O-Glycan-Altered Extracellular Vesicles: A Specific Serum Marker Elevated in Pancreatic Cancer
Simple Summary
Abstract
1. Introduction
2. Results
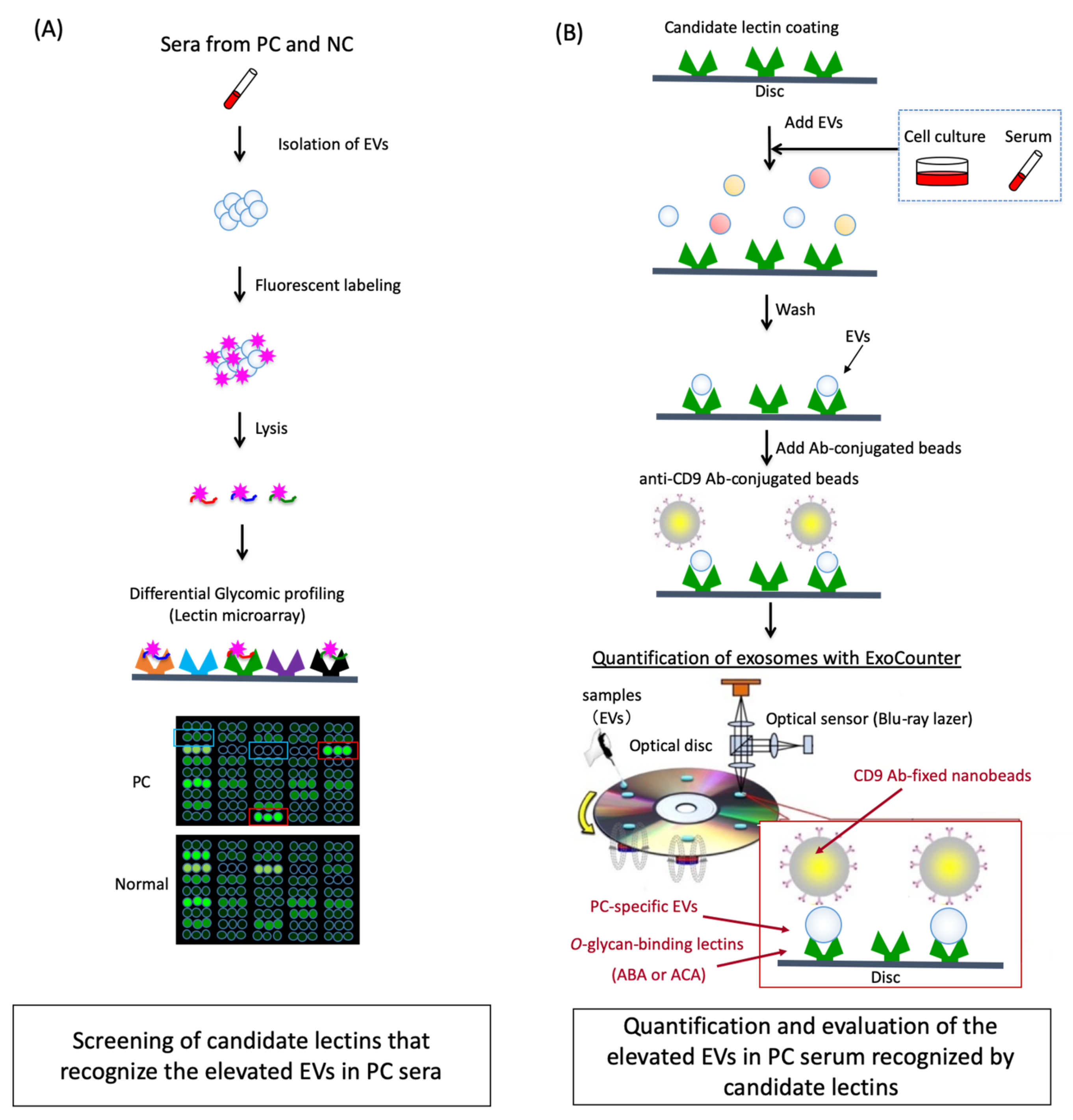
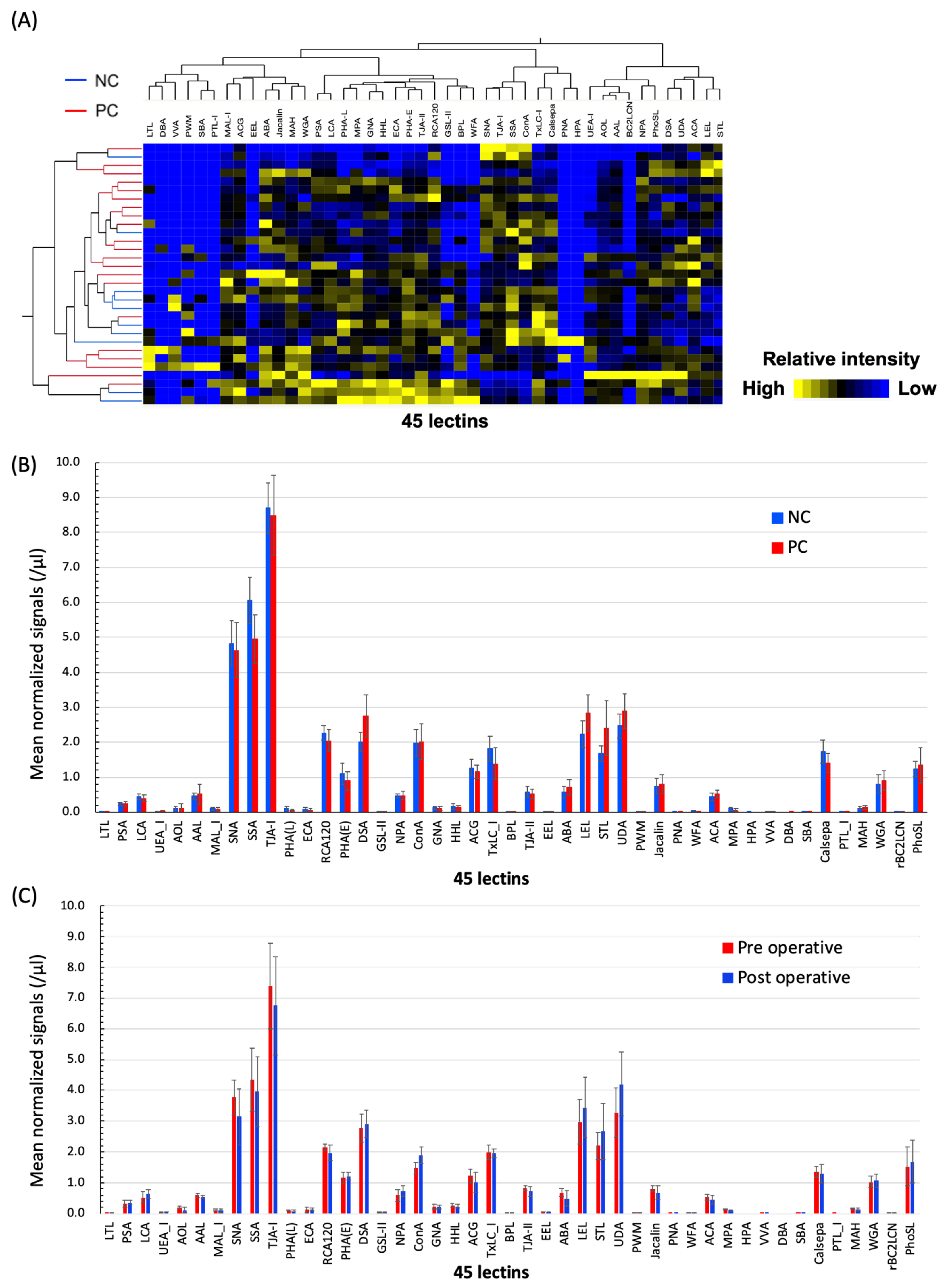
2.1. Differential Glycomic Profiling of Specific EVs in PC Sera Using Lectin Microarray
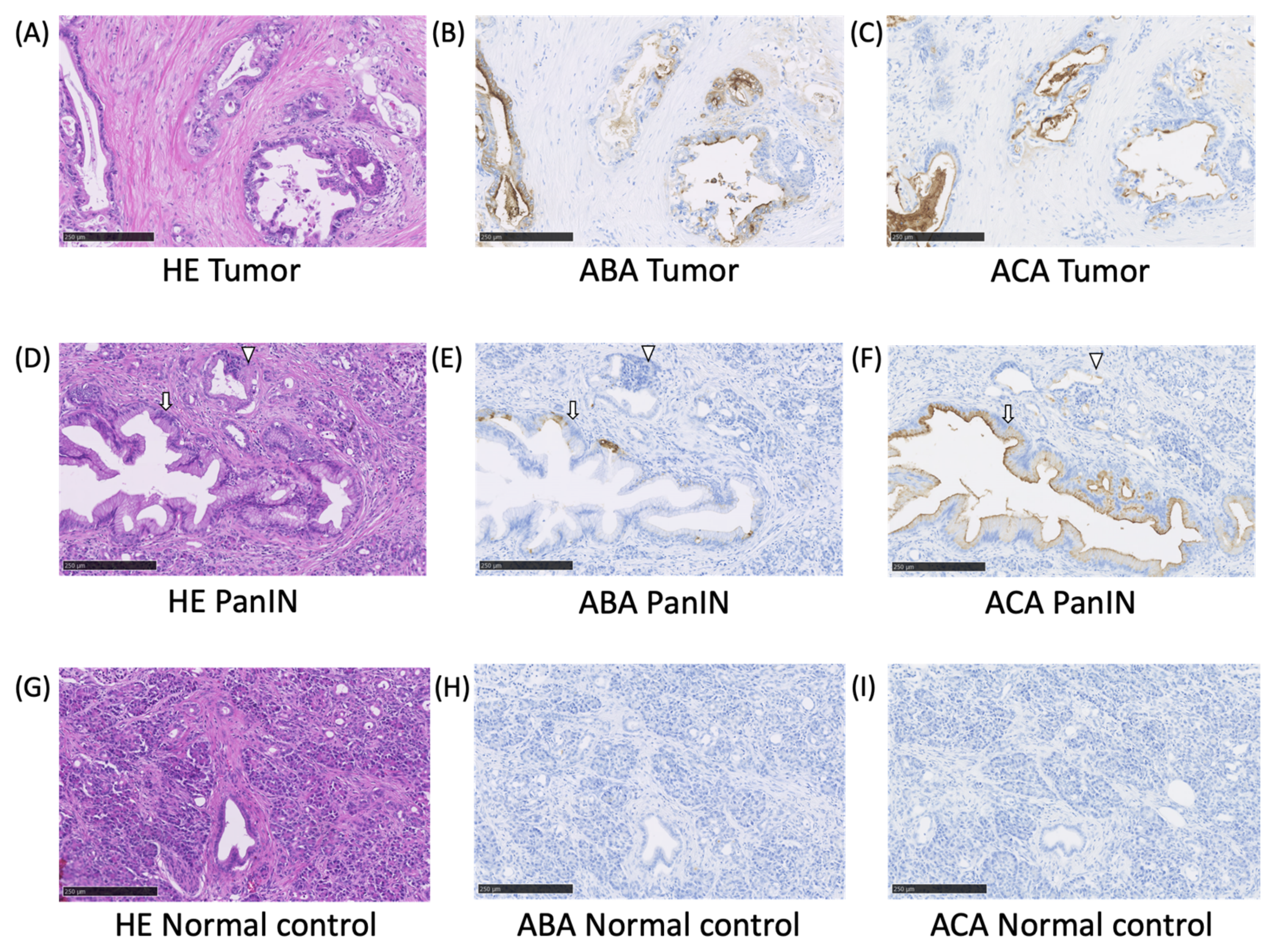
2.2. The ABA- and ACA-Positive Glycans are Strongly Localized in the Pathological Lesion of PC
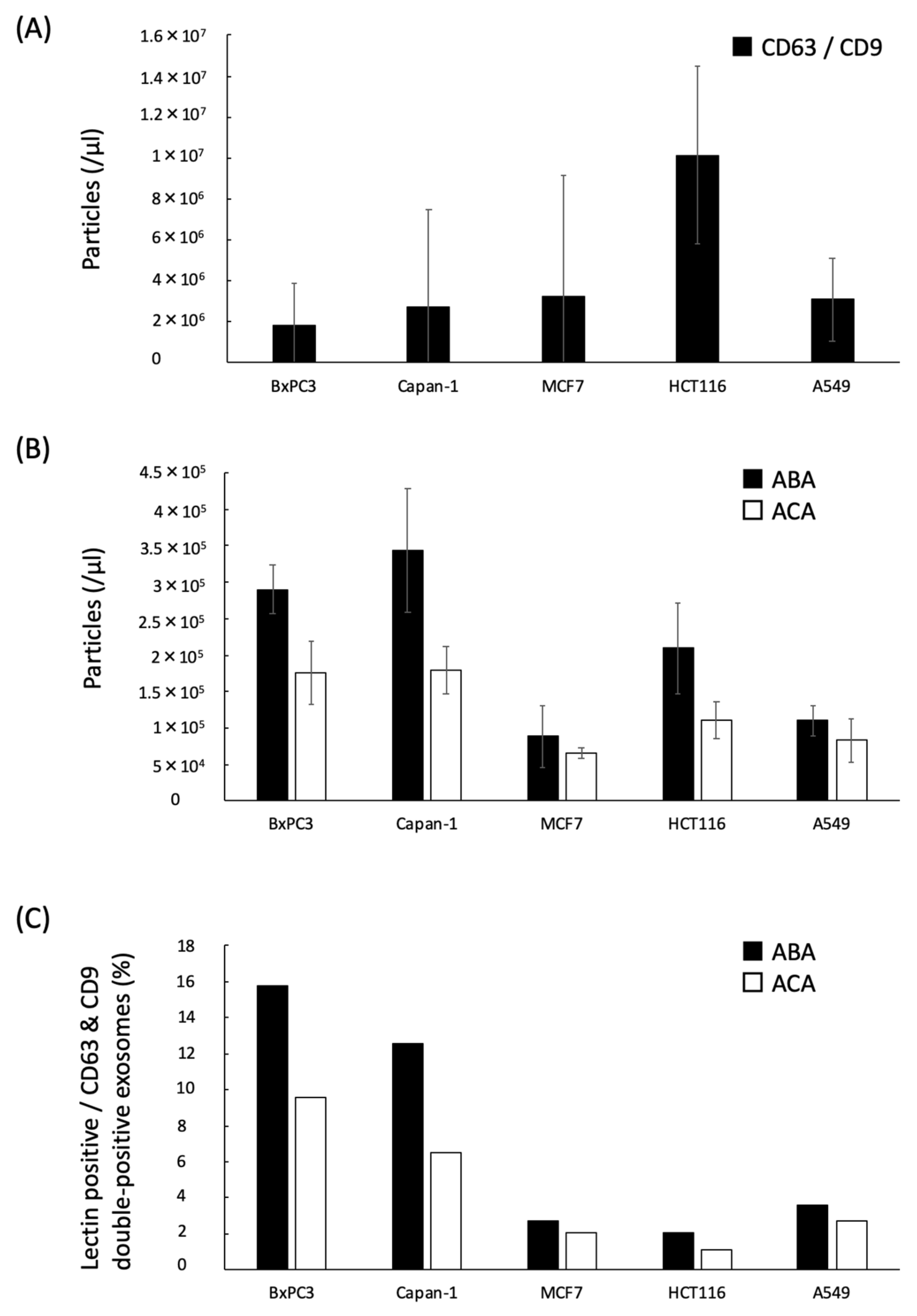
2.3. Quantification of the ABA- and ACA-Positive EVs Derived from Cancer Cell Lines with ExoCounter
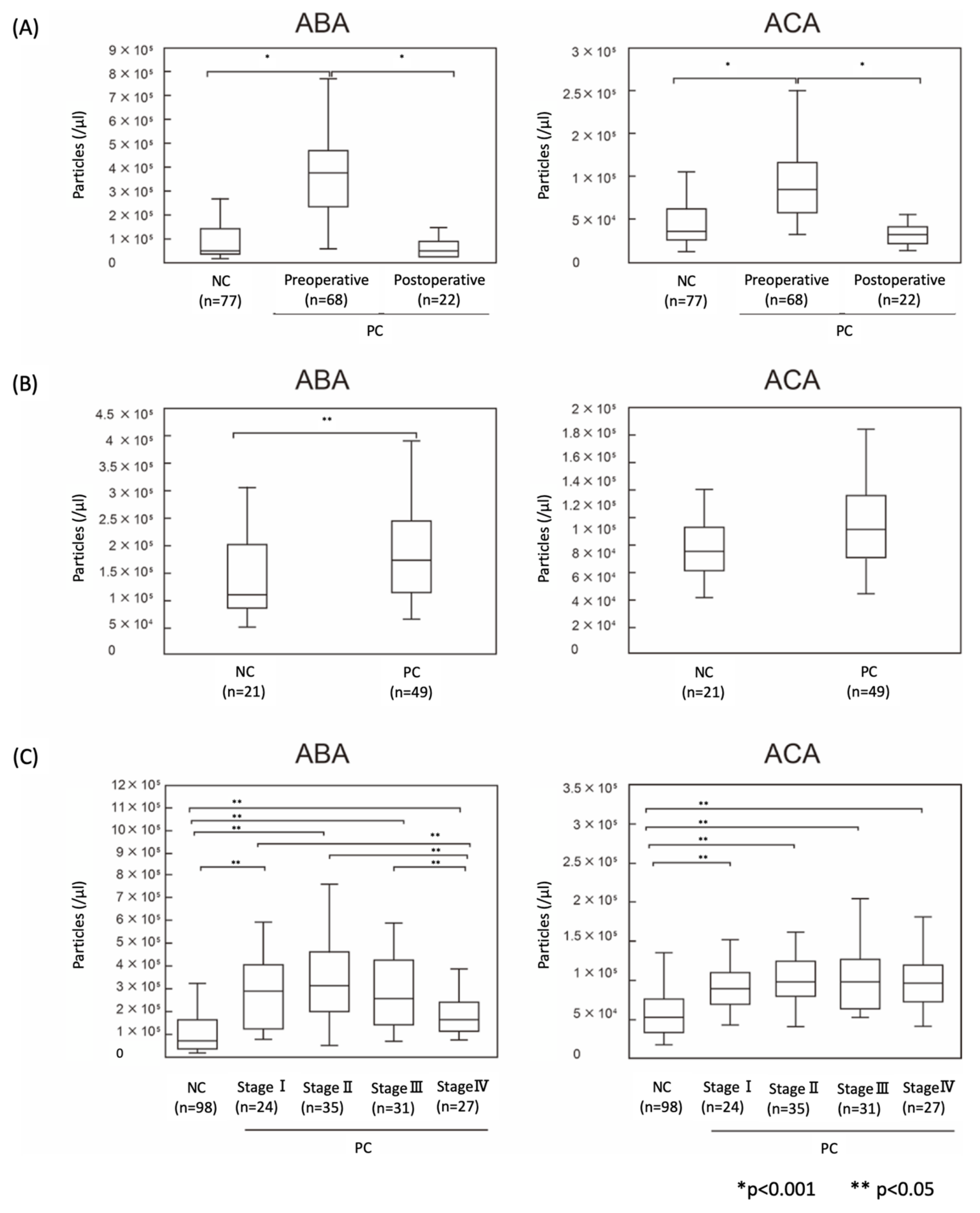
2.4. The Numbers of ABA- and ACA-Positive EVs were Elevated in Sera from PC Patients
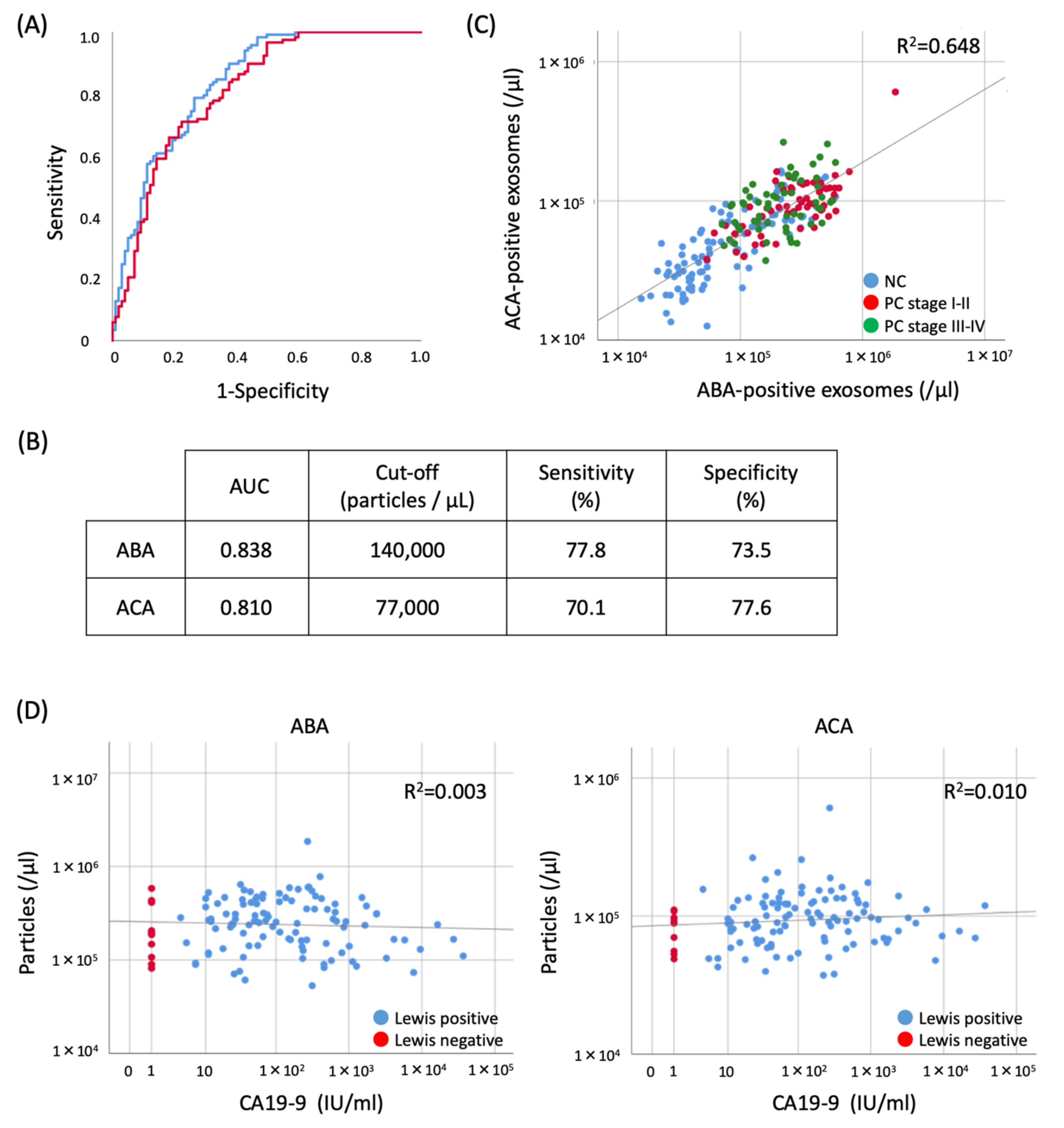
2.5. Evaluation of the Diagnostic Utility of ABA- and ACA-Positive EV Counts Elevated in PC Sera
3. Discussion
4. Materials and Methods
4.1. Antibody and Lectin
4.2. Cell Culture
4.3. Clinical Samples
4.4. Analysis with Lectin Microarray
4.5. Lectin-Staining of PC Tissue Sections
4.6. Preparation of Antibody-Conjugated Nanobeads
4.7. EV Quantification with ExoCounter
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sant, M.; Allemani, C.; Santaquilani, M.; Knijn, A.; Marchesi, F.; Capocaccia, R. EUROCARE-4. Survival of cancer patients diagnosed in 1995–1999. Results and commentary. Eur. J. Cancer 2009, 45, 931–991. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA A Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Mori, H.; Matsumoto, S.; Kiyosue, H.; Hori, Y.; Hongo, N. Pancreatic adenocarcinoma versus chronic pancreatitis: Differentiation with triple-phase helical CT. Abdom. Imaging 2010, 35, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.M.; Delbeke, D.; Beauchamp, R.D.; Chapman, W.C.; Sandler, M.P.; Sharp, K.W.; Richards, W.O.; Wright, J.K.; Frexes, M.E.; Pinson, C.W.; et al. 18Fluorodeoxyglucose-positron emission tomography in the management of patients with suspected pancreatic cancer. Ann. Surg. 1999, 229, 729–737, discussion 737–728. [Google Scholar] [CrossRef] [PubMed]
- Koyama, K.; Okamura, T.; Kawabe, J.; Nakata, B.; Chung, K.H.; Ochi, H.; Yamada, R. Diagnostic usefulness of FDG PET for pancreatic mass lesions. Ann. Nucl. Med. 2001, 15, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.B.; Sahai, A.V.; Aabakken, L.; Penman, I.D.; van Velse, A.; Webb, J.; Wilson, M.; Hoffman, B.J.; Hawes, R.H. Endoscopic ultrasound guided fine needle aspiration biopsy: A large single centre experience. Gut 1999, 44, 720–726. [Google Scholar] [CrossRef]
- Fritscher-Ravens, A.; Brand, L.; Knöfel, W.T.; Bobrowski, C.; Topalidis, T.; Thonke, F.; de Werth, A.; Soehendra, N. Comparison of endoscopic ultrasound-guided fine needle aspiration for focal pancreatic lesions in patients with normal parenchyma and chronic pancreatitis. Am. J. Gastroenterol. 2002, 97, 2768–2775. [Google Scholar] [CrossRef]
- Itoi, T.; Tsuchiya, T.; Itokawa, F.; Sofuni, A.; Kurihara, T.; Tsuji, S.; Ikeuchi, N. Histological diagnosis by EUS-guided fine-needle aspiration biopsy in pancreatic solid masses without on-site cytopathologist: A single-center experience. Dig. Endosc. 2011, 23 Suppl 1, 34–38. [Google Scholar] [CrossRef]
