor lysate-pulsed DCs + auto-CIKs Group 2: IFN-α Group 3: no adjuvant therapy | Increased CD4+/CD8+ ratio, decreased CD4+CD25high cells and significantly higher 3- and 5-year OS rates | Controllable fever and fatigue in DC-CIK group | Postoperative immunotherapy with tumor lysate-pulsed DC-CIK cells may prevent recurrence/metastasis and increase OS rates |
| Zhang et al. [20] | 40 (10 RCC) | Auto-CIKs | 6-month, 1-year and 3-year OS rates were 70.0, 60.0 and 57.5%, respectively; median OS was 34.9 months; 6 recurrence, 12 metastasis and 15 deaths in all patients (2 recurrence, 5 metastasis and 3 deaths in RCC) | Controllable fever and poor appetite | CIK cell therapy can be an effective adjuvant instrument of the routine anti-tumor treatment |
| Cui et al. [27] | 121 (7 RCC) | Tumor lysate-pulsed DCs + auto-CIKs | Improvements in the physical strength, appetite and sleeping status | Controllable fever, insomnia, anorexia, joint soreness and skin rashes | Tumor lysate-pulsed DC-CIK cells were safe without serious adverse side-effects |
| Zhang et al. [19] | 20 (20 RCC) | Group 1: auto-CIKs Group 2: control | Significantly longer median PFS; 6 CR, 4 SD in CIK group and 5 CR, 3 SD, 2 PD in control group | Mild arthralgia, laryngeal edema, fatigue, and low-grade fever | CIK cell treatment could prolong survival in patients with RCC after radical nephrectomy |
| Wang et al. [28] | 28 (28 RCC) | GmDCs + auto-CIKs | 4 CR, 7 PR, 10 SD, 6 PD and 1 death | Flu-like symptoms with fever | DCR was significantly related with cycles of treatment |
| Wang et al. [24] | 29 (29 RCC) | Auto-CIKs + IL-2 | 4 PR, 18 SD and 7 PD; 1-year survival was 82.8%; median PFS and OS were 7.7 months and 12.6 months, respectively | Grade 1 and 2 fever and grade 2 diarrhea | Auto-CIKs can induce regression of RCC; MDSCs can serve as a potential marker for the prognosis of patients receiving a CIK-based therapy |
| Li et al. [22] | 20 (1 RCC) | RetroNectin-activated auto-CIKs + conventional therapies | 1 CR, 5 PR, 9 SD and 5 PD; median PFS and OS were 7.7 months and 12.6 months, respectively (1 CR with an OS of 14 months and PFS of 8 months in RCC) | Fever | RetroNectin-activated CIKs combined with conventional therapies could improve the prognosis of metastatic brain tumor patients |
| Zheng et al. [30] | 410 (410 RCC) | Group 1: tumor lysate-pulsed DCs + auto-CIKs Group 2: IFN-α | Significantly increased 3-, 5-year OS rates and PFS in DC-CIK group | Transient low-grade fever and fatigue | Adjuvant DC-CIK treatment after surgery prolonged PFS and reduced mortality |
| Zhao et al. [29] | 122 (122 RCC) | In operable patients: Group 1: tumor lysate-pulsed DCs + auto-CIKs Group 2: control in inoperable patients: Group 1: auto-CIKs Group 2: control | Significantly higher 3-year DFS and decreased risk of post-operative disease progression and relapse in operable patients; Significantly higher 3-year OS rate, median OS and PFS in inoperable patients | Flu-like symptoms such as fever and fatigue | DC-CIK cells might be more efficient and personalized for the patients with tumor resection, and CIK cells could improve the prognosis for inoperable patients |
| Yang et al. [32] | 1 (1 RCC) | Auto-CIKs + sorafenib | Metastasis remained stable | No serious adverse reactions | CIK cells combined with sorafenib could result in a synergistic effect |
| Mai et al. [33] | 34 (34 RCC) | Group 1: sunitinib/sorafenib monotherapy Group 2: DCs + auto-CIKs + sunitinib/sorafenib | Significantly higher median PFS and 3-year OS rate; more SD and less PD and death | No serious adverse reactions in group 2; bone marrow suppression, oral ulcer, fatigue, and hand-foot syndrome | Sunitinib/sorafenib combined with CIK cells could significantly prolong the median PFS and 3-year OS |
| Wang et al. [35] | 2 (1 RCC) | Auto-CIKs + pembrolizumab | 1 CR (RCC) and 1 near-CR | No serious adverse reactions associated with CIK cells | Pembrolizumab in combination with CIK cells led to potent anti-tumor activity in RCC; CD3+ T cell infiltration in baseline tumor biopsies is a potential predictive biomarker |
| Chen et al. [34] | 37 (8 RCC) | DCs + auto-CIKs + pembrolizumab | 2 CR, 5 PR, 13 SD and 11 PD (1 CR, 1 PR, 4 SD, 2 PD in RCC); median OS and PFS were 270 and 162 days | All treatment-related adverse reactions were reversible or controllable; grade 3 or 4 toxicities, including fever and chills, were observed in two patients | Pembrolizumab-activated autologous DC-CIK cells were safe and effective in advanced solid tumors |
| Li et al. [31] | 228 (12 RCC) | Group 1: tumor lysate-pulsed DCs + auto-CIKs Group 2: tumor lysate-pulsed DCs + cytotoxic T cells | Elevated percentage of CD3+ HLA-DR+ T cells, NK cells and cytokines such as IL-2, IL-6 in group 1; Elevated total CD3+ T cells, CD8+ T cells, CD3+ HLA-DR+ cells and IL-12 in group 2 | -- | DCs combined with cytotoxic T cells have more dominance to induce Th1 cytokine response instead of skewing toward the Th2 cytokine profile |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Ellinger, J.; Ritter, M.; Schmidt-Wolf, I.G.H. Clinical Studies Applying Cytokine-Induced Killer Cells for the Treatment of Renal Cell Carcinoma. Cancers 2020, 12, 2471. https://doi.org/10.3390/cancers12092471
Zhang Y, Ellinger J, Ritter M, Schmidt-Wolf IGH. Clinical Studies Applying Cytokine-Induced Killer Cells for the Treatment of Renal Cell Carcinoma. Cancers. 2020; 12(9):2471. https://doi.org/10.3390/cancers12092471
Chicago/Turabian StyleZhang, Ying, Jörg Ellinger, Manuel Ritter, and Ingo G. H. Schmidt-Wolf. 2020. "Clinical Studies Applying Cytokine-Induced Killer Cells for the Treatment of Renal Cell Carcinoma" Cancers 12, no. 9: 2471. https://doi.org/10.3390/cancers12092471
APA StyleZhang, Y., Ellinger, J., Ritter, M., & Schmidt-Wolf, I. G. H. (2020). Clinical Studies Applying Cytokine-Induced Killer Cells for the Treatment of Renal Cell Carcinoma. Cancers, 12(9), 2471. https://doi.org/10.3390/cancers12092471






