Real World Outcomes of Ipilimumab and Nivolumab in Patients with Metastatic Melanoma
Abstract
:1. Introduction
2. Methods
2.1. Statistical Analysis
2.2. Ethics
3. Results
3.1. Patient Demographics
3.2. Efficacy
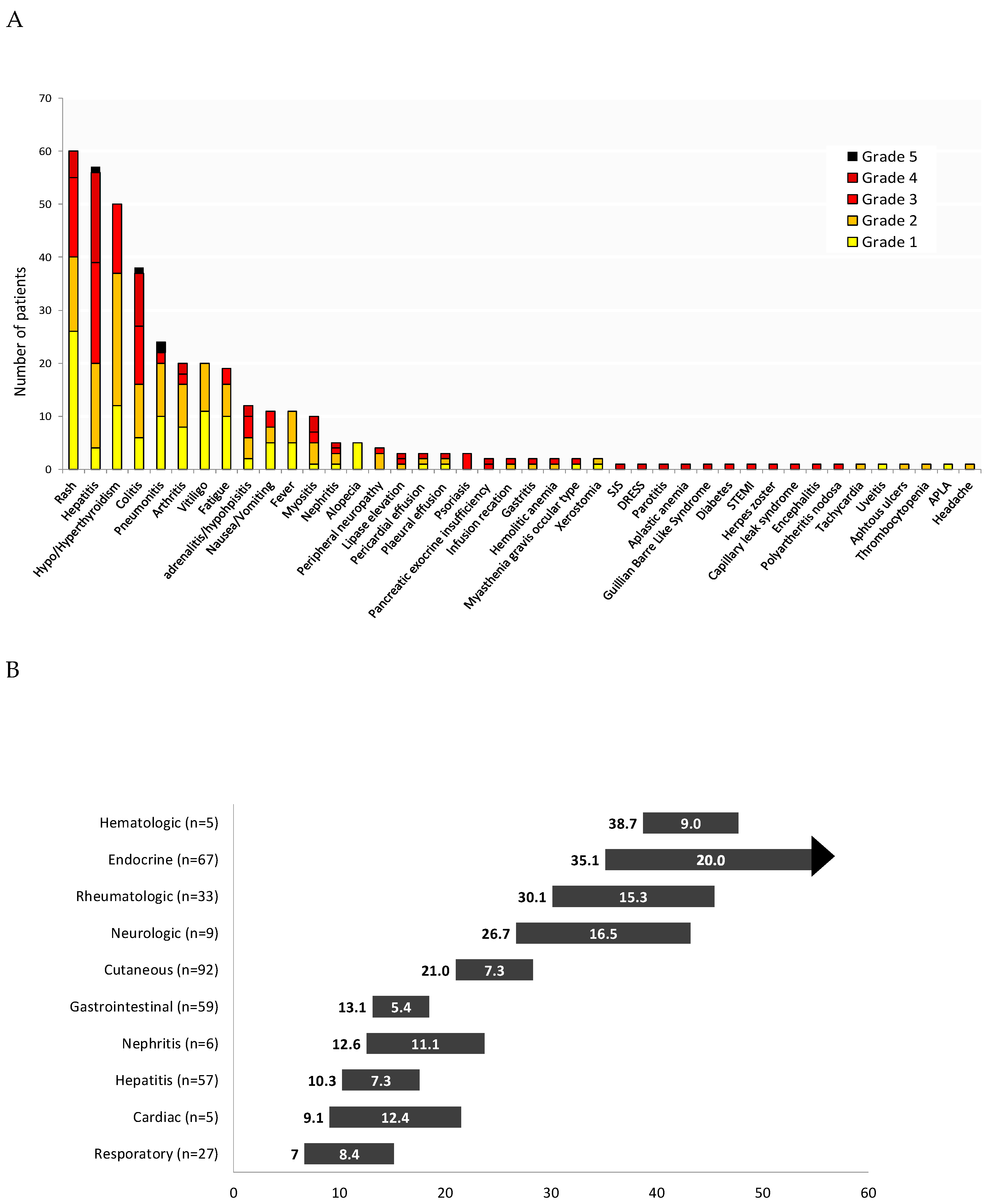
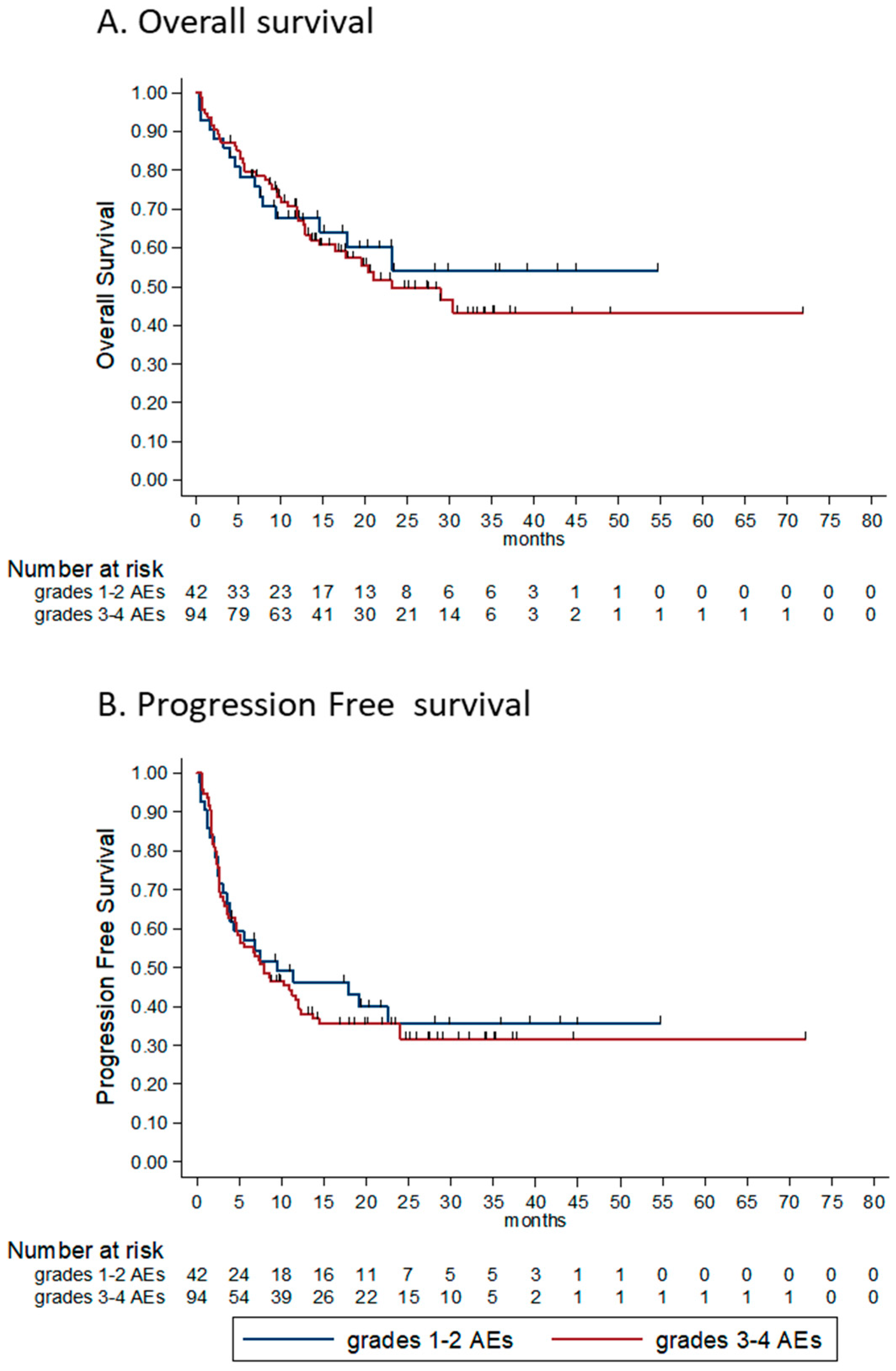
3.3. Toxicity
3.4. Factors Associated with Outcome
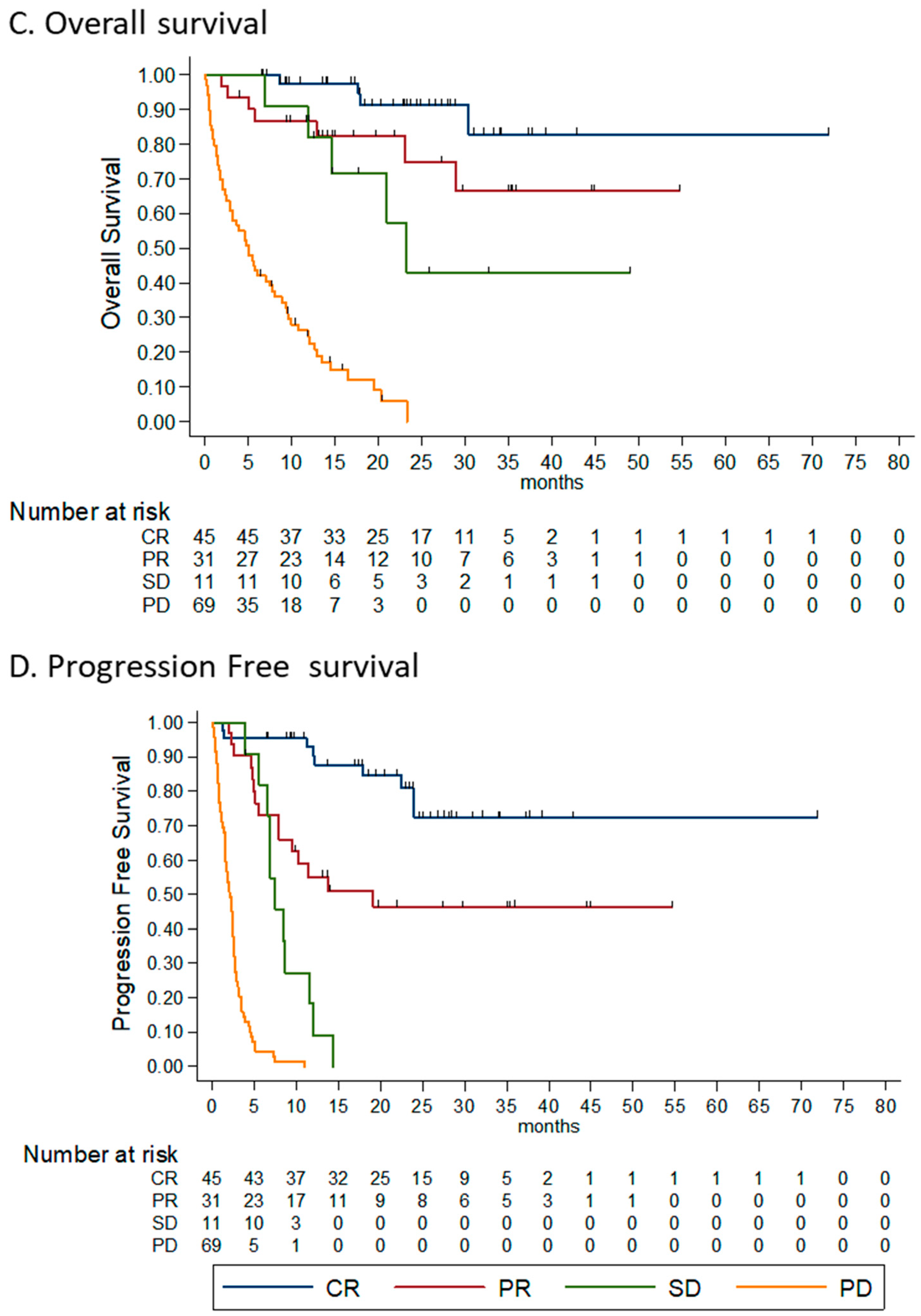
3.4.1. Histology Subtype
3.4.2. Disease Burden
3.4.3. ECOG Performance Status
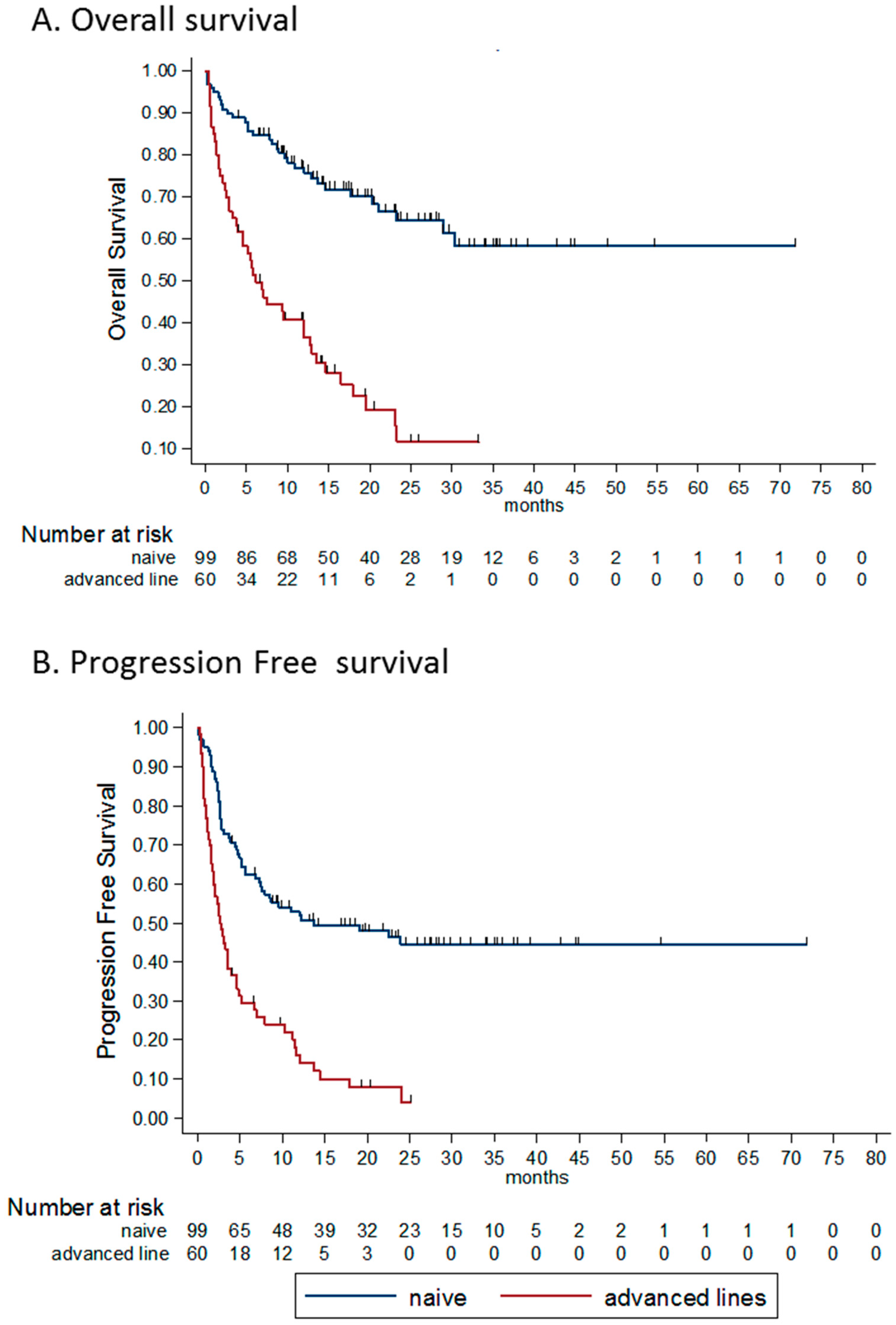
3.4.4. Line of Treatment
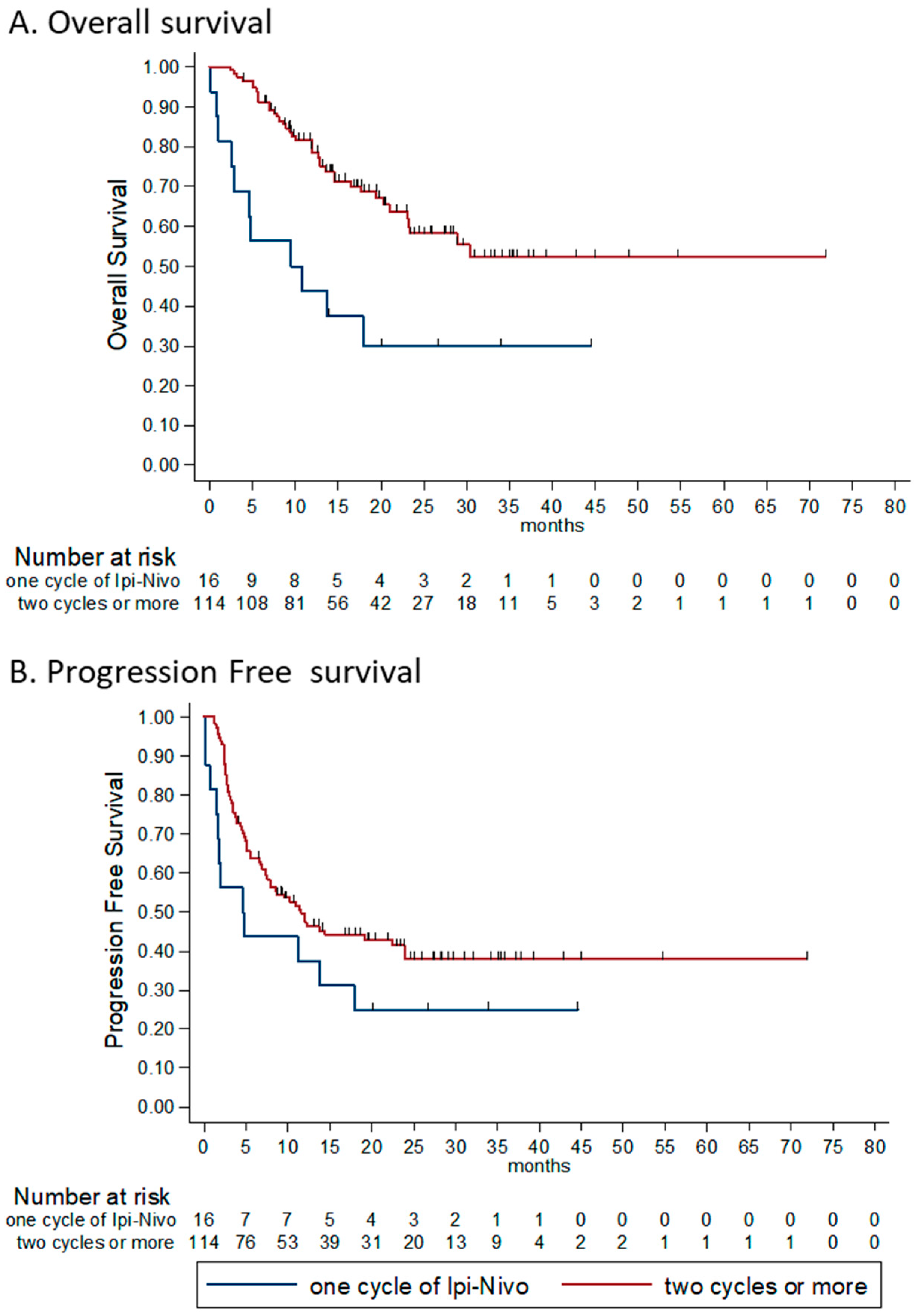
3.4.5. Number of Combinations Administered
3.4.6. BRAF Status
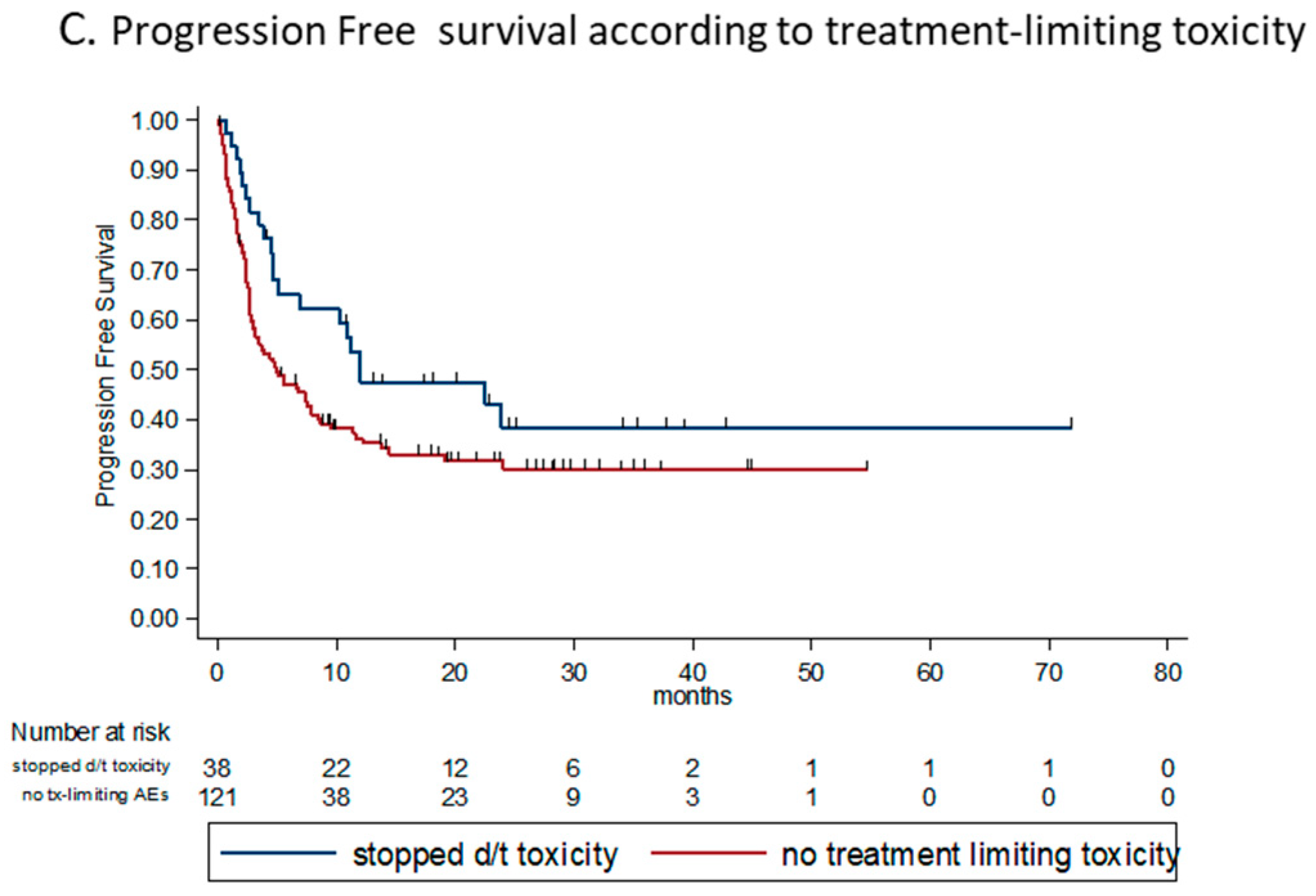
3.4.7. Toxicity and Steroid Treatment
3.4.8. Uni- and Multivariable Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schadendorf, D.; Dummer, R.; Hauschild, A.; Robert, C.; Hamid, O.; Daud, A.I.; Eertwegh, A.V.D.; Cranmer, L.D.; O’Day, S.J.; Puzanov, I.; et al. Health-related quality of life in the randomised KEYNOTE-002 study of pembrolizumab versus chemotherapy in patients with ipilimumab-refractory melanoma. Eur. J. Cancer 2016, 67, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Schadendorf, D.; Larkin, J.; Wolchok, J.; Hodi, F.S.; Sileni, V.C.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.; et al. Health-related quality of life results from the phase III CheckMate 067 study. Eur. J. Cancer 2017, 82, 80–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrella, T.M.; Robert, C.; Richtig, E.; Miller, W.H.; Masucci, G.; Walpole, E.; Lebbé, C.; Steven, N.M.; Middleton, M.R.; Hille, D.; et al. Patient-reported outcomes in KEYNOTE-006, a randomised study of pembrolizumab versus ipilimumab in patients with advanced melanoma. Eur. J. Cancer 2017, 86, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Mamoor, M.; Postow, M.A.; Lavery, J.A.; Baxi, S.S.; Khan, N.; Mao, J.J.; Rogak, L.J.; Sidlow, R.; Thom, B.; Wolchok, J.A.; et al. Quality of life in long-term survivors of advanced melanoma treated with checkpoint inhibitors. J. Immunother. Cancer 2020, 8, e000260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogiers, A.; Boekhout, A.; Schwarze, J.K.; Awada, G.; Blank, C.U.; Neyns, B. Long-Term Survival, Quality of Life, and Psychosocial Outcomes in Advanced Melanoma Patients Treated with Immune Checkpoint Inhibitors. J. Oncol. 