Hazard Curves for Tumor Recurrence and Tumor-Related Death Following Esophagectomy for Esophageal Cancer
Abstract
1. Introduction
2. Results
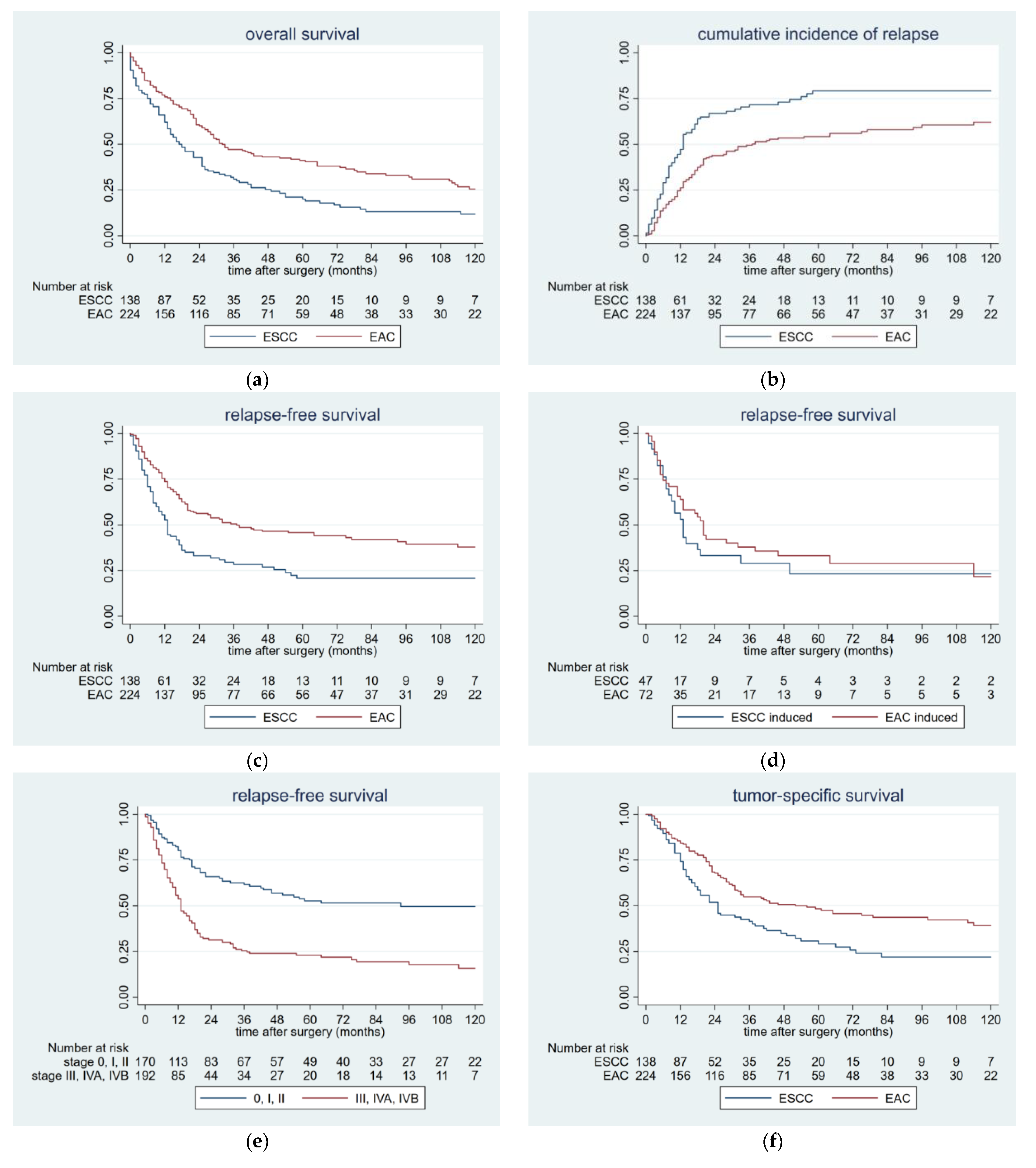
2.1. Survival Data
2.2. Patterns of Postoperative Tumor Recurrence and Tumor-Specific Survival
2.3. Risk Factors for Postoperative Tumor Recurrence
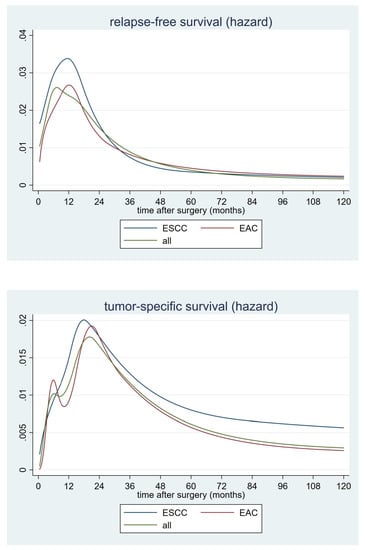
2.4. Hazard Rates for Postoperative Tumor Recurrence
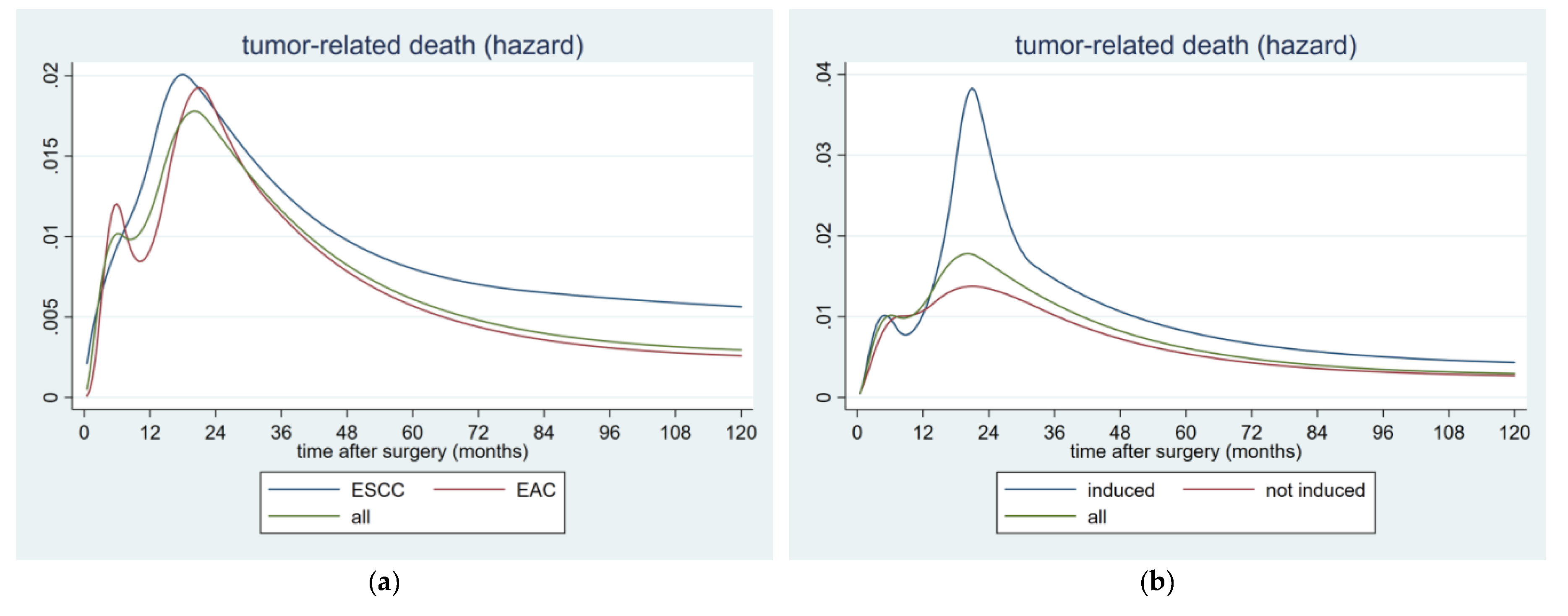
2.5. Hazard Rates for Postoperative Tumor-Related Death
3. Discussion
4. Materials and Methods
4.1. Surgery
4.2. Postoperative Follow-Up
4.3. Data Management
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Conteduca, V.; Sansonno, D.; Ingravallo, G.; Marangi, S.; Russi, S.; Lauletta, G.; Dammacco, F. Barrett’s oesophagus and oesophageal cancer: An overview. Int. J. Oncol. 2012, 41, 414–424. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Patil, D.T.; Blackstone, E.H. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: Application to clinical practice. Ann. Cardiothorac. Surg. 2017, 6, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Markar, S.R.; Noordman, B.J.; Mackenzie, H.; Findlay, J.M.; Boshier, P.R.; Ni, M.; Steyerberg, E.W.; van der Gaast, A.; Hulshof, M.C.C.M.; Maynard, N. Multimodality treatment for oesophageal adenocaricnoma: Multi-center propensity-score matched study. Ann. Oncol. 2017, 28, 519–527. [Google Scholar] [CrossRef] [PubMed]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. Preoperative chemoradiotherapy for oesophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Ma, H.F.; Lv, G.X.; Cai, Z.F.; Zhang, D.H. Comparison of the prognosis of neoadjuvant chemoradiotherapy treatment with surgery alone in oesophageal carcinoma: A meta-analysis. Onco Targets Ther. 2018, 11, 3441–3447. [Google Scholar] [CrossRef]
- Sjoquist, K.M.; Burmeister, B.H.; Smithers, B.M.; Zalcberg, J.R.; Simes, R.J.; Barbour, A.; Gebski, V. Australasian Gastro-Intestinal Trials Group. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable ooesophageal carcinoma: An updated meta-analysis. Lancet Oncol. 2011, 12, 681–692. [Google Scholar] [CrossRef]
