A Mathematical Model to Predict Diagnostic Periods for Secondary Distant Metastases in Patients with ER/PR/HER2/Ki-67 Subtypes of Breast Cancer
Abstract
1. Introduction
2. Results
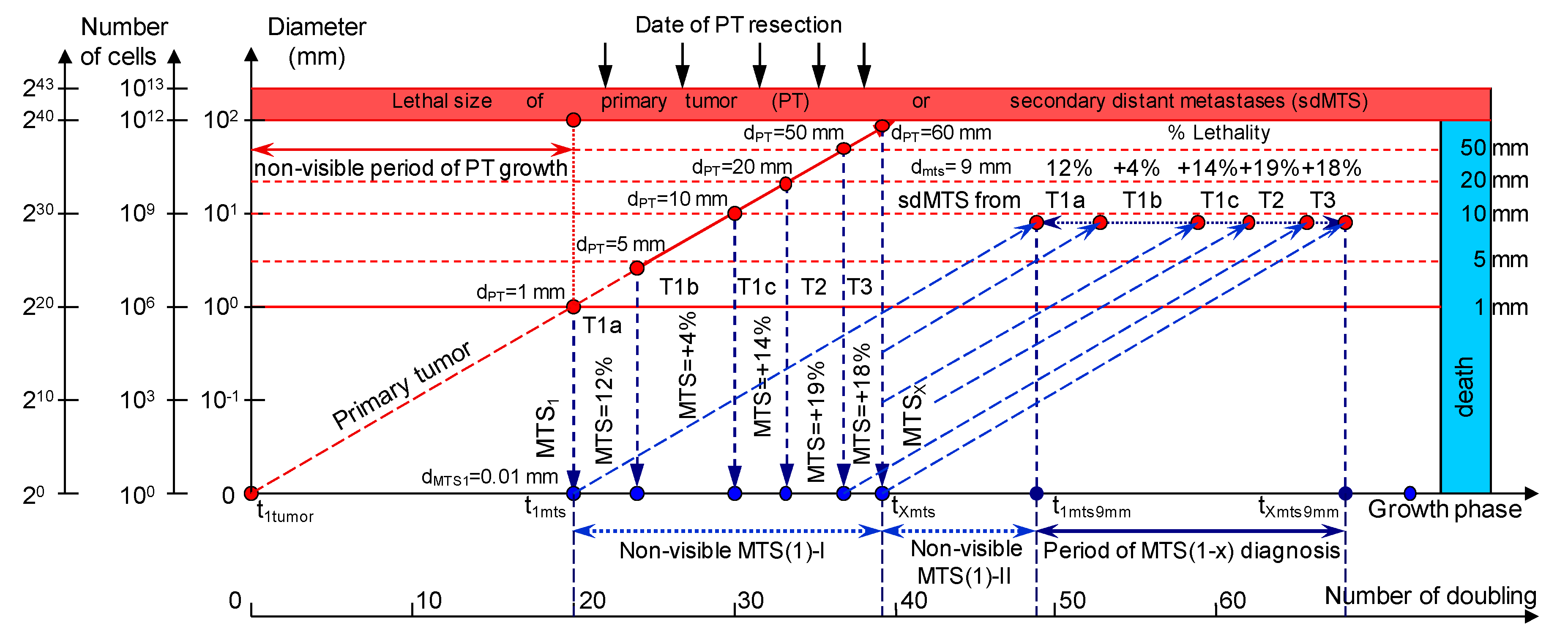
2.1. Consolidated Mathematical Growth Model of Primary Tumor and Secondary Distant Metastases (CoMPaS) in Patients with ER/PR/HER2/Ki-67 Subtypes and Stage I/II/III of Breast Cancer with or without Metastases in the Lymph Nodes
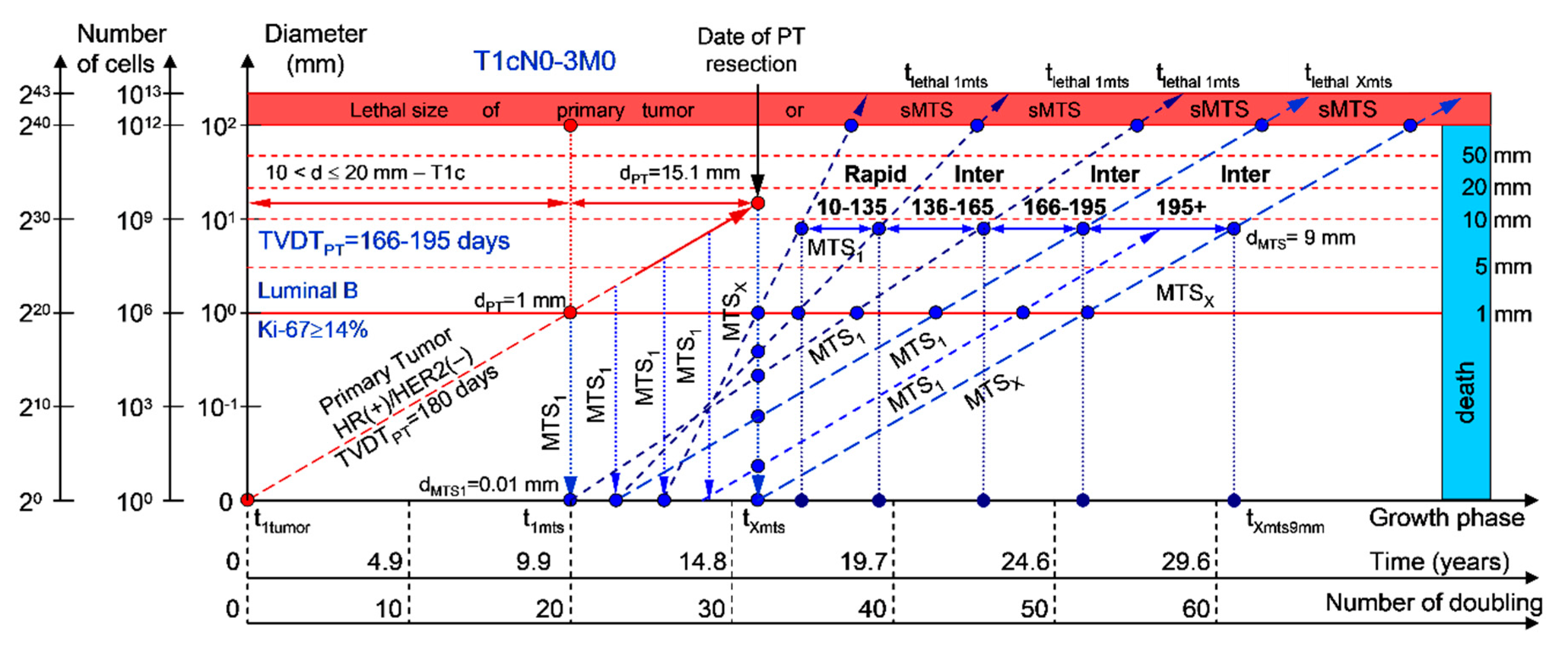
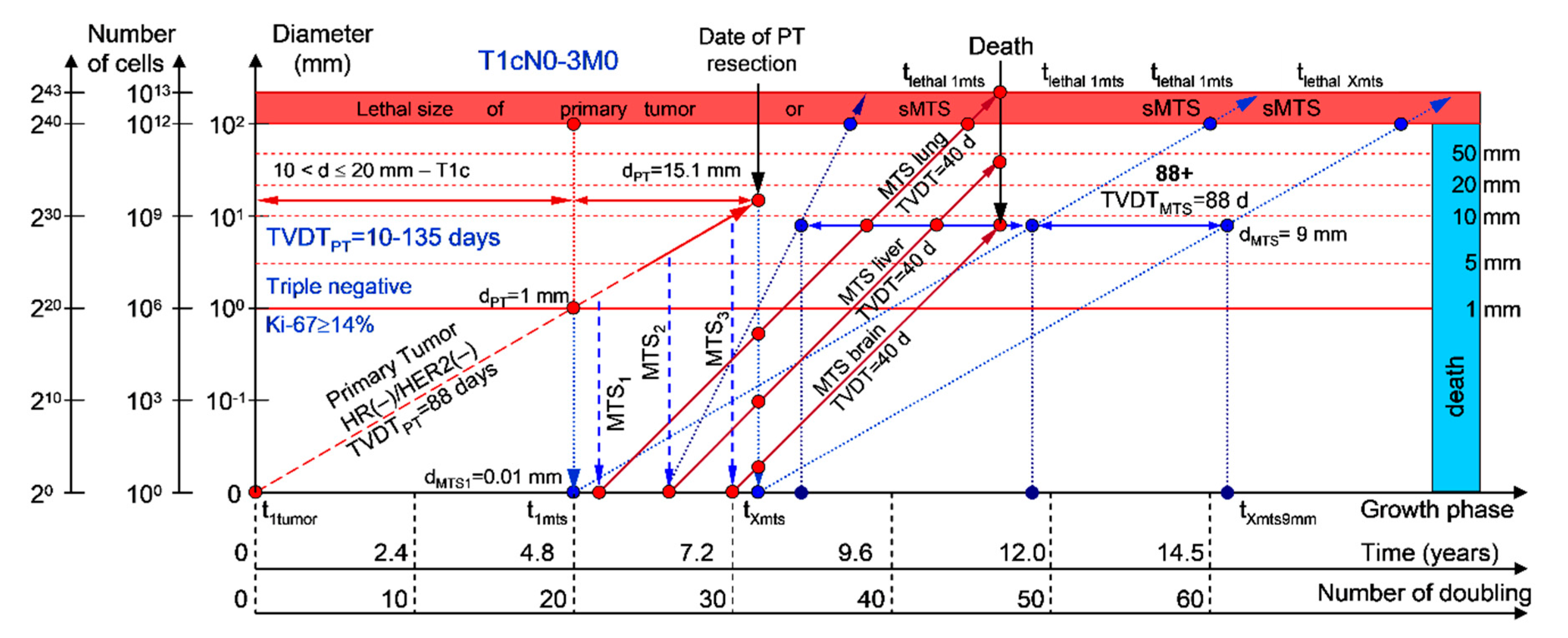
2.1.1. T1cN0-3M0. The Whole Natural History of the PT (Triple-Negative, HR(−)/HER2(−), Ki-67 ≥ 14) and sdMTS
2.1.2. T1cN0-3M0. The Whole Natural History of the PT (HER2-Positive, HR(−)/HER2(+), Ki-67 ≥ 14) and sdMTS
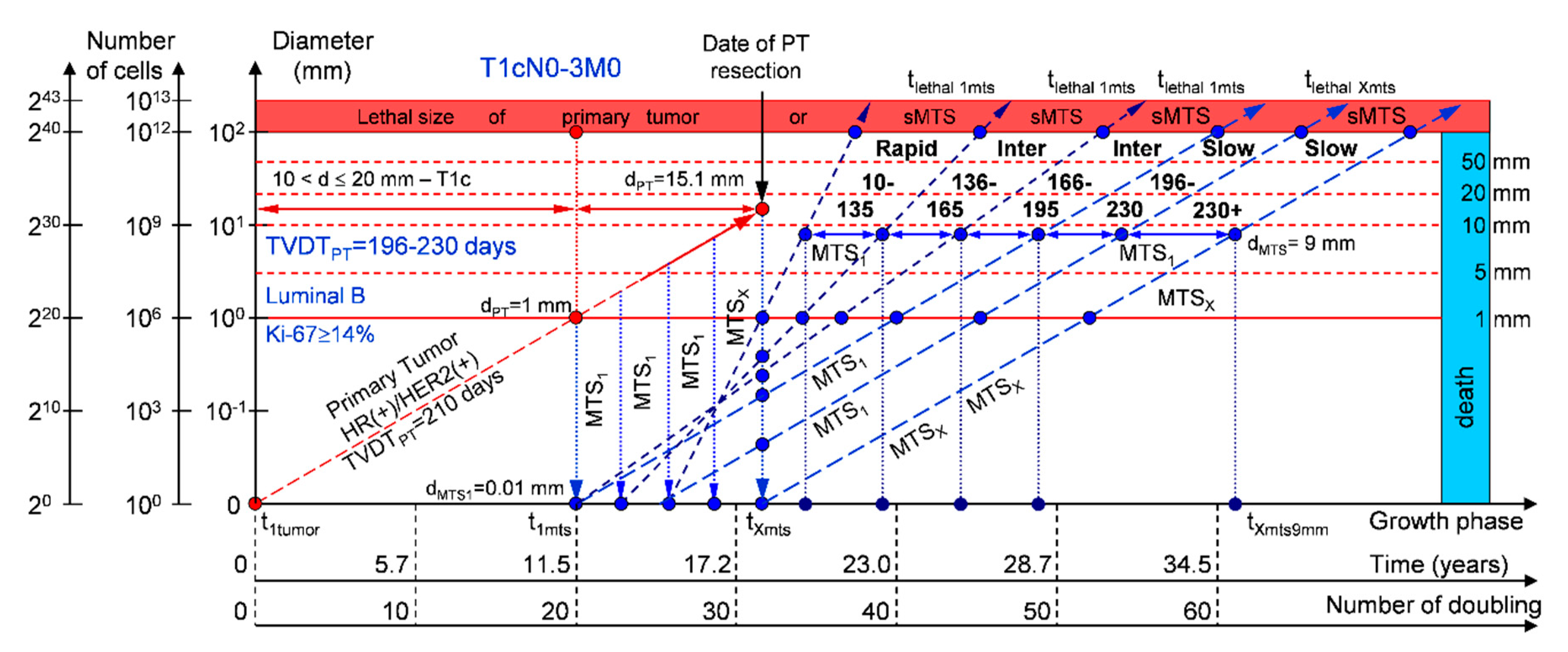
2.1.3. T1cN0-3M0. The Whole Natural History of the PT (Luminal B, HR(+)/HER2(−), Ki-67 ≥ 14) and sdMTS
2.1.4. T1cN0-3M0. The Whole Natural History of the PT (Luminal B, HR(+)/Her2(+), Ki-67 ≥ 14) and sdMTS
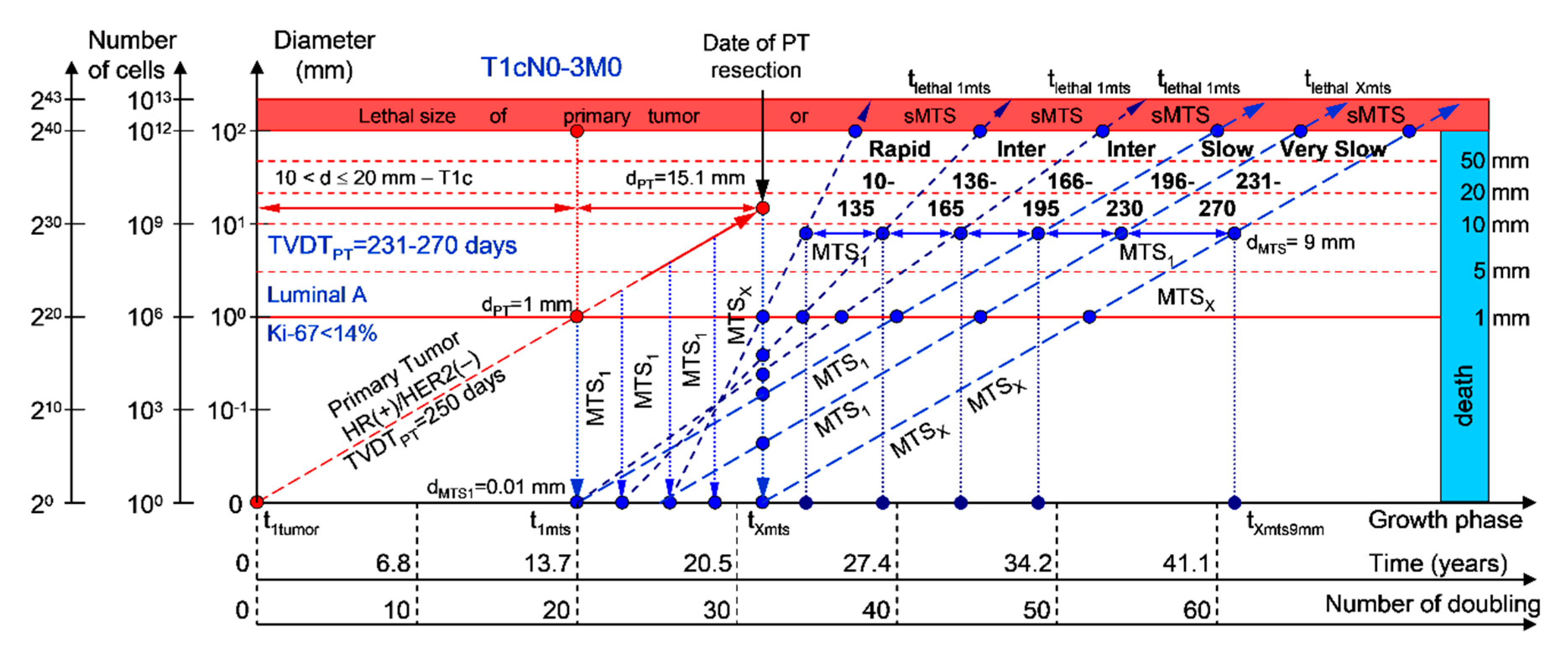
2.1.5. T1cN0-3M0. The Whole Natural History of the PT (Luminal A, HR(+)/Her2(−), Ki-67 < 14) and sdMTS
2.1.6. Examinations during the MTS-Free Period
2.2. Application of Consolidated Mathematical Growth Model of Primary Tumor and Secondary Distant Metastases (CoMPaS) in Patients with ER/PR/HER2/Ki-67 Subtypes and Stage I/II/III Breast Cancer in Clinical Practices
2.3. Software Tool for Personalized Scheduling of Multimodal Examinations
3. Discussion
3.1. The Relationship between the Size of the PT and an Appearance of the sdMTSs and TVDT
3.2. The Relationship between the TVDT and the Subtypes ER/PR/HER2/Ki-67 of BC
3.3. The Relationship between the Different Diagnostic Periods and Subtypes ER/PR/HER2/Ki-67 of BC
3.4. The Relationship between the Mortality and the Convalescence and Subtypes ER/PR/HER2/Ki-67 of BC
4. Materials and Methods
4.1. Consolidated Mathematical Growth Model of Primary Tumor and Secondary Distant Metastases (CoMPaS)
4.2. A Mathematical Model to Predict the Earliest Diagnostic Periods of Secondary Distant Metastases in Patients with ER/PR/HER2/Ki-67 Subtypes of Breast Cancer
4.3. Limitations
4.4. Implementation Software
4.5. Calculation Method
5. Conclusions
6. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Shah, R.; Rosso, K.; Nathanson, S.D. Pathogenesis, prevention, diagnosis and treatment of breast cancer. World J. Clin. Oncol. 2014, 5, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Slack, N.H.; Blumenson, L.E.; Bross, I.D. Therapeutic implications from a mathematical model characterizing the course of breast cancer. Cancer 1969, 24, 960–971. [Google Scholar] [CrossRef]
- Pearlman, A.W. Breast cancer: Influence of growth rate on prognosis and treatment evaluation. Cancer 1976, 38, 1826–1833. [Google Scholar] [CrossRef]
- Koscielny, S.; Tubiana, M.; Valleron, A.-J. A simulation model of the natural history of human breast cancer. Br. J. Cancer. 1985, 52, 515–524. [Google Scholar] [CrossRef]
- Koscielny, S.; Le, M.G.; Tubiana, M. The natural history of human breast cancer. The relationship between involvement of axillary lymph nodes and the initiation of distant metastases. Br. J. Cancer. 1989, 59, 775–782. [Google Scholar] [CrossRef]
- Tubiana, M.; Courdi, A. Cell proliferation kinetics in human solid tumors: Relation to probability of metastatic dissemination and long-term survival. Radiother. Oncol. 1989, 15, 1–18. [Google Scholar] [CrossRef]
- Friberg, S.; Mattson, S. On the growth rates of human malignant tumors: Implications for medical decision making. J. Surg. Oncol. 1997, 65, 284–297. [Google Scholar] [CrossRef]
- Michaelson, J.S.; Silverstein, M.; Wyatt, J.; Weber, G.; Moore, R.; Halpern, E.; Kopans, D.B.; Hughes, K. Predicting the survival of patients with breast carcinoma using tumor size. Cancer 2002, 95, 713–723. [Google Scholar] [CrossRef]
