TERT Promoter Mutation and Extent of Thyroidectomy in Patients with 1–4 cm Intrathyroidal Papillary Carcinoma
Abstract
:1. Introduction
2. Results
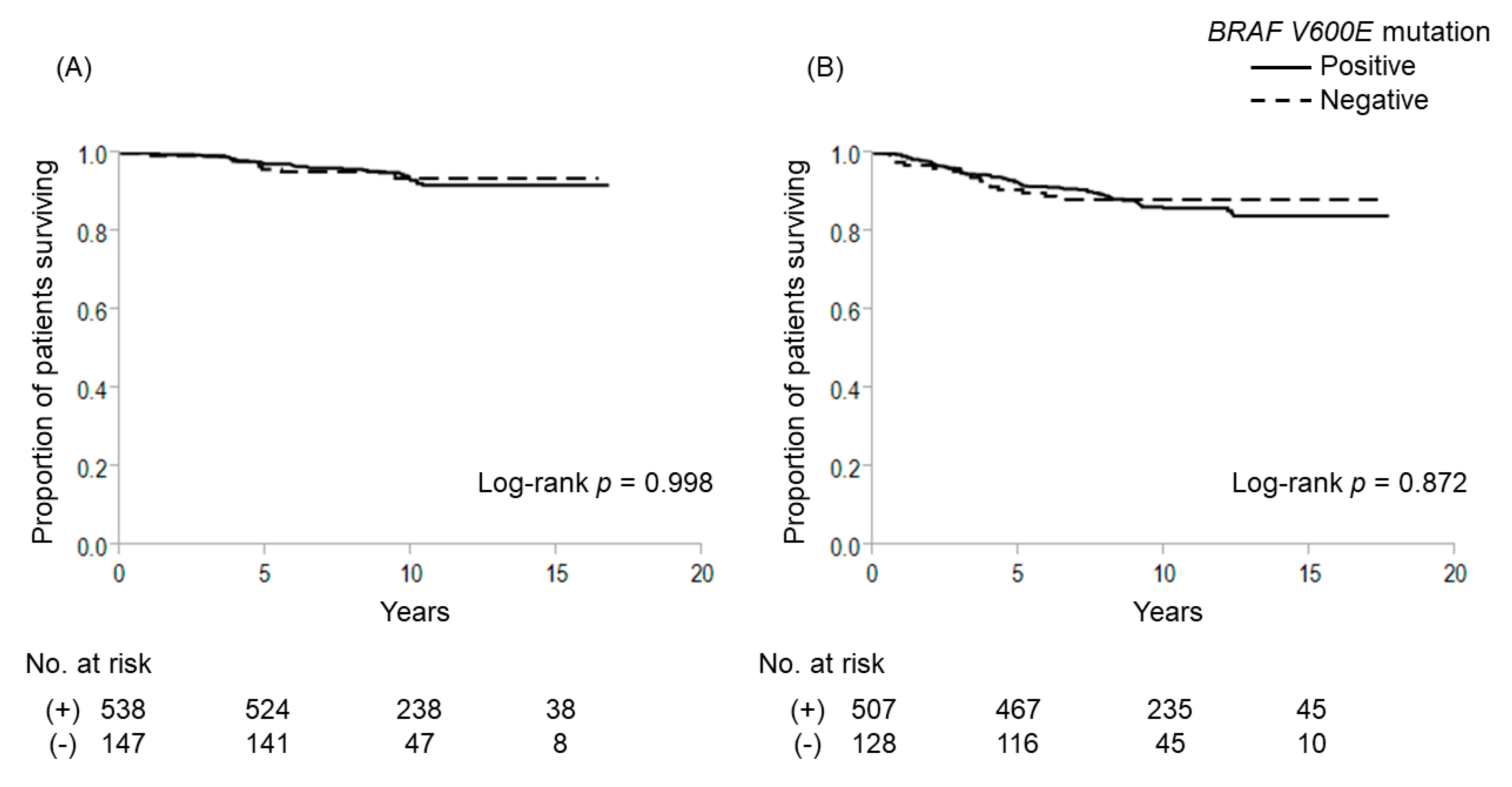
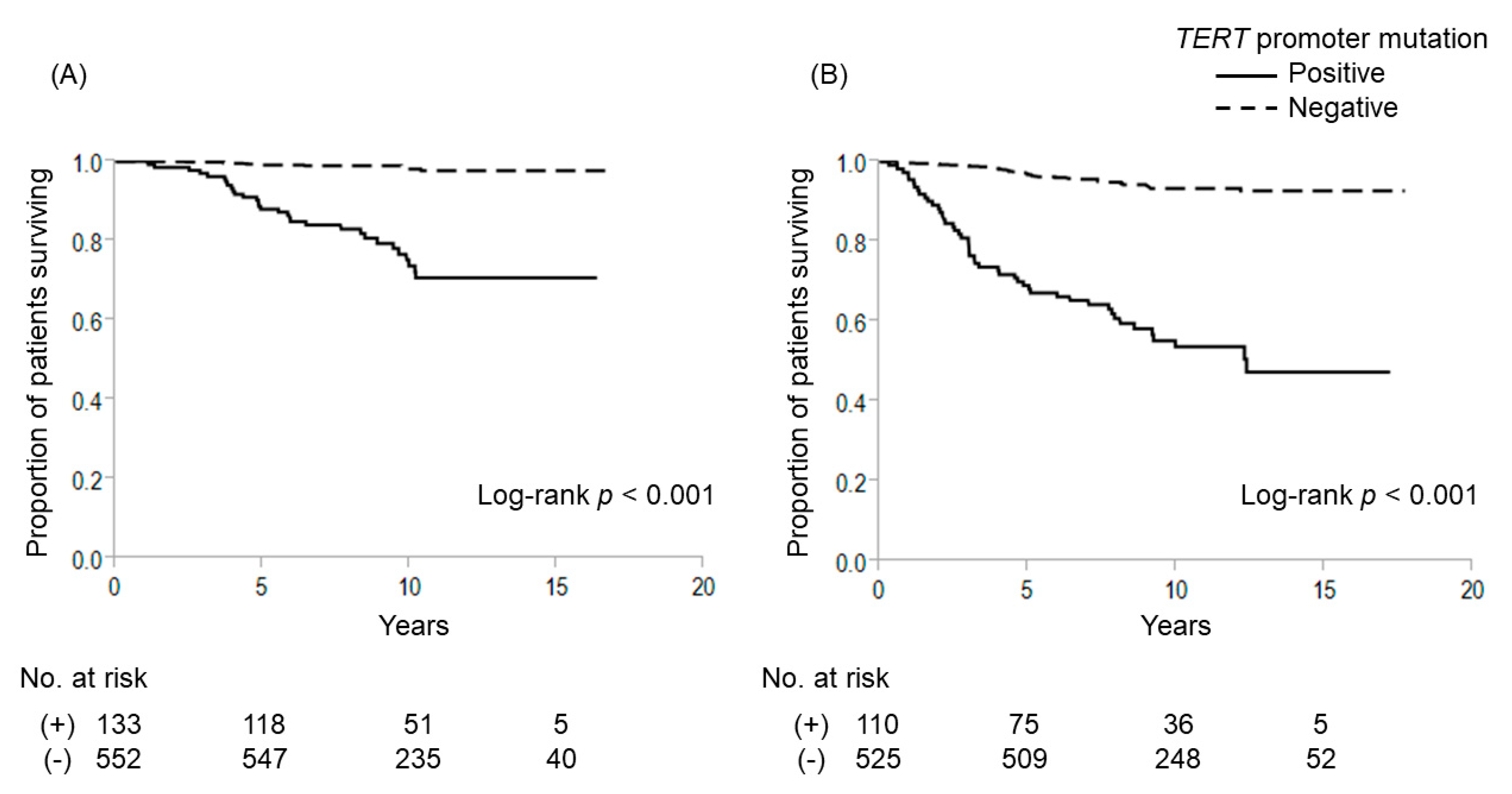
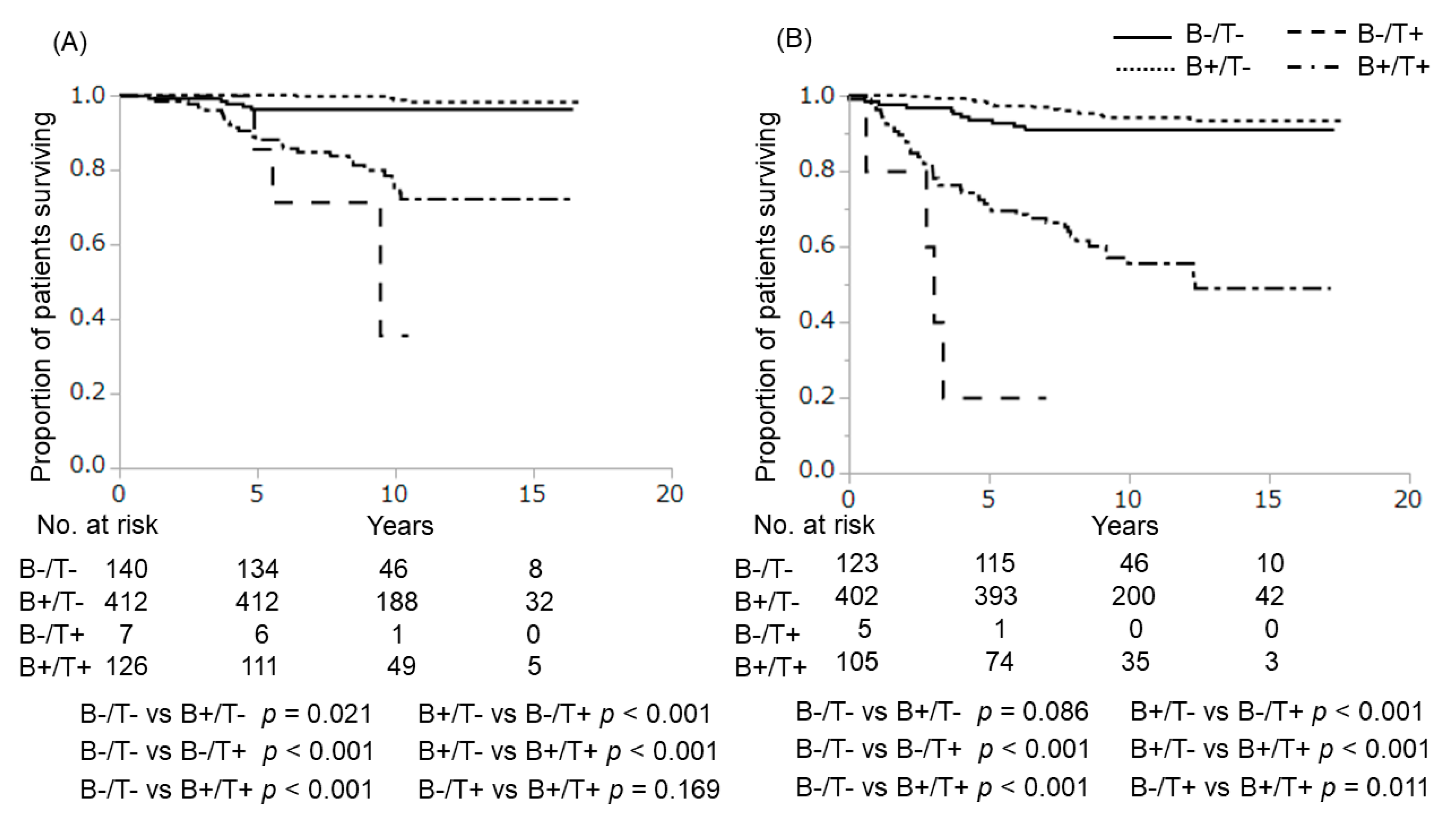
2.1. Gene Mutations and Outcome
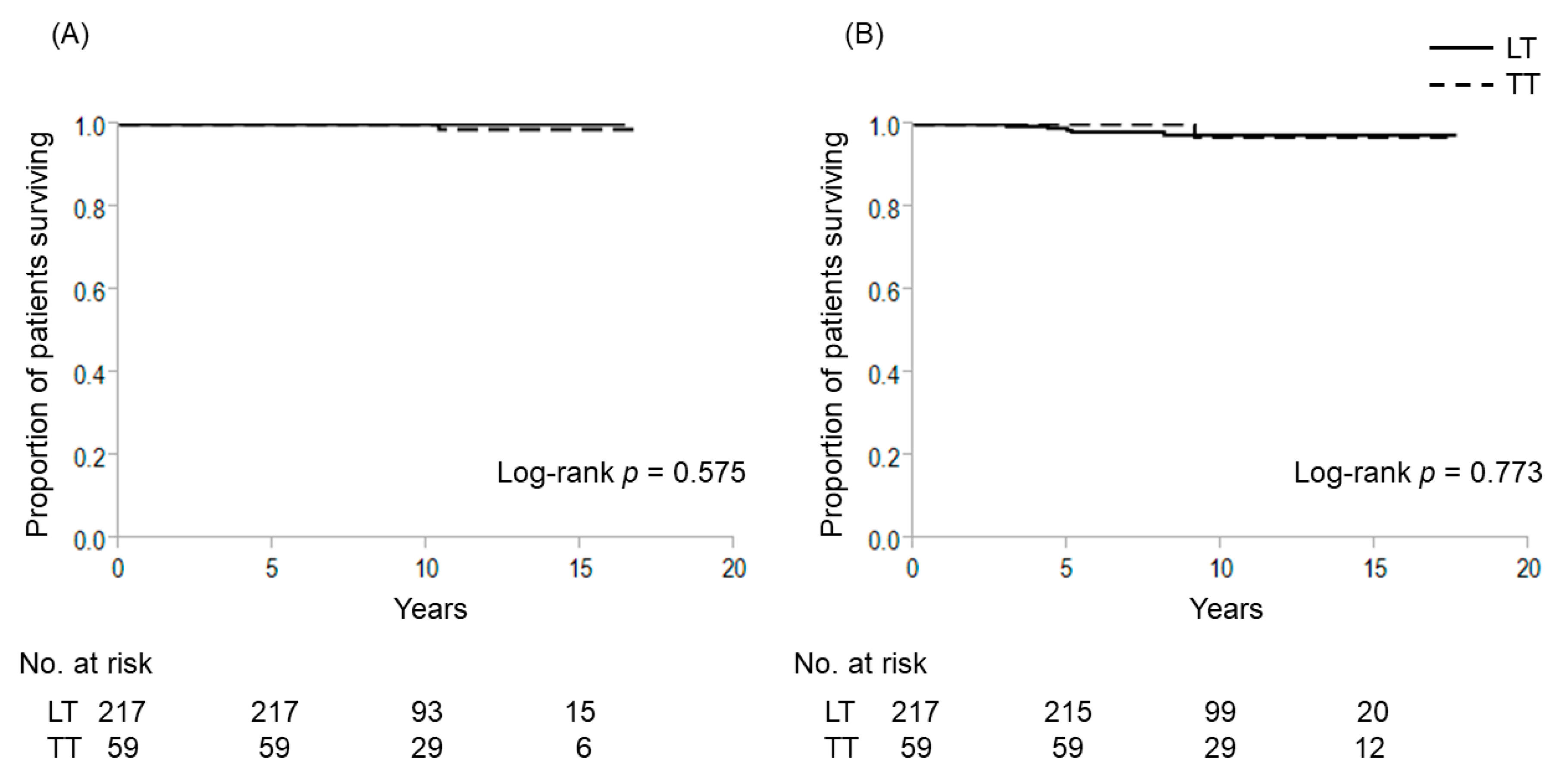
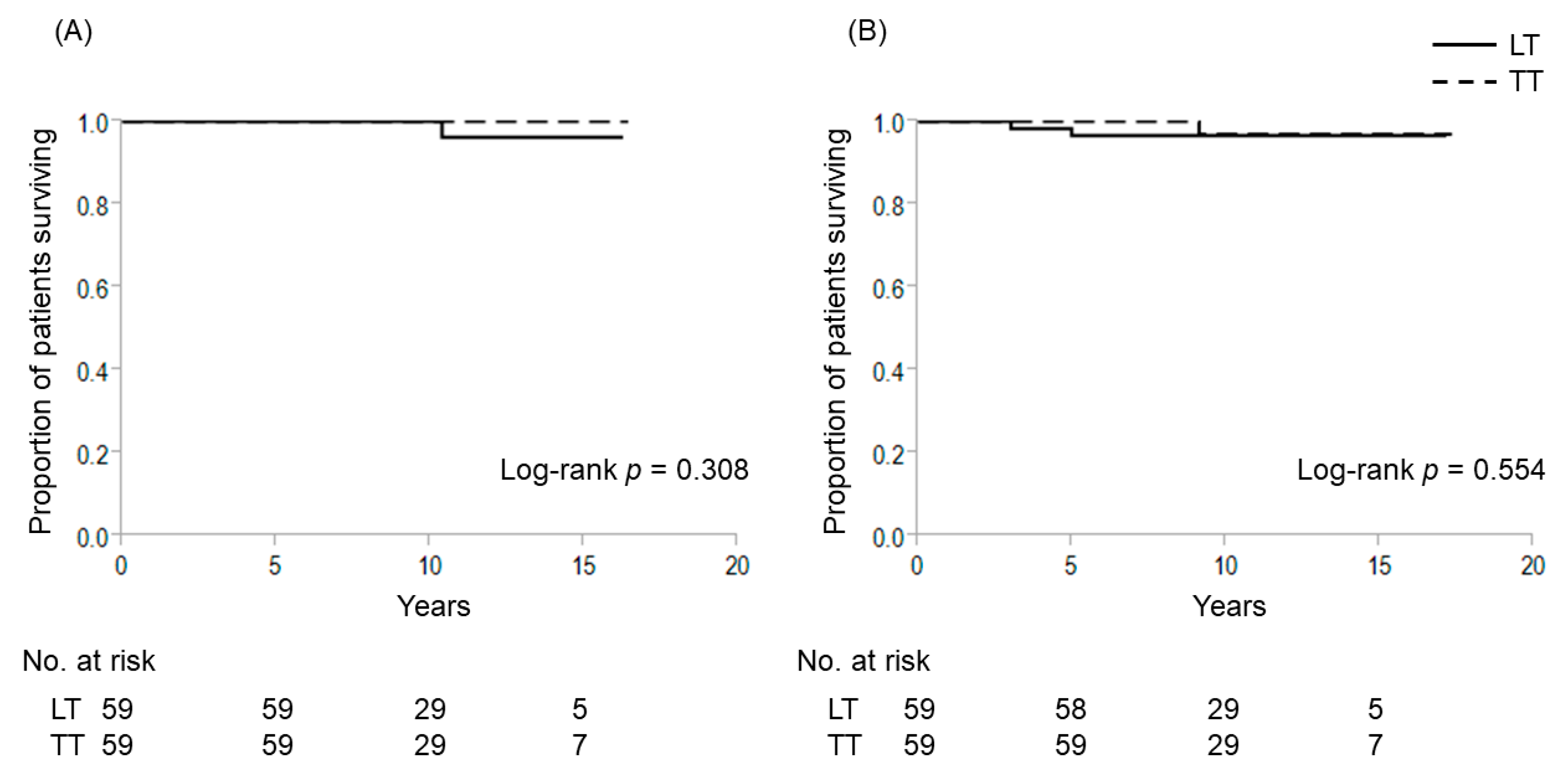
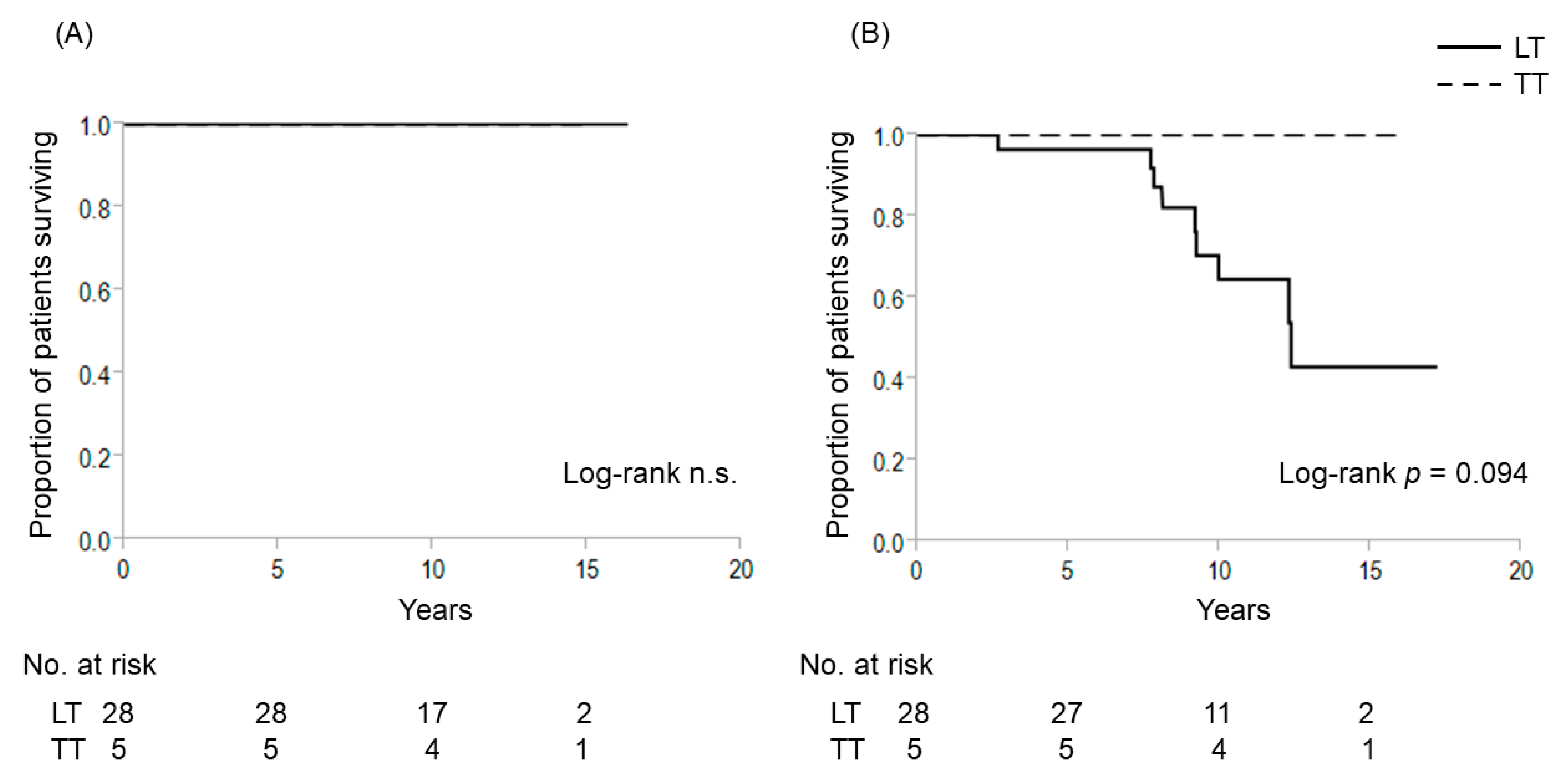
2.2. Extent of Thyroidectomy and TERT Promoter Mutations for Patients with 1–4 cm Intrathyroidal PTC
3. Discussion
4. Materials and Methods
4.1. Study Design and Patients
4.2. DNA Extraction and Mutation Screening
4.3. Digital PCR for TERT Promoter Mutations
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Davies, L.; Welch, H.G. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA 2006, 295, 2164–2167. [Google Scholar] [CrossRef] [Green Version]
- Cady, B.; Rossi, R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery 1988, 104, 947–953. [Google Scholar]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [Green Version]
- Ullmann, T.M.; Gray, K.D.; Stefanova, D.; Limberg, J.; Buicko, J.L.; Finnerty, B.; Zarnegar, R.; Fahey, T.J., III; Beninato, T. The 2015 American Thyroid Association guidelines are associated with an increasing rate of hemithyroidectomy for thyroid cancer. Surgery 2019, 166, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Welch, H.G.; Doherty, G.M. Saving Thyroids—Overtreatment of Small Papillary Cancers. N. Engl. J. Med. 2018, 379, 310–312. [Google Scholar] [CrossRef]
- Nikiforova, M.N.; Kimura, E.T.; Gandhi, M.; Biddinger, P.W.; Knauf, J.A.; Basolo, F.; Zhu, Z.; Giannini, R.; Salvatore, G.; Fusco, A.; et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J. Clin. Endocrinol. Metab. 2003, 88, 5399–5404. [Google Scholar] [CrossRef]
- Landa, I.; Ganly, I.; Chan, T.A.; Mitsutake, N.; Matsuse, M.; Ibrahimpasic, T.; Ghossein, R.A.; Fagin, J.A. Frequent somatic TERT promoter mutations in thyroid cancer: Higher prevalence in advanced forms of the disease. J. Clin. Endocrinol. Metab. 2013, 98, E1562–E1566. [Google Scholar] [CrossRef] [Green Version]