- Mantziari, S.; Gronnier, C.; Renaud, F.; Duhamel, A.; Théreaux, J.; Brigand, C.; Carrère, N.; Lefevre, J.H.; Pasquer, A.; Demartines, N.; et al. FREGAT working group—FRENCH–AFC. Survival Benefit of Neoadjuvant Treatment in Clinical T3N0M0 Esophageal Cancer: Results From a Retrospective Multicenter European Study. Ann. Surg. 2017, 266, 805–813. [Google Scholar] [CrossRef]
- Fiorica, F.; Di, B.D.; Schepis, F.; Licata, A.; Shahied, L.; Venturi, A.; Falchi, A.M.; Craxì, A.; Cammà, C. Preoperative chemoradiotherapy for oesophageal cancer: A systematic review and meta-analysis. Gut 2004, 53, 925–930. [Google Scholar] [CrossRef]
- Pasquali, S.; Yim, G.; Vohra, R.S.; Mocellin, S.; Nyanhongo, D.; Marriott, P.; Geh, J.I.; Griffiths, E.A. Survival After Neoadjuvant and Adjuvant Treatments Compared to Surgery Alone for Resectable Esophageal Carcinoma: A Network Meta-analysis. Ann. Surg. 2017, 265, 481–491. [Google Scholar] [CrossRef]
- Buderi, S.I.; Shackcloth, M.; Page, R.D. Does neoadjuvant chemoradiotherapy increase survival in patients with resectable oesophageal cancer? Interact. Cardiovasc. Thorac. Surg. 2017, 24, 115–120. [Google Scholar] [CrossRef][Green Version]
- Berger, A.C.; Farma, J.; Scott, W.J.; Freedman, G.; Weiner, L.; Cheng, J.D.; Wang, H.; Goldberg, M. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J. Clin. Oncol. 2005, 23, 4330–4337. [Google Scholar] [CrossRef]
- Blum, M.M.; Xiao, L.; Patel, V.R.; Maru, D.M.; Correa, A.M.; Amlashi, F.G.; Liao, Z.; Komaki, R.; Lin, S.H.; Skinner, H.D.; et al. Pathological complete response in patients with esophageal cancer after the trimodality approach: The association with baseline variables and survival-The University of Texas MD Anderson Cancer Center experience. Cancer 2017, 123, 4106–4113. [Google Scholar] [CrossRef] [PubMed]
- Hiyoshi, Y.; Yoshida, N.; Watanabe, M.; Kurashige, J.; Karashima, R.; Iwagami, S.; Baba, Y.; Baba, H. Late Recurrence After Radical Resection of Esophageal Cancer. World J. Surg. 2016, 40, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Steffen, T.; Dietrich, D.; Schnider, A.; Kettelhack, C.; Huber, O.; Marti, W.R.; Furrer, M.; Gloor, B.; Schiesser, M.; Thierstein, S.; et al. Swiss Group for Clinical Cancer Research (SAKK). Recurrence Patterns and Long-term Results After Induction Chemotherapy, Chemoradiotherapy, and Curative Surgery in Patients with Locally Advanced Esophageal Cancer. Ann. Surg. 2019, 269, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Ajani, J.A.; D’Amico, T.A.; Bentrem, D.J.; Chao, J.; Corvera, C.; Das, P.; Denlinger, C.S.; Enzinger, P.C.; Fanta, P.; Farjah, F.; et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2019, 17, 855–883. [Google Scholar] [CrossRef]