2019, 2019, 5269062. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Thomas, L.; Bondarenko, I.N.; O’Day, S.; Weber, J.; Garbe, C.; Lebbé, C.; Baurain, J.F.; Testori, A.; Grob, J.J.; et al. Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. N. Engl. J. Med. 2011, 364, 2517–2526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Schadendorf, D.; Hodi, F.S.; Robert, C.; Weber, J.S.; Margolin, K.; Hamid, O.; Patt, D.; Chen, T.T.; Berman, D.M.; Wolchok, J.D. Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of Ipilimumab in Unresectable or Metastatic Melanoma. J. Clin. Oncol. 2015, 33, 1889–1894. [Google Scholar] [CrossRef] [Green Version]
- Ribas, A.; Puzanov, I.; Dummer, R.; Schadendorf, D.; Hamid, O.; Robert, C.; Hodi, F.S.; Schachter, J.; Pavlick, A.C.; Lewis, K.; et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): A randomised, controlled, phase 2 trial. Lancet Oncol. 2015, 16, 908–918. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in Previously Untreated Melanoma without BRAF Mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Schachter, J.; Ribas, A.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.I.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus ipilimumab for advanced melanoma: Final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006). Lancet 2017, 390, 1853–1862. [Google Scholar] [CrossRef]
- Eggermont, A.M.M.; Blank, C.U.; Mandalà, M.; Long, G.V.; Atkinson, V.; Dalle, S.; Haydon, A.; Lichinitser, M.; Khattak, A.; Carlino, M.S.; et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018, 378, 1789–1801. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Mandalà, M.; Del Vecchio, M.; Gogas, H.; Arance, A.; Cowey, C.L.; Dalle, S.; Schenker, M.; Sileni, V.C.; Marquez-Rodas, I.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017, 377, 1824–1835. [Google Scholar] [CrossRef] [PubMed]
