Lymphoepithelial Carcinoma of Larynx and Hypopharynx: A Rare Clinicopathological Entity
Abstract
1. Introduction
2. Materials and Methods
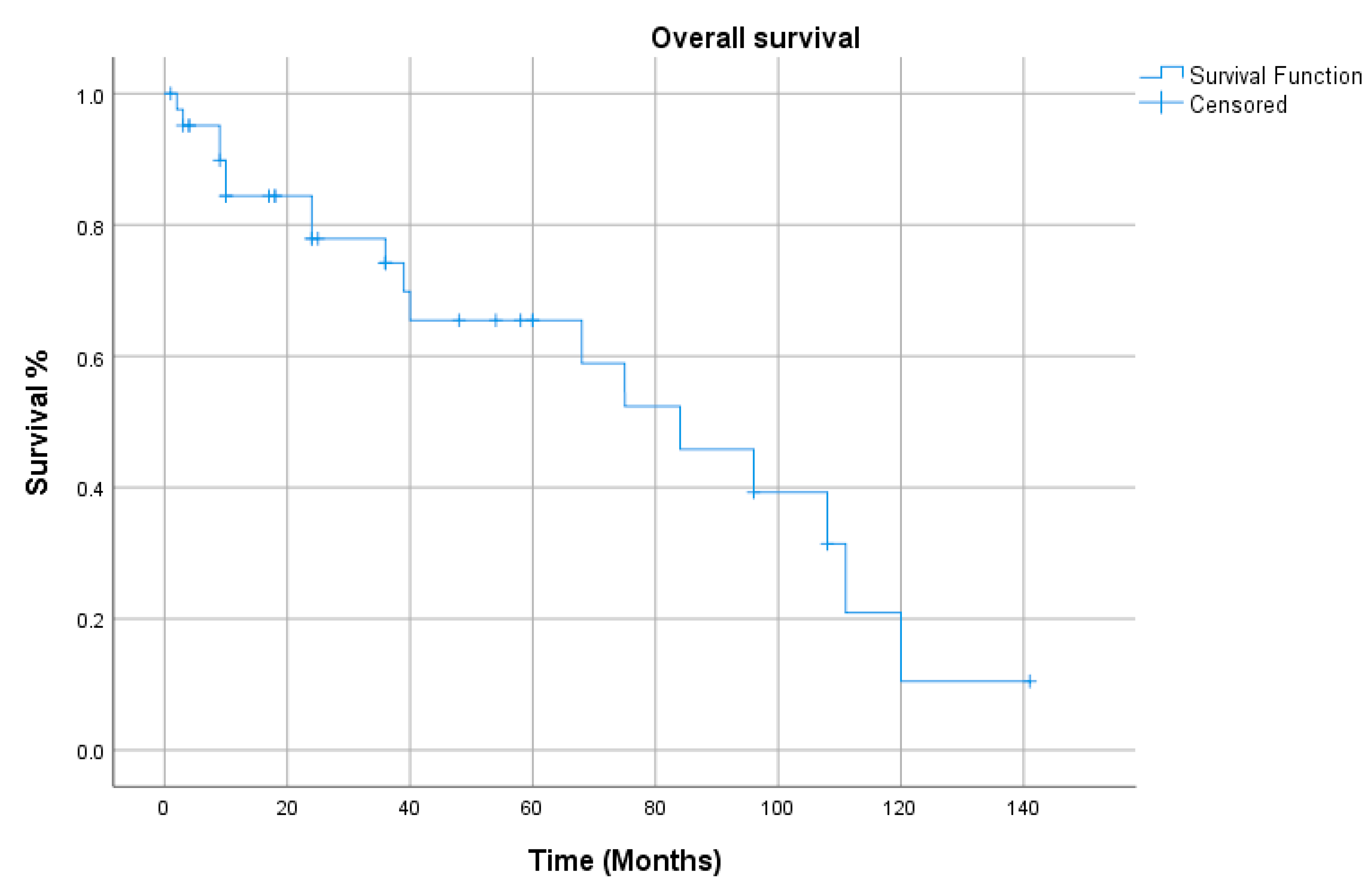
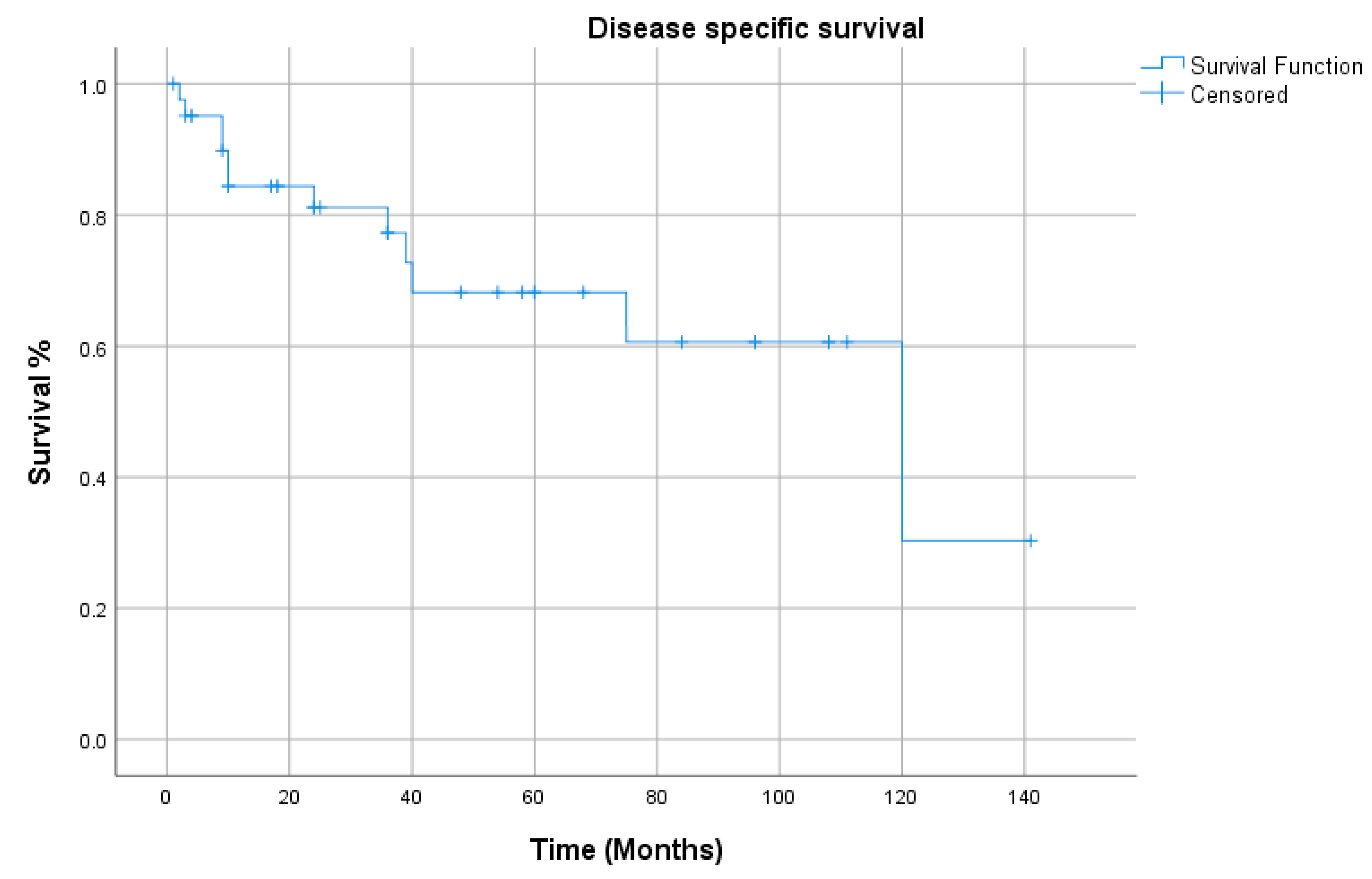
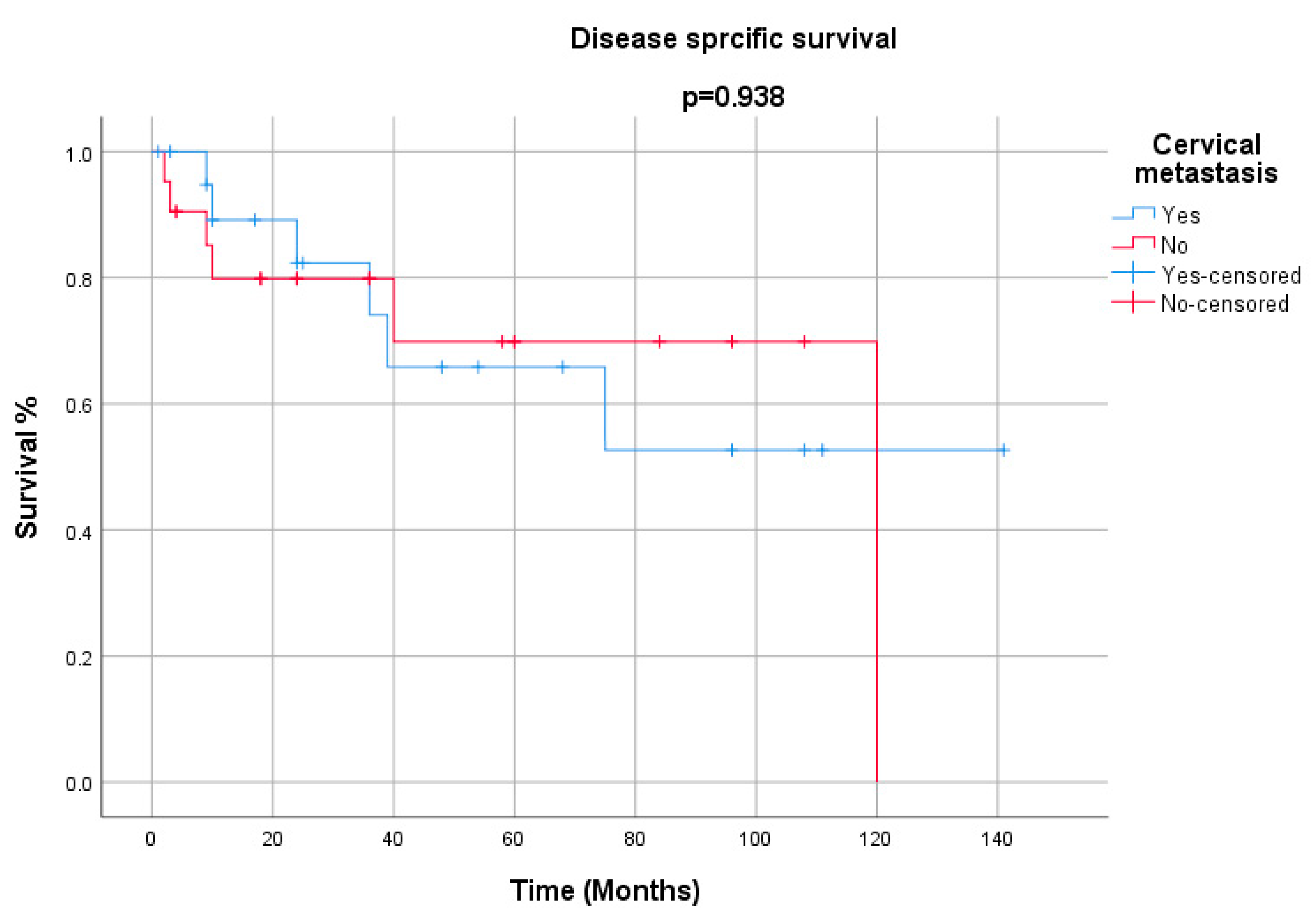
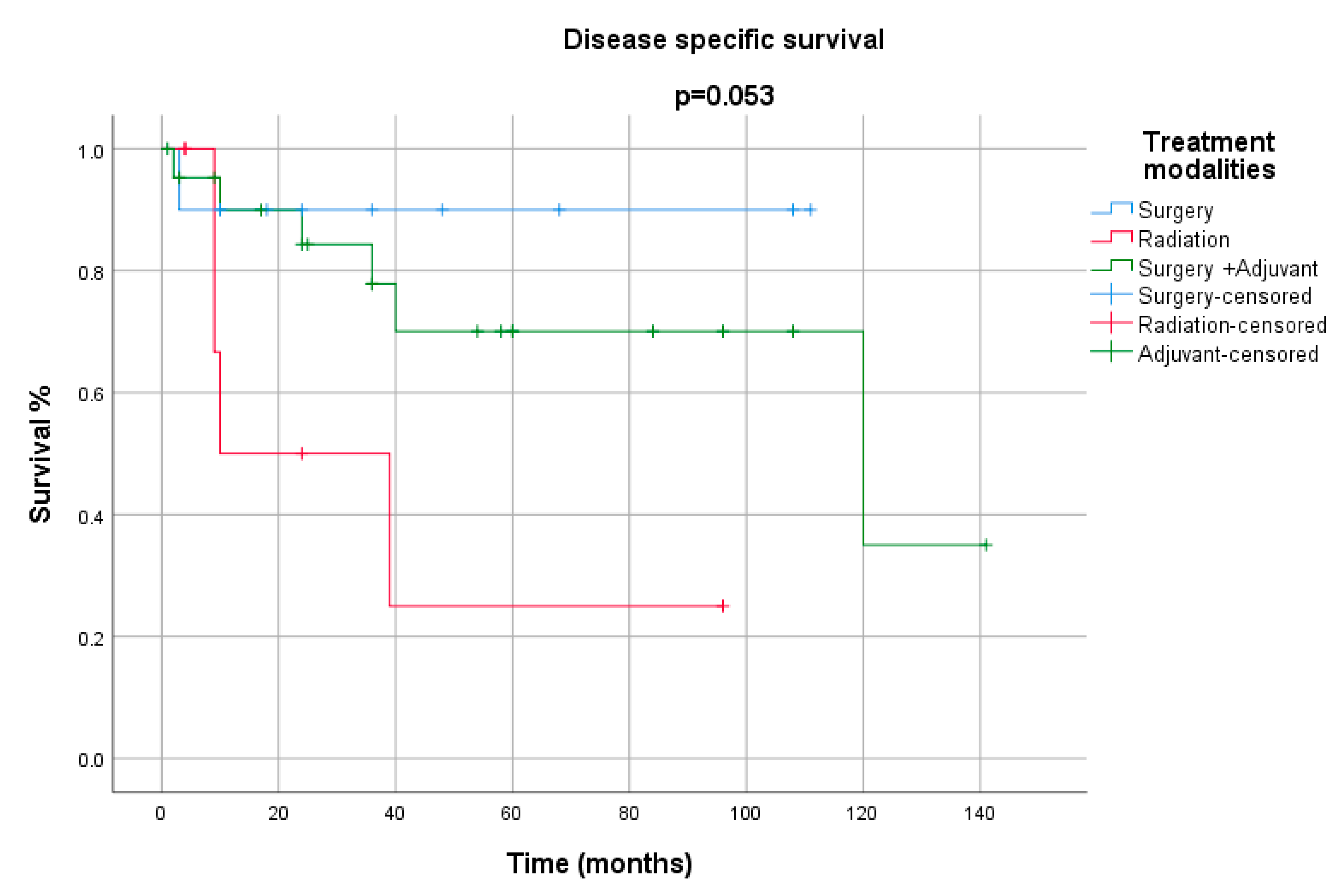
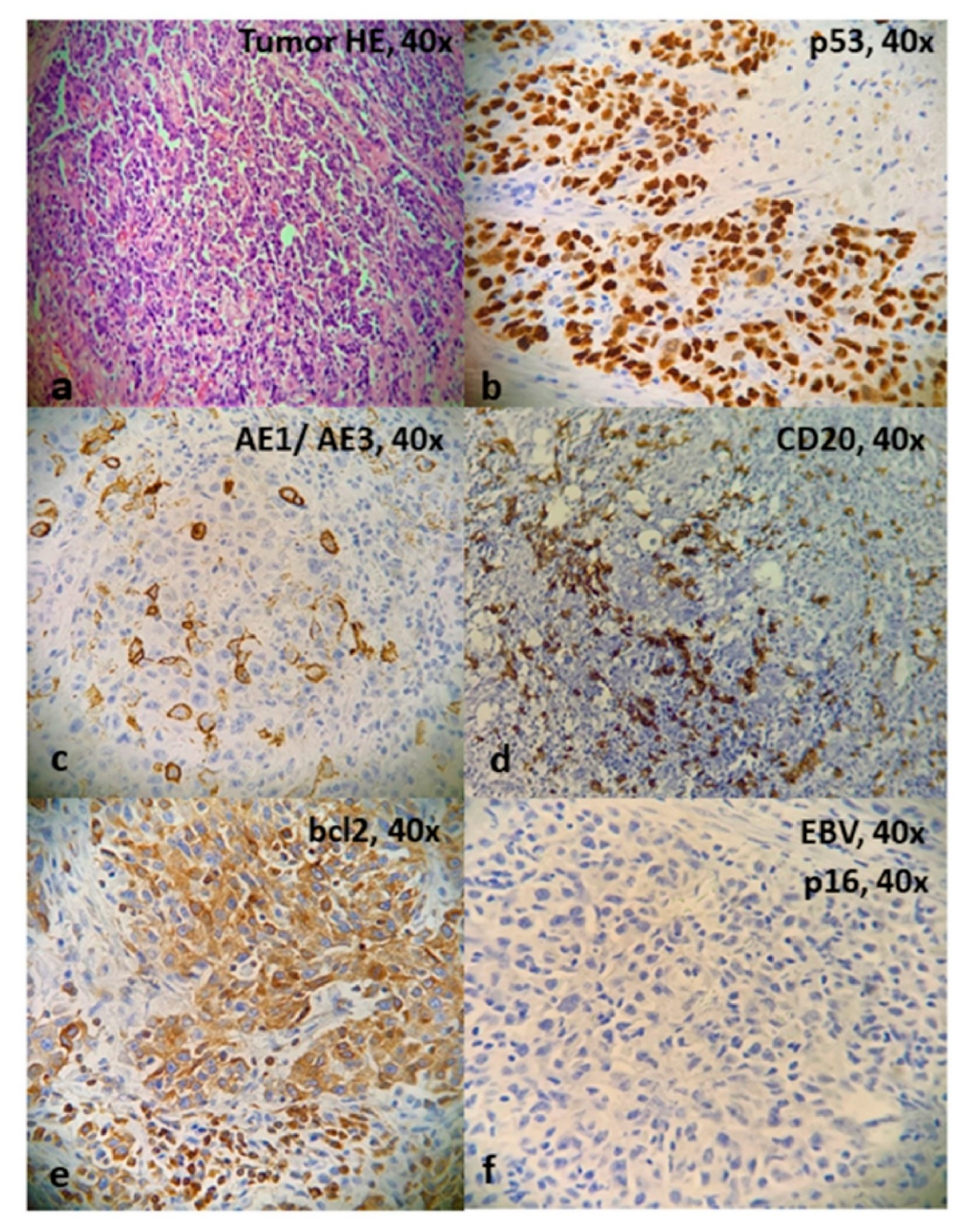
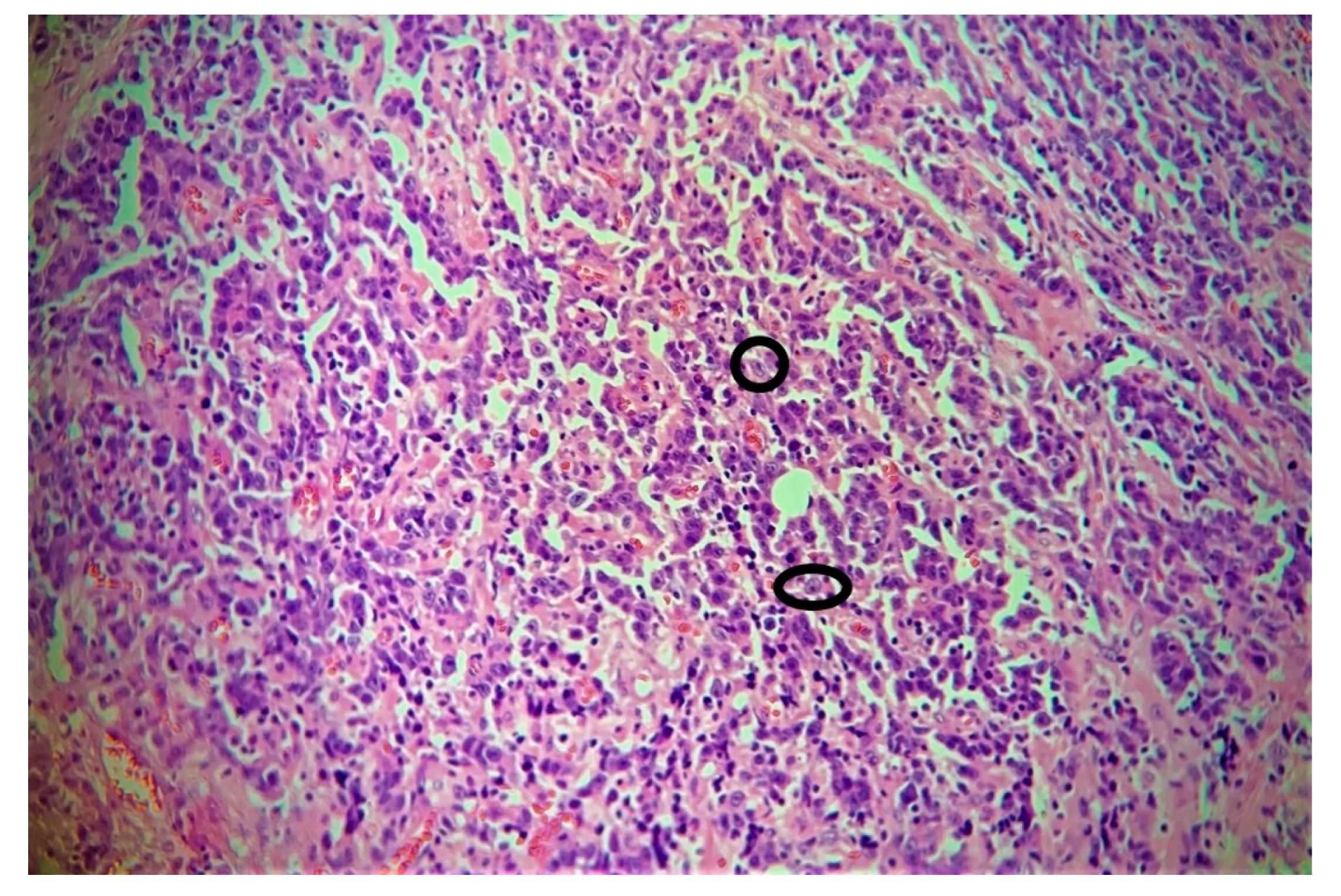
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Marioni, G.; Mariuzzi, L.; Gaio, E.; Portaleone, S.; Pertoldi, B.; Staffieri, A. Lymphoepithelial carcinoma of the larynx. Acta Otolaryngol. 2002, 122, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Sone, M.; Nakashima, T.; Nagasaka, T.; Itoh, A.; Yanagita, N. Lymphoepithelioma-like carcinoma of the larynx associated with an Epstein-Barr viral infection. Otolaryngol. Head Neck Surg. 1998, 119, 134–137. [Google Scholar] [CrossRef]
- Gaulard, P.; Bishop, J.; Gillison, M. Lymphoepithelial carcinoma. In WHO Classification of Head and Neck Tumours, 4th ed.; El-Naggar, A.K., Chan, J.K.C., Grandis, J.R., Takata, T., Slootweg, P.J., Eds.; International Agency for Research in Cancer: Lyon, France, 2017; p. 90. [Google Scholar]
- Singhi, A.D.; Stelow, E.B.; Mills, S.E.; Westra, W.H. Lymphoepithelial like carcinoma of the oropharynx: A morphologic variant of HPV related head and neck carcinoma. Am. J. Surg. Pathol. 2010, 34, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.H.; El Mofty, S.K.; Lewis, J.S., Jr. Undifferentiated carcinoma of the oropharynx: A human papillomavirus-associated tumor with a favorable prognosis. Mod. Pathol. 2011, 24, 1306–1312. [Google Scholar] [CrossRef]
