Phase II Clinical Trial of Pembrolizumab in Patients with Progressive Metastatic Pheochromocytomas and Paragangliomas
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Study Design
2.3. Intervention
2.4. Procedures
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
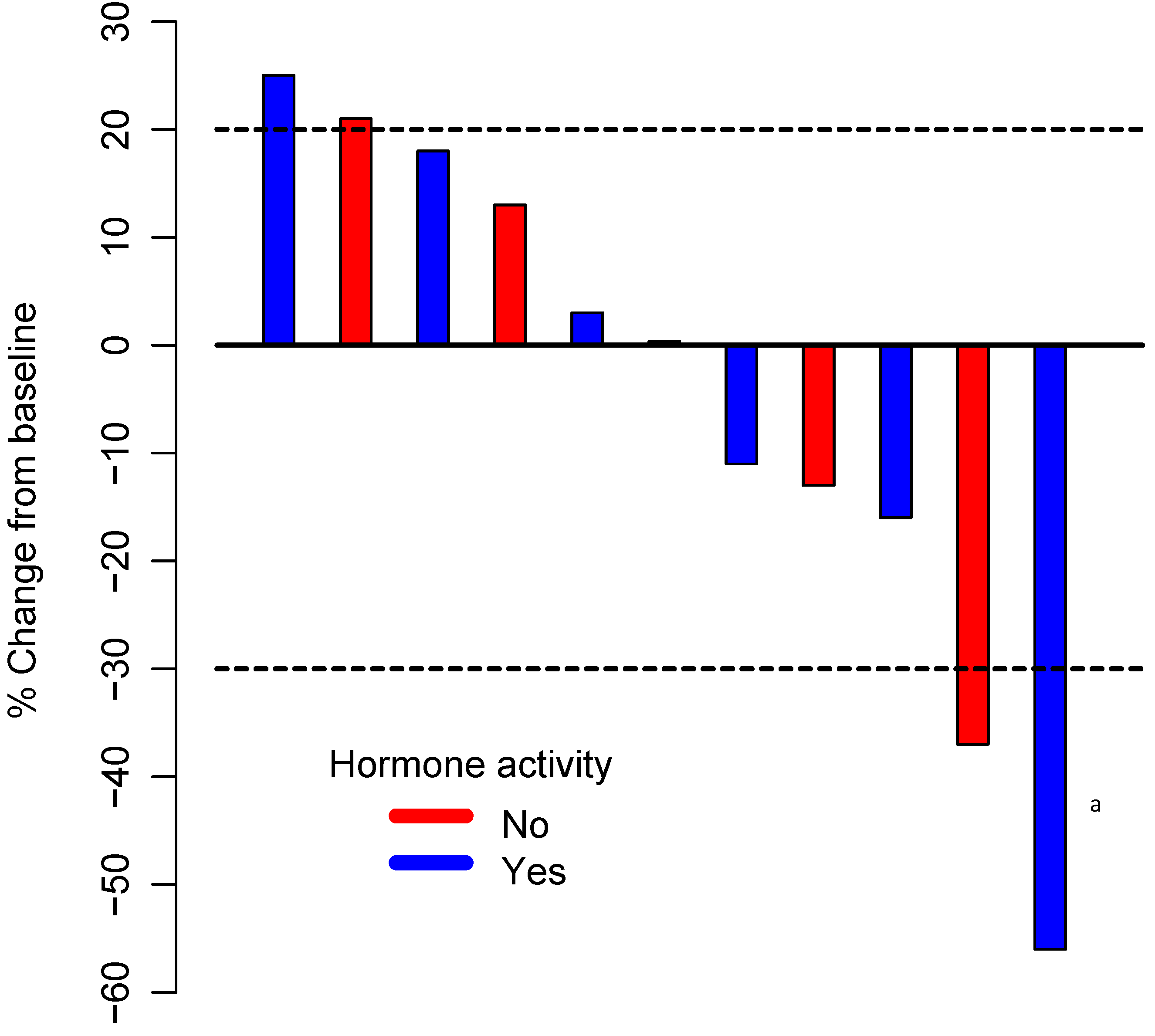
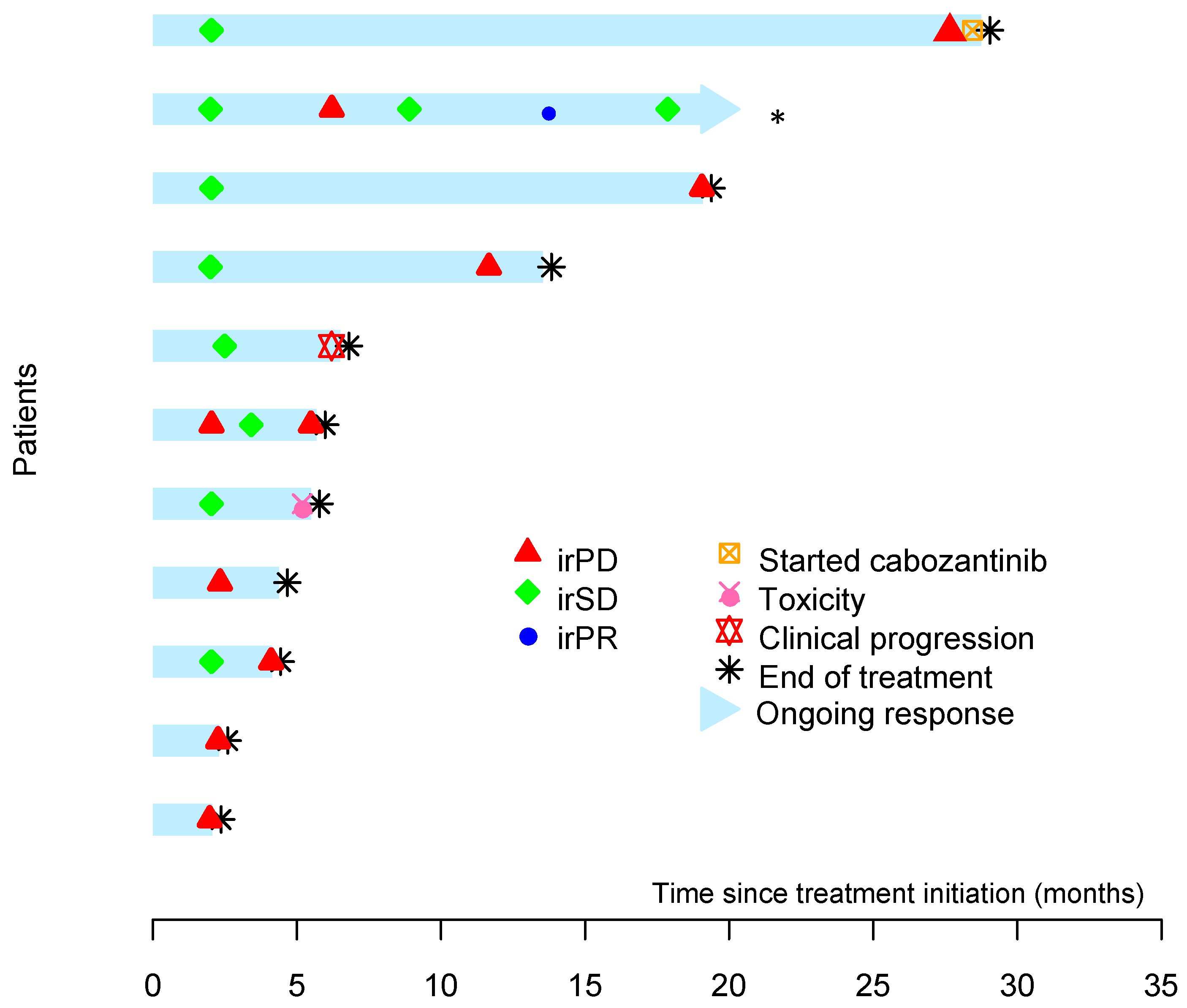
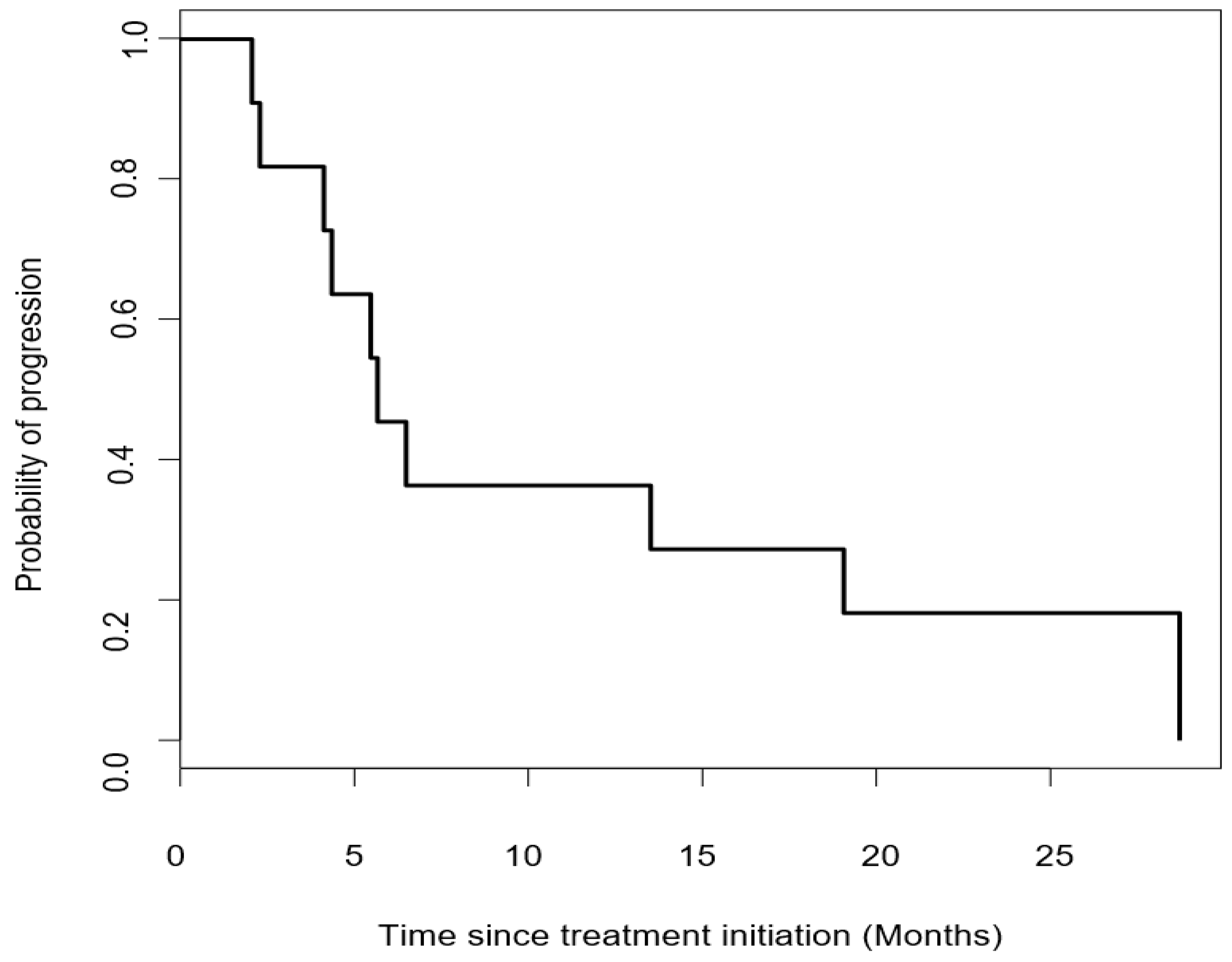
3.2. Efficacy
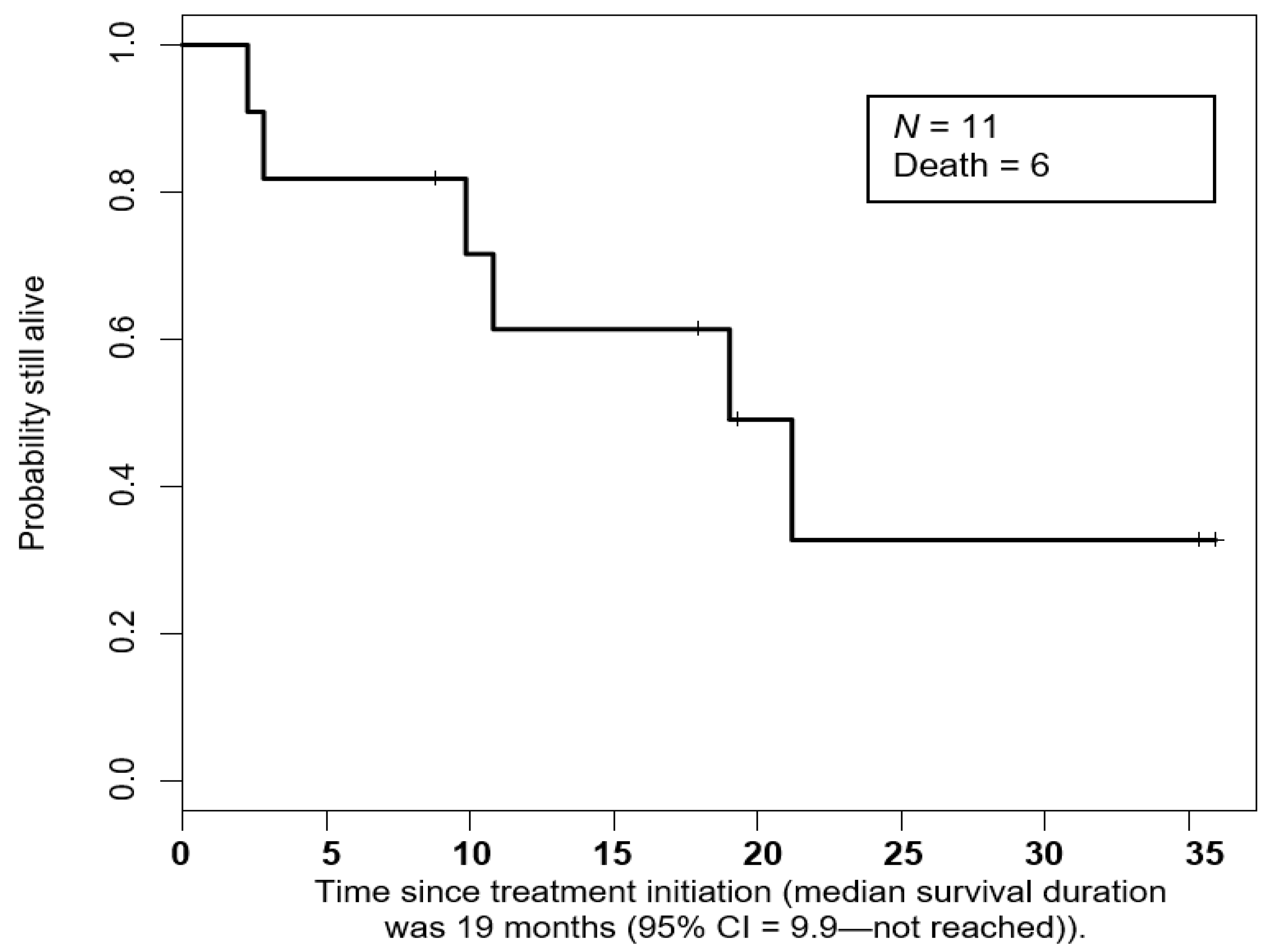
3.3. Duration of Response and Overall Survival
3.4. Safety
3.5. PDL-1 Membrane Expression and TILs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thosani, S.; Ayala-Ramirez, M.; Roman-Gonzalez, A.; Zhou, S.; Thosani, N.; Bisanz, A.; Jimenez, C. Constipation: An overlooked, unmanaged symptom of patients with pheochromocytoma and sympathetic paraganglioma. Eur. J. Endocrinol. 2015, 173, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Butz, J.J.; Yan, Q.; McKenzie, T.J.; Weingarten, T.N.; Cavalcante, A.N.; Bancos, I.; Young, W.F., Jr.; Schroeder, D.R.; Martin, D.P.; Sprung, J. Perioperative outcomes of syndromic paraganglioma and pheochromocytoma resection in patients with von Hippel-Lindau disease, multiple endocrine neoplasia type 2, or neurofibromatosis type 1. Surgery 2017, 162, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ramirez, M.; Feng, L.; Johnson, M.M.; Ejaz, S.; Habra, M.A.; Rich, T.; Busaidy, N.; Cote, G.J.; Perrier, N.; Phan, A.; et al. Clinical risk factors for malignancy and overall survival in patients with pheochromocytomas and sympathetic paragangliomas: Primary tumor size and primary tumor location as prognostic indicators. J. Clin. Endocrinol. Metab. 