- Michaelson, J.S.; Silverstein, M.; Sgroi, D.; Cheongsiatmoy, J.A.; Taghian, A.; Powell, S.; Hughes, K.; Comegno, A.; Tanabe, K.K.; Smith, B. The effect of tumor size and lymph node status on breast carcinoma lethality. Cancer 2003, 98, 2133–2143. [Google Scholar] [CrossRef]
- Engel, J.; Eckel, R.; Aydemir, U.; Aydemir, S.; Kerr, J.; Schlesinger-Raab, A.; Dirschedl, P.; Hölzel, D. Determinants and prognoses of locoregional and distant progression in breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 1186–1195. [Google Scholar] [CrossRef]
- Engel, J.; Eckel, R.; Kerr, J.; Schmidt, M.; Fürstenberger, G.; Richter, R.; Sauer, H.; Senn, H.J.; Hölzel, D. The process of metastasisation for breast cancer. Eur. J. Cancer 2003, 39, 1794–1806. [Google Scholar] [CrossRef]
- Klein, C.A.; Hölzel, D. Systemic cancer progression and tumor dormancy: Mathematical models meet single cell genomics. Cell Cycle 2006, 5, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Michaelson, J.S.; Chen, L.L.; Silverstein, M.J.; Cheongsiatmoy, J.A.; Mihm, M.C., Jr.; Sober, A.J.; Tanabe, K.K.; Smith, B.L.; Younger, J. Why cancer at primary site and in the lymph nodes contributes to the risk of cancer death. Cancer 2009, 115, 5084–5094. [Google Scholar] [CrossRef]
- Hölzel, D.; Eckel, R.; Emeny, R.T.; Engel, J. Distant metastases do not metastasize. Cancer Metastasis Rev. 2010, 29, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.; Emeny, R.T.; Hölzel, D. Positive lymph nodes do not metastasize. Cancer Metastasis Rev. 2012, 31, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Clare, S.E.; Nakhlis, F.; Panetta, J.C. Molecular biology of breast cancer metastasis: The use of mathematical models to determine relapse and to predict response to chemotherapy in breast cancer. Breast Cancer Res. 2000, 2, 430–435. [Google Scholar] [CrossRef]
- Withers, H.R.; Lee, S.P. Modeling growth kinetics and statistical distribution of oligometastases. Semin. Radiat. Oncol. 2006, 16, 111–119. [Google Scholar] [CrossRef]
- Weedon-Fekjaer, H.; Lindqvist, B.H.; Vatten, L.J.; Aalen, O.O.; Tretli, S. Breast cancer tumor growth estimated through mammography screening data. Breast Cancer Res. 2008, 10, R41. [Google Scholar] [CrossRef]
- Molina-Pena, R.; Alvarez, M.M. A simple mathematical model based on the cancer stem cell hypothesis suggests kinetic commonalities in solid tumor growth. PLoS ONE 2012, 7, e26233. [Google Scholar] [CrossRef]
- Coumans, F.A.; Siesling, S.; Terstappen, L.W. Detection of cancer before distant metastases. BMC Cancer 2013, 13, 283. [Google Scholar] [CrossRef]
- Tyuryumina, E.; Neznanov, A. Consolidated mathematical growth model of the primary tumor and sdMTS of breast cancer (CoMPaS). PLoS ONE 2018, 13, e0200148. [Google Scholar] [CrossRef] [PubMed]
- Parise, C.A.; Caggiano, V. Breast cancer survival defined by the ER/PR/HER2 subtypes and a surrogate classification according to tumor grade and immunohistochemical biomarkers. J. Cancer Epidemiol. 2014, 2014, 469251. [Google Scholar] [CrossRef] [PubMed]
- Chavez-MacGregor, M.; Mittendorf, E.A.; Clarke, C.A.; Lichtensztajn, D.Y.; Hunt, K.K.; Giordano, S.H. Incorporating tumor characteristics to the American joint committee on cancer breast cancer staging system. Oncologist 2017, 22, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in breast cancer survival by molecular subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 619–626. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Lawrenson, R.; Lao, C.; Campbell, I.; Harvey, V.; Seneviratne, S.; Elwood, M.; Sarfati, D.; Kuper-Hommel, M. The impact of different tumor subtypes on management and survival of New Zealand women with stage I-III breast cancer. N. Z. Med. J. 2018, 131, 51–60. [Google Scholar]