- MacMillan, C.; Kapadia, S.B.; Finkelstein, S.D.; Nalesnik, M.A.; Barnes, L. Lymphoepithelial carcinoma of the larynx and hypopharynx: Study of eight cases with relationship to Epstein-Barr virus and p53 gene alterations, and review of the literature. Hum. Pathol. 1996, 27, 1172–1179. [Google Scholar] [CrossRef]
- Dockerty, M.B.; Parkhill, E.M.; Dahlin, D.C.; Woolner, L.B.; Soule, E.H.; Harrison, E.G. Atlas of tumor pathology. Tumors of the Oral Cavity and Pharynx. Sect IV, Fasc 106; Armed Forces Institute of Pathology: Washington, DC, USA, 1968. [Google Scholar]
- Ferlito, A.; Fiore Donati, L. “Malignant lymphoepithelial lesions” (undifferentiated ductal carcinomas of the parotid gland). Three case reports and review of the literature. J. Laryngol. Otol. 1977, 91, 869–885. [Google Scholar] [CrossRef]
- Frank, D.K.; Cheron, F.; Cho, H.; Sclafani, A.P. Nonnasopharyngeal lymphoepitheliomas (undifferentiated carcinomas) of the upper aerodigestive tract. Ann. Otol. Rhinol. Laryngol. 1995, 104, 305–310. [Google Scholar] [CrossRef]
- Andryk, J.; Freije, J.E.; Schultz, C.J.; Campbell, B.H.; Komorowski, R.A. Lymphoepithelioma of the larynx. Am. J. Otolaryngol. 1996, 17, 61–63. [Google Scholar] [CrossRef]
- Acuna, G.; Goma, M.; Salvador, J.T.; Garcia-Bragado, F.; Alós, L.; Ordi, J.; Cardesa, A.; Nadal, A. Human papillomavirus in laryngeal and hypopharyngeal lymphoepithelial carcinoma. Mod. Pathol. 2019, 32, 621–626. [Google Scholar] [CrossRef]