- Magnani, J.L.; Steplewski, Z.; Koprowski, H.; Ginsburg, V. Identification of the gastrointestinal and pancreatic cancer-associated antigen detected by monoclonal antibody 19-9 in the sera of patients as a mucin. Cancer Res. 1983, 43, 5489–5492. [Google Scholar]
- Ferrone, C.R.; Finkelstein, D.M.; Thayer, S.P.; Muzikansky, A.; Fernandez-delCastillo, C.; Warshaw, A.L. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J. Clin. Oncol. 2006, 24, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Tessler, D.A.; Catanzaro, A.; Velanovich, V.; Havstad, S.; Goel, S. Predictors of cancer in patients with suspected pancreatic malignancy without a tissue diagnosis. Am. J. Surg. 2006, 191, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Ballehaninna, U.K.; Chamberlain, R.S. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J. Gastrointest. Oncol. 2012, 3, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Kitago, M.; Matsuda, S.; Nakamura, Y.; Fujita, Y.; Imai, S.; Shinoda, M.; Yagi, H.; Abe, Y.; Hibi, T.; et al. KRAS mutations in cell-free DNA from preoperative and postoperative sera as a pancreatic cancer marker: A retrospective study. Br. J. Cancer 2018, 118, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Luchini, C.; Veronese, N.; Nottegar, A.; Cappelletti, V.; Daidone, M.G.; Smith, L.; Parris, C.; Brosens, L.A.A.; Caruso, M.G.; Cheng, L.; et al. Liquid Biopsy as Surrogate for Tissue for Molecular Profiling in Pancreatic Cancer: A Meta-Analysis Towards Precision Medicine. Cancers 2019, 11. [Google Scholar] [CrossRef]