- Lafranconi, A.; Pylkkanen, L.; Deandrea, S.; Bramesfeld, A.; Lerda, D.; Neamtiu, L.; Saz-Parkinson, Z.; Posso, M.; Rigau, D.; Sola, I.; et al. Intensive follow-up for women with breast cancer: Review of clinical, economic and patient’s preference domains through evidence to decision framework. Health Qual. Life Outcomes 2017, 15, 206. [Google Scholar] [CrossRef]
- Smith, T.J. Breast cancer surveillance guidelines. J. Oncol. Pract. 2013, 9, 65–67. [Google Scholar] [CrossRef]
- Puglisi, F.; Fontanella, C.; Numico, G.; Sini, V.; Evangelista, L.; Monetti, F.; Gori, S.; Del Mastro, L. Follow-up of patients with early breast cancer: Is it time to rewrite the story? Crit. Rev. Oncol. Hematol. 2014, 91, 130–141. [Google Scholar] [CrossRef]
- Stevens, G.M.; Weigen, J.F. Mammography survey for breast cancer detection. A 2-year study of 1,223 clinically negative asymptomatic women over 40. Cancer. 1966, 19, 51–59. [Google Scholar] [CrossRef]
- Khatcheressian, J.L.; Wolff, A.C.; Smith, T.J.; Grunfeld, E.; Muss, H.B.; Vogel, V.G.; Halberg, F.; Somerfield, M.R.; Davidson, N.E. American Society of Clinical Oncology 2006 updates of the breast cancer follow-up and management guidelines in the adjuvant setting. J. Clin. Oncol. 2006, 24, 5091–5097. [Google Scholar] [CrossRef] [PubMed]
- Khatcheressian, J.L.; Hurley, P.; Bantug, E.; Esserman, L.J.; Grunfeld, E.; Halberg, F.; Hantel, A.; Henry, N.L.; Muss, H.B.; Smith, T.J.; et al. Breast cancer follow-up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 2013, 31, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Collins, V.P.; Loeffler, R.K.; Tivey, H. Observations on growth rates of human tumors. Am. J. Roentgen. 1956, 76, 988–1000. [Google Scholar]
- Schwartz, M. A biomathematical approach to clinical tumor growth. Cancer 1961, 14, 1272–1294. [Google Scholar] [CrossRef]
- Bloom, H.J.; Richarson, W.W.; Harries, E.J. Natural history of untreated breast cancer (1805–1933). Comparison of untreated and treated cases according to histological grade of malignancy. Br. Med. J. 1962, 2, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Skipper, H.E. Kinetics of mammary tumor cell growth and implications for therapy. Cancer 1971, 28, 1479–1499. [Google Scholar] [CrossRef]
- Silvestrini, R.; Sanfilippo, O.; Tedesco, G. Kinetics of human mammary carcinomas and their correlation with the cancer and the host characteristics. Cancer 1974, 34, 1252–1258. [Google Scholar] [CrossRef]
- Lundgren, B. Observations on growth rate of breast carcinomas and its possible implications for lead time. Cancer 1977, 40, 1722–1725. [Google Scholar] [CrossRef]
- Spratt, J.A.; von Fournier, D.; Spratt, J.S.; Weber, E.E. Mammographic assessment of human breast cancer growth and duration. Cancer 1993, 71, 2020–2026. [Google Scholar] [CrossRef]
- Moiseenko, V.M. The natural history of breast cancer growth. Pract. Oncol. 2002, 3, 6–14. (In Russian) [Google Scholar]
- Rodriguez-Brenes, I.A.; Komarova, N.L.; Wodarz, D. Tumor growth dynamics: Insights into evolutionary processes. Trends Ecol. Evol. 2013, 28, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Benzekry, S.; Lamont, C.; Beheshti, A.; Tracz, A.; Ebos, J.M.; Hlatky, L.; Hahnfeldt, P. Classical mathematical models for description and prediction of experimental tumor growth. PLoS Comput. Biol. 2014, 10, e1003800. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ye, Y.; Barcenas, C.H.; Chow, W.H.; Meng, Q.H.; Chavez-MacGregor, M.; Hildebrandt, M.A.; Zhao, H.; Gu, X.; Deng, Y.; et al. Personalized prognostic prediction models for breast cancer recurrence and survival incorporating multidimensional data. J. Natl. Cancer Inst. 2017, 109, djw314. [Google Scholar] [CrossRef] [PubMed]
- Gershon-Cohen, J.; Berger, S.M.; Klickstein, H.S. Roentgenography of breast cancer moderating concept of “Biologic predeterminism”. Cancer 1963, 16, 961–964. [Google Scholar] [CrossRef]
