Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis
Abstract
1. Introduction
2. Cannabinoids
2.1. Delta9-tetrahydrocannabinol (THC)
2.1.1. Breast Cancer
2.1.2. Glioma
2.1.3. Leukemia
2.1.4. Lung Cancer
2.1.5. Melanoma
2.1.6. Myeloma
2.1.7. Hepatocellular Carcinoma
2.1.8. Pancreatic Cancer
2.1.9. Prostate Cancer
2.1.10. Colon Cancer
2.1.11. Endometrial and Cervical Cancers
2.1.12. Oral Cancer
2.1.13. Clinical Results
2.2. Cannabidiol (CBD)
2.2.1. Breast Cancer
2.2.2. Lung Cancer
2.2.3. Glioma and Neuroblastoma
2.2.4. Myeloma
2.2.5. Colon Cancer
2.2.6. Prostate Cancer
2.2.7. Other Cancers
2.2.8. Clinical Results
2.3. Cannabigerol (CBG)
2.4. Cannabichromene (CBC)
2.5. Cannabidivarin (CBDV)
2.6. Cannabinol (CBN)
2.7. Cannabivarin (CBV)
2.8. Tetrahydrocannabivarin (THCV)
3. Terpenes
3.1. Myrcene
3.2. Beta-Caryophyllene and Metabolite Caryophyllene Oxide
3.3. Humulene
3.4. Limonene
3.5. Pinene
3.6. Linalool
3.7. Bisabolol
3.8. Elemene
3.9. Eudesmols
3.10. Eucalyptol
3.11. Borneol
3.12. Terpineol
3.13. Terpinene Isomers
3.14. Valencene
3.15. Geraniol
3.16. Nerolidol
3.17. Guaiol
3.18. Camphene
3.19. Alpha-Phellandrene
3.20. Delta-3-Carene
3.21. Cadinenes
3.22. Thujone
3.23. p-Cymene
3.24. Gurjunene
3.25. Farnesene
4. Flavonoids
4.1. Kaempferol
4.2. Apigenin
4.3. Cannflavins
4.4. Silymarin
4.5. Luteolin
4.6. Orientin
4.7. Vitexin and Isovitexin
4.8. Quercetin
5. Entourage Effect
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pisanti, S.; Bifulco, M. Medical Cannabis: A plurimillennial history of an evergreen. J. Cell. Physiol. 2019, 234, 8342–8351. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, E.C.D.; Baldasso, G.M.; Bicca, M.A.; Paes, R.S.; Capasso, R.; Dutra, R.C. Terpenoids, cannabimimetic ligands, beyond the cannabis plant. Molecules 2020, 25, 1567. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Bifulco, M.; Petrocellis, L.D. The endocannabinoid system and its therapeutic exploitation. Nat. Rev. Drug Discov. 2004, 3, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.C.; Merlin, M.D. Cannabis: Evolution and Ethnobotany; University of California Press: Berkeley, CA, USA, 2016; ISBN 978-0-520-29248-2. [Google Scholar]
- Ramer, R.; Schwarz, R.; Hinz, B. Modulation of the endocannabinoid system as a potential anticancer strategy. Front. Pharmacol. 2019, 10, 430. [Google Scholar] [CrossRef]
- Laezza, C.; Pagano, C.; Navarra, G.; Pastorino, O.; Proto, M.C.; Fiore, D.; Piscopo, C.; Gazzerro, P.; Bifulco, M. The Endocannabinoid system: A target for cancer treatment. Int. J. Mol. Sci. 2020, 21, 747. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical use of cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Sobocińska, A.A.; Czarnecka, A.M.; Król, M.; Botta, B.; Szczylik, C. The therapeutic aspects of the endocannabinoid system (ECS) for cancer and their development: From nature to laboratory. Curr. Pharm. Des. 2016, 22, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.; Ramer, R.; Hinz, B. Targeting the endocannabinoid system as a potential anticancer approach. Drug Metab. Rev. 2018, 50, 26–53. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Hinz, B. Antitumorigenic targets of cannabinoids—Current status and implications. Expert Opin. Ther. Targets 2016, 20, 1219–1235. [Google Scholar] [CrossRef] [PubMed]
- Michalski, C.W.; Oti, F.E.; Erkan, M.; Sauliunaite, D.; Bergmann, F.; Pacher, P.; Batkai, S.; Müller, M.W.; Giese, N.A.; Friess, H.; et al. Cannabinoids in pancreatic cancer: Correlation with survival and pain. Int. J. Cancer 2008, 122, 742–750. [Google Scholar] [CrossRef]
- Chung, S.C.; Hammarsten, P.; Josefsson, A.; Stattin, P.; Granfors, T.; Egevad, L.; Mancini, G.; Lutz, B.; Bergh, A.; Fowler, C.J. A high cannabinoid CB1 receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur. J. Cancer 2009, 45, 174–182. [Google Scholar] [CrossRef]
- Messalli, E.M.; Grauso, F.; Luise, R.; Angelini, A.; Rossiello, R. Cannabinoid receptor type 1 immunoreactivity and disease severity in human epithelial ovarian tumors. Am. J. Obstet. Gynecol. 2014, 211, 234.e1–234.e6. [Google Scholar] [CrossRef]
- Jung, C.K.; Kang, W.K.; Park, J.M.; Ahn, H.J.; Kim, S.W.; Taek Oh, S.; Choi, K.Y. Expression of the cannabinoid type I receptor and prognosis following surgery in colorectal cancer. Oncol. Lett. 2013, 5, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chu, H.-J.; Liang, Y.-C.; Huang, J.-M.; Shang, C.-L.; Tan, H.; Liu, D.; Zhao, Y.-H.; Liu, T.-Y.; Yao, S.-Z. FABP5 correlates with poor prognosis and promotes tumor cell growth and metastasis in cervical cancer. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 14873–14883. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Gómez, E.; Andradas, C.; Blasco-Benito, S.; Caffarel, M.M.; García-Taboada, E.; Villa-Morales, M.; Moreno, E.; Hamann, S.; Martín-Villar, E.; Flores, J.M.; et al. Role of cannabinoid receptor CB2 in HER2 pro-oncogenic signaling in breast cancer. JNCI J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Klein Nulent, T.J.W.; Van Diest, P.J.; van der Groep, P.; Leusink, F.K.J.; Kruitwagen, C.L.J.J.; Koole, R.; Van Cann, E.M. Cannabinoid receptor-2 immunoreactivity is associated with survival in squamous cell carcinoma of the head and neck. Br. J. Oral Maxillofac. Surg. 2013, 51, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; Bisogno, T.; Matias, I.; De Petrocellis, L.; Cascio, M.G.; Cosenza, V.; D’argenio, G.; Scaglione, G.; Bifulco, M.; Sorrentini, I.; et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology 2003, 125, 677–687. [Google Scholar] [CrossRef]
- Pagotto, U.; Marsicano, G.; Fezza, F.; Theodoropoulou, M.; Grübler, Y.; Stalla, J.; Arzberger, T.; Milone, A.; Losa, M.; Di Marzo, V.; et al. Normal human pituitary gland and pituitary adenomas express cannabinoid receptor type 1 and synthesize endogenous cannabinoids: First evidence for a direct role of cannabinoids on hormone modulation at the human pituitary level. J. Clin. Endocrinol. Metab. 2001, 86, 2687–2696. [Google Scholar] [CrossRef][Green Version]
- Chen, L.; Chen, H.; Li, Y.; Li, L.; Qiu, Y.; Ren, J. Endocannabinoid and ceramide levels are altered in patients with colorectal cancer. Oncol. Rep. 2015, 34, 447–454. [Google Scholar] [CrossRef]
- Petersen, G.; Moesgaard, B.; Schmid, P.C.; Schmid, H.H.O.; Broholm, H.; Kosteljanetz, M.; Hansen, H.S. Endocannabinoid metabolism in human glioblastomas and meningiomas compared to human non-tumour brain tissue. J. Neurochem. 2005, 93, 299–309. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Bisogno, T.; Ligresti, A.; Bifulco, M.; Melck, D.; Di Marzo, V. Effect on cancer cell proliferation of palmitoylethanolamide, a fatty acid amide interacting with both the cannabinoid and vanilloid signalling systems. Fundam. Clin. Pharmacol. 2002, 16, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Melck, D.; De Petrocellis, L.; Orlando, P.; Bisogno, T.; Laezza, C.; Bifulco, M.; Di Marzo, V. Suppression of nerve growth factor Trk receptors and prolactin receptors by endocannabinoids leads to inhibition of human breast and prostate cancer cell proliferation. Endocrinology 2000, 141, 118–126. [Google Scholar] [CrossRef]