- Hakomori, S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc. Natl. Acad. Sci. USA 2002, 99, 10231–10233. [Google Scholar] [CrossRef]
- Fukuda, M. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 1996, 56, 2237–2244. [Google Scholar]
- Hirabayashi, J. Concept, strategy and realization of lectin-based glycan profiling. J. Biochem. 2008, 144, 139–147. [Google Scholar] [CrossRef]
- Kuno, A.; Uchiyama, N.; Koseki-Kuno, S.; Ebe, Y.; Takashima, S.; Yamada, M.; Hirabayashi, J. Evanescent-field fluorescence-assisted lectin microarray: A new strategy for glycan profiling. Nat. Methods 2005, 2, 851–856. [Google Scholar] [CrossRef]
- Uchiyama, N.; Kuno, A.; Tateno, H.; Kubo, Y.; Mizuno, M.; Noguchi, M.; Hirabayashi, J. Optimization of evanescent-field fluorescence-assisted lectin microarray for high-sensitivity detection of monovalent oligosaccharides and glycoproteins. Proteomics 2008, 8, 3042–3050. [Google Scholar] [CrossRef]
- Matsuda, A.; Kuno, A.; Kawamoto, T.; Matsuzaki, H.; Irimura, T.; Ikehara, Y.; Zen, Y.; Nakanuma, Y.; Yamamoto, M.; Ohkohchi, N.; et al. Wisteria floribunda agglutinin-positive mucin 1 is a sensitive biliary marker for human cholangiocarcinoma. Hepatology 2010, 52, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Kuno, A.; Nakagawa, T.; Ikehara, Y.; Irimura, T.; Yamamoto, M.; Nakanuma, Y.; Miyoshi, E.; Nakamori, S.; Nakanishi, H.; et al. Lectin Microarray-Based Sero-Biomarker Verification Targeting Aberrant O-Linked Glycosylation on Mucin 1. Anal. Chem. 2015, 87, 7274–7281. [Google Scholar] [CrossRef] [PubMed]