- Nixon, I.J.; Ganly, I.; Patel, S.G.; Palmer, F.L.; Whitcher, M.M.; Tuttle, R.M.; Shaha, A.; Shah, J.P. Thyroid lobectomy for treatment of well differentiated intrathyroid malignancy. Surgery 2012, 151, 571–579. [Google Scholar] [CrossRef]
- Matsuzu, K.; Sugino, K.; Masudo, K.; Nagahama, M.; Kitagawa, W.; Shibuya, H.; Ohkuwa, K.; Uruno, T.; Suzuki, A.; Magoshi, S.; et al. Thyroid lobectomy for papillary thyroid cancer: Long-term follow-up study of 1,088 cases. World J. Surg. 2014, 38, 68–79. [Google Scholar] [CrossRef]
- Hauch, A.; Al-Qurayshi, Z.; Randolph, G.; Kandil, E. Total thyroidectomy is associated with increased risk of complications for low- and high-volume surgeons. Ann. Surg. Oncol. 2014, 21, 3844–3852. [Google Scholar] [CrossRef]
- Sugitani, I.; Fujimoto, Y. Management of low-risk papillary thyroid carcinoma: Unique conventional policy in Japan and our efforts to improve the level of evidence. Surg. Today 2010, 40, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, N.; Takami, H.; Kubo, A. Unique treatment policy for well-differentiated thyroid cancer in Japan: Results of a questionnaire distributed to members of the Japanese Society of Thyroid Surgery and the International Association of Endocrine Surgeons. Endocr. J. 2006, 53, 829–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugitani, I.; Kasai, N.; Fujimoto, Y.; Yanagisawa, A. A novel classification system for patients with PTC: Addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery 2004, 135, 139–148. [Google Scholar] [CrossRef]
- Ebina, A.; Sugitani, I.; Fujimoto, Y.; Yamada, K. Risk-adapted management of papillary thyroid carcinoma according to our own risk group classification system: Is thyroid lobectomy the treatment of choice for low-risk patients? Surgery 2014, 156, 1579–1589. [Google Scholar] [CrossRef]
- Nikiforov, Y.E.; Nikiforova, M.N. Molecular genetics and diagnosis of thyroid cancer. Nat. Rev. Endocrinol. 2011, 7, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 2013, 13, 184–199. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, W.B.; Rhee, Y.S.; Song, J.Y.; Kim, J.M.; Gong, G.; Lee, S.; Kim, S.Y.; Kim, S.C.; Hong, S.J.; et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin. Endocrinol. 2006, 65, 364–368. [Google Scholar] [CrossRef]