- Hammas, N.; Benmansour, N.; El Amine, M.N.; Chbani, L.; El Fatemi, H. Lymphoepithelial carcinoma: A case report of a rare tumor of the larynx. BMC Clin. Pathol. 2017, 17, 24. [Google Scholar] [CrossRef]
- Kermani, W.; Belcadhi, M.; Sriha, B.; Abdelkéfi, M. Epstein-Barr virus-associated lymphoepithelial carcinoma of the larynx. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2015, 132, 231–233. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Micheau, C.; Luboinski, B.; Schwaab, G.; Richard, J.; Cachin, Y. Lymphoepitheliomas of the larynx (undifferentiated carcinomas of nasopharyngeal type). Clin. Otolaryngol. 1979, 4, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zbaren, P.; Borisch, B.; Lang, H.; Greiner, R. Undifferentiated carcinoma of nasopharyngeal type of the laryngopharyngeal region. Otolaryngol. Head Neck Surg. 1997, 117, 688–693. [Google Scholar] [CrossRef]
- Dray, T.; Vargas, H.; Weidner, N.; Sofferman, R.A. Lymphoepitheliomas of the laryngohypopharynx. Am. J. Otolaryngol. 1998, 19, 263–266. [Google Scholar] [CrossRef]
- Coskun, B.U.; Cinar, U.; Sener, B.M.; Dadas, B. Lymphoepithelial carcinoma of the larynx. Auris Nasus Larynx 2005, 32, 189–193. [Google Scholar] [CrossRef]
- Bansal, S.; Shankar, A.; Gupta, A.K. Undifferentiated carcinoma of larynx of nasopharyngeal type. Online J. Health Allied Sci. 2011, 10, 1–2. [Google Scholar]
- Ibrahimov, M.; Yilmaz, M.; Celal, M.H.; Mamanov, M.; Yollu, U.; Ozek, H. Lymphoepithelial carcinoma of the larynx. J. Craniofac. Surg. 2013, 24, 1049. [Google Scholar] [CrossRef]