- Kalra, H.; Drummen, G.P.; Mathivanan, S. Focus on Extracellular Vesicles: Introducing the Next Small Big Thing. Int. J. Mol. Sci. 2016, 17, 170. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Valadi, H.; Ekström, K.; Bossios, A.; Sjöstrand, M.; Lee, J.J.; Lötvall, J.O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 2007, 9, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, A.; Costa-Silva, B.; Shen, T.L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]
- Millimaggi, D.; Mari, M.; D’Ascenzo, S.; Carosa, E.; Jannini, E.A.; Zucker, S.; Carta, G.; Pavan, A.; Dolo, V. Tumor vesicle-associated CD147 modulates the angiogenic capability of endothelial cells. Neoplasia 2007, 9, 349–357. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Kosaka, N.; Konishi, Y.; Ohta, H.; Okamoto, H.; Sonoda, H.; Nonaka, R.; Yamamoto, H.; Ishii, H.; Mori, M.; et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat. Commun. 2014, 5, 3591. [Google Scholar] [CrossRef]
- Dai, S.; Wan, T.; Wang, B.; Zhou, X.; Xiu, F.; Chen, T.; Wu, Y.; Cao, X. More efficient induction of HLA-A*0201-restricted and carcinoembryonic antigen (CEA)-specific CTL response by immunization with exosomes prepared from heat-stressed CEA-positive tumor cells. Clin. Cancer Res. 2005, 11, 7554–7563. [Google Scholar] [CrossRef]