- Fugazzola, L.; Puxeddu, E.; Avenia, N.; Romei, C.; Cirello, V.; Cavaliere, A.; Faviana, P.; Mannavola, D.; Moretti, S.; Rossi, S.; et al. Correlation between B-RAFV600E mutation and clinico-pathologic parameters in papillary thyroid carcinoma: Data from a multicentric Italian study and review of the literature. Endocr. Relat. Cancer 2006, 13, 455–464. [Google Scholar] [CrossRef]
- Ito, Y.; Yoshida, H.; Maruo, R.; Morita, S.; Takano, T.; Hirokawa, M.; Yabuta, T.; Fukushima, M.; Inoue, H.; Tomoda, C.; et al. BRAF mutation in papillary thyroid carcinoma in a Japanese population: Its lack of correlation with high-risk clinicopathological features and disease-free survival of patients. Endocr. J. 2009, 56, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Bullock, M.; Ren, Y.; O’Neill, C.; Gill, A.; Aniss, A.; Sywak, M.; Sidhu, S.; Delbridge, L.; Learoyd, D.; de Vathaire, F.; et al. TERT promoter mutations are a major indicator of recurrence and death due to papillary thyroid carcinomas. Clin. Endocrinol. 2016, 85, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Shen, Y.; Hu, D.; He, W.; Zhou, J.; Cao, Y.; Mao, Y.; Dou, Y.; Xiong, W.; Xiao, Q.; et al. Co-existence of BRAFV600E and TERT promoter mutations in papillary thyroid carcinoma is associated with tumor aggressiveness, but not with lymph node metastasis. Cancer Manag. Res. 2018, 10, 1005–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasirden, A.; Saito, T.; Fukumura, Y.; Hara, K.; Akaike, K.; Kurisaki-Arakawa, A.; Asahina, M.; Yamashita, A.; Tomomasa, R.; Hayashi, T.; et al. In Japanese patients with papillary thyroid carcinoma, TERT promoter mutation is associated with poor prognosis, in contrast to BRAFV600E mutation. Virchows Arch. 2016, 469, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, G.; Ragazzi, M.; Frasoldati, A.; Piana, S.; Ciarrocchi, A.; Sancisi, V. TERT promoter mutations are associated with distant metastases in papillary thyroid carcinoma. Eur. J. Endocrinol. 