- Kouadir, A.; El Mazghi, A.; Hassouni, K. Lymphoepithelial carcinoma of the larynx. J. Case Rep. Images Oncol. 2017, 3, 17–21. [Google Scholar]
- Monteiro, F.; Baldaia, H.; Ribeiro, L.; Sousa, M.; Oliveira, P.; Ferreira, E.; De Almeida, M.G.; Condé, A. Epstein-Barr Virus-Associated With Lymphoepithelial Carcinoma: A Rare Tumor of the Larynx. Clin. Med. Insights Ear. Nose Throat. 2019, 12, 1179550619865551. [Google Scholar] [CrossRef]
- Schmincke A: Uber lympho-eitheliale geschwulste. Beitr. Pathol. Anat. Allg. Pathol. 1921, 68, 161–170.
- Regaud, C.; Reverchon, L. Sur un d’epithelioma—Epidermoide developpe dans le massif maxillaire superieur, et endu aux teguments de la face, aux cavities buccale, nasale et orbitaire, ainsiqua’aux ganglions de cou, gueri par la curietherapie. Rev. Laryngol. Otol. Rhinol. (Bord.) 1921, 42, 369–378. [Google Scholar]
- Marx, H. Über “lympho-epitheliale” Geschwülste des Kehlkopfes. Z Hals Nasen Ohrenheilkd 1926, 15, 392–394. [Google Scholar]
- Calvet, P.J.; Claux, J.; Birague, C.; Coll, J. Les sarcomes du larynx étude générale à propos de 7 observations. J. Fr. Otorhinolaryngol. 1968, 17, 21–29. [Google Scholar]
- Iezzoni, J.C.; Gaffey, M.J.; Weiss, L.M. The role of Epstein-Barr virus in lymphoepithelioma-like carcinomas. Am. J. Clin. Pathol. 1995, 103, 308–315. [Google Scholar] [CrossRef]
- Narozny, W.; Betlejewski, A.; Stankiewicz, C.; Kaminski, M. Ventriculosaccular lymphoe pithelioma of the larynx: Case report and literature review. Head Neck 1998, 20, 425–429. [Google Scholar] [CrossRef]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. World Health Organization Classification of Head and Neck Tumours, 4th ed.; WHO/IARC Classification of Tumours: Lyon, France, 2017; Volume 9. [Google Scholar]
