Phenotypic and Functional Characterization of NK Cells in αβT-Cell and B-Cell Depleted Haplo-HSCT to Cure Pediatric Patients with Acute Leukemia
Abstract
1. Introduction
2. Results
2.1. Criteria for Donor Selection
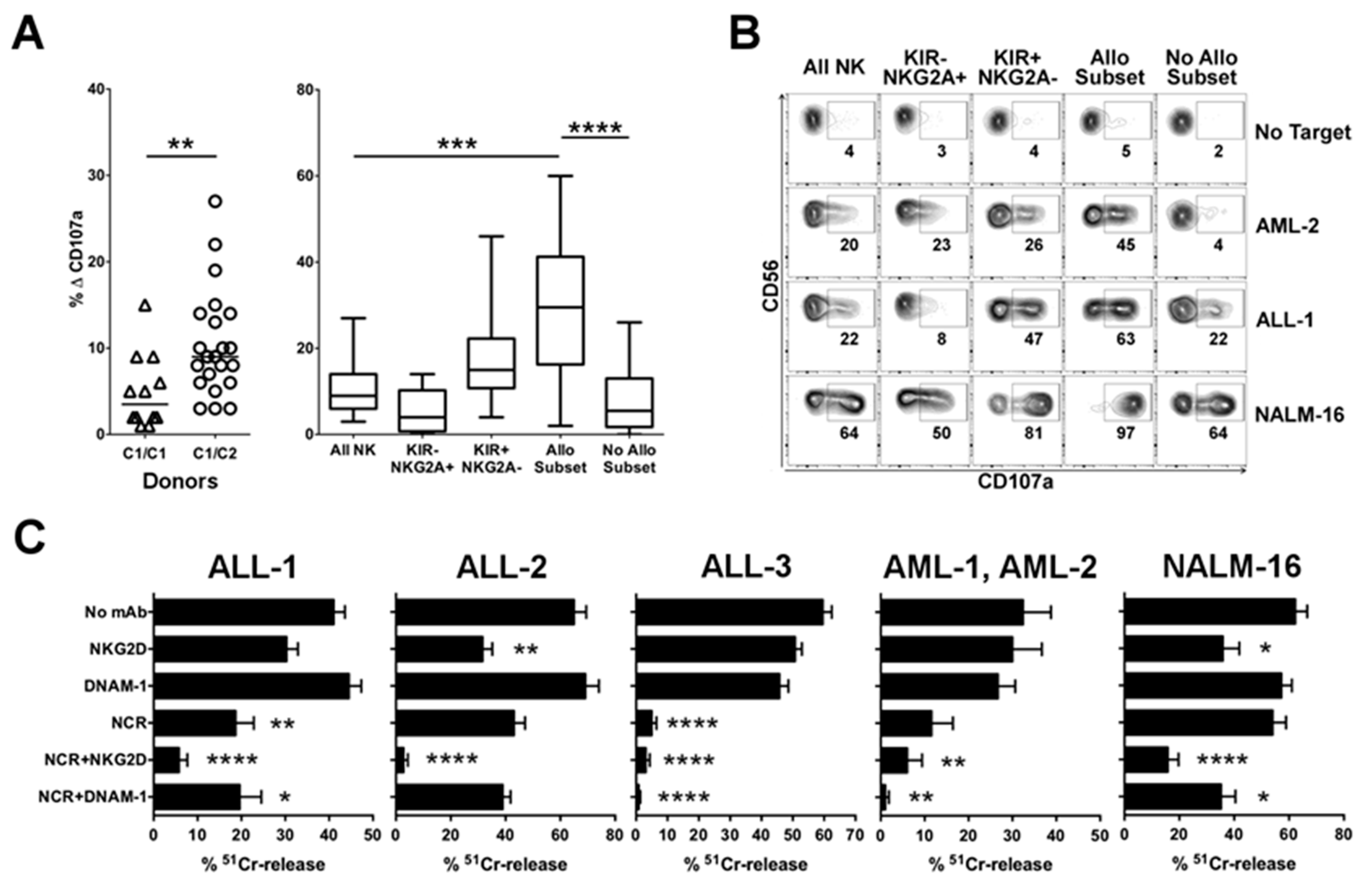
2.1.1. Advantage of NK Alloreactivity in the Anti-Leukemia Effect
2.1.2. Relevance of Activating NK Cell Receptors
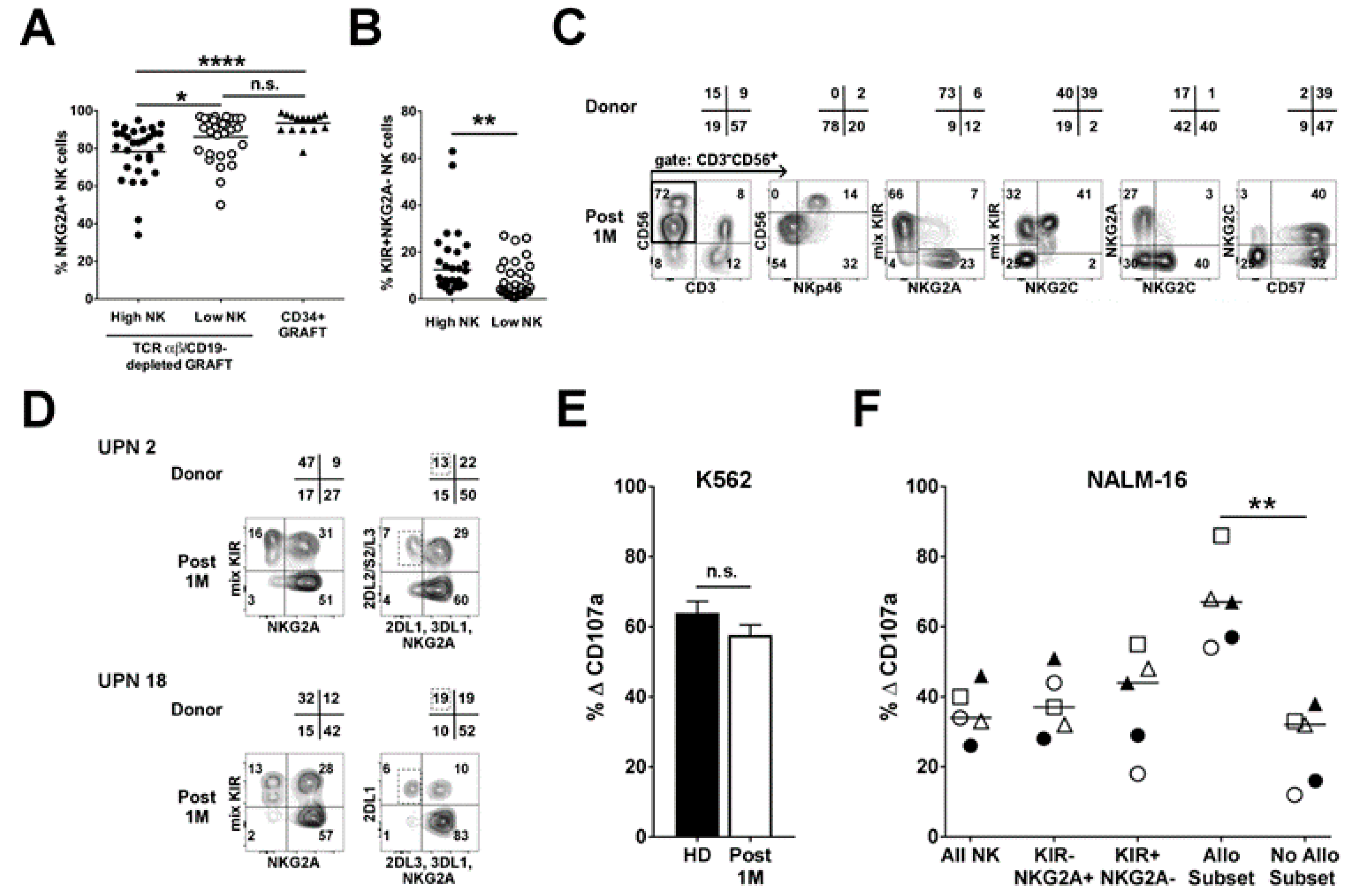
2.2. Infused NK Cells Persist and Are Functional up to One-Month Post-Transplant
2.3. Analysis of NK Alloreactivity in the Reconstituted Repertoire after Transplantation
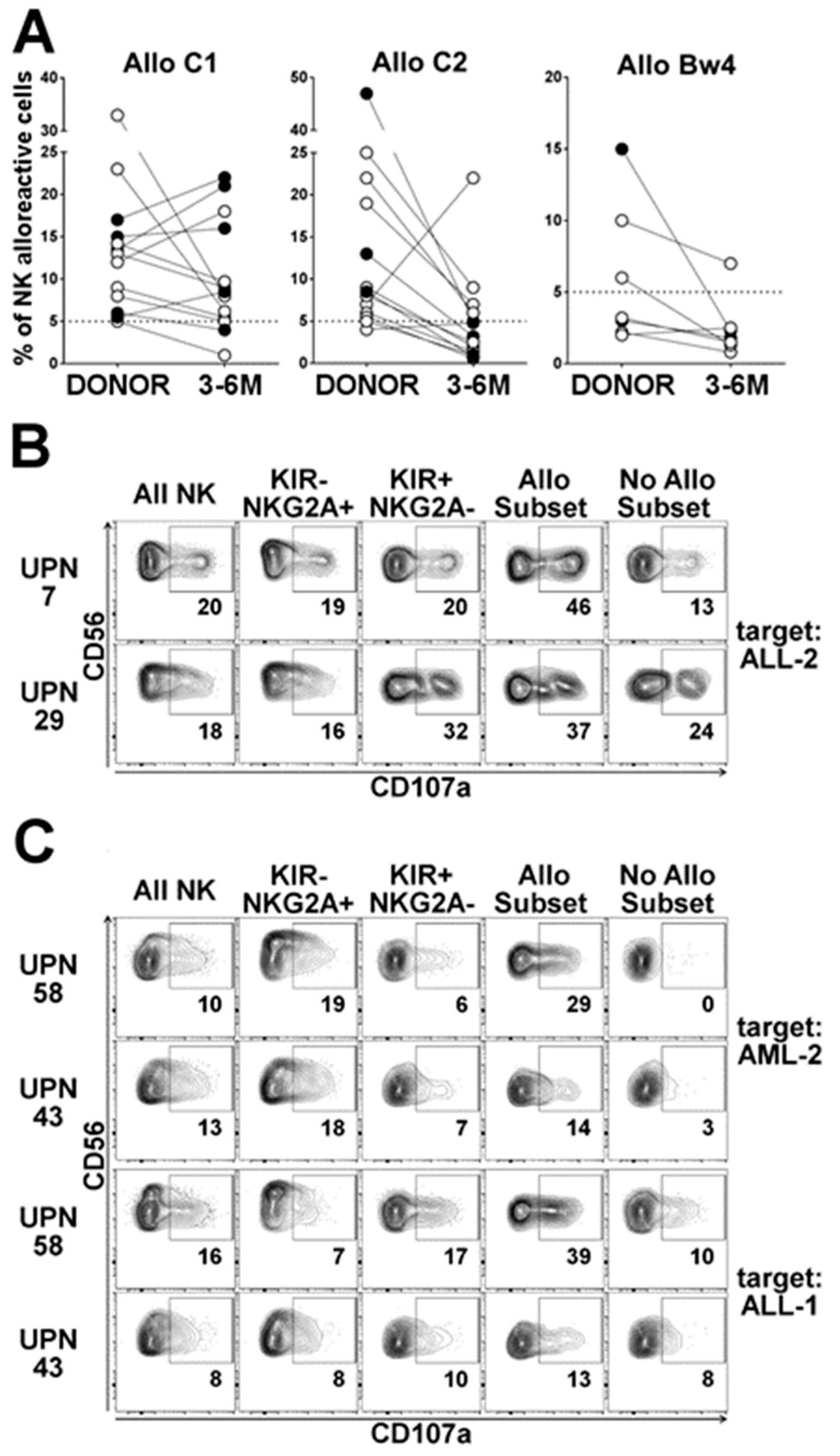
2.3.1. Dynamics of the Alloreactive NK Cell Subset
2.3.2. Influence of HCMV Reactivation on Alloreactive NK-Cell Phenotype
2.3.3. Anti-Leukemia Activity
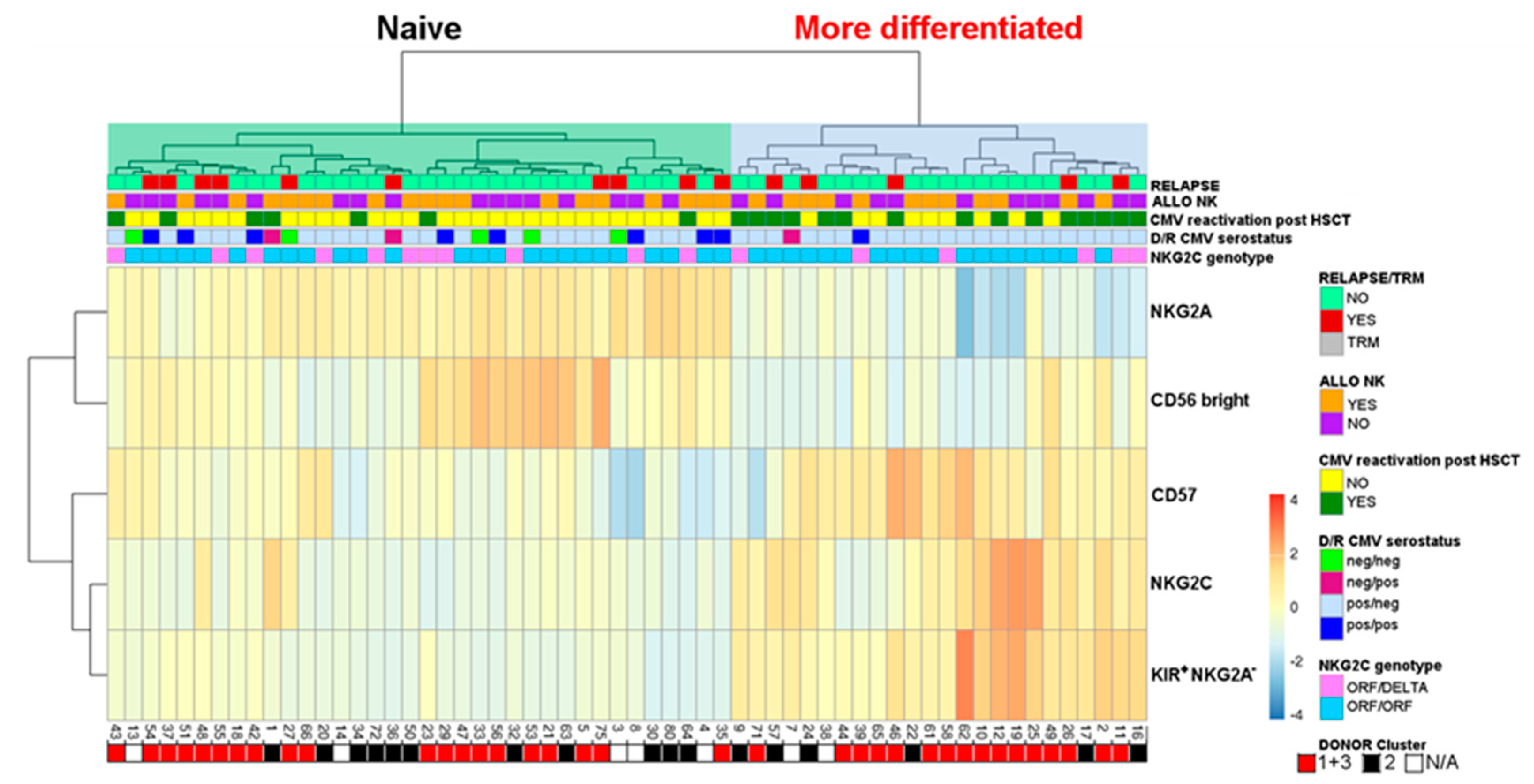
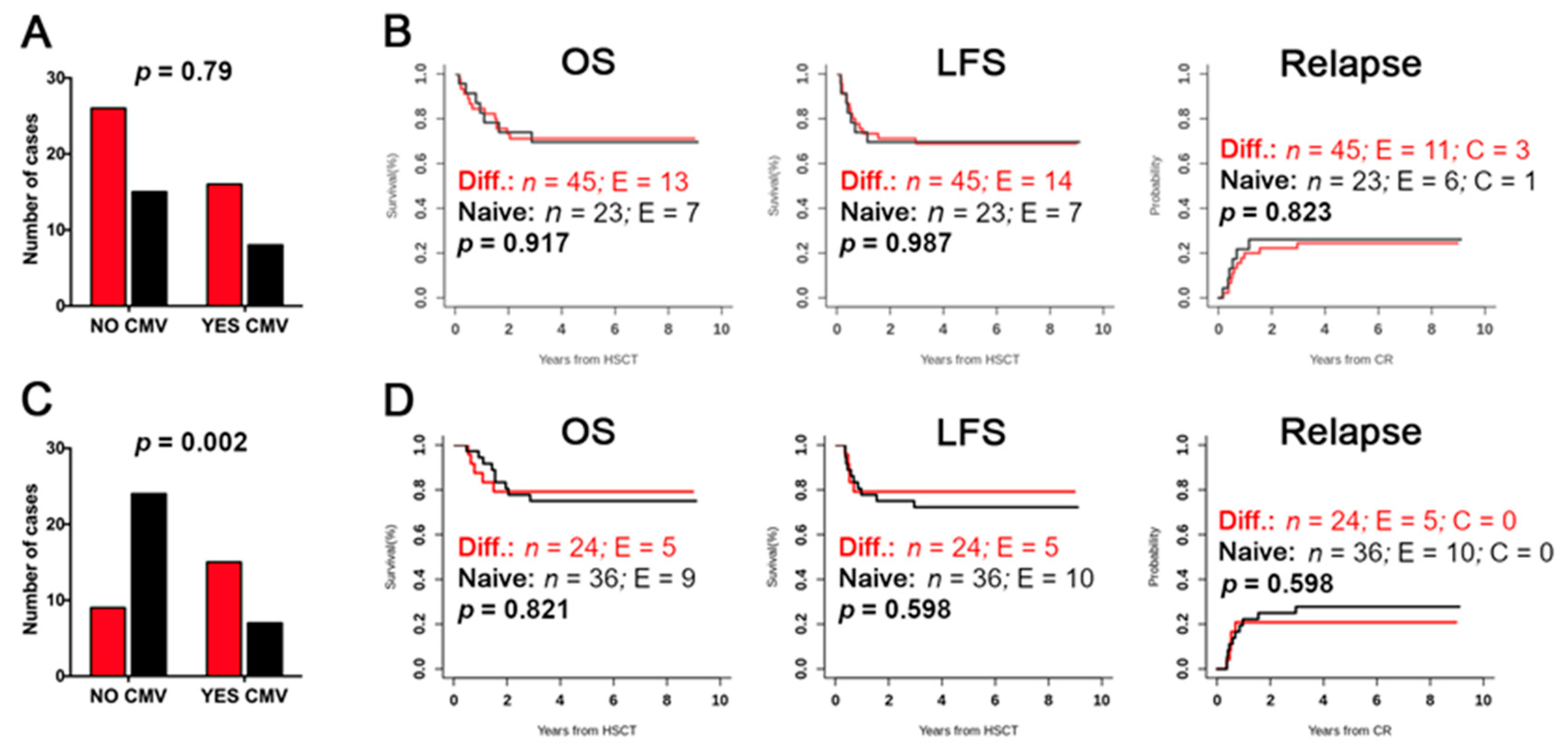
2.4. Evaluation of Differentiated vs. Naïve NK-Cell Repertoires and Possible Correlation with the Clinical Outcome
3. Discussion
4. Materials and Methods
4.1. Patients and Donors
4.2. KIR, KIR-Ligand, and NKG2C Analyses
4.3. NK Cell Phenotype of Donors and Post-Transplant Patients
4.4. Leukemia Cells
4.5. Functional Assays
4.6. Unsupervised Hierarchical Cluster Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mancusi, A.; Ruggeri, L.; Velardi, A. Haploidentical hematopoietic transplantation for the cure of leukemia: From its biology to clinical translation. Blood 2016, 128, 2616–2623. [Google Scholar] [CrossRef] [PubMed]
- Martelli, M.F.; Aversa, F. Haploidentical transplants using ex vivo T-cell depletion. Semin. Hematol. 2016, 53, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, L.; Capanni, M.; Urbani, E.; Perruccio, K.; Shlomchik, W.D.; Tosti, A.; Posati, S.; Rogaia, D.; Frassoni, F.; Aversa, F.; et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 2002, 295, 2097–2100. [Google Scholar] [CrossRef] [PubMed]
- Leung, W.; Iyengar, R.; Turner, V.; Lang, P.; Bader, P.; Conn, P.; Niethammer, D.; Handgretinger, R. Determinants of antileukemia effects of allogeneic NK cells. J. Immunol. 2004, 172, 644–650. [Google Scholar] [CrossRef]
- Ruggeri, L.; Mancusi, A.; Capanni, M.; Urbani, E.; Carotti, A.; Aloisi, T.; Stern, M.; Pende, D.; Perruccio, K.; Burchielli, E.; et al. Donor natural killer cell allorecognition of missing self in haploidentical hematopoietic transplantation for acute myeloid leukemia: Challenging its predictive value. Blood 2007, 110, 433–440. [Google Scholar] [CrossRef]
- Symons, H.J.; Leffell, M.S.; Rossiter, N.D.; Zahurak, M.; Jones, R.J.; Fuchs, E.J. Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol. Blood Marrow Transplant. 2010, 16, 533–542. [Google Scholar] [CrossRef]
- Solomon, S.R.; Aubrey, M.T.; Zhang, X.; Piluso, A.; Freed, B.M.; Brown, S.; Jackson, K.C.; Morris, L.E.; Holland, H.K.; Solh, M.M.; et al. Selecting the Best Donor for Haploidentical Transplant: Impact of HLA, Killer Cell Immunoglobulin-Like Receptor Genotyping, and Other Clinical Variables. Biol. Blood Marrow Transplant. 2018, 24, 789–798. [Google Scholar] [CrossRef]
- Wanquet, A.; Bramanti, S.; Harbi, S.; Furst, S.; Legrand, F.; Faucher, C.; Granata, A.; Calmels, B.; Lemarie, C.; Picard, C.; et al. Killer Cell Immunoglobulin-Like Receptor-Ligand Mismatch in Donor versus Recipient Direction Provides Better Graft-versus-Tumor Effect in Patients with Hematologic Malignancies Undergoing Allogeneic T Cell-Replete Haploidentical Transplantation Followed by Post-Transplant Cyclophosphamide. Biol. Blood Marrow Transplant. 2018, 24, 549–554. [Google Scholar]
- Huang, X.J.; Zhao, X.Y.; Liu, D.H.; Liu, K.Y.; Xu, L.P. Deleterious effects of KIR ligand incompatibility on clinical outcomes in haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion. Leukemia 2007, 21, 848–851. [Google Scholar] [CrossRef]
- Shimoni, A.; Labopin, M.; Lorentino, F.; Van Lint, M.T.; Koc, Y.; Gulbas, Z.; Tischer, J.; Bruno, B.; Blaise, D.; Pioltelli, P.; et al. Killer cell immunoglobulin-like receptor ligand mismatching and outcome after haploidentical transplantation with post-transplant cyclophosphamide. Leukemia 2019, 33, 230–239. [Google Scholar] [CrossRef]
- Willem, C.; Makanga, D.R.; Guillaume, T.; Maniangou, B.; Legrand, N.; Gagne, K.; Peterlin, P.; Garnier, A.; Bene, M.C.; Cesbron, A.; et al. Impact of KIR/HLA Incompatibilities on NK Cell Reconstitution and Clinical Outcome after T Cell-Replete Haploidentical Hematopoietic Stem Cell Transplantation with Posttransplant Cyclophosphamide. J. Immunol. 2019, 202, 2141–2152. [Google Scholar] [CrossRef] [PubMed]
- Kongtim, P.; Ciurea, S.O. Who is the best donor for haploidentical stem cell transplantation? Semin. Hematol. 2019, 56, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Pende, D.; Falco, M.; Della Chiesa, M.; Moretta, A.; Moretta, L. NK Cells Mediate a Crucial Graft-versus-Leukemia Effect in Haploidentical-HSCT to Cure High-Risk Acute Leukemia. Trends Immunol. 2018, 39, 577–590. [Google Scholar] [CrossRef]
- Moretta, A.; Bottino, C.; Vitale, M.; Pende, D.; Biassoni, R.; Mingari, M.C.; Moretta, L. Receptors for HLA class-I molecules in human natural killer cells. Annu. Rev. Immunol. 1996, 14, 619–648. [Google Scholar] [CrossRef]
- Uhrberg, M.; Valiante, N.M.; Shum, B.P.; Shilling, H.G.; Lienert-Weidenbach, K.; Corliss, B.; Tyan, D.; Lanier, L.L.; Parham, P. Human diversity in killer cell inhibitory receptor genes. Immunity 1997, 7, 753–763. [Google Scholar] [CrossRef]
- Vilches, C.; Parham, P. KIR: Diverse, rapidly evolving receptors of innate and adaptive immunity. Annu. Rev. Immunol. 2002, 20, 217–251. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Poursine-Laurent, J.; Truscott, S.M.; Lybarger, L.; Song, Y.J.; Yang, L.; French, A.R.; Sunwoo, J.B.; Lemieux, S.; Hansen, T.H.; et al. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature 2005, 436, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, N.; Andre, P.; Guia, S.; Falk, C.S.; Roetynck, S.; Stewart, C.A.; Breso, V.; Frassati, C.; Reviron, D.; Middleton, D.; et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 2006, 25, 331–342. [Google Scholar] [CrossRef]
- Fauriat, C.; Ivarsson, M.A.; Ljunggren, H.G.; Malmberg, K.J.; Michaelsson, J. Education of human natural killer cells by activating killer cell immunoglobulin-like receptors. Blood 2010, 115, 1166–1174. [Google Scholar] [CrossRef]
- Braud, V.M.; Allan, D.S.; O’Callaghan, C.A.; Soderstrom, K.; D’Andrea, A.; Ogg, G.S.; Lazetic, S.; Young, N.T.; Bell, J.I.; Phillips, J.H.; et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 1998, 391, 795–799. [Google Scholar] [CrossRef]
- Pende, D.; Spaggiari, G.M.; Marcenaro, S.; Martini, S.; Rivera, P.; Capobianco, A.; Falco, M.; Lanino, E.; Pierri, I.; Zambello, R.; et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: Evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112). Blood 2005, 105, 2066–2073. [Google Scholar] [CrossRef] [PubMed]
- Brandt, C.S.; Baratin, M.; Yi, E.C.; Kennedy, J.; Gao, Z.; Fox, B.; Haldeman, B.; Ostrander, C.D.; Kaifu, T.; Chabannon, C.; et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 2009, 206, 1495–1503. [Google Scholar] [CrossRef]
- Torelli, G.F.; Peragine, N.; Raponi, S.; Pagliara, D.; De Propris, M.S.; Vitale, A.; Bertaina, A.; Barberi, W.; Moretta, L.; Basso, G.; et al. Recognition of adult and pediatric acute lymphoblastic leukemia blasts by natural killer cells. Haematologica 2014, 99, 1248–1254. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barrow, A.D.; Martin, C.J.; Colonna, M. The Natural Cytotoxicity Receptors in Health and Disease. Front. Immunol. 2019, 10, 909. [Google Scholar] [CrossRef] [PubMed]
- Parham, P. MHC class I molecules and KIRs in human history, health and survival. Nat. Rev. Immunol. 2005, 5, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Falco, M.; Vitale, M.; Cantoni, C.; Vitale, C.; Munari, E.; Bertaina, A.; Moretta, F.; Del Zotto, G.; Pietra, G.; et al. Killer Ig-Like Receptors (KIRs): Their Role in NK Cell Modulation and Developments Leading to Their Clinical Exploitation. Front. Immunol. 2019, 10, 1179. [Google Scholar] [CrossRef] [PubMed]
- Guma, M.; Angulo, A.; Vilches, C.; Gomez-Lozano, N.; Malats, N.; Lopez-Botet, M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004, 104, 3664–3671. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, T.; Hwang, I.; Kim, A.; Nitschke, L.; Kim, M.; Scott, J.M.; Kamimura, Y.; Lanier, L.L.; Kim, S. Epigenetic Modification and Antibody-Dependent Expansion of Memory-like NK Cells in Human Cytomegalovirus-Infected Individuals. Immunity 2015, 42, 431–442. [Google Scholar] [CrossRef]
- Schlums, H.; Cichocki, F.; Tesi, B.; Theorell, J.; Beziat, V.; Holmes, T.D.; Han, H.; Chiang, S.C.; Foley, B.; Mattsson, K.; et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015, 42, 443–456. [Google Scholar] [CrossRef]
- Foley, B.; Cooley, S.; Verneris, M.R.; Pitt, M.; Curtsinger, J.; Luo, X.; Lopez-Verges, S.; Lanier, L.L.; Weisdorf, D.; Miller, J.S. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2011, 119, 2665–2674. [Google Scholar] [CrossRef]
- Della Chiesa, M.; Falco, M.; Podesta, M.; Locatelli, F.; Moretta, L.; Frassoni, F.; Moretta, A. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: A role for human cytomegalovirus? Blood 2012, 119, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Muccio, L.; Bertaina, A.; Falco, M.; Pende, D.; Meazza, R.; Lopez-Botet, M.; Moretta, L.; Locatelli, F.; Moretta, A.; Della Chiesa, M. Analysis of memory-like natural killer cells in human cytomegalovirus-infected children undergoing alphabeta+T and B cell-depleted hematopoietic stem cell transplantation for hematological malignancies. Haematologica 2016, 101, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Marcenaro, S.; Falco, M.; Martini, S.; Bernardo, M.E.; Montagna, D.; Romeo, E.; Cognet, C.; Martinetti, M.; Maccario, R.; et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: Evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood 2009, 113, 3119–3129. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, F.; Merli, P.; Pagliara, D.; Li Pira, G.; Falco, M.; Pende, D.; Rondelli, R.; Lucarelli, B.; Brescia, L.P.; Masetti, R.; et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after alphabeta T-cell and B-cell depletion. Blood 2017, 130, 677–685. [Google Scholar] [CrossRef]
- Cooley, S.; Weisdorf, D.J.; Guethlein, L.A.; Klein, J.P.; Wang, T.; Le, C.T.; Marsh, S.G.; Geraghty, D.; Spellman, S.; Haagenson, M.D.; et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 2010, 116, 2411–2419. [Google Scholar] [CrossRef] [PubMed]
- Chewning, J.H.; Gudme, C.N.; Hsu, K.C.; Selvakumar, A.; Dupont, B. KIR2DS1-Positive NK Cells Mediate Alloresponse against the C2 HLA-KIR Ligand Group in Vitro. J. Immunol. 2007, 179, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Venstrom, J.M.; Pittari, G.; Gooley, T.A.; Chewning, J.H.; Spellman, S.; Haagenson, M.; Gallagher, M.M.; Malkki, M.; Petersdorf, E.; Dupont, B.; et al. HLA-C-dependent prevention of leukemia relapse by donor activating KIR2DS1. N. Engl. J. Med. 2012, 367, 805–816. [Google Scholar] [CrossRef]
- Norell, H.; Moretta, A.; Silva-Santos, B.; Moretta, L. At the Bench: Preclinical rationale for exploiting NK cells and gammadelta T lymphocytes for the treatment of high-risk leukemias. J. Leukoc. Biol. 2013, 94, 1123–1139. [Google Scholar] [CrossRef]
- Oevermann, L.; Michaelis, S.U.; Mezger, M.; Lang, P.; Toporski, J.; Bertaina, A.; Zecca, M.; Moretta, L.; Locatelli, F.; Handgretinger, R. KIR B haplotype donors confer a reduced risk for relapse after haploidentical transplantation in children with ALL. Blood 2014, 124, 2744–2747. [Google Scholar] [CrossRef]
- Foley, B.; Cooley, S.; Verneris, M.R.; Curtsinger, J.; Luo, X.; Waller, E.K.; Anasetti, C.; Weisdorf, D.; Miller, J.S. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J. Immunol. 2012, 189, 5082–5088. [Google Scholar] [CrossRef]
- Bjorklund, A.T.; Clancy, T.; Goodridge, J.P.; Beziat, V.; Schaffer, M.; Hovig, E.; Ljunggren, H.G.; Ljungman, P.T.; Malmberg, K.J. Naive Donor NK Cell Repertoires Associated with Less Leukemia Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. J. Immunol. 2016, 196, 1400–1411. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.; Locatelli, F. Searching for alternative hematopoietic stem cell donors for pediatric patients. Bone Marrow Transplant. 2007, 41, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Handgretinger, R. New approaches to graft engineering for haploidentical bone marrow transplantation. Semin. Oncol. 2012, 39, 664–673. [Google Scholar] [CrossRef]
- Aversa, F.; Pierini, A.; Ruggeri, L.; Martelli, M.F.; Velardi, A. The Evolution of T Cell Depleted Haploidentical Transplantation. Front. Immunol. 2019, 10, 2769. [Google Scholar] [CrossRef]
- Baumeister, S.H.C.; Rambaldi, B.; Shapiro, R.M.; Romee, R. Key Aspects of the Immunobiology of Haploidentical Hematopoietic Cell Transplantation. Front. Immunol. 2020, 11, 191. [Google Scholar] [CrossRef]
- Bertaina, A.; Merli, P.; Rutella, S.; Pagliara, D.; Bernardo, M.E.; Masetti, R.; Pende, D.; Falco, M.; Handgretinger, R.; Moretta, F.; et al. HLA-haploidentical stem cell transplantation after removal of alphabeta+ T and B cells in children with nonmalignant disorders. Blood 2014, 124, 822–826. [Google Scholar] [CrossRef]
- Locatelli, F.; Pende, D.; Mingari, M.C.; Bertaina, A.; Falco, M.; Moretta, A.; Moretta, L. Cellular and molecular basis of haploidentical hematopoietic stem cell transplantation in the successful treatment of high-risk leukemias: Role of alloreactive NK cells. Front. Immunol. 2013, 4, 15. [Google Scholar] [CrossRef]
- Airoldi, I.; Bertaina, A.; Prigione, I.; Zorzoli, A.; Pagliara, D.; Cocco, C.; Meazza, R.; Loiacono, F.; Lucarelli, B.; Bernardo, M.E.; et al. gammadelta T cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-alphabeta+/CD19+ lymphocytes. Blood 2015, 125, 2349–2358. [Google Scholar] [CrossRef]
- Russo, A.; Oliveira, G.; Berglund, S.; Greco, R.; Gambacorta, V.; Cieri, N.; Toffalori, C.; Zito, L.; Lorentino, F.; Piemontese, S.; et al. NK cell recovery after haploidentical HSCT with posttransplant cyclophosphamide: Dynamics and clinical implications. Blood 2018, 131, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Galaverna, F.; Guolo, F.; Pende, D.; Dominietto, A.; Meazza, R.; Falco, M.; Di Grazia, C.; Raiola, A.M.; Ghiso, A.; Varaldo, R.; et al. Natural killer (NK) alloreactivity seems not to play a role in preventing leukemia relapse in unmanipulated haploidentical bone marrow transplantation with post-transplant cyclophosphamide. Blood 2015, 126, 2033. [Google Scholar] [CrossRef]
- Della Chiesa, M.; Falco, M.; Bertaina, A.; Muccio, L.; Alicata, C.; Frassoni, F.; Locatelli, F.; Moretta, L.; Moretta, A. Human Cytomegalovirus Infection Promotes Rapid Maturation of NK Cells Expressing Activating Killer Ig-Like Receptor in Patients Transplanted with NKG2C-/- Umbilical Cord Blood. J. Immunol. 2014, 192, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Weisdorf, D.; Cooley, S.; Wang, T.; Trachtenberg, E.; Vierra-Green, C.; Spellman, S.; Sees, J.A.; Spahn, A.; Vogel, J.; Fehniger, T.A.; et al. KIR B donors improve the outcome for AML patients given reduced intensity conditioning and unrelated donor transplantation. Blood Adv. 2020, 4, 740–754. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Cooley, S.; Davis, Z.; DeFor, T.E.; Schlums, H.; Zhang, B.; Brunstein, C.G.; Blazar, B.R.; Wagner, J.; Diamond, D.J.; et al. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia 2016, 30, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, F.; Taras, E.; Chiuppesi, F.; Wagner, J.E.; Blazar, B.R.; Brunstein, C.; Luo, X.; Diamond, D.J.; Cooley, S.; Weisdorf, D.J.; et al. Adaptive NK cell reconstitution is associated with better clinical outcomes. JCI Insight 2019, 4, e125553. [Google Scholar] [CrossRef]
- Fischer, J.C.; Ottinger, H.; Ferencik, S.; Sribar, M.; Punzel, M.; Beelen, D.W.; Schwan, M.A.; Grosse-Wilde, H.; Wernet, P.; Uhrberg, M. Relevance of C1 and C2 epitopes for hemopoietic stem cell transplantation: Role for sequential acquisition of HLA-C-specific inhibitory killer Ig-like receptor. J. Immunol. 2007, 178, 3918–3923. [Google Scholar] [CrossRef]
- Zhao, X.; Weinhold, S.; Brands, J.; Hejazi, M.; Degistirici, O.; Kogler, G.; Meisel, R.; Uhrberg, M. NK cell development in a human stem cell niche: KIR expression occurs independently of the presence of HLA class I ligands. Blood Adv. 2018, 2, 2452–2461. [Google Scholar] [CrossRef]
- Bertaina, A.; Zorzoli, A.; Petretto, A.; Barbarito, G.; Inglese, E.; Merli, P.; Lavarello, C.; Brescia, L.P.; De Angelis, B.; Tripodi, G.; et al. Zoledronic acid boosts gammadelta T-cell activity in children receiving alphabeta(+) T and CD19(+) cell-depleted grafts from an HLA-haplo-identical donor. Oncoimmunology 2017, 6, e1216291. [Google Scholar] [CrossRef]
- Zhou, X.; Dotti, G.; Krance, R.A.; Martinez, C.A.; Naik, S.; Kamble, R.T.; Durett, A.G.; Dakhova, O.; Savoldo, B.; Di Stasi, A.; et al. Inducible caspase-9 suicide gene controls adverse effects from alloreplete T cells after haploidentical stem cell transplantation. Blood 2015, 125, 4103–4113. [Google Scholar] [CrossRef]
- Stabile, H.; Nisti, P.; Fionda, C.; Pagliara, D.; Gaspari, S.; Locatelli, F.; Santoni, A.; Gismondi, A. NK Cell Reconstitution in Paediatric Leukemic Patients after T-Cell-Depleted HLA-Haploidentical Haematopoietic Stem Cell Transplantation Followed by the Reinfusion of iCasp9-Modified Donor T Cells. J. Clin. Med. 2019, 8, 1904. [Google Scholar] [CrossRef]
- Tumino, N.; Besi, F.; Di Pace, A.L.; Mariotti, F.R.; Merli, P.; Li Pira, G.; Galaverna, F.; Pitisci, A.; Ingegnere, T.; Pelosi, A.; et al. PMN-MDSC are a new target to rescue graft-versus-leukemia activity of NK cells in haplo-HSC transplantation. Leukemia 2020, 34, 932–937. [Google Scholar] [CrossRef]
- Babor, F.; Peters, C.; Manser, A.R.; Glogova, E.; Sauer, M.; Pötschger, U.; Ahlmann, M.; Cario, G.; Feuchtinger, T.; Gruhn, B.; et al. Presence of centromeric but absence of telomeric group B KIR haplotypes in stem cell donors improve leukaemia control after HSCT for childhood ALL. Bone Marrow Transplant. 2019, 54, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Moesta, A.K.; Norman, P.J.; Yawata, M.; Yawata, N.; Gleimer, M.; Parham, P. Synergistic polymorphism at two positions distal to the ligand-binding site makes KIR2DL2 a stronger receptor for HLA-C than KIR2DL3. J. Immunol. 2008, 180, 3969–3979. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.; Ruggeri, L.; Capanni, M.; Mancusi, A.; Velardi, A. Human leukocyte antigens A23, A24, and A32 but not A25 are ligands for KIR3DL1. Blood 2008, 112, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Falco, M.; Romeo, E.; Marcenaro, S.; Martini, S.; Vitale, M.; Bottino, C.; Mingari, M.C.; Moretta, L.; Moretta, A.; Pende, D. Combined genotypic and phenotypic killer cell Ig-like receptor analyses reveal KIR2DL3 alleles displaying unexpected monoclonal antibody reactivity: Identification of the amino acid residues critical for staining. J. Immunol. 2010, 185, 433–441. [Google Scholar] [CrossRef]
- Kubler, A.; Woiterski, J.; Witte, K.E.; Buhring, H.J.; Hartwig, U.F.; Ebinger, M.; Oevermann, L.; Mezger, M.; Herr, W.; Lang, P.; et al. Both mature KIR+ and immature KIR- NK cells control pediatric acute B-cell precursor leukemia in NOD.Cg-Prkdcscid IL2rgtmWjl/Sz mice. Blood 2014, 124, 3914–3923. [Google Scholar] [CrossRef][Green Version]
| ALLO * | UPN | Diagnosis | Donor | HCMV Serology D/R | NKG2C | B cont | Permissive iKIR * | aKIR (2DS1, 2DS2) # | Viral Infections ∞ | Acute GvHD | Relapse/TRM | Alive | Infused NK Cells (×106/kg) § | Alloreactive Subset ‡ | Cluster Phenotype ¶ | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donor | Recipient Post-1M | Recipient Post-3/6M | Donor | Recipient | ||||||||||||||
| C1 | 1 | AML (M4) | Father | neg/pos | ORF/ORF | 2 | 2DL2/L3 | 2DS1,2DS2 | HCMV | NO | NO | YES | 33.9 | 5 | 2 | 8 | Naive | Naive |
| 2 | T-ALL | Mother | pos/pos | ORF/ORF | 1 | 2DL2/L3 | 2DS2 | HCMV | NO | NO | YES | 82.83 | 13 | 7 | 21 | Naive | Adaptive | |
| 6 | BCP-ALL | Father | neg/pos | ORF/ORF | 1 | 2DL3 | 2DS1 | NO | NO | Relapse | NO | 75.3 | 12 | 5 | 18 | nd | nd | |
| 7 | BCP-ALL | Mother | neg/pos | ORF/ORF | 1 | 2DL3 | 2DS1 | HCMV | NO | NO | YES | 65.95 | 17 | nd | 22 | nd | Adaptive | |
| 10 | BCP-ALL | Mother | pos/pos | ORF/ORF | 1 | 2DL2/L3 | 2DS2 | BK | NO | NO | YES | 94.76 | 9 | nd | 5 | Adaptive | Adaptive | |
| 22 | BCP-ALL | Father | pos/pos | ORF/ORF | 1 | 2DL2/L3 | 2DS2 | NO | Grade II | NO | YES | 22.4 | 8 | 4 | 5 | Naive | Adaptive | |
| 29 | AML (M1) | Father | pos/neg | ORF/delta | 3 | 2DL2/L3 | 2DS1,2DS2 | NO | NO | NO | YES | 22.6 | 14 | 2 | 9 | Naive | Naive | |
| 35 | T-ALL | Mother | pos/neg | ORF/ORF | 2 | 2DL2 | 2DS2 | NO | Grade I | Relapse | NO | 16.4 | 23 | 2 | 6 | Naive | Naive | |
| 40 | T-ALL | Father | pos/pos | delta/delta | 0 | 2DL3 | NO | NO | NO | NO | YES | 13.4 | 33 | 10 | 8 | NA | NA | |
| 45 | AML (M7) | Mother | pos/pos | ORF/ORF | 1 | 2DL3 | 2DS1 | HCMV, ADV | NO | Relapse | NO | 56.1 | 20 | 25 | NA | Naive | NA | |
| 38 | AML (M4) | Mother | pos/pos | ORF/ORF | 0 | 2DL3 | NO | HCMV, VRS | NO | NO | YES | 23.3 | 15 | 15 | 16 | nd | Adaptive | |
| 73 | B-ALL | Father | pos/pos | ORF/delta | 3 | 2DL2/L3 | 2DS1,2DS2 | HHV6 | Grade II | TRM | NO | 30 | 20 | nd | NA | Naive | NA | |
| 75 | AML (M7) | Father | pos/pos | ORF/ORF | 1 | 2DL2/L3 | 2DS2 | NO | NO | Relapse | NO | 57.5 | 5 | 0 | 1 | Adaptive | Naive | |
| 5 | T-ALL | Father | pos/pos | ORF/ORF | 2 | 2DL2/L3, 3DL1 | 2DS1 | BK | NO | NO | YES | 86.6 | 13 | 4 | 9 | Naive | Naive | |
| 64 | B-ALL | Mother | pos/pos | ORF/delta | 2 | 2DL3, 3DL1 | 2DS1,2DS2 | HCMV, BK | NO | Relapse | NO | 57.6 | 6 | 4 | 4 | Naive | Naive | |
| C2 | 4 | BCP-ALL | Mother | pos/neg | ORF/ORF | 0 | 2DL1 | NO | NO | NO | NO | YES | 53.9 | 5 | 1 | 1 | nd | Naive |
| 18 | BCP-ALL | Father | pos/pos | ORF/ORF | 1 | 2DL1 | 2DS1 | NO | NO | NO | YES | 87.9 | 19 | 6 | 7 | Naive | Naive | |
| 23 | BCP-ALL | Mother | pos/pos | ORF/delta | 0 | 2DL1 | NO | HCMV | Grade II | NO | YES | 16.7 | 13 | 5 | 3 | Naive | Naive | |
| 24 | AML (M2) | Father | pos/pos | ORF/ORF | 2 | 2DL1 | 2DS1,2DS2 | BK | NO | Relapse | NO | 66.7 | (7) | (15) | (22) | nd | nd | |
| 27 | AML (M0) | Mother | neg/neg | ORF/ORF | 1 | 2DL1 | 2DS2 | NO | NO | Relapse | NO | 21.5 | (9) | (1.6) | (2) | Naive | Naive | |
| 30 | BCP-ALL | Mother | pos/pos | ORF/ORF | 0 | 2DL1 | NO | NO | Grade I | NO | YES | 13.6 | 6 | 0.7 | 1 | Naive | Naive | |
| 39 | BCP-ALL | Father | pos/neg | ORF/delta | 3 | 2DL1 | 2DS1,2DS2 | NO | NO | NO | YES | 60.2 | (4) | (2.5) | (5) | Naive | Adaptive | |
| 43 | AML (M4) | Father | pos/pos | ORF/delta | 0 | 2DL1 | NO | HCMV | NO | NO | YES | 54.1 | 47 | 4 | 5 | Adaptive | Naive | |
| 50 | AML (M4) | Father | pos/pos | ORF/delta | 3 | 2DL1 | 2DS1,2DS2 | NO | NO | NO | YES | 6.8 | (5) | (0) | (1) | Naive | Naive | |
| 58 | AML (M5) | Father | pos/pos | ORF/delta | 1 | 2DL1 | 2DS2 | NO | Grade II | NO | YES | 31.2 | (22) | (5) | (6) | Adaptive | Adaptive | |
| 61 | B-ALL | Mother | pos/pos | ORF/ORF | 1 | 2DL1 | 2DS2 | H1N1 | NO | NO | YES | 51.67 | (25) | (7) | (9) | Adaptive | Adaptive | |
| 66 | T-ALL | Mother | pos/pos | ORF/ORF | 3 | 2DL1 | 2DS1,2DS2 | HHV6 | Grade I | NO | YES | 12.77 | (8) | (3) | (2) | Naive | Naive | |
| 71 | BCP-ALL | Mother | pos/pos | ORF/ORF | 1 | 2DL1 | 2DS2 | HCMV, HHV6 | NO | NO | YES | 12.4 | (8) | nd | (1) | Adaptive | Adaptive | |
| 79 | AML (M4) | Mother | pos/neg | ORF/ORF | 0 | 2DL1 | NO | NO | NO | NO | YES | 28.3 | 6 | 1 | nd | Naive | nd | |
| Bw4 | 12 | BCP-ALL | Mother | pos/pos | ORF/ORF | 1 | 3DL1 | 2DS2 | HCMV | Grade II | NO | YES | 90.2 | (15) | (2) | (2) | Naive | Adaptive |
| 26 | BCP-ALL | Father | pos/pos | ORF/ORF | 2 | 3DL1 | 2DS2 | HCMV | NO | Relapse | NO | 35 | (3) | (2) | (2) | Adaptive | Adaptive | |
| 20 | AML (M3) | Mother | pos/pos | ORF/delta | 3 | 3DL1 | 2DS1,2DS2 | NO | NO | NO | YES | 82.3 | (6) | nd | (2) | Naive | Naive | |
| 21 | BCP-ALL | Mother | pos/pos | ORF/ORF | 2 | 3DL1 | 2DS1,2DS2 | NO | NO | NO | YES | 36.5 | (3) | nd | (2) | Naive | Naive | |
| 47 | ALL-bifen | Mother | pos/pos | ORF/ORF | 0 | 3DL1 | NO | NO | NO | NO | YES | 7.87 | 2 | 1 | 1 | Naive | Naive | |
| 51 | T-ALL | Mother | pos/neg | ORF/ORF | 0 | 3DL1 | NO | NO | NO | NO | YES | 44.7 | 10 | 2 | 7 | Adaptive | Naive | |
| 72 | T-ALL | Father | pos/pos | ORF/delta | 2 | 3DL1 | 2DS1,2DS2 | ADV | Grade II | NO | YES | 82.6 | (2) | nd | (2) | Naive | Naive | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meazza, R.; Falco, M.; Loiacono, F.; Canevali, P.; Della Chiesa, M.; Bertaina, A.; Pagliara, D.; Merli, P.; Indio, V.; Galaverna, F.; et al. Phenotypic and Functional Characterization of NK Cells in αβT-Cell and B-Cell Depleted Haplo-HSCT to Cure Pediatric Patients with Acute Leukemia. Cancers 2020, 12, 2187. https://doi.org/10.3390/cancers12082187
Meazza R, Falco M, Loiacono F, Canevali P, Della Chiesa M, Bertaina A, Pagliara D, Merli P, Indio V, Galaverna F, et al. Phenotypic and Functional Characterization of NK Cells in αβT-Cell and B-Cell Depleted Haplo-HSCT to Cure Pediatric Patients with Acute Leukemia. Cancers. 2020; 12(8):2187. https://doi.org/10.3390/cancers12082187
Chicago/Turabian StyleMeazza, Raffaella, Michela Falco, Fabrizio Loiacono, Paolo Canevali, Mariella Della Chiesa, Alice Bertaina, Daria Pagliara, Pietro Merli, Valentina Indio, Federica Galaverna, and et al. 2020. "Phenotypic and Functional Characterization of NK Cells in αβT-Cell and B-Cell Depleted Haplo-HSCT to Cure Pediatric Patients with Acute Leukemia" Cancers 12, no. 8: 2187. https://doi.org/10.3390/cancers12082187
APA StyleMeazza, R., Falco, M., Loiacono, F., Canevali, P., Della Chiesa, M., Bertaina, A., Pagliara, D., Merli, P., Indio, V., Galaverna, F., Algeri, M., Moretta, F., Colomar-Carando, N., Muccio, L., Sivori, S., Pession, A., Mingari, M. C., Moretta, L., Moretta, A., ... Pende, D. (2020). Phenotypic and Functional Characterization of NK Cells in αβT-Cell and B-Cell Depleted Haplo-HSCT to Cure Pediatric Patients with Acute Leukemia. Cancers, 12(8), 2187. https://doi.org/10.3390/cancers12082187