- Pyszniak, M.; Tabarkiewicz, J.; Łuszczki, J. Endocannabinoid system as a regulator of tumor cell malignancy—Biological pathways and clinical significance. Oncotargets Ther. 2016, 9, 4323–4326. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Huang, M.-Q.; Bao, J.-L.; Chen, X.-P.; Wang, Y.-T. Terpenoids: Natural products for cancer therapy. Expert Opin. Investig. Drugs 2012, 21, 1801–1818. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Samuel, S.; Varghese, E.; Varghese, S.; Kubatka, P.; Liskova, A.; Büsselberg, D. Flavonoids in cancer and apoptosis. Cancers 2018, 11, 28. [Google Scholar] [CrossRef]
- Afrin, F.; Chi, M.; Eamens, A.L.; Duchatel, R.J.; Douglas, A.M.; Schneider, J.; Gedye, C.; Woldu, A.S.; Dun, M.D. Can hemp help? Low-THC cannabis and non-THC cannabinoids for the treatment of cancer. Cancers 2020, 12, 1033. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. In Phytocannabinoids; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Progress in the Chemistry of Organic Natural Products; Springer International Publishing: Cham, Switzerland, 2017; Volume 103, pp. 1–36. ISBN 978-3-319-45539-6. [Google Scholar]
- Bauer, R.; Woelkart, K.; Salo-Ahen, O. CB receptor ligands from plants. Curr. Top. Med. Chem. 2008, 8, 173–186. [Google Scholar] [CrossRef]
- Ligresti, A.; Moriello, A.S.; Starowicz, K.; Matias, I.; Pisanti, S.; De Petrocellis, L.; Laezza, C.; Portella, G.; Bifulco, M.; Di Marzo, V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006, 318, 1375–1387. [Google Scholar] [CrossRef]
- Takeda, S.; Yoshida, K.; Nishimura, H.; Harada, M.; Okajima, S.; Miyoshi, H.; Okamoto, Y.; Amamoto, T.; Watanabe, K.; Omiecinski, C.J.; et al. Δ9-Tetrahydrocannabinol disrupts estrogen-signaling through up-regulation of estrogen receptor β (ERβ). Chem. Res. Toxicol. 2013, 26, 1073–1079. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Moreno-Bueno, G.; Cerutti, C.; Palacios, J.; Guzman, M.; Mechta-Grigoriou, F.; Sanchez, C. JunD is involved in the antiproliferative effect of Delta9-tetrahydrocannabinol on human breast cancer cells. Oncogene 2008, 27, 5033–5044. [Google Scholar] [CrossRef] [PubMed]
- von Bueren, A.O.; Schlumpf, M.; Lichtensteiger, W. Delta(9)-tetrahydrocannabinol inhibits 17beta-estradiol-induced proliferation and fails to activate androgen and estrogen receptors in MCF7 human breast cancer cells. Anticancer Res. 2008, 28, 85–89. [Google Scholar]
- Takeda, S.; Yamaori, S.; Motoya, E.; Matsunaga, T.; Kimura, T.; Yamamoto, I.; Watanabe, K. Delta(9)-tetrahydrocannabinol enhances MCF-7 cell proliferation via cannabinoid receptor-independent signaling. Toxicology 2008, 245, 141–146. [Google Scholar] [CrossRef]
- McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J. Immunol. Baltim. Md 1950 2005, 174, 3281–3289. [Google Scholar] [CrossRef]
- Caffarel, M.M.; Sarrió, D.; Palacios, J.; Guzmán, M.; Sánchez, C. Delta9-tetrahydrocannabinol inhibits cell cycle progression in human breast cancer cells through Cdc2 regulation. Cancer Res. 2006, 66, 6615–6621. [Google Scholar] [CrossRef] [PubMed]
- Hirao-Suzuki, M.; Takeda, S.; Watanabe, K.; Takiguchi, M.; Aramaki, H. Δ9-tetrahydrocannabinol upregulates fatty acid 2-hydroxylase (FA2H) via PPARα induction: A possible evidence for the cancellation of PPARβ/δ-mediated inhibition of PPARα in MDA-MB-231 cells. Arch. Biochem. Biophys. 2019, 662, 219–225. [Google Scholar] [CrossRef]
- Blasco-Benito, S.; Seijo-Vila, M.; Caro-Villalobos, M.; Tundidor, I.; Andradas, C.; García-Taboada, E.; Wade, J.; Smith, S.; Guzmán, M.; Pérez-Gómez, E.; et al. Appraising the “entourage effect”: Antitumor action of a pure cannabinoid versus a botanical drug preparation in preclinical models of breast cancer. Biochem. Pharmacol. 2018, 157, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Tournier, N.; Chevillard, L.; Megarbane, B.; Pirnay, S.; Scherrmann, J.-M.; Declèves, X. Interaction of drugs of abuse and maintenance treatments with human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2). Int. J. Neuropsychopharmacol. 2010, 13, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Caffarel, M.M.; Andradas, C.; Mira, E.; Pérez-Gómez, E.; Cerutti, C.; Moreno-Bueno, G.; Flores, J.M.; García-Real, I.; Palacios, J.; Mañes, S.; et al. Cannabinoids reduce ErbB2-driven breast cancer progression through Akt inhibition. Mol. Cancer 2010, 9, 196. [Google Scholar] [CrossRef]
- Blasco-Benito, S.; Moreno, E.; Seijo-Vila, M.; Tundidor, I.; Andradas, C.; Caffarel, M.M.; Caro-Villalobos, M.; Urigüen, L.; Diez-Alarcia, R.; Hernández, L.; et al. Therapeutic targeting of HER2-CB2R heteromers in HER2-positive breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 6505. [Google Scholar] [CrossRef]
- McAllister, S.D.; Chan, C.; Taft, R.J.; Luu, T.; Abood, M.E.; Moore, D.H.; Aldape, K.; Yount, G. Cannabinoids selectively inhibit proliferation and induce death of cultured human glioblastoma multiforme cells. J. Neurooncol. 2005, 74, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Marcu, J.P.; Christian, R.T.; Lau, D.; Zielinski, A.J.; Horowitz, M.P.; Lee, J.; Pakdel, A.; Allison, J.; Limbad, C.; Moore, D.H.; et al. Cannabidiol enhances the inhibitory effects of delta9-tetrahydrocannabinol on human glioblastoma cell proliferation and survival. Mol. Cancer Ther. 2010, 9, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Jacobsson, S.O.; Rongård, E.; Stridh, M.; Tiger, G.; Fowler, C.J. Serum-dependent effects of tamoxifen and cannabinoids upon C6 glioma cell viability. Biochem. Pharmacol. 2000, 60, 1807–1813. [Google Scholar] [CrossRef]
- Goncharov, I.; Weiner, L.; Vogel, Z. Delta9-tetrahydrocannabinol increases C6 glioma cell death produced by oxidative stress. Neuroscience 2005, 134, 567–574. [Google Scholar] [CrossRef]
- Sánchez, C.; Galve-Roperh, I.; Canova, C.; Brachet, P.; Guzmán, M. Delta9-tetrahydrocannabinol induces apoptosis in C6 glioma cells. FEBS Lett. 1998, 436, 6–10. [Google Scholar] [CrossRef]
- Carracedo, A.; Lorente, M.; Egia, A.; Blázquez, C.; García, S.; Giroux, V.; Malicet, C.; Villuendas, R.; Gironella, M.; González-Feria, L.; et al. The stress-regulated protein p8 mediates cannabinoid-induced apoptosis of tumor cells. Cancer Cell 2006, 9, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Tiedra, S.; Fabriàs, G.; Dávila, D.; Salanueva, Í.J.; Casas, J.; Montes, L.R.; Antón, Z.; García-Taboada, E.; Salazar-Roa, M.; Lorente, M.; et al. Dihydroceramide accumulation mediates cytotoxic autophagy of cancer cells via autolysosome destabilization. Autophagy 2016, 12, 2213–2229. [Google Scholar] [CrossRef] [PubMed]
- Galve-Roperh, I.; Sánchez, C.; Cortés, M.L.; Gómez del Pulgar, T.; Izquierdo, M.; Guzmán, M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000, 6, 313–319. [Google Scholar] [CrossRef]
- Hart, S.; Fischer, O.M.; Ullrich, A. Cannabinoids induce cancer cell proliferation via tumor necrosis factor alpha-converting enzyme (TACE/ADAM17)-mediated transactivation of the epidermal growth factor receptor. Cancer Res. 2004, 64, 1943–1950. [Google Scholar] [CrossRef]