- Samandari, M.; Julia, M.G.; Rice, A.; Chronopoulos, A.; Del Rio Hernandez, A.E. Liquid biopsies for management of pancreatic cancer. Transl. Res. 2018, 201, 98–127. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Lucien, F.; Lac, V.; Billadeau, D.D.; Borgida, A.; Gallinger, S.; Leong, H.S. Glypican-1 and glycoprotein 2 bearing extracellular vesicles do not discern pancreatic cancer from benign pancreatic diseases. Oncotarget 2019, 10, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Kabe, Y.; Suematsu, M.; Sakamoto, S.; Hirai, M.; Koike, I.; Hishiki, T.; Matsuda, A.; Hasegawa, Y.; Tsujita, K.; Ono, M.; et al. Development of a Highly Sensitive Device for Counting the Number of Disease-Specific Exosomes in Human Sera. Clin. Chem. 2018, 64, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Kabe, Y.; Sakamoto, S.; Hatakeyama, M.; Yamaguchi, Y.; Suematsu, M.; Itonaga, M.; Handa, H. Application of high-performance magnetic nanobeads to biological sensing devices. Anal. Bioanal. Chem. 2019, 411, 1825–1837. [Google Scholar] [CrossRef]
- Matsuda, A.; Kuno, A.; Yoshida, M.; Wagatsuma, T.; Sato, T.; Miyagishi, M.; Zhao, J.; Suematsu, M.; Kabe, Y.; Narimatsu, H. Comparative Glycomic Analysis of Exosome Subpopulations Derived from Pancreatic Cancer Cell Lines. J. Proteome Res. 2020, 19, 2516–2524. [Google Scholar] [CrossRef]
- Pan, D.; Chen, J.; Feng, C.; Wu, W.; Wang, Y.; Tong, J.; Zhou, D. Preferential Localization of MUC1 Glycoprotein in Exosomes Secreted by Non-Small Cell Lung Carcinoma Cells. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tateno, H.; Dohra, H.; Moriwaki, K.; Miyoshi, E.; Hirabayashi, J.; Kawagishi, H. A novel core fucose-specific lectin from the mushroom Pholiota squarrosa. J. Biol. Chem. 2012, 287, 33973–33982. [Google Scholar] [CrossRef]