- Lordick, F.; Mariette, C.; Haustermans, K.; Obermannová, R.; Arnold, D. ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, 50–v57. [Google Scholar] [CrossRef] [PubMed]
- Barbetta, A.; Sihag, S.; Nobel, T.; Hsu, M.; Tan, K.S.; Bains, M.; Jones, D.R.; Molena, D. Patterns and risk of recurrence in patients with esophageal cancer with a pathologic complete response after chemoradiotherapy followed by surgery. J. Thorac. Cardiovasc. Surg. 2018, 157, 1249–1259. [Google Scholar] [CrossRef] [PubMed]
- Robb, W.B.; Messager, M.; Dahan, L.; Mornex, F.; Maillard, E.; D’Journo, X.B.; Triboulet, J.P.; Bedenne, L.; Seitz, J.F.; Mariette, C.; et al. Patterns of recurrence in early-stage oesophageal cancer after chemoradiotherapy and surgery compared with surgery alone. Br. J. Surg. 2016, 103, 117–125. [Google Scholar] [CrossRef]
- Xi, M.; Yang, Y.; Zhang, L.; Yang, H.; Merrell, K.W.; Hallemeier, C.L.; Shen, R.K.; Haddock, M.G.; Hofstetter, W.L.; Maru, D.M.; et al. Multi-institutional Analysis of Recurrence and Survival After Neoadjuvant Chemoradiotherapy of Esophageal Cancer: Impact of Histology on Recurrence Patterns and Outcomes. Ann. Surg. 2019, 269, 663–670. [Google Scholar] [CrossRef]
- Maeda, H.; Kashiwabara, K.; Aoyama, T.; Oba, K.; Honda, M.; Mayanagi, S.; Kanda, M.; Hamada, C.; Sadahiro, S.; Sakamoto, J.; et al. Hazard rate of tumor recurrence over time in patients with colon cancer: Implications for postoperative surveillance from three Japanese Foundation for Multidisciplinary Treatment of Cancer (JFMC) clinical trials. J. Cancer 2017, 8, 4057–4064. [Google Scholar] [CrossRef]
- Dignam, J.J.; Dukic, V.; Anderson, S.J.; Mamounas, E.P.; Wickerham, D.L.; Wolmark, N. Hazard of recurrence and adjuvant treatment effects over time in lymph node-negative breast cancer. Breast Cancer Res. Treat. 