- Stanley, R.J.; Weiland, L.H.; DeSanto, L.W.; Neel, H.B. Lymphoepithelioma (undifferentiated carcinoma) of the laryngohypopharynx. Laryngoscope 1985, 95, 1077–1081. [Google Scholar] [CrossRef]
- Chan, J.Y.K.; Wong, E.W.Y.; Ng, S.-K.; Vlantis, A.C. Nonnasopharyngeal head and neck lymphoepithelioma-like carcinoma in the United States: A population-based study. Head Neck 2016, 38, 1294–1300. [Google Scholar] [CrossRef]
- Greene, L.; Brundage, W.; Cooper, K. Large cell neuroendocrine carcinoma of the larynx: A case report and a review of the classification of this neoplasm. J. Clin. Pathol. 2005, 58, 658–661. [Google Scholar] [CrossRef]
- Lewis, J.S., Jr.; Spence, D.C.; Chiosea, S.; Barnes, E.L., Jr.; Brandwein-Gensler, M.; El-Mofty, S.K. Large cell neuroendocrine carcinoma of the larynx: Definition of an entity. Head Neck Pathol. 2010, 4, 198–207. [Google Scholar] [CrossRef]

| Variables | Number of Patients N (%) |
|---|---|
| Gender | |
| Male | 41(91) |
| Female | 04(9) |
| Site | |
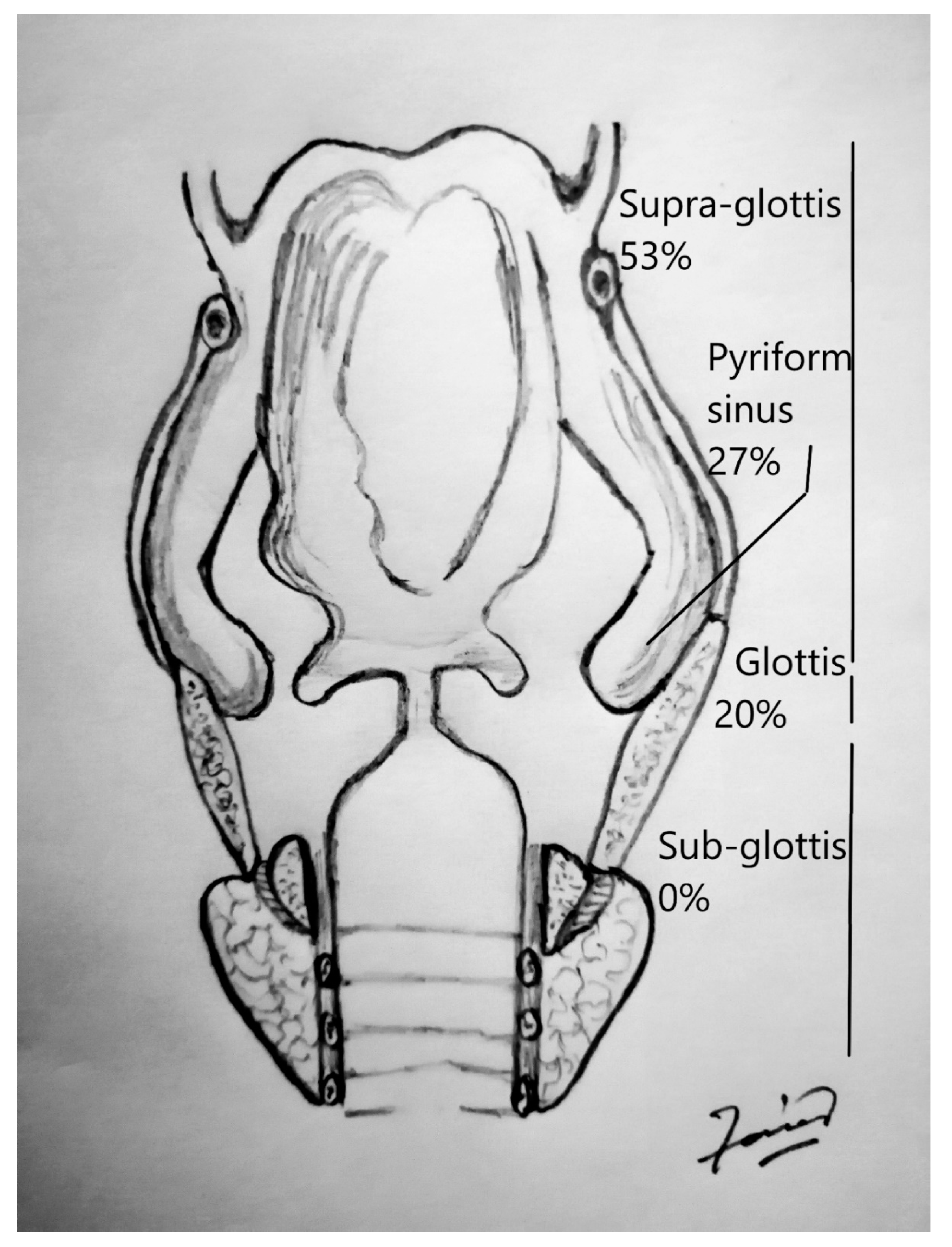
| Larynx | |
| –Supraglottis | 24 |
| –Glottis | 9 |
| –Subglottis | 0 |
| Hypopharynx | |
| –Pyriform sinus | 12 |
| Associated factors | |
| Smoking | 12 |
| Alcohol | 4 |
| Not reported | 28 |
| Symptoms | |
| Hoarseness | 17 |
| Dysphagia | 10 |
| Neck mass | 10 |
| Dyspnea | 2 |
| Odynophagia | 1 |
| Others (Globus sensation, vomiting, sore throat) | 5 |
| Treatment | |