- Shimomura, O.; Oda, T.; Tateno, H.; Ozawa, Y.; Kimura, S.; Sakashita, S.; Noguchi, M.; Hirabayashi, J.; Asashima, M.; Ohkohchi, N. A Novel Therapeutic Strategy for Pancreatic Cancer: Targeting Cell Surface Glycan Using rBC2LC-N Lectin-Drug Conjugate (LDC). Mol. Cancer Ther. 2018, 17, 183–195. [Google Scholar] [CrossRef]
- Khan, S.; Jutzy, J.M.; Valenzuela, M.M.; Turay, D.; Aspe, J.R.; Ashok, A.; Mirshahidi, S.; Mercola, D.; Lilly, M.B.; Wall, N.R. Plasma-derived exosomal survivin, a plausible biomarker for early detection of prostate cancer. PLoS ONE 2012, 7, e46737. [Google Scholar] [CrossRef]
- Shimoda, A.; Tahara, Y.; Sawada, S.I.; Sasaki, Y.; Akiyoshi, K. Glycan profiling analysis using evanescent-field fluorescence-assisted lectin array: Importance of sugar recognition for cellular uptake of exosomes from mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2017, 491, 701–707. [Google Scholar] [CrossRef]
- Saito, S.; Hiemori, K.; Kiyoi, K.; Tateno, H. Glycome analysis of extracellular vesicles derived from human induced pluripotent stem cells using lectin microarray. Sci. Rep. 2018, 8, 3997. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Nowack, D.D.; Omenn, G.S.; Haab, B.B. Mucin glycosylation is altered by pro-inflammatory signaling in pancreatic-cancer cells. J. Proteome Res. 2009, 8, 1876–1886. [Google Scholar] [CrossRef] [PubMed]
- Tohoku Medical MegaBank Organization in Japan. Available online: http://www.megabank.tohoku.ac.jp/english/ (accessed on 25 July 2020).
- Nakai, W.; Yoshida, T.; Diez, D.; Miyatake, Y.; Nishibu, T.; Imawaka, N.; Naruse, K.; Sadamura, Y.; Hanayama, R. A novel affinity-based method for the isolation of highly purified extracellular vesicles. Sci. Rep. 2016, 6, 33935. [Google Scholar] [CrossRef]
- Sakamoto, S.; Kabe, Y.; Hatakeyama, M.; Yamaguchi, Y.; Handa, H. Development and application of high-performance affinity beads: Toward chemical biology and drug discovery. Chem. Rec. 2009, 9, 66–85. [Google Scholar] [CrossRef]
- Kabe, Y.; Nakane, T.; Koike, I.; Yamamoto, T.; Sugiura, Y.; Harada, E.; Sugase, K.; Shimamura, T.; Ohmura, M.; Muraoka, K.; et al. Haem-dependent dimerization of PGRMC1/Sigma-2 receptor facilitates cancer proliferation and chemoresistance. Nat. Commun. 2016, 7, 11030. [Google Scholar] [CrossRef]
| Lectins 1 | Study 1 | Study 2 | ||||
|---|---|---|---|---|---|---|
| Tumor/Normal Ratio | p Value 2 | Pre-/Post-Operative Ratio | ||||
| DSA | 1.375 | <0.001 | 3 | 4 | 0.953 | |
| STL | 1.440 | <0.001 | 3 | 4 | 0.822 | |
| LEL | 1.276 | 0.001 | 3 | 4 | 0.866 | |
| UDA | 1.170 | 0.011 | 3 | 4 | 0.780 | |
| ACA | 1.199 | 0.058 | 3 | 4 | 1.175 | 6 |
| ABA | 1.239 | 0.063 | 3 | 4 | 1.387 | 6 |
| SSA | 0.820 | <0.001 | 3 | 5 | 1.097 | |
| TxLC I | 0.764 | 0.009 | 3 | 5 | 1.019 | |
| Calsepa | 0.800 | 0.012 | 3 | 5 | 1.054 | |
| PHA(E) | 0.814 | 0.085 | 5 | 0.959 | ||
| ACG | 0.899 | 0.157 | ||||
| LCA | 0.898 | 0.186 | ||||
| WGA | 1.131 | 0.322 | ||||
| AAL | 1.119 | 0.443 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yokose, T.; Kabe, Y.; Matsuda, A.; Kitago, M.; Matsuda, S.; Hirai, M.; Nakagawa, T.; Masugi, Y.; Hishiki, T.; Nakamura, Y.; et al. O-Glycan-Altered Extracellular Vesicles: A Specific Serum Marker Elevated in Pancreatic Cancer. Cancers 2020, 12, 2469. https://doi.org/10.3390/cancers12092469
Yokose T, Kabe Y, Matsuda A, Kitago M, Matsuda S, Hirai M, Nakagawa T, Masugi Y, Hishiki T, Nakamura Y, et al. O-Glycan-Altered Extracellular Vesicles: A Specific Serum Marker Elevated in Pancreatic Cancer. Cancers. 2020; 12(9):2469. https://doi.org/10.3390/cancers12092469
Chicago/Turabian StyleYokose, Takahiro, Yasuaki Kabe, Atsushi Matsuda, Minoru Kitago, Sachiko Matsuda, Miwa Hirai, Tomomi Nakagawa, Yohei Masugi, Takako Hishiki, Yuki Nakamura, and et al. 2020. "O-Glycan-Altered Extracellular Vesicles: A Specific Serum Marker Elevated in Pancreatic Cancer" Cancers 12, no. 9: 2469. https://doi.org/10.3390/cancers12092469
APA StyleYokose, T., Kabe, Y., Matsuda, A., Kitago, M., Matsuda, S., Hirai, M., Nakagawa, T., Masugi, Y., Hishiki, T., Nakamura, Y., Shinoda, M., Yagi, H., Abe, Y., Oshima, G., Hori, S., Nakano, Y., Honda, K., Kashiro, A., Morizane, C., ... Kitagawa, Y. (2020). O-Glycan-Altered Extracellular Vesicles: A Specific Serum Marker Elevated in Pancreatic Cancer. Cancers, 12(9), 2469. https://doi.org/10.3390/cancers12092469