2009, 116, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Fink-Neuboeck, N.; Lindenmann, J.; Porubsky, C.; Fediuk, M.; Anegg, U.; Maier, A.; Smolle, J.; Lamont, E.; Smolle-Juettner, F.M. Hazards of recurrence, second primary or other tumour during ten years after surgery for non-small cell lung cancer. Clin. Lung. Cancer 2020, 26, S1525-730430036-X. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, I.; Okamoto, K.; Tsukada, T.; Kinoshita, J.; Oyama, K.; Fushida, S.; Osugi, H.; Ohta, T. Recurrence patterns and risk factors following thoracoscopic esophagectomy with radical lymph node dissection for thoracic esophageal squamous cell carcinoma. Mol. Clin. Oncol. 2016, 4, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, L.; Xia, W.; Wu, L.; Wang, F. The impact of adjuvant therapies on patient survival and the recurrence patterns for resected stage IIa-IVa lower thoracic oesophageal squamous cell carcinoma. World J. Surg. Oncol. 2018, 16, 216. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.K.; Chen, H.S.; Huang, C.S.; Liu, C.C.; Hsieh, C.C.; Hsu, H.S.; Wu, Y.C.; Wu, S.C. Patterns of recurrence after oesophagectomy and postoperative chemoradiotherapy versus surgery alone for oesophageal squamous cell carcinoma. Br. J. Surg. 2017, 104, 90–97. [Google Scholar] [CrossRef]
- Guo, X.; Mao, T.; Gu, Z.; Ji, C.; Fang, W. Clinical study on postoperative recurrence in patients with pN1 esophageal squamous cell carcinoma. Thorac. Cancer 2015, 6, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, S.; Zhang, L.; Guo, S.; Shen, J.; Li, Q.; Yang, H.; Feng, Y.; Liu, M.; Lin, S.H.; et al. Recurrence Risk Based on Pathologic Stage After Neoadjuvant Chemoradiotherapy in Esophageal Squamous Cell Carcinoma: Implications for Risk-Based Postoperative Surveillance Strategies. Ann. Surg. Oncol. 2018, 25, 3639–3646. [Google Scholar] [CrossRef]
- Hamai, Y.; Emi, M.; Ibuki, Y.; Murakami, Y.; Nishibuchi, I.; Nagata, Y.; Furukawa, T.; Kurokawa, T.; Ohsawa, M.; Okada, M. Early Recurrence and Cancer Death After Trimodal Therapy for Esophageal Squamous Cell Carcinoma. Anticancer. Res. 2019, 39, 1433–1440. [Google Scholar] [CrossRef]
- Nobel, T.B.; Livschitz, J.; Xing, X.X.; Barbetta, A.; Hsu, M.; Tan, K.S.; Sihag, S.; Jones, D.R.; Molena, D. Surveillance Implications of Recurrence Patterns in Early Node-Negative Esophageal Adenocarcinoma. Ann. Thorac. Surg. 2019, 108, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Moehler, M.; Baltin, C.T.; Ebert, M.; Fischbach, W.; Gockel, I.; Grenacher, L.; Hölscher, A.H.; Lordick, F.; Malfertheiner, P.; Messmann, H.; et al. International comparison of the German evidence-based S3-guidelines on the diagnosis and multimodal treatment of early and locally advanced gastric cancer, including adenocarcinoma of the lower esophagus. Gastric. Cancer 2015, 18, 550–563. [Google Scholar] [CrossRef]
- D’Amico, T.A. Mckeown esophagogastrectomy. J. Thorac. Dis. 2014, 6 (Suppl. 3), S322–S324. [Google Scholar] [PubMed]
- Orringer, M.B.; Marshall, B.; Iannettoni, M.D. Transhiatal esophagectomy for treatment of benign and malignant esophageal disease. World J. Surg. 2001, 25, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Lindenmann, J.; Fink-Neuboeck, N.; Avian, A.; Pichler, M.; Habitzruther, M.; Maier, A.; Smolle-Juettner, F.M. Preoperative Glasgow Prognostic Score as additional independent prognostic parameter for patients with esophageal cancer after curative esophagectomy. Eur. J. Surg. Oncol. 2017, 43, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.G.; Zhang, W.W.; He, Z.Y.; Sun, J.Y.; Chen, Y.X.; Guo, L. Sites of metastasis and overall survival in esophageal cancer: A population-based study. Cancer Manag. Res. 2017, 9, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Lambert, P.C.; Royston, P. Further development of flexible parametric models for survival analysis. Stata J. 2009, 9, 265–290. [Google Scholar] [CrossRef]

| Characteristic | Overall | EAC | ESCC | p Value |
|---|---|---|---|---|
| Number (percent) | 362 (100%) | 224 (61.9%) | 138 (38.1%) | |
| Age (median; range; years) | 62 (22–88) | 64 (22–88) | 61 (31–82) | 0.004 |
| Gender | 0.003 | |||
| Male | 313 (86.5%) | 203 (90.6%) | 110 (79.7%) | |
| Female | 49 (13.5%) | 21 (9.4%) | 28 (20.3%) | |
| BMI (median; range; kg/m2) | 25.3 (14.9–40.9) | 25.7 (14.9–40.9) | 24.4 (14.8–35.0) | 0.0001 |
| ASA | 0.316 | |||
| 1 | 16 (4.4%) | 10 (4.5%) | 6 (4.3%) | |
| 2 | 147 (40.6%) | 100 (44.6%) | 47 (34.1%) | |
| 3 | 169 (46.7%) | 96 (42.9%) | 73 (52.9%) | |
| 4 | 30 (8.3%) | 18 (8.0%) | 12 (8.7%) | |
| CRP (median; range; mg/L) | 3.2 (0.5–260.5) | 2.9 (0.5–229.0) | 3.55 (0.4–260.5) | 0.4648 |
| Tumor location | 0.001 | |||
| Upper third | 30 (8.3%) | 0 (0.0%) | 30 (21.7%) | |
| Middle third | 83 (22.9%) | 13 (5.8%) | 70 (50.7%) | |
| Lower third/cardia | 249 (68.7%) | 211 (94.2%) | 38 (27.6%) | |
| Tumor differentiation | 0.034 | |||
| G1 | 17 (4.8%) | 15 (6.8%) | 2 (1.5%) | |
| G2 | 162 (45.4%) | 93 (41.9%) | 69 (51.1%) | |
| G3 | 178 (49.8%) | 114 (51.3%) | 64 (47.4%) | |
| N/A | 5 | 2 | 3 | |
| Tumor infiltration | 0.003 | |||
| pT0 | 24 (6.6%) | 10 (4.5%) | 14 (10.1%) | |
| pT1 | 83 (22.9%) | 59 (26.3%) | 24 (17.4%) | |
| pT2 | 79 (21.8%) | 53 (23.7%) | 26 (18.8%) | |
| pT3 | 161 (44.5%) | 98 (43.7%) | 63 (45.7%) | |
| pT4 | 15 (4.2%) | 4 (1.8%) | 11 (8.0%) | |
| Lymph node involvement | 0.143 | |||
| pN0 | 185 (51.1%) | 108 (48.2%) | 77 (55.8%) | |
| pN1 | 106 (29.3%) | 65 (29.0%) | 41 (29.7%) | |
| pN2 | 41 (11.3%) | 27 (12.1%) | 14 (10.1%) | |
| pN3 | 30 (8.3%) | 24 (10.7%) | 6 (4.4%) | |
| Tumor stage | 0.001 | |||
| 0 | 9 (2.5%) | 9 (4.0%) | 0 (0.0%) | |
| I | 88 (24.3%) | 51 (22.8%) | 37 (26.8%) | |
| II | 73 (20.2%) | 26 (11.6%) | 47 (34.0%) | |
| III | 112 (30.9%) | 79 (35.3%) | 33 (23.9%) | |
| IVA | 60 (16.6%) | 49 (21.9%) | 11 (8.0%) | |
| IVB | 20 (5.5%) | 10 (4.4%) | 10 (7.3%) | |
| Resection margin | 0.078 | |||
| R0 | 293 (86.9%) | 186 (89.4%) | 107 (82.9%) | |
| R1 | 39 (11.6%) | 21 (10.1%) | 18 (14.0%) | |
| R2 | 5 (1.5%) | 1 (0.5%) | 4 (3.1%) | |
| N/A | 25 | 16 | 9 | |
| Neo-adjuvant treatment | 0.706 | |||
| Yes | 119 (32.9%) | 72 (32.1%) | 47 (34.1%) | |
| No | 243 (67.1%) | 125 (67.9%) | 91 (65.9%) | |
| Adjuvant treatment | 0.017 | |||
| Yes | 61 (17.3%) | 30 (13.6%) | 31 (23.5%) | |
| No | 292 (87.7%) | 191 (86.4%) | 101 (76.5%) | |
| N/A | 9 | 3 | 6 | |
| Tumor recurrence | 0.005 | |||
| Total number of recurrences | 192 (53%) | 107 (29.6%) | 85 (23.5%) | |
| Loco-regional | 32 (8.8%) | 12 (6.3%) | 20 (10.4%) | 0.002 |
| Distant metastases | 160 (44.2%) | 95 (49.5%) | 65 (33.9%) | 0.438 |
| Characteristic | HR | SE | 95% CI | p Value |
|---|---|---|---|---|
| RFS | ||||
| Univariate Analysis | ||||
| Age | 0.999 | 0.006 | 0.986–1.013 | 0.968 |
| Gender | 1.302 | 0.295 | 0.834–2.030 | 0.244 |
| BMI | 0.980 | 0.016 | 0.947–1.013 | 0.247 |
| ASA | 1.259 | 0.134 | 1.002–1.551 | 0.030 |
| CRP | 1.010 | 0.002 | 1.004–1.015 | <0.001 |
| Albumin | 0.689 | 0.100 | 0.518–0.916 | 0.011 |
| Histology | 1.874 | 0.275 | 1.405–2.499 | <0.001 |
| Tumor location | 0.594 | 0.063 | 0.482–0.733 | <0.001 |
| Tumor differentiation | 1.705 | 0.219 | 1.324–2.194 | <0.001 |
| Tumor infiltration | 1.675 | 0.134 | 1.432–1.960 | <0.001 |
| Lymph node involvement | 1.763 | 0.125 | 1.533–2.027 | <0.001 |
| Tumor stage | 1.580 | 0.097 | 1.400–1.784 | <0.001 |
| Resection margin | 2.312 | 0.370 | 1.688–3.166 | <0.001 |
| Neo-adjuvant treatment | 1.341 | 0.207 | 0.990–1.817 | 0.057 |
| Adjuvant treatment | 1.653 | 0.284 | 1.181–2.314 | 0.003 |
| Multivariate Analysis | ||||
| Histology | 1.956 | 0.315 | 1.426–2.683 | <0.001 |
| Tumor stage | 1.592 | 0.110 | 1.389–1.824 | <0.001 |
| Tumor differentiation | 1.426 | 0.200 | 1.082–1.879 | 0.012 |
| Resection margin | 1.635 | 0.277 | 1.782–2.280 | 0.004 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindenmann, J.; Fediuk, M.; Fink-Neuboeck, N.; Porubsky, C.; Pichler, M.; Brcic, L.; Anegg, U.; Balic, M.; Dandachi, N.; Maier, A.; et al. Hazard Curves for Tumor Recurrence and Tumor-Related Death Following Esophagectomy for Esophageal Cancer. Cancers 2020, 12, 2066. https://doi.org/10.3390/cancers12082066
Lindenmann J, Fediuk M, Fink-Neuboeck N, Porubsky C, Pichler M, Brcic L, Anegg U, Balic M, Dandachi N, Maier A, et al. Hazard Curves for Tumor Recurrence and Tumor-Related Death Following Esophagectomy for Esophageal Cancer. Cancers. 2020; 12(8):2066. https://doi.org/10.3390/cancers12082066
Chicago/Turabian StyleLindenmann, Joerg, Melanie Fediuk, Nicole Fink-Neuboeck, Christian Porubsky, Martin Pichler, Luka Brcic, Udo Anegg, Marija Balic, Nadia Dandachi, Alfred Maier, and et al. 2020. "Hazard Curves for Tumor Recurrence and Tumor-Related Death Following Esophagectomy for Esophageal Cancer" Cancers 12, no. 8: 2066. https://doi.org/10.3390/cancers12082066
APA StyleLindenmann, J., Fediuk, M., Fink-Neuboeck, N., Porubsky, C., Pichler, M., Brcic, L., Anegg, U., Balic, M., Dandachi, N., Maier, A., Smolle, M., Smolle, J., & Smolle-Juettner, F. M. (2020). Hazard Curves for Tumor Recurrence and Tumor-Related Death Following Esophagectomy for Esophageal Cancer. Cancers, 12(8), 2066. https://doi.org/10.3390/cancers12082066