- Salazar, M.; Carracedo, A.; Salanueva, I.J.; Hernández-Tiedra, S.; Lorente, M.; Egia, A.; Vázquez, P.; Blázquez, C.; Torres, S.; García, S.; et al. Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J. Clin. Investig. 2009, 119, 1359–1372. [Google Scholar] [CrossRef]
- Salazar, M.; Carracedo, A.; Salanueva, I.J.; Hernández-Tiedra, S.; Egia, A.; Lorente, M.; Vázquez, P.; Torres, S.; Iovanna, J.L.; Guzmán, M.; et al. TRB3 links ER stress to autophagy in cannabinoid anti-tumoral action. Autophagy 2009, 5, 1048–1049. [Google Scholar] [CrossRef]
- Blázquez, C.; Salazar, M.; Carracedo, A.; Lorente, M.; Egia, A.; González-Feria, L.; Haro, A.; Velasco, G.; Guzmán, M. Cannabinoids inhibit glioma cell invasion by down-regulating matrix metalloproteinase-2 expression. Cancer Res. 2008, 68, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- López-Valero, I.; Saiz-Ladera, C.; Torres, S.; Hernández-Tiedra, S.; García-Taboada, E.; Rodríguez-Fornés, F.; Barba, M.; Dávila, D.; Salvador-Tormo, N.; Guzmán, M.; et al. Targeting Glioma Initiating Cells with A combined therapy of cannabinoids and temozolomide. Biochem. Pharmacol. 2018, 157, 266–274. [Google Scholar] [CrossRef] [PubMed]
- López-Valero, I.; Torres, S.; Salazar-Roa, M.; García-Taboada, E.; Hernández-Tiedra, S.; Guzmán, M.; Sepúlveda, J.M.; Velasco, G.; Lorente, M. Optimization of a preclinical therapy of cannabinoids in combination with temozolomide against glioma. Biochem. Pharmacol. 2018, 157, 275–284. [Google Scholar] [CrossRef]
- Lorente, M.; Torres, S.; Salazar, M.; Carracedo, A.; Hernández-Tiedra, S.; Rodríguez-Fornés, F.; García-Taboada, E.; Meléndez, B.; Mollejo, M.; Campos-Martín, Y.; et al. Stimulation of the midkine/ALK axis renders glioma cells resistant to cannabinoid antitumoral action. Cell Death Differ. 2011, 18, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Hernán Pérez de la Ossa, D.; Lorente, M.; Gil-Alegre, M.E.; Torres, S.; García-Taboada, E.; Aberturas, M.D.R.; Molpeceres, J.; Velasco, G.; Torres-Suárez, A.I. Local delivery of cannabinoid-loaded microparticles inhibits tumor growth in a murine xenograft model of glioblastoma multiforme. PLoS ONE 2013, 8, e54795. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, M.; Hendson, G.; Sargent, M.A.; Steinbok, P. Spontaneous regression of septum pellucidum/forniceal pilocytic astrocytomas--possible role of Cannabis inhalation. Childs Nerv. Syst. ChNS Off. J. Int. Soc. Pediatr. Neurosurg. 2011, 27, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, M.; Duarte, M.J.; Blázquez, C.; Ravina, J.; Rosa, M.C.; Galve-Roperh, I.; Sánchez, C.; Velasco, G.; González-Feria, L. A pilot clinical study of Delta9-tetrahydrocannabinol in patients with recurrent glioblastoma multiforme. Br. J. Cancer 2006, 95, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, C.; González-Feria, L.; Alvarez, L.; Haro, A.; Casanova, M.L.; Guzmán, M. Cannabinoids inhibit the vascular endothelial growth factor pathway in gliomas. Cancer Res. 2004, 64, 5617–5623. [Google Scholar] [CrossRef]
- Allen, D. Dronabinol therapy: Central nervous system adverse events in adults with primary brain tumors. Clin. J. Oncol. Nurs. 2019, 23, 23–26. [Google Scholar] [CrossRef]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. Anticancer effects of phytocannabinoids used with chemotherapy in leukaemia cells can be improved by altering the sequence of their administration. Int. J. Oncol. 2017, 51, 369–377. [Google Scholar] [CrossRef]
- Liu, W.M.; Scott, K.A.; Shamash, J.; Joel, S.; Powles, T.B. Enhancing the in vitro cytotoxic activity of Delta9-tetrahydrocannabinol in leukemic cells through a combinatorial approach. Leuk. Lymphoma 2008, 49, 1800–1809. [Google Scholar] [CrossRef]