- Kusama, S.; Spratt, J.S., Jr.; Donegan, W.L.; Watson, F.R.; Cunningham, C. The cross rates of growth of human mammary carcinoma. Cancer 1972, 30, 594–599. [Google Scholar] [CrossRef]
- von Fournier, D.V.; Weber, E.; Hoeffken, W.; Bauer, M.; Kubli, F.; Barth, V. Growth rate of 147 mammary carcinomas. Cancer 1980, 45, 2198–2207. [Google Scholar] [CrossRef]
- Peer, P.G.; van Dijck, J.A.; Hendriks, J.H.; Holland, R.; Verbeek, A.L. Age-dependent growth rate of primary breast cancer. Cancer 1993, 71, 3547–3551. [Google Scholar] [CrossRef]
- Gruber, I.V.; Rueckert, M.; Kagan, K.O.; Staebler, A.; Siegmann, K.C.; Hartkopf, A.; Wallwiener, D.; Hahn, M. Measurement of tumor size with mammography, sonography and magnetic resonance imaging as compared to histological tumor size in primary breast cancer. BMC Cancer 2013, 13, 328. [Google Scholar] [CrossRef]
- Ryu, E.B.; Chang, J.M.; Seo, M.; Kim, S.A.; Lim, J.H.; Moon, W.K. Tumor volume doubling time of molecular breast cancer subtypes assessed by serial breast ultrasound. Eur. Radiol. 2014, 24, 2227–2235. [Google Scholar] [CrossRef]
- Yoo, T.K.; Min, J.W.; Kim, M.K.; Lee, E.; Kim, J.; Lee, H.B.; Kang, Y.J.; Kim, Y.G.; Moon, H.G.; Moon, W.K.; et al. In vivo tumor growth rate measured by US in preoperative period and long term disease outcome in breast cancer patients. PLoS ONE 2015, 10, e0144144. [Google Scholar] [CrossRef]
- Cortadellas, T.; Argacha, P.; Acosta, J.; Rabasa, J.; Peiró, R.; Gomez, M.; Rodellar, L.; Gomez, S.; Navarro-Golobart, A.; Sanchez-Mendez, S.; et al. Estimation of tumor size in breast cancer comparing clinical examination, mammography, ultrasound and MRI–correlation with the pathological analysis of the surgical specimen. Gland Surg. 2017, 6, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ding, Y.; Zhou, Q.; Wang, C.; Wu, P.; Dong, J. Correlation factors analysis of breast cancer tumor volume doubling time measured by 3D-Ultrasound. Med. Sci. Monit. 2017, 23, 3147–3153. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Uematsu, T.; Takahashi, K.; Nishimura, S.; Tadokoro, Y.; Hayashi, T.; Sugino, T. Does breast cancer growth rate really depend on tumor subtype? Measurement of tumor doubling time using serial ultrasonography between diagnosis and surgery. Breast Cancer 2019, 26, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, Y.S.; Han, W.; Ryu, H.S.; Chang, J.M.; Cho, N.; Moon, W.K. Tumor growth rate of invasive breast cancers during wait times for surgery assessed by ultrasonography. Medicine (Baltim.) 2016, 95, e4874. [Google Scholar] [CrossRef] [PubMed]
- Friberg, S. On the growth rates of human malignant tumors: Implications for medical decision making. J. Oncol. 2005, 55, 1–22. [Google Scholar]
- Klein, C.A. Parallel progression of primary tumors and metastases. Nat. Rev. Cancer. 2009, 9, 302–312. [Google Scholar] [CrossRef]
- Spratt, J.S.; Spratt, T.L. Rates of growth of pulmonary metastases and host survival. Ann. Surg. 1964, 159, 61–171. [Google Scholar] [CrossRef]
- Philippe, E.; Le Gal, Y. Growth of 78 recurrent mammary cancers. Cancer 1968, 21, 461–467. [Google Scholar] [CrossRef]
- Lee, Y.T.; Spratt, J.S., Jr. Rate of growth of soft tissue metastases of breast cancer. Cancer 1972, 29, 344–348. [Google Scholar] [CrossRef]
- Gullino, P.M. Natural history of breast cancer. Progression from hyperplasia to neoplasia as predicted by angiogenesis. Cancer 1977, 39, 2697–2703. [Google Scholar] [CrossRef]
- Retsky, M.W.; Swartzendruber, D.E.; Wardwell, R.H.; Bame, P.D. Is Gompertzian or exponential kinetics a valid description of individual human cancer growth? Med. Hypotheses 1990, 33, 95–106. [Google Scholar] [CrossRef]
- Spratt, J.S.; Kaltenbach, M.L.; Spratt, J.A. Cytokinetic definition of acute and chronic breast cancer. Cancer Res. 1977, 37, 226–230. [Google Scholar] [PubMed]