- Selby, M.J.; Engelhardt, J.J.; Johnston, R.J.; Lu, L.S.; Han, M.; Thudium, K.; Yao, D.; Quigley, M.; Valle, J.; Wang, C.; et al. Preclinical Development of Ipilimumab and Nivolumab Combination Immunotherapy: Mouse Tumor Models, in Vitro Functional Studies, and Cynomolgus Macaque Toxicology. PLoS ONE 2016, 11, e0161779. [Google Scholar]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef] [Green Version]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.A.; Reed, K.; et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and Ipilimumab versus Ipilimumab in Untreated Melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef] [Green Version]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef] [Green Version]
- Larkin, J.; Chiarionsileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Wolchok, J.D.; Sileni, V.C.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef]
- Hodi, F.S.; Sileni, V.C.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawbi, H.; Forsyth, P.A.; Algazi, A.P.; Hamid, O.; Hodi, F.S.; Moschos, S.J.; Khushalani, N.I.; Lewis, K.; Lao, C.D.; Postow, M.A.; et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018, 379, 722–730. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Lo, S.; Sandhu, S.; Guminski, A.D.; Brown, M.P.; Wilmott, J.S.; Edwards, J.; González, M.; Scolyer, R.A.; et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: A multicentre randomised phase 2 study. Lancet Oncol. 2018, 19, 672–681. [Google Scholar] [CrossRef]
- Long, G.V.; Atkinson, V.; Cebon, J.S.; Jameson, M.B.; Fitzharris, B.M.; McNeil, C.M.; Hill, A.G.; Ribas, A.; Atkins, M.; Thompson, J.A.; et al. Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): An open-label, phase 1b trial. Lancet Oncol. 2017, 18, 1202–1210. [Google Scholar] [CrossRef] [Green Version]
- Carlino, M.S.; Menzies, A.M.; Atkinson, V.G.; Cebon, J.S.; Jameson, M.B.; Fitzharris, B.M.; McNeil, C.M.; Hill, A.G.; Ribas, A.; Atkins, M.B.; et al. Long-Term Follow-Up of Standard-Dose Pembrolizumab Plus Reduced-Dose Ipilimumab in Patients with Advanced Melanoma: KEYNOTE-029 Part 1B. Clin. Cancer Res. 2020. [Google Scholar] [CrossRef]
- Moser, J.C.; Chen, D.; Hu-Lieskovan, S.; Grossmann, K.F.; Patel, S.; Colonna, S.V.; Ying, J.; Hyngstrom, J.R. Real-world survival of patients with advanced BRAF V600 mutated melanoma treated with front-line BRAF/MEK inhibitors, anti-PD-1 antibodies, or nivolumab/ipilimumab. Cancer Med. 2019, 8, 7637–7643. [Google Scholar] [CrossRef] [Green Version]