| Surgery | 12 |
| RT | 9 |
| Surgery + Radiation | 17 |
| Surgery + Chemoradiotherapy | 7 |
| Cervical metastasis | |
| Yes | 24 |
| No | 20 |
| Not reported | 1 |
| Distant metastasis | |
| Yes | 9 |
| No | 32 |
| Not reported | 4 |
| EBV | |
| Positive | 5 |
| Negative | 24 |
| Not reported | 16 |
| HPV | |
| Positive | 4 |
| Negative | 4 |
| Not reported | 37 |
| P53 | |
| Positive | 10 |
| Negative | 4 |
| Not reported | 31 |
| Author | Year | Age | Gender | Site | Smoking | Alcohol | Symptoms | Follow |
|---|---|---|---|---|---|---|---|---|
| up | ||||||||
| Dockerty | 1968 | 69 | M | Hypopharynx | – | – | 10 | |
| Ferlito | 1977 | 60 | M | Pyriform sinus | – | – | Neck mass | 10 |
| Toker | 1978 | 69 | F | Glottis | – | – | Hoarseness | 108 |
| Micheau | 1979 | 40 | M | Epiglottis | – | – | Dysphagia, otalgia | 54 |
| 62 | M | Epiglottis | – | – | Dysphagia, Hoarseness | 2 | ||
| 68 | M | Glottis | – | – | Neck mass | 60 | ||
| Stanley | 1985 | 52 | M | Glottis | – | – | Dyspnea | 9 |
| 50 | F | Supraglottis | – | – | 75 | |||
| 63 | M | Supraglottis | – | – | Neck mass | 39 | ||
| 53 | F | Supraglottis | – | – | Dysphagia/Hoarseness | 96 | ||
| Navarrete | 1989 | 64 | M | Glottis | – | – | 36 | |
| Frank | 1995 | 67 | M | Pyriform | – | – | NA | |
| Sinus | ||||||||
| Narozny | 1995 | 59 | M | Supraglottis | – | – | Hoarseness, Cervical mass | 24 |
| Andryk | 1996 | 60 | M | Pyriform sinus | – | – | Dysphagia/ | 4 |
| Cough | ||||||||
| MacMillan | 1996 | 77 | M | Pyriform sinus | Yes | – | Hoarseness | 58 |
| 54 | M | Pyriform sinus | – | Yes | Sore throat | 10 | ||
| 59 | M | Pyriform sinus | – | – | Hoarseness | 3 | ||
| 59 | M | Pyriform sinus | – | – | Hoarseness/ | 1 | ||
| Neck mass/ | ||||||||
| Dysphagia | ||||||||
| 75 | M | Glottis | Yes | Yes | Lost | |||
| 53 | F | Epiglottis | Yes | Yes | 25 | |||
| 51 | M | Epiglottis | Yes | 17 | ||||
| 82 | M | Glottis | Yes | 10 | ||||
| Zbaren | 1997 | 53 | M | Glottis | NR | NR | Hoarseness, Neck mass | 120 |
| 71 | M | Pyriform fossa | NR | NR | Dysphagia, Neck mass | 36 | ||
| 66 | M | Supraglottis | NR | NR | Hoarseness, Neck mass | 60 | ||
| 73 | M | Glottis | NR | NR | Hoarseness | 36 | ||
| Dray | 1998 | 70 | M | Supraglottis | Yes | – | Globus sensation | 36 |
| Sone | 1998 | 71 | M | Supraglottis | Dysphagia/Hoarseness | 3 | ||
| Marioni | 2002 | 67 | M | Supraglottis | Yes | – | Hoarseness | 48 |
| Coskun | 2005 | 60 | M | Epiglottis | Yes | – | Neck mass | 24 |
| Bansal | 2011 | 60 | M | Supraglottis | Yes | Yes | Hoarseness, dysphagia, Odynophagia | 4 |
| Ibrahimov | 2013 | 58 | M | Glottis | No | No | Hoarseness | 18 |
| Kermani | 2015 | 73 | M | Glottis | – | – | Dysphonea, Dyspnea | 18 |
| Asma | 2017 | 81 | M | Glottis, Supraglottis | Yes | No | Dysphonia, Dyspnea, Dysphagia | 9 |
| Hammas | 2017 | 81 | M | Supraglottic | Yes | No | Dysphonia, Dyspnea, Dysphagia | – |
| Glottic | ||||||||
| Subglottic | ||||||||
| Acuna | 2018 | 68 | M | Pyriform sinus | NR | 24 | ||
| 71 | M | Pyriform sinus | NR | 111 | ||||
| 80 | M | Pyriform sinus | NR | 68 | ||||
| 76 | M | Supraglottis | NR | 40 | ||||
| 58 | M | Glottis | NR | NA | ||||
| 49 | M | Supraglottis | NR | 24 | ||||
| 46 | M | Supraglottis | NR | 141 | ||||
| 53 | M | Supraglottis | NR | 96 | ||||
| 74 | M | Glottis | NR | 84 | ||||
| 65 | M | Supraglottis | NR | 108 | ||||
| Monteiro | 2019 | 59 | M | Supraglottis | Yes | No | Odynophagia, dysphonia | 9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisal, M.; Hartenbach, S.; Schratter, A.; Köstler, W.J.; Kaufmann, H.; Seemann, R.; Lill, C.; Hamzavi, S.; Wutzl, A.; Erovic, B.M. Lymphoepithelial Carcinoma of Larynx and Hypopharynx: A Rare Clinicopathological Entity. Cancers 2020, 12, 2431. https://doi.org/10.3390/cancers12092431
Faisal M, Hartenbach S, Schratter A, Köstler WJ, Kaufmann H, Seemann R, Lill C, Hamzavi S, Wutzl A, Erovic BM. Lymphoepithelial Carcinoma of Larynx and Hypopharynx: A Rare Clinicopathological Entity. Cancers. 2020; 12(9):2431. https://doi.org/10.3390/cancers12092431
Chicago/Turabian StyleFaisal, Muhammad, Sabrina Hartenbach, Annemarie Schratter, Wolfgang J. Köstler, Hannes Kaufmann, Rudolf Seemann, Claudia Lill, Sasan Hamzavi, Arno Wutzl, and Boban M. Erovic. 2020. "Lymphoepithelial Carcinoma of Larynx and Hypopharynx: A Rare Clinicopathological Entity" Cancers 12, no. 9: 2431. https://doi.org/10.3390/cancers12092431
APA StyleFaisal, M., Hartenbach, S., Schratter, A., Köstler, W. J., Kaufmann, H., Seemann, R., Lill, C., Hamzavi, S., Wutzl, A., & Erovic, B. M. (2020). Lymphoepithelial Carcinoma of Larynx and Hypopharynx: A Rare Clinicopathological Entity. Cancers, 12(9), 2431. https://doi.org/10.3390/cancers12092431