- Boyd, N.F.; Meakin, J.W.; Hayward, J.L.; Brown, T.C. Clinical estimation of the growth rate of breast cancer. Cancer 1981, 48, 1037–1042. [Google Scholar] [CrossRef]
- Galante, E.; Gallus, G.; Guzzon, A.; Bono, A.; Bandieramonte, G.; Di Pietro, S. Growth rate of primary breast cancer and prognosis: Observations on a 3- to 7-year follow-up in 180 breast cancers. Br. J. Cancer. 1986, 54, 833–836. [Google Scholar] [CrossRef] [PubMed]
- Kuroishi, T.; Tominaga, S.; Morimoto, T.; Tashiro, H.; Itoh, S.; Watanabe, H.; Fukuda, M.; Ota, J.; Horino, T.; Ishida, T.; et al. Tumor growth rate and prognosis of breast cancer mainly detected by mass screening. Jpn. J. Cancer Res. 1990, 81, 454–462. [Google Scholar] [CrossRef]
- Spratt, J.A.; von Fournier, D.; Spratt, J.S.; Weber, E.E. Decelerating growth and human breast cancer. Cancer 1993, 71, 2013–2019. [Google Scholar] [CrossRef]
- Friberg, S.; Nyström, A. Cancer metastases: Early dissemination and late recurrences. Cancer Growth Metastasis 2015, 8, 43–49. [Google Scholar] [CrossRef]
- Tabbane, F.; Bahi, J.; Rahal, K.; May, A.E.; Riahi, M.; Cammoun, M.; Hechiche, M.; Jaziri, M.; Mourali, N. Inflammatory symptoms in breast cancer. Correlations with growth rate, clinicopathologic variables, and evolution. Cancer 1989, 64, 2081–2089. [Google Scholar] [CrossRef]
- Hortobagyi, G.; Connolly, J.L.; D’Orsi, C.J.; Edge, S.B.; Mittendorf, E.A.; Rugo, H.S.; Solin, L.J.; Weaver, D.L.; Winchester, D.J.; Giuliano, A. Breast. In AJCC Cancer Staging Manual, 8th ed.; Amin, M.B., Edge, S., Greene, F., Byrd, D.R., Brookland, R.K., Washington, M.K., Gershenwald, J.E., Compton, C.C., Hess, K.R., Sullivan, D.C., et al., Eds.; American Joint Committee on Cancer (AJCC): New York, NY, USA; Springer: Berlin/Heidelberg, Germany, 2017; pp. 587–628. ISBN 978-3-319-40617-6. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Edge, S.B.; Giuliano, A. New and important changes in the TNM staging system for breast cancer. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 457–467. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, B.; Jin, F. The assessment of 8th edition AJCC prognostic staging system and a simplified staging system for breast cancer: The analytic results from the SEER database. Breast J. 2019, 25, 838–847. [Google Scholar] [CrossRef]
- He, J.; Tsang, J.Y.; Xu, X.; Li, J.; Li, M.; Chao, X.; Xu, Y.; Luo, R.; Tse, G.M.; Sun, P. AJCC 8th edition prognostic staging provides no better discriminatory ability in prognosis than anatomical staging in triple negative breast cancer. BMC Cancer 2020, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Koscielny, S.; Tubiana, M.; Lê, M.G.; Valleron, A.J.; Mouriesse, H.; Contesso, G.; Sarrazin, D. Breast cancer: Relationship between the size of the primary tumor and the probability of metastatic dissemination. Br. J. Cancer 1984, 49, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Koscielny, S.; Tubiana, M. The link between local recurrence and distant metastases in human breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 1999, 43, 11–24. [Google Scholar] [CrossRef]
- Tabar, L.; Duffy, S.W.; Vitak, B.; Chen, H.H.; Prevost, T.C. The natural history of breast carcinoma: What have we learned from screening? Cancer 1999, 86, 449–462. [Google Scholar] [CrossRef]
- Tubiana, M.; Koscielny, S. The natural history of breast cancer and the link between local recurrence and distant metastases: Implications for therapy. Rep. Pract. Oncol. Radiother. 2001, 6, 181–195. [Google Scholar] [CrossRef][Green Version]
- Koscielny, S.; Arriagada, R.; Adolfsson, J.; Fornander, T.; Bergh, J. Impact of tumor size on axillary involvement and distant dissemination in breast cancer. Br. J. Cancer 2009, 101, 902–907. [Google Scholar] [CrossRef]
- Yu, K.D.; Jiang, Y.Z.; Chen, S.; Cao, Z.G.; Wu, J.; Shen, Z.Z.; Shao, Z.M. Effect of large tumor size on cancer-specific mortality in node-negative breast cancer. Mayo Clin. Proc. 2012, 87, 1171–1180. [Google Scholar] [CrossRef]
- Lin, R.S.; Plevritis, S.K. Comparing the benefits of screening for breast cancer and lung cancer using a novel natural history model. Cancer Causes Control 2012, 23, 175–185. [Google Scholar] [CrossRef]
- Narod, S.A. Tumor size predicts long-term survival among women with lymph node-positive breast cancer. Curr. Oncol. 2012, 19, 249–253. [Google Scholar] [CrossRef]
- Narod, S.A.; Iqbal, J.; Jakubowska, A.; Huzarski, T.; Sun, P.; Cybulski, C.; Gronwald, J.; Byrski, T.; Lubinski, J. Are two-centimeter breast cancers large or small? Curr. Oncol. 2013, 20, 205–211. [Google Scholar] [CrossRef]
- Elkin, E.B.; Hudis, C.; Begg, C.B.; Schrag, D. The effect of changes in tumor size on breast carcinoma survival in the U.S.: 1975–1999. Cancer 2005, 104, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Tevaarwerk, A.J.; Gray, R.J.; Schneider, B.P.; Smith, M.L.; Wagner, L.I.; Fetting, J.H.; Davidson, N.; Goldstein, L.J.; Miller, K.D.; Sparano, J.A. Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: Little evidence of improvement over the past 30 years. Cancer 2013, 119, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual Report to the Nation on the Status of Cancer, 1975–2014, Featuring Survival. J. Natl. Cancer Inst. 2017, 109, djx030. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.Z.; Wang, L.; Hu, X.; Shao, Z.M. Effect of tumor size on breast cancer-specific survival stratified by joint hormone receptor status in a SEER population-based study. Oncotarget 2015, 6, 22985–22995. [Google Scholar] [CrossRef]
- Sopik, V.; Narod, S.A. The relationship between tumor size, nodal status and distant metastases: On the origins of breast cancer. Breast Cancer Res. Treat. 2018, 170, 647–656. [Google Scholar] [CrossRef]
- Orang, E.; Marzony, E.T.; Afsharfard, A. Predictive role of tumor size in breast cancer with axillary lymph node involvement—Can size of primary tumor be used to omit an unnecessary axillary lymph node dissection? Asian Pac. J. Cancer Prev. 2013, 14, 717–722. [Google Scholar] [CrossRef]
- Jung, J.; Suh, Y.J.; Ko, B.K.; Lee, E.S.; Kim, E.K.; Paik, N.S.; Byun, K.D.; Hwang, K.T. Clinical implication of subcategorizing T2 category into T2a and T2b in TNM staging of breast cancer. Cancer Med. 2018, 7, 5514–5524. [Google Scholar] [CrossRef]
- Narod, S.A.; Giannakeas, V.; Sopik, V. Time to death in breast cancer patients as an indicator of treatment response. Breast Cancer Res. Treat. 2018, 172, 659–669. [Google Scholar] [CrossRef]
- Kirch, R.L.; Klein, M. Prospective evaluation of periodic breast examination programs. Cancer 1976, 38, 265–272. [Google Scholar] [CrossRef]
- Heuser, L.; Spratt, J.S., Jr.; Polk, H.C., Jr.; Buchanan, J. Relation between mammary cancer growth kinetics and the intervals between screenings. Cancer 1979, 43, 857–862. [Google Scholar] [CrossRef]
- Heuser, L.; Spratt, J.S.; Polk, H.C., Jr. Growth rates of primary breast cancers. Cancer 1979, 43, 1888–1894. [Google Scholar] [CrossRef]
- Spratt, J.S.; Heuser, L.; Kuhns, J.G.; Reiman, H.M.; Buchanan, J.B.; Polk, H.C., Jr.; Sandoz, J. Association between the actual doubling times of primary breast cancer with histopathologic characteristics and Wolfe’s parenchymal mammographic patterns. Cancer 1981, 47, 2265–2268. [Google Scholar] [CrossRef]
- Begg, A.C.; McNally, N.J.; Shrieve, D.C.; Karcher, H. A method to measure the duration of DNA synthesis and the potential doubling time from a single sample. Cytometry 1985, 6, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Arnerlöv, C.; Emdin, S.O.; Lundgren, B.; Roos, G.; Söderström, J.; Bjersing, L.; Norberg, C.; Angquist, K.A. Breast carcinoma growth rate described by mammographic doubling time and S-phase fraction. Correlations to clinical and histopathologic factors in a screened population. Cancer 1992, 70, 1928–1934. [Google Scholar] [CrossRef]
- Arnerlöv, C.; Emdin, S.O.; Lundgren, B.; Roos, G.; Söderström, J.; Bjersing, L.; Norberg, C.; Angquist, K.A. Mammographic growth rate, DNA ploidy, and S-phase fraction analysis in breast carcinoma. A prognostic evaluation in a screened population. Cancer 1992, 70, 1935–1942. [Google Scholar] [CrossRef]
- Bailey, S.L.; Sigal, B.M.; Plevritis, S.K. A simulation model investigating the impact of tumor volume doubling time and mammographic tumor detectability on screening outcomes in women aged 40–49 years. J. Natl. Cancer Inst. 2010, 102, 1263–1271. [Google Scholar] [CrossRef]
- Vieira, I.T.; de Senna, V.; Harper, P.R.; Shahani, A.K. Tumor doubling times and the length bias in breast cancer screening programmes. Health Care Manag. Sci. 2011, 14, 203–211. [Google Scholar] [CrossRef]
- Fornvik, D.; Lang, K.; Andersson, I.; Dustler, M.; Borgquist, S.; Timberg, P. Estimates of breast cancer growth rate from mammograms and its relation to tumor characteristics. Radiat. Prot. Dosim. 2016, 169, 151–157. [Google Scholar] [CrossRef]
- Seely, J.M.; Alhassan, T. Screening for breast cancer in 2018-what should we be doing today? Curr. Oncol. 2018, 25, S115–S124. [Google Scholar] [CrossRef]
- Wu, Q.; Li, J.; Zhu, S.; Wu, J.; Chen, C.; Liu, Q.; Wei, W.; Zhang, Y.; Sun, S. Breast cancer subtypes predict the preferential site of distant metastases: A SEER based study. Oncotarget 2017, 8, 27990–27996. [Google Scholar] [CrossRef]
- Wu, S.G.; Li, H.; Tang, L.Y.; Sun, J.Y.; Zhang, W.W.; Li, F.Y.; Chen, Y.X.; He, Z.Y. The effect of distant metastases sites on survival in de novo stage-IV breast cancer: A SEER database analysis. Tumor Biol. 2017, 39, 1010428317705082. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, C.; Zhang, J.; Kong, L.; Zhu, H.; Yu, J. The prognosis analysis of different metastasis pattern in patients with different breast cancer subtypes: A SEER based study. Oncotarget 2017, 8, 26368–26379. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Zheng, S.; Yang, A.; Zhang, X.; Zou, Y.; Tang, H.; Xie, X. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: A population-based study. Cancer Manag. Res. 2018, 10, 5329–5338. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chu, Y.; Xu, B.; Hu, Q.; Song, Q. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci. Rep. 2019, 39, BSR20190288. [Google Scholar] [CrossRef]
- Chen, Q.F.; Huang, T.; Shen, L.; Wu, P.; Huang, Z.L.; Li, W. Prognostic factors and survival according to tumor subtype in newly diagnosed breast cancer with liver metastases: A competing risk analysis. Mol. Clin. Oncol. 2019, 11, 259–269. [Google Scholar] [CrossRef]



| T1c (mm) 10 < d ≤ 20 | T1c (mm) 10 < d ≤ 20 | T1c (mm) 10 < d ≤ 20 | T1c (mm) 10 < d ≤ 20 | T1c (mm) 10 < d ≤ 20 | |
|---|---|---|---|---|---|
| dPT at surgery (mm) | 15.1 | 15.1 | 15.1 | 15.1 | 15.1 |
| PTlog(V) | 31.7 | 31.7 | 31.7 | 31.7 | 31.7 |
| TVDTPT (days) | 80–110–135 | 136–150–165 | 166–180–195 | 196–210–230 | 231–250–270 |
| TVDTPT (days) mean | 110 | 150 | 180 | 210 | 250 |
| V subtype | IV subtype | III subtype | II subtype | I subtype | |
| Good prognosis | I subtype | ||||
| Very slow growth rate | HR(+)/Her2(−) | ||||
| TVDTMTS (days) | 230(+) | 231–270 | |||
| Mean TVDTMTS (days) | 230(+) | 116–135 | |||
| dMTS (mm) | 9.0 | 9.0 | |||
| Non-visible MTS(1-X)-I | 7.41–14.80 | 7.43–8.69 | |||
| Non-visible MTS(1-X)-II | 11.19–18.58 | 11.23–13.13 | |||
| Visible MTS(1-X) (years) | 6.66–14.05 | 6.68–7.81 | |||
| Survival MTS(1-X) (years) | 17.84–25.23 | 17.91–20.93 | |||
| Period of MTS(1-X) diagnosis | 7.40 | 1.95 | |||
| Screening time | 12 mo | 12 mo | |||
| Intermediate prognosis | II subtype | ||||
| Slow growth rate | HR(+)/Her2(+) | ||||
| TVDTMTS (days) | 195(+) | 196–230 | 196–230 | ||
| Mean TVDTMTS (days) | 195(+) | 98–115 | 98–115 | ||
| dMTS (mm) | 9.0 | 9.0 | 9.0 | ||
| Non-visible MTS(1-X)-I | 6.28–12.55 | 6.31–7.40 | 6.31–7.40 | ||
| Non-visible MTS(1-X)-II | 9.49–15.76 | 9.53–11.18 | 9.53–11.18 | ||
| Visible MTS(1-X) (years) | 5.65–11.91 | 5.67–6.65 | 5.67–6.65 | ||
| Survival MTS(1-X) (years) | 15.13–21.39 | 15.20–17.83 | 15.20–17.83 | ||
| Period of MTS(1-X) diagnosis | 6.27 | 1.70 | 1.70 | ||
| Screening time | 8 mo | 8 mo | 8 mo | ||
| Intermediate prognosis | III subtype | ||||
| Intermediate growth rate | HR(+)/HER2(−) | ||||
| TVDTMTS (days) | 165(+) | 166–195 | 166–195 | 166–195 | |
| Mean TVDTMTS (days) | 165(+) | 83–97 | 83–97 | 83–97 | |
| dMTS (mm) | 9.0 | 9.0 | 9.0 | 9.0 | |
| Non-visible MTS(1-X)-I | 5.32–10.62 | 5.34–6.27 | 5.34–6.27 | 5.34–6.27 | |
| Non-visible MTS(1-X)-II | 8.03–13.33 | 8.07–9.48 | 8.07–9.48 | 8.07–9.48 | |
| Visible MTS(1-X) (years) | 4.78–10.08 | 4.80–5.64 | 4.80–5.64 | 4.80–5.64 | |
| Survival MTS(1-X) (years) | 12.80–18.10 | 12.87–15.12 | 12.87–15.12 | 12.87–15.12 | |
| Period of MTS(1-X) diagnosis | 5.31 | 1.46 | 1.46 | 1.46 | |
| Screening time | 6 mo | 6 mo | 6 mo | 6 mo | |
| Intermediate prognosis | IV subtype | ||||
| Intermediate growth rate | HR(−)/HER2(+) | ||||
| TVDTMTS (days) | 135(+) | 136–165 | 136–165 | 136–165 | 136–165 |
| Mean TVDTMTS (days) | 135(+) | 68–82 | 68–82 | 68–82 | 68–82 |
| dMTS (mm) | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 |
| Non-visible MTS(1-X)-I | 4.35–8.69 | 4.38–5.31 | 4.38–5.31 | 4.38–5.31 | 4.38–5.31 |
| Non-visible MTS(1-X)-II | 6.57–10.91 | 6.61–8.02 | 6.61–8.02 | 6.61–8.02 | 6.61–8.02 |
| Visible MTS(1-X) (years) | 3.91–8.25 | 3.93–4.77 | 3.93–4.77 | 3.93–4.77 | 3.93–4.77 |
| Survival MTS(1-X) (years) | 10.48–14.81 | 10.54–12.79 | 10.54–12.79 | 10.54–12.79 | 10.54–12.79 |
| Period of MTS(1-X) diagnosis | 4.34 | 1.46 | 1.46 | 1.46 | 1.46 |
| Screening time | 5 mo | 5 mo | 5 mo | 5 mo | 5 mo |
| Poor prognosis | V subtype | ||||
| Rapid growth rate | HR(−)/HER2(−) | ||||
| TVDTMTS (days) | 10–135 | 10–135 | 10–135 | 10–135 | 10–135 |
| Mean TVDTMTS (days) | 40–67 | 40–67 | 40–67 | 40–67 | 40–67 |
| dMTS (mm) | 9.0 | 9.0 | 9.0 | 9.0 | 9.0 |
| Non-visible MTS(1-X)-I | 0.55–4.34 | 0.55–4.34 | 0.55–4.34 | 0.55–4.34 | 0.55–4.34 |
| Non-visible MTS(1-X)-II | 0.26–6.56 | 0.26–6.56 | 0.26–6.56 | 0.26–6.56 | 0.26–6.56 |
| Visible MTS(1-X) (years) | 0.29–3.90 | 0.29–3.90 | 0.29–3.90 | 0.29–3.90 | 0.29–3.90 |
| Survival MTS(1-X) (years) | 0.55–10.47 | 0.55–10.47 | 0.55–10.47 | 0.55–10.47 | 0.55–10.47 |
| Period of MTS(1-X) diagnosis | 6.30 | 6.30 | 6.30 | 6.30 | 6.30 |
| Screening time | 3 mo | 3 mo | 3 mo | 3 mo | 3 mo |
| Total period MTS(1-X) diagnosis | 10.64 | 13.07 | 15.49 | 18.32 | 12.87 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyuryumina, E.Y.; Neznanov, A.A.; Turumin, J.L. A Mathematical Model to Predict Diagnostic Periods for Secondary Distant Metastases in Patients with ER/PR/HER2/Ki-67 Subtypes of Breast Cancer. Cancers 2020, 12, 2344. https://doi.org/10.3390/cancers12092344
Tyuryumina EY, Neznanov AA, Turumin JL. A Mathematical Model to Predict Diagnostic Periods for Secondary Distant Metastases in Patients with ER/PR/HER2/Ki-67 Subtypes of Breast Cancer. Cancers. 2020; 12(9):2344. https://doi.org/10.3390/cancers12092344
Chicago/Turabian StyleTyuryumina, Ella Ya., Alexey A. Neznanov, and Jacob L. Turumin. 2020. "A Mathematical Model to Predict Diagnostic Periods for Secondary Distant Metastases in Patients with ER/PR/HER2/Ki-67 Subtypes of Breast Cancer" Cancers 12, no. 9: 2344. https://doi.org/10.3390/cancers12092344
APA StyleTyuryumina, E. Y., Neznanov, A. A., & Turumin, J. L. (2020). A Mathematical Model to Predict Diagnostic Periods for Secondary Distant Metastases in Patients with ER/PR/HER2/Ki-67 Subtypes of Breast Cancer. Cancers, 12(9), 2344. https://doi.org/10.3390/cancers12092344