2015, 172, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, A.; Matsuse, M.; Saenko, V.; Nakao, T.; Yamanouchi, K.; Sakimura, C.; Yano, H.; Nishihara, E.; Hirokawa, M.; Suzuki, K.; et al. TERT mRNA Expression as a Novel Prognostic Marker in Papillary Thyroid Carcinomas. Thyroid 2019, 29, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, Y.E.; Ahn, S.; Kim, J.Y.; Ki, C.S.; Oh, Y.L.; Kim, K.; Yun, J.W.; Park, W.Y.; Choe, J.H.; et al. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr. Relat. Cancer 2016, 23, 813–823. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Bishop, J.; Shan, Y.; Pai, S.; Liu, D.; Murugan, A.K.; Sun, H.; El-Naggar, A.K.; Xing, M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer 2013, 20, 603–610. [Google Scholar] [CrossRef] [Green Version]
- Pestana, A.; Batista, R.; Celestino, R.; Canberk, S.; Sobrinho-Simoes, M.; Soares, P. Comprehensive Assessment of TERT mRNA Expression across a Large Cohort of Benign and Malignant Thyroid Tumours. Cancers 2020, 12, 1846. [Google Scholar] [CrossRef]
- Xing, M.; Liu, R.; Liu, X.; Murugan, A.K.; Zhu, G.; Zeiger, M.A.; Pai, S.; Bishop, J. BRAFV600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J. Clin. Oncol. 2014, 32, 2718–2726. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.; Song, Y.S.; Kim, Y.A.; Lim, J.A.; Cho, S.W.; Moon, J.H.; Hahn, S.; Park, D.J.; Park, Y.J. Effects of Coexistent BRAFV600E and TERT Promoter Mutations on Poor Clinical Outcomes in Papillary Thyroid Cancer: A Meta-Analysis. Thyroid 2017, 27, 651–660. [Google Scholar] [CrossRef]
- Vuong, H.G.; Altibi, A.M.A.; Duong, U.N.P.; Hassell, L. Prognostic implication of BRAF and TERT promoter mutation combination in papillary thyroid carcinoma—A meta-analysis. Clin. Endocrinol. 2017, 87, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikiforova, M.N.; Mercurio, S.; Wald, A.I.; Barbi de Moura, M.; Callenberg, K.; Santana-Santos, L.; Gooding, W.E.; Yip, L.; Ferris, R.L.; Nikiforov, Y.E. Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer 2018, 124, 1682–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugitani, I.; Fujimoto, Y.; Yamada, K.; Yamamoto, N. Prospective outcomes of selective lymph node dissection for papillary thyroid carcinoma based on preoperative ultrasonography. World J. Surg. 2008, 32, 2494–2502. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | n = 685 |
|---|---|
| Follow-up duration, years (range) | 10 ± 3 (1–17) |
| Age, years (range) | 52 ± 14 (15–86) |
| Age, n (%) | |
| ≥55 years | 322 (47.0%) |
| <55 years | 363 (53.0%) |
| Sex, female, n (%) | 520 (75.9%) |
| T, n (%) AJCC/UICC 8th edition | |
| 1b | 211 (30.8%) |
| 2 | 82 (12.0%) |
| 3a | 20 (2.9%) |
| 3b | 229 (33.5%) |
| 4a | 142 (20.7%) |
| 4b | 1 (0.1%) |
| N, n (%) AJCC/UICC 8th edition | |
| 0 | 362 (52.9%) |
| 1a | 87 (12.7%) |
| 1b | 236 (34.4%) |
| M, n (%) AJCC/UICC 8th edition | |
| 0 | 635 (92.7%) |
| 1 | 50 (7.3%) |
| Stage classification AJCC/UICC 8th edition, n (%) | |
| I | 409 (59.6%) |
| II | 170 (24.9%) |
| III | 78 (11.4%) |
| IVA | 0 (0%) |
| IVB | 28 (4.1%) |
| Tumor size, n (%) | |
| <4 cm | 583 (85.1%) |
| ≥4 cm | 102 (14.9%) |
| Total thyroidectomy, n (%) | 225 (32.8%) |
| Radioactive iodine therapy, ≥30 mCi, n (%) | 102 (14.9%) |
| Mutations | Cause-Specific Death | Recurrence | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| No mutations | 1 | 1 | ||||
| BRAF V600E mutation only | 0.56 | 0.28–3.12 | 0.131 | 0.4 | 0.07–3.10 | 0.347 |
| TERT promoter mutation only | 22.83 | 6.25–67.91 | <0.001 | 14.34 | 0.67–149.97 | 0.078 |
| BRAF V600E + TERT promoter mutations | 5.8 | 3.12–11.79 | <0.001 | 9.18 | 2.63–57.91 | <0.001 |
| Characteristics | BRAFV600E Status, n (%) | ||
|---|---|---|---|
| Wild Type | Mutated | p Value | |
| N | 147 | 538 | |
| Follow-up duration, years (range) | 9 ± 3 (1–16) | 10 ± 3 (1–17) | <0.001 |
| Age, years (range) | 46 ± 15 (15–81) | 54 ± 14 (15–86) | <0.001 |
| Age, n (%) | |||
| ≥55 years | 44 (29.9%) | 270 (50.2%) | <0.001 |
| <55 years | 103 (70.1%) | 268 (49.8%) | |
| Sex, female, n (%) | 103 (70.1%) | 417 (77.5%) | 0.066 |
| T, n (%) AJCC/UICC 8th edition | |||
| 1 | 53 (36.1%) | 158 (29.4%) | <0.001 |
| 2 | 30 (20.4%) | 52 (9.7%) | |
| 3a | 9 (6.1%) | 11 (2.0%) | |
| 3b | 34 (23.1%) | 195 (36.2%) | |
| 4a | 21 (14.3%) | 121 (22.5%) | |
| 4b | 0 (0%) | 1 (0.2%) | |
| N, n (%) AJCC/UICC 8th edition | |||
| 0 | 70 (47.6%) | 292 (54.3%) | 0.045 |
| 1a | 14 (9.5%) | 73 (13.6%) | |
| 1b | 63 (42.9%) | 173 (32.2%) | |
| M, n (%) AJCC/UICC 8th edition | |||
| 0 | 128 (87.1%) | 507 (94.2%) | 0.006 |
| 1 | 19 (12.9%) | 31 (5.8%) | |
| Stage classification AJCC/UICC 8th edition, n (%) | |||
| I | 107 (72.8%) | 302 (56.1%) | <0.001 |
| II | 24 (16.4%) | 146 (27.1%) | |
| III | 8 (5.4%) | 70 (13.1%) | |
| IVA | 0 (0%) | 0 (0%) | |
| IVB | 8 (5.4%) | 20 (3.7%) | |
| Tumor size, n (%) | |||
| <4 cm | 120 (81.6%) | 463 (86.1%) | 0.191 |
| ≥4 cm | 27 (18.4%) | 75 (13.9%) | |
| Total thyroidectomy, n (%) | 49 (33.3%) | 176 (32.7%) | 0.887 |
| Radioactive iodine therapy, ≥30 mCi, n (%) | 27 (18.4%) | 75 (13.9%) | 0.191 |
| Characteristics | TERT Status, n (%) | ||
|---|---|---|---|
| Wild Type | Mutated | p Value | |
| N | 552 | 133 | |
| Follow-up duration, years (range) | 12 ± 5 (1–25) | 9 ± 3 (1–17) | 0.03 |
| Age, years (range) | 52 ± 13 (15–86) | 64 ± 10 (27–86) | <0.001 |
| Age, n (%) | |||
| ≥55 years | 214 (38.8%) | 108 (81.2%) | <0.001 |
| <55 years | 338 (61.2%) | 25 (18.8%) | |
| Sex, female, n (%) | 435 (78.8%) | 85 (63.9%) | <0.001 |
| T, n (%) AJCC/UICC 8th edition | |||
| 1 | 206 (37.3%) | 5 (3.8%) | <0.001 |
| 2 | 65 (11.8%) | 17 (12.8%) | |
| 3a | 14 (2.6%) | 6 (4.4%) | |
| 3b | 189 (34.2%) | 40 (30.1%) | |
| 4a | 78 (14.1%) | 64 (48.1%) | |
| 4b | 0 (0%) | 1 (0.8%) | |
| N, n (%) AJCC/UICC 8th edition | |||
| 0 | 308 (55.8%) | 54 (40.6%) | 0.003 |
| 1a | 70 (12.7%) | 17 (12.8%) | |
| 1b | 174 (31.5%) | 62 (46.6%) | |
| M, n (%) AJCC/UICC 8th edition | |||
| 0 | 525 (95.1%) | 110 (82.7%) | <0.001 |
| 1 | 27 (4.9%) | 23 (17.3%) | |
| Stage classification AJCC/UICC 8th edition, n (%) | |||
| I | 382 (69.1%) | 27 (20.3%) | <0.001 |
| II | 123 (22.4%) | 47 (35.3%) | |
| III | 38 (6.9%) | 40 (30.1%) | |
| IVA | 0 (0%) | 0 (0%) | |
| IVB | 9 (1.6%) | 19 (14.3%) | |
| Tumor size, n (%) | |||
| <4 cm | 495 (89.7%) | 88 (66.2%) | <0.001 |
| ≥4 cm | 57 (10.3%) | 45 (33.8%) | |
| Total thyroidectomy, n (%) | 163 (29.5%) | 72 (54.1%) | <0.001 |
| Radioactive iodine therapy, ≥30 mCi, n (%) | 57 (10.3%) | 45 (33.8%) | <0.001 |
| Characteristics | Cause-Specific Death | Recurrence | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p Value | HR | 95%CI | p Value | |
| Age ≥55 years | 2.47 | 1.09–6.38 | 0.03 | 1.93 | 1.13–3.35 | 0.014 |
| Sex | 1.51 | 0.78–2.91 | 0.216 | 1.22 | 0.75–1.97 | 0.41 |
| T ≥4a | 1.3 | 0.56–3.14 | 0.55 | 1.06 | 0.63–1.79 | 0.82 |
| Tumor size >4 cm | 3.9 | 1.89–8.42 | <0.001 | 3.72 | 2.31–5.93 | <0.001 |
| N1b | 3.27 | 1.30–8.82 | 0.011 | 3.52 | 2.10–5.95 | <0.001 |
| M1 | 2.46 | 1.17–5.15 | 0.018 | |||
| TERT promoter mutation | 5.23 | 2.33–12.78 | <0.001 | 6.46 | 3.90–10.83 | <0.001 |
| Characteristics | TERT (−) | ||||||
|---|---|---|---|---|---|---|---|
| All | Before Matching | After Matching | |||||
| TT | LT | p Value | TT | LT | p Value | ||
| N | 276 | 59 | 217 | 59 | 59 | ||
| Follow-up duration, y | 9.7 ± 3.2 | 10.2 ± 3.4 | 9.6 ± 3.0 | 0.307 | 10.2 ± 0.4 | 9.6 ± 0.4 | 0.439 |
| Age ≥55 years, n (%) | 115 (41.7%) | 33 (55.9%) | 82 (33.2%) | 0.017 | 33 (55.9%) | 34 (57.6%) | 0.645 |
| Sex, female, n (%) | 234 (84.8%) | 51 (86.4%) | 183 (84.3%) | 0.839 | 51 (86.4%) | 53 (89.8%) | 0.777 |
| Tumor dimeter, mm | 18.0 ± 5.1 | 18.1 ± 5.1 | 18.0 ± 6.8 | 0.257 | 18.1 ± 5.1 | 18.4 ± 5.6 | 0.944 |
| Bilateral tumor | 40 (14.5%) | 40 (67.8%) | 0 (0%) | <0.001 | 40 (67.8%) | 0 (0%) | <0.001 |
| T3b, n (%) | 98 (35.5%) | 28 (47.5%) | 70 (32.3%) | 0.033 | 28 (47.5%) | 27 (45.8%) | 1.000 |
| Pathological lymph node metastasis, n (range) | 0.8 ± 1.4 (0–8) | 1.0 ± 1.7 (0–8) | 0.8 ± 1.4 (0–8) | 0.354 | 1.0 ± 0.7 (0–8) | 0.7±1.1 (0–4) | 0.355 |
| Outcome | |||||||
| cause-specific death | 1 (1.7%) | 1 (1.7%) | 0 (0%) | 1.000 | 1 (1.7%) | 0 (0%) | 1.000 |
| recurrence | 6 (2.2%) | 1 (1.7%) | 5 (2.3%) | 1.000 | 1 (1.7%) | 2 (3.4%) | 1.000 |
| Complication | |||||||
| Hypoparathyroidism | |||||||
| temporary | 16 (5.8%) | 16 (27.1%) | 0 (0%) | <0.001 | 16 (27.1%) | 0 (0%) | <0.001 |
| permanent | 5 (1.8%) | 5 (8.5%) | 0 (0%) | <0.001 | 5 (8.5%) | 0 (0%) | 0.057 |
| Recurrent laryngeal nerve paralysis | |||||||
| temporary | 14 (5.1%) | 3 (5.1%) | 11 (5.1%) | 1.000 | 3 (5.1%) | 2 (3.4%) | 1.000 |
| permanent | 5 (1.8%) | 2 (3.4%) | 3 (1.4%) | 0.291 | 2 (3.4%) | 0 (0%) | 0.496 |
| (A) For Genomic PCR | ||||
| Gene | Primer name | Primer sequence (5′→3′) | Product size (bp) | Concentration (μM) |
| BRAF | BRAF-int14_5F | GCAGGTTATATAGGCTAAATAGAACTAATC | 334 | 0.2 |
| BRAF-int15_R | TAGCCTCAATTCTTACCATCCAC | 0.2 | ||
| TERT promotor | TERT promotor_1F | CGTCCTGCCCCTTCACCTTC | 119 | 0.1 |
| TERT promotor_1R | GAAAGGAAGGGGAGGGGCTG | 0.1 | ||
| (B) For SNaPshot | ||||
| Target mutation | Primer name | Primer sequence (5′→3′) | Concentration (μM) | |
| BRAF V600E | BRAF_c.1799T > A_F_60mer | ATGCATGCATGCATGCATGCATGCATGCATGCATGCATGGTGATTTTGGTCTAGCTACAG | 0.3 | |
| TERT promotor C250T | TERT promotor_C250T_30mer | ATGCGCGGACCCCGCCCCGTCCCGACCCCT | 0.2 | |
| TERT promotor C228T | TERT promotor_C228T_45mer | ATGCATGCATGCATGCATGCATGCCCGGGTCCCCGGCCCAGCCCC | 0.2 | |
| (C) For direct sequencing | ||||
| Gene | Primer name | Primer sequence (5′→3′) | ||
| BRAF | BRAF-int14_5F | GCAGGTTATATAGGCTAAATAGAACTAATC | ||
| TERT promotor | TERT promotor_2F | CACCTTCCAGCTCCGCCTCCTC | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebina, A.; Togashi, Y.; Baba, S.; Sato, Y.; Sakata, S.; Ishikawa, M.; Mitani, H.; Takeuchi, K.; Sugitani, I. TERT Promoter Mutation and Extent of Thyroidectomy in Patients with 1–4 cm Intrathyroidal Papillary Carcinoma. Cancers 2020, 12, 2115. https://doi.org/10.3390/cancers12082115
Ebina A, Togashi Y, Baba S, Sato Y, Sakata S, Ishikawa M, Mitani H, Takeuchi K, Sugitani I. TERT Promoter Mutation and Extent of Thyroidectomy in Patients with 1–4 cm Intrathyroidal Papillary Carcinoma. Cancers. 2020; 12(8):2115. https://doi.org/10.3390/cancers12082115
Chicago/Turabian StyleEbina, Aya, Yuki Togashi, Satoko Baba, Yukiko Sato, Seiji Sakata, Masashi Ishikawa, Hiroki Mitani, Kengo Takeuchi, and Iwao Sugitani. 2020. "TERT Promoter Mutation and Extent of Thyroidectomy in Patients with 1–4 cm Intrathyroidal Papillary Carcinoma" Cancers 12, no. 8: 2115. https://doi.org/10.3390/cancers12082115
APA StyleEbina, A., Togashi, Y., Baba, S., Sato, Y., Sakata, S., Ishikawa, M., Mitani, H., Takeuchi, K., & Sugitani, I. (2020). TERT Promoter Mutation and Extent of Thyroidectomy in Patients with 1–4 cm Intrathyroidal Papillary Carcinoma. Cancers, 12(8), 2115. https://doi.org/10.3390/cancers12082115