- National Cancer Institute (NCI). DREAMseq (Doublet, Randomized Evaluation in Advanced Melanoma Sequencing) a Phase III Trial; Report No.: NCT02224781; ClinicalTrials.gov; U.S. Available online: https://clinicaltrials.gov/ct2/show/NCT02224781 (accessed on 24 July 2020).
- Sequential Combo Immuno and Target Therapy (SECOMBIT) Study—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02631447 (accessed on 24 July 2020).
- Da Silva, I.P.; Ahmed, T.; Lo, S.; Reijers, I.L.; Weppler, A.; Warner, A.B.; Patrinely, J.R.; Serra-Bellver, P.; Lebbe, C.; Mangana, J.; et al. Ipilimumab (IPI) alone or in combination with anti-PD-1 (IPI+PD1) in patients (pts) with metastatic melanoma (MM) resistant to PD1 monotherapy. J. Clin. Oncol. 2020, 38, 10005. [Google Scholar] [CrossRef]
- Olson, D.; Luke, J.J.; Poklepovic, A.S.; Bajaj, M.; Higgs, E.; Carll, T.C.; Labadie, B.; Krausz, T.; Zha, Y.; Karrison, T.; et al. Significant antitumor activity for low-dose ipilimumab (IPI) with pembrolizumab (PEMBRO) immediately following progression on PD1 Ab in melanoma (MEL) in a phase II trial. J. Clin. Oncol. 2020, 38, 10004. [Google Scholar] [CrossRef]
- Mason, R.; Dearden, H.C.; Nguyen, B.; Soon, J.A.; Smith, J.L.; Randhawa, M.; Mant, A.; Warburton, L.; Lo, S.; Meniawy, T.; et al. Combined ipilimumab and nivolumab first-line and after BRAF-targeted therapy in advanced melanoma. Pigment. Cell Melanoma Res. 2019, 33, 358–365. [Google Scholar] [CrossRef]
- Tarhini, A.; Kudchadkar, R.R. Predictive and on-treatment monitoring biomarkers in advanced melanoma: Moving toward personalized medicine. Cancer Treat. Rev. 2018, 71, 8–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrelli, F.; Ardito, R.; Merelli, B.; Lonati, V.; Cabiddu, M.; Seghezzi, S.; Barni, S.; Ghidini, A. Prognostic and predictive role of elevated lactate dehydrogenase in patients with melanoma treated with immunotherapy and BRAF inhibitors. Melanoma Res. 2019, 29, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Robert, C.; Larkin, J.; Ascierto, P.A.; Long, G.V.; Hassel, J.C.; Schadendorf, D.; Hodi, F.S.; Lebbe, C.; Grob, J.J.; Grossmann, K.F.; et al. 1213OCharacterization of complete responses (CRs) in patients with advanced melanoma (MEL) who received the combination of nivolumab (NIVO) and ipilimumab (IPI), NIVO or IPI alone. Ann. Oncol. 2017, 28. [Google Scholar] [CrossRef] [Green Version]
- Van Zeijl, M.; Wouters, M.W.J.M.; Eertwegh, A.V.D.; De Wreede, L.; Aarts, M.; Van Akkooi, A.; Berkmortel, F.W.P.J.V.D.; De Groot, J.; Boers-Sonderen, M.; Van Der Hoeven, J.; et al. Real-world outcomes of ipilimumab plus nivolumab for advanced melanoma in the Netherlands. Ann. Oncol. 2019, 30, v552. [Google Scholar] [CrossRef]
- Boon, I.S.; Marples, M. Real-world experience of combined ipilimumab and nivolumab for advanced melanoma in a single UK institution. Clin. Oncol. 2018, 30, e2. [Google Scholar] [CrossRef]
- Hodi, F.S.; Postow, M.A.; Chesney, J.A.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; et al. Overall survival in patients with advanced melanoma (MEL) who discontinued treatment with nivolumab (NIVO) plus ipilimumab (IPI) due to toxicity in a phase II trial (CheckMate 069). J. Clin. Oncol. 2016, 34, 9518. [Google Scholar] [CrossRef]
- Schadendorf, D.; Wolchok, J.D.; Hodi, F.S.; Sileni, V.C.; Gonzalez, R.; Rutkowski, P.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Chesney, J.; et al. Efficacy and Safety Outcomes in Patients with Advanced Melanoma Who Discontinued Treatment with Nivolumab and Ipilimumab Because of Adverse Events: A Pooled Analysis of Randomized Phase II and III Trials. J. Clin. Oncol. 2017, 35, 3807–3814. [Google Scholar] [CrossRef] [Green Version]
- Hogg, D.; Chapman, P.B.; Sznol, M.; Lao, C.D.; Gonzalez, R.; Daniels, G.A.; Smylie, M.; Kudchadkar, R.; Thompson, J.A.; Sharfman, W.H.; et al. Overall survival (OS) analysis from an expanded access program (EAP) of nivolumab (NIVO) in combination with ipilimumab (IPI) in patients with advanced melanoma (MEL). J. Clin. Oncol. 2017, 35, 9522. [Google Scholar] [CrossRef]
- Karadurmus, N.; Sendur, M.A.N.; Karaca, B.; Olmez, O.F.; Hacibekiroglu, I.; Coskun, H.S.; Degirmencioglu, S.; Kemal, Y.; Kilickap, S.; Sumbul, A.T.; et al. Experience from Turkish centers participating in the Early Access Program (EAP): Preliminary real-world safety data of nivolumab (nivo) combined with ipilimumab (ipi) in pre-treated advanced melanoma patients. J. Oncol. Sci. 2018, 4, 125–129. [Google Scholar] [CrossRef]
- Corrie, P.; Chao, D.; Board, R.E.; Sarah, L.; Smittenaar, R. Metastatic Melanoma Patient Outcomes since Introduction of Immune Checkpoint Inhibitors in England between 2014 and 2018. J. Clin. Oncol. 2020, 38, 55. Available online: https://meetinglibrary.asco.org/record/184098/abstract (accessed on 29 March 2020). [CrossRef]
- Soldatos, T.G.; Dimitrakopoulou-Strauss, A.; Larribere, L.; Hassel, J.C.; Sachpekidis, C. Retrospective Side Effect Profiling of the Metastatic Melanoma Combination Therapy Ipilimumab-Nivolumab Using Adverse Event Data. Diagnostics 2018, 8, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, R.W.; Shillington, A.; Lee, T.A.; Macahilig, C.P.; Diede, S.J.; Dave, V.; Harshaw, Q.; Scherrer, E.; Liu, F.X. Hospitalization and emergency department utilization in patients with advanced melanoma receiving pembrolizumab versus ipilimumab plus nivolumab in US academic centers. J. Med. Econ. 2019, 23, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parakh, S.; Randhawa, M.; Nguyen, B.H.; Warburton, L.; Hussain, M.A.; Cebon, J.; Millward, M.; Yip, D.; Ali, S. Real-world efficacy and toxicity of combined nivolumab and ipilimumab in patients with metastatic melanoma. Asia-Pacific J. Clin. Oncol. 2018, 15, 26–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | All Patients N = 172 | Non Responders (SD/PD), n = 90 | Responders (CR/PR), n = 79 | p-Value |
|---|---|---|---|---|
| Age (median, range) | 59 (12–80) | 59 (12–80) | 60 (12–80) | 0.635 |
| Male (%) | 99 (58%) | 52 (58%) | 46 (58%) | 0.953 |
| Melanoma subtype (%) | ||||
| Cutaneous | 116 (67%) | 58 (50%) | 58 (50%) | - |
| Mucosal | 8 (5%) | 7 (88%) | 1 (12%) | 0.04 * |
| ocular | 13 (8%) | 10 (77%) | 3 (23%) | 0.065 * |
| Unknown primary | 35 (20%) | 15 (43%) | 17 (49%) | 0.754 * |
| Disease presentation | ||||
| Advanced upfront Recurrent disease | 46 (28%) 124 (72%) | 21 (46%) 69 (56%) | 22 (48%) 55 (44%) | 0.235 |
| BRAF wild-type | 81 (47%) | 43 (53%) | 38 (47%) | - |
| BRAF V600 mutant | 85 (49%) | 44 (52%) | 38 (45%) | 0.984 |
| V600E mutation | 50 (30%) | 26 (52%) | 24 (48%) | |
| V600K mutation | 3 (2%) | 1 (33%) | 2 (67%) | |
| V600 unspecified | 29 (17%) | 17 (59%) | 12 (41%) | |
| Unknown status | 6 (3%) | 3 (50%) | 3 (50%) | |
| LDH | ||||
| Ratio (mean±SD) > ULN (%) | 1.59 ± 2.08 65 (38%) | 2.12 ± 2.71 41 (63%) | 1.0 ± 0.6 23 (35%) | 0.003 |
| AJCC 8th edition (%) | ||||
| IIIC | 2 (1%) | 0 | 2 (3%) | - |
| M1a | 32 (19%) | 16 (50%) | 16 (50%) | |
| M1b | 32 (19%) | 16 (50%) | 16 (50%) | 1.000 |
| M1c | 68 (39%) | 37 (54%) | 29 (43%) | 0.573 |
| M1d | 38 (22%) | 21 (55%) | 16 (42%) | 0.575 |
| Number of disease sites (mean ± SD) | 2.5 ± 1.7 | 2.86 ± 1.93 | 2.13 ± 1.33 | 0.005 |
| ECOG PS (%) | ||||
| 0 | 114 (66%) | 46 (40%) | 66 (58%) | - |
| 1 | 35 (20%) | 23 (66%) | 11 (31%) | 0.008 ** |
| ≥2 | 13 (8%) | 12 (92%) | 1 (8%) | 0.017 ** |
| unknown | 10 (6%) | 9 (90%) | 1 (10%) | |
| Treatment naïve | 110 (64%) | 43 (39%) | 64 (58%) | |
| Previously treated | 62 (36%) | 47 (76%) | 14 (23%) | <0.001 |
| Previous treatments | - | |||
| Immunotherapy | 30 (17%) | 23 (77%) | 7 (23%) | |
| BRAF inhibitors | 47 (27%) | 36 (77%) | 11 (23%) | |
| N° of comb Ipi-Nivo (%) | ||||
| All four | 67 (40%) | 27 (40%) | 40 (60%) | |
| 2–3 | 72 (42%) | 38 (53%) | 34 (47%) | - |
| Only one | 33 (19%) | 25 (76%) | 5 (15%) | - |
| Weeks on treatment (median, range) | 18 (1–190) | 9 (1–57) | 49 (1–190) | - |
| Response | All Patients (n = 159) | Treatment Naïve (n = 99) | Advanced Lines (n = 60) |
|---|---|---|---|
| CR | 45 (28%) | 36 (36%) | 9 (15%) |
| PR | 31 (20%) | 25 (25%) | 6 (10%) |
| SD | 11 (7%) | 6 (6%) | 5 (8%) |
| PD | 69 (43%) | 29 (29%) | 40 (67%) |
| NE | 3 (2%) | 3 (3%) | 0 |
| DCR | 55% | 67% | 33% |
| ORR | 48% | 61% | 25% |
| Characteristics | All Patients, N = 172 | Non Responders (SD/PD), n = 90 | Responders (CR/PR), n = 79 | p-Value |
|---|---|---|---|---|
| Maximal severity of AE *, (%) | ||||
| None | 17 (10%) | 15 (17%) | 1 (1%) | - |
| Grade 1–2 | 45 (28%) | 22 (24%) | 23 (29%) | - |
| Grade 3–4 | 103 (60%) | 52 (58%) | 50 (66%) | 0.901 ¥ |
| Grade 5 | 4 (2%) | 1(1%) | 3 (4%) | - |
| Steroid treatment, (%) | 102 (59%) | 51 (57%) | 50 (63%) | 0.381 |
| Duration of steroid treatment, weeks—median (range) | 12 (1–153) | 12 (1–106) | 16 (1–153) | 0.039 |
| Maximal dose of steroids, mg/kg **—mean ± SD | 1.7 ± 2.3 | 2.1 ± 3.0 | 1.3 ± 1.2 | 0.060 |
| Advanced immune suppression † (%) | 11 (6%) | 5 (6%) | 6 (8%) | 0.592 |
| Variable | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR for Death (95% CI) | p-Value | HR for Death (95% CI) | p-Value | |
| Histology subtype (mucosal vs. cutaneous) | 3.13 (1.41–6.92) | 0.005 | 1.84 (0.69–5.04) | 0.217 |
| LDH ratio | 1.20 (1.13–1.28) | 0.003 | 1.07 (0.97–1.18) | 0.176 |
| Number of metastatic sites | 1.26 (1.13–1.41) | <0.0001 | 1.14 (0.98–1.34) | 0.092 |
| ECOG PS | 3.02 (2.27–4.01) | <0.0001 | 2.00 (1.37–2.90) | <0.0001 |
| Line of treatment | 4.06 (2.56–6.46) | <0.0001 | 2.70 (1.41–5.15) | 0.003 |
| Number of combinations administered | 0.62 (0.51–7.66) | <0.0001 | 0.68 (0.52–0.89) | 0.005 |
| BRAF status (V600 mutant vs. WT) | 0.5 (0.22–1.13) | 0.096 | ||
| Best tumor response (NR vs. R) | 12.42 (6.43–24.00) | <0.0001 | 8.67 (3.69–20.39) | <0.0001 |
| Grade of AEs (3–4 vs. 1–2) | 1.09 (0.62–1.95) | 0.746 | ||
| Exposure to steroids | 0.67 (0.40–1.10) | 0.116 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asher, N.; Ben-Betzalel, G.; Lev-Ari, S.; Shapira-Frommer, R.; Steinberg-Silman, Y.; Gochman, N.; Schachter, J.; Meirson, T.; Markel, G. Real World Outcomes of Ipilimumab and Nivolumab in Patients with Metastatic Melanoma. Cancers 2020, 12, 2329. https://doi.org/10.3390/cancers12082329
Asher N, Ben-Betzalel G, Lev-Ari S, Shapira-Frommer R, Steinberg-Silman Y, Gochman N, Schachter J, Meirson T, Markel G. Real World Outcomes of Ipilimumab and Nivolumab in Patients with Metastatic Melanoma. Cancers. 2020; 12(8):2329. https://doi.org/10.3390/cancers12082329
Chicago/Turabian StyleAsher, Nethanel, Guy Ben-Betzalel, Shaked Lev-Ari, Ronnie Shapira-Frommer, Yael Steinberg-Silman, Neta Gochman, Jacob Schachter, Tomer Meirson, and Gal Markel. 2020. "Real World Outcomes of Ipilimumab and Nivolumab in Patients with Metastatic Melanoma" Cancers 12, no. 8: 2329. https://doi.org/10.3390/cancers12082329
APA StyleAsher, N., Ben-Betzalel, G., Lev-Ari, S., Shapira-Frommer, R., Steinberg-Silman, Y., Gochman, N., Schachter, J., Meirson, T., & Markel, G. (2020). Real World Outcomes of Ipilimumab and Nivolumab in Patients with Metastatic Melanoma. Cancers, 12(8), 2329. https://doi.org/10.3390/cancers12082329