2011, 96, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr. Pathol. 2017, 28, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ramirez, M.; Palmer, J.L.; Hofmann, M.C.; de la Cruz, M.; Moon, B.S.; Waguespack, S.G.; Habra, M.A.; Jimenez, C. Bone metastases and skeletal-related events in patients with malignant pheochromocytoma and sympathetic paraganglioma. J. Clin. Endocrinol. Metab. 2013, 98, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Rohren, E.; Habra, M.A.; Rich, T.; Jimenez, P.; Ayala-Ramirez, M.; Baudin, E. Current and future treatments for malignant pheochromocytoma and sympathetic paraganglioma. Curr. Oncol. Rep. 2013, 15, 356–371. [Google Scholar] [CrossRef]
- Hamidi, O.; Young, W.F., Jr.; Iniguez-Ariza, N.M.; Kittah, N.E.; Gruber, L.; Bancos, C.; Tamhane, S.; Bancos, I. Malignant Pheochromocytoma and Paraganglioma: 272 Patients Over 55 Years. J. Clin. Endocrinol. Metab. 2017, 102, 3296–3305. [Google Scholar] [CrossRef]
- Jasim, S.; Jimenez, C. Metastatic pheochromocytoma and paraganglioma: Management of endocrine manifestations, surgery and ablative procedures, and systemic therapies. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 34, 101354. [Google Scholar] [CrossRef]
- Hescot, S.; Leboulleux, S.; Amar, L.; Vezzosi, D.; Borget, I.; Bournaud-Salinas, C.; de la Fouchardiere, C.; Libé, R.; Do Cao, C.; Niccoli, P.; et al. One-year progression-free survival of therapy-naive patients with malignant pheochromocytoma and paraganglioma. J. Clin. Endocrinol. Metab. 2013, 98, 4006–4012. [Google Scholar] [CrossRef]
- Pryma, D.A.; Chin, B.B.; Noto, R.B.; Dillon, J.S.; Perkins, S.; Solnes, L.; Kostakoglu, L.; Serafini, A.N.; Pampaloni, M.H.; Jensen, J.; et al. Efficacy and Safety of High-Specific-Activity (131)I-MIBG Therapy in Patients with Advanced Pheochromocytoma or Paraganglioma. J. Nucl. Med. 2019, 60, 623–630. [Google Scholar] [CrossRef]
- Jimenez, C.; Erwin, W.; Chasen, B. Targeted Radionuclide Therapy for Patients with Metastatic Pheochromocytoma and Paraganglioma: From Low-Specific-Activity to High-Specific-Activity Iodine-131 Metaiodobenzylguanidine. Cancers 2019, 11, 1018. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Nunez, R.; Wendt, R. High-specific-activity iodine 131 metaiodobenzylguanidine for the treatment of metastatic pheochromocytoma or paraganglioma: A novel therapy for an orphan disease. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Ramirez, M.; Feng, L.; Habra, M.A.; Rich, T.; Dickson, P.V.; Perrier, N.; Phan, A.; Waguespack, S.; Patel, S.; Jimenez, C. Clinical benefits of systemic chemotherapy for patients with metastatic pheochromocytomas or sympathetic extra-adrenal paragangliomas: Insights from the largest single-institutional experience. Cancer 2012, 118, 2804–2812. [Google Scholar] [CrossRef] [PubMed]
- Niemeijer, N.D.; Alblas, G.; van Hulsteijn, L.T.; Dekkers, O.M.; Corssmit, E.P. Chemotherapy with cyclophosphamide, vincristine and dacarbazine for malignant paraganglioma and pheochromocytoma: Systematic review and meta-analysis. Clin. Endocrinol. 2014, 81, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Fazeli, S.; Roman-Gonzalez, A. Antiangiogenic therapies for pheochromocytoma and paraganglioma. Endocr. Relat. Cancer 2020, 27, R239–R254. [Google Scholar] [CrossRef] [PubMed]
- Forrer, F.; Riedweg, I.; Maecke, H.R.; Mueller-Brand, J. Radiolabeled DOTATOC in patients with advanced paraganglioma and pheochromocytoma. Q. J. Nucl. Med. Mol. Imaging 2008, 52, 334–340. [Google Scholar] [PubMed]
- van Essen, M.; Krenning, E.P.; Kooij, P.P.; Bakker, W.H.; Feelders, R.A.; de Herder, W.W.; Wolbers, J.G.; Kwekkeboom, D.J. Effects of therapy with [177Lu-DOTA0, Tyr3]octreotate in patients with paraganglioma, meningioma, small cell lung carcinoma, and melanoma. J. Nucl. Med. 2006, 47, 1599–1606. [Google Scholar]
- Puranik, A.D.; Kulkarni, H.R.; Singh, A.; Baum, R.P. Peptide receptor radionuclide therapy with (90)Y/(177)Lu-labelled peptides for inoperable head and neck paragangliomas (glomus tumours). Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1223–1230. [Google Scholar] [CrossRef]
- Mak, I.Y.F.; Hayes, A.R.; Khoo, B.; Grossman, A. Peptide Receptor Radionuclide Therapy as a Novel Treatment for Metastatic and Invasive Phaeochromocytoma and Paraganglioma. Neuroendocrinology 2019, 109, 287–298. [Google Scholar] [CrossRef]
- Dahia, P.; Clifton-Bligh, R.; Gimenez-Roqueplo, A.P.; Robledo, M.; Jimenez, C. Metastatic pheochromocytoma and paraganglioma: Proceedings of the MEN2019 workshop. Endocr. Relat. Cancer 2020, 27, T41–T52. [Google Scholar] [CrossRef]
- Jimenez, C. Treatment for Patients with Malignant Pheochromocytomas and Paragangliomas: A Perspective From the Hallmarks of Cancer. Front. Endocrinol. 2018, 9, 277. [Google Scholar] [CrossRef] [PubMed]
- Dahia, P.L.; Ross, K.N.; Wright, M.E.; Hayashida, C.Y.; Santagata, S.; Barontini, M.; Kung, A.L.; Sanso, G.; Powers, J.F.; Tischler, A.S.; et al. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005, 1, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Hypoxia-/HIF-1alpha-Driven Factors of the Tumor Microenvironment Impeding Antitumor Immune Responses and Promoting Malignant Progression. Adv. Exp. Med. Biol. 2018, 1072, 171–175. [Google Scholar] [PubMed]
- Pinato, D.J.; Black, J.R.; Trousil, S.; Dina, R.E.; Trivedi, P.; Mauri, F.A.; Sharma, R. Programmed cell death ligands expression in phaeochromocytomas and paragangliomas: Relationship with the hypoxic response, immune evasion and malignant behavior. Oncoimmunology 2017, 6, e1358332. [Google Scholar] [CrossRef]
- Naing, A.; Meric-Bernstam, F.; Stephen, B.; Karp, D.D.; Hajjar, J.; Rodon Ahnert, J.; Piha-Paul, S.A.; Colen, R.R.; Jimenez, C.; Raghav, K.P.; et al. Phase 2 study of pembrolizumab in patients with advanced rare cancers. J. Immunother. Cancer 2020, 8, e000347. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef]
- Jasim, S.; Suman, V.J.; Jimenez, C.; Harris, P.; Sideras, K.; Burton, J.K.; Worden, F.P.; Auchus, R.J.; Bible, K.C. Phase II trial of pazopanib in advanced/progressive malignant pheochromocytoma and paraganglioma. Endocrine 2017, 57, 220–225. [Google Scholar] [CrossRef]
- Gonias, S.; Goldsby, R.; Matthay, K.K.; Hawkins, R.; Price, D.; Huberty, J.; Damon, L.; Linker, C.; Sznewajs, A.; Shiboski, S.; et al. Phase II study of high-dose [131I]metaiodobenzylguanidine therapy for patients with metastatic pheochromocytoma and paraganglioma. J. Clin. Oncol. 2009, 27, 4162–4168. [Google Scholar] [CrossRef]
- O’Kane, G.M.; Ezzat, S.; Joshua, A.M.; Bourdeau, I.; Leibowitz-Amit, R.; Olney, H.J.; Krzyzanowska, M.; Reuther, D.; Chin, S.; Wang, L.; et al. A phase 2 trial of sunitinib in patients with progressive paraganglioma or pheochromocytoma: The SNIPP trial. Br. J. Cancer 2019, 120, 1113–1119. [Google Scholar] [CrossRef]
- Huang, A.C.; Orlowski, R.J.; Xu, X.; Mick, R.; George, S.M.; Yan, P.K.; Manne, S.; Kraya, A.A.; Wubbenhorst, B.; Dorfman, L.; et al. A single dose of neoadjuvant PD-1 blockade predicts clinical outcomes in resectable melanoma. Nat. Med. 2019, 25, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, V.; Spencer, C.N.; Nezi, L.; Reuben, A.; Andrews, M.C.; Karpinets, T.V.; Prieto, P.A.; Vicente, D.; Hoffman, K.; Wei, S.C.; et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018, 359, 97–103. [Google Scholar] [CrossRef]
- Dahia, P.L. Pheochromocytoma and paraganglioma pathogenesis: Learning from genetic heterogeneity. Nat. Rev. Cancer 2014, 14, 108–119. [Google Scholar] [CrossRef]
- Spranger, S.; Luke, J.J.; Bao, R.; Zha, Y.; Hernandez, K.M.; Li, Y.; Gajewski, A.P.; Andrade, J.; Gajewski, T.F. Density of immunogenic antigens does not explain the presence or absence of the T-cell-inflamed tumor microenvironment in melanoma. Proc. Natl. Acad. Sci. USA 2016, 113, E7759–E7768. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
| Characteristic | n (%) |
|---|---|
| Sex | |
| Female | 4 (36) |
| Male | 7 (64) |
| Germline mutation | |
| No | 7 (64) |
| SDHB | 2 (18) |
| SDHD | 1 (9) |
| PMS2 | 1 (9) |
| Primary tumor location | |
| Adrenal (pheochromocytoma) | 4 (36) |
| Sympathetic abdominal paraganglia | 6 (55) |
| Head and neck (parasympathetic) | 1 (9) |
| Hormonal activity | |
| Yes | 7 (64) |
| No | 4 (36) |
| MIBG uptake | |
| Yes | 7 (64) |
| No | 3 (27) |
| Unknown | 1 (9) |
| Prior systemic therapy | |
| Naïve | 3 (28) |
| High-specific activity MIBG | 1 (9) |
| Cabozantinib | 1 (9) |
| CVD | 1 (9) |
| CVD, cabozantinib, lenvatinib | 1 (9) |
| Surgery, cabozantinib | 2 (18) |
| Surgery, CVD | 1 (9) |
| Surgery, CVD, cabozantinib | 1 (9) |
| Eastern Cooperative Oncology Group | |
| 0 | 4 (36) |
| 1 | 7 (64) |
| Adverse Event | Overall, n (%) | Grade 1, n (%) | Grade 2, n (%) | Grade 3, n (%) |
|---|---|---|---|---|
| Total | 26 | 18 | 4 | 4 |
| Laboratory abnormalities | ||||
| Increased ALT/AST | 4 (16) | 3 (17) | 0 (0) | 1 (25) |
| Increased alkaline phosphatase | 3 (12) | 3 (17) | 0 (0) | 0 (0) |
| Increased bilirubin | 1 (4) | 0 | 1 (25) | 0 |
| Increased creatinine | 1 (4) | 0 | 1 (25) | 0 |
| Anemia | 5 (19) | 2 (11) | 1 (25) | 2 (50) |
| General disorders | ||||
| Anorexia | 1 (4) | 1 (6) | 0 | 0 |
| Dysgeusia | 1 (4) | 0 | 1 (25) | 0 |
| Fatigue | 3 (12) | 3 (17) | 0 | 0 |
| Genital edema | 1 (4) | 1 (6) | 0 | 0 |
| Limb edema | 2 (8) | 2 (11) | 0 | 0 |
| Hyperthyroidism | 1 (4) | 1 (6) | 0 | 0 |
| Neurological disorders | ||||
| Peripheral sensory neuropathy | 1 (4) | 1 (6) | 0 | 0 |
| III cranial nerve paralysis | 1 (4) | 0 | 0 | 1 (25) |
| Rash macula-papular | 1 (4) | 1 (6) | 0 | 0 |
| Patient | NPR at 27 Weeks (Met or No Met) | PDL-1 H-Score | TILs | Best IrRECIST Response a | Alive at Last Follow-Up |
|---|---|---|---|---|---|
| 1 | Met | 75 | 1 | SD (−13%) | Yes |
| 2 | Met | N/A | N/A | SD (−11%) | Yes |
| 3 | no Met | 0 | 2 | - | No |
| 4 | no Met | 0 | 2 | - | No |
| 5 | Met | 0 | 0 | SD b | No |
| 6 | no Met | 0 | 0 | - c | No |
| 7 | no Met | 35 | 1 | - | Yes |
| 8 | no Met | N/A | N/A | - | No |
| 9 | Met | 0 | 3 | PR (−37%) | Yes |
| 10 | no Met | 0 | 3 | - | No |
| 11 | Not evaluable | 73 | 1 | - | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jimenez, C.; Subbiah, V.; Stephen, B.; Ma, J.; Milton, D.; Xu, M.; Zarifa, A.; Akhmedzhanov, F.O.; Tsimberidou, A.; Habra, M.A.; et al. Phase II Clinical Trial of Pembrolizumab in Patients with Progressive Metastatic Pheochromocytomas and Paragangliomas. Cancers 2020, 12, 2307. https://doi.org/10.3390/cancers12082307
Jimenez C, Subbiah V, Stephen B, Ma J, Milton D, Xu M, Zarifa A, Akhmedzhanov FO, Tsimberidou A, Habra MA, et al. Phase II Clinical Trial of Pembrolizumab in Patients with Progressive Metastatic Pheochromocytomas and Paragangliomas. Cancers. 2020; 12(8):2307. https://doi.org/10.3390/cancers12082307
Chicago/Turabian StyleJimenez, Camilo, Vivek Subbiah, Bettzy Stephen, Junsheng Ma, Denai Milton, Mingxuan Xu, Abdualrazzak Zarifa, Fechukwu Omolara Akhmedzhanov, Apostolia Tsimberidou, Mouhammed Amir Habra, and et al. 2020. "Phase II Clinical Trial of Pembrolizumab in Patients with Progressive Metastatic Pheochromocytomas and Paragangliomas" Cancers 12, no. 8: 2307. https://doi.org/10.3390/cancers12082307
APA StyleJimenez, C., Subbiah, V., Stephen, B., Ma, J., Milton, D., Xu, M., Zarifa, A., Akhmedzhanov, F. O., Tsimberidou, A., Habra, M. A., Rodon Anhert, J., Fu, S., & Naing, A. (2020). Phase II Clinical Trial of Pembrolizumab in Patients with Progressive Metastatic Pheochromocytomas and Paragangliomas. Cancers, 12(8), 2307. https://doi.org/10.3390/cancers12082307