- Holland, M.L.; Panetta, J.A.; Hoskins, J.M.; Bebawy, M.; Roufogalis, B.D.; Allen, J.D.; Arnold, J.C. The effects of cannabinoids on P-glycoprotein transport and expression in multidrug resistant cells. Biochem. Pharmacol. 2006, 71, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Kampa-Schittenhelm, K.M.; Haverkamp, T.; Bonin, M.; Tsintari, V.; Bühring, H.J.; Haeusser, L.; Blumenstock, G.; Dreher, S.T.; Ganief, T.; Akmut, F.; et al. Epigenetic activation of O-linked β-N-acetylglucosamine transferase overrides the differentiation blockage in acute leukemia. EBioMedicine 2020, 54, 102678. [Google Scholar] [CrossRef]
- Kampa-Schittenhelm, K.M.; Salitzky, O.; Akmut, F.; Illing, B.; Kanz, L.; Salih, H.R.; Schittenhelm, M.M. Dronabinol has preferential antileukemic activity in acute lymphoblastic and myeloid leukemia with lymphoid differentiation patterns. BMC Cancer 2016, 16, 25. [Google Scholar] [CrossRef][Green Version]
- Jia, W.; Hegde, V.L.; Singh, N.P.; Sisco, D.; Grant, S.; Nagarkatti, M.; Nagarkatti, P.S. Delta9-tetrahydrocannabinol-induced apoptosis in Jurkat leukemia T cells is regulated by translocation of Bad to mitochondria. Mol. Cancer Res. MCR 2006, 4, 549–562. [Google Scholar] [CrossRef]
- Lombard, C.; Nagarkatti, M.; Nagarkatti, P.S. Targeting cannabinoid receptors to treat leukemia: Role of cross-talk between extrinsic and intrinsic pathways in Delta9-tetrahydrocannabinol (THC)-induced apoptosis of Jurkat cells. Leuk. Res. 2005, 29, 915–922. [Google Scholar] [CrossRef]
- Singh, Y.; Bali, C. Cannabis extract treatment for terminal acute lymphoblastic leukemia with a Philadelphia chromosome mutation. Case Rep. Oncol. 2013, 6, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Baram, L.; Peled, E.; Berman, P.; Yellin, B.; Besser, E.; Benami, M.; Louria-Hayon, I.; Lewitus, G.M.; Meiri, D. The heterogeneity and complexity of Cannabis extracts as antitumor agents. Oncotarget 2019, 10, 4091–4106. [Google Scholar] [CrossRef] [PubMed]
- Preet, A.; Ganju, R.K.; Groopman, J.E. Delta9-Tetrahydrocannabinol inhibits epithelial growth factor-induced lung cancer cell migration in vitro as well as its growth and metastasis in vivo. Oncogene 2008, 27, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Milian, L.; Mata, M.; Alcacer, J.; Oliver, M.; Sancho-Tello, M.; Martín de Llano, J.J.; Camps, C.; Galbis, J.; Carretero, J.; Carda, C. Cannabinoid receptor expression in non-small cell lung cancer. Effectiveness of tetrahydrocannabinol and cannabidiol inhibiting cell proliferation and epithelial-mesenchymal transition in vitro. PLoS ONE 2020, 15, e0228909. [Google Scholar] [CrossRef]
- Burnette-Curley, D.; Cabral, G.A. Differential inhibition of RAW264.7 macrophage tumoricidal activity by delta 9tetrahydrocannabinol. Proc. Soc. Exp. Biol. Med. N. Y. 1995, 210, 64–76. [Google Scholar] [CrossRef]
- Martín-Banderas, L.; Muñoz-Rubio, I.; Prados, J.; Álvarez-Fuentes, J.; Calderón-Montaño, J.M.; López-Lázaro, M.; Arias, J.L.; Leiva, M.C.; Holgado, M.A.; Fernández-Arévalo, M. In vitro and in vivo evaluation of Δ9-tetrahidrocannabinol/PLGA nanoparticles for cancer chemotherapy. Int. J. Pharm. 2015, 487, 205–212. [Google Scholar] [CrossRef]
- Zhu, L.X.; Sharma, S.; Stolina, M.; Gardner, B.; Roth, M.D.; Tashkin, D.P.; Dubinett, S.M. Delta-9-tetrahydrocannabinol inhibits antitumor immunity by a CB2 receptor-mediated, cytokine-dependent pathway. J. Immunol. Baltim. Md 1950 2000, 165, 373–380. [Google Scholar] [CrossRef]
- Blázquez, C.; Carracedo, A.; Barrado, L.; Real, P.J.; Fernández-Luna, J.L.; Velasco, G.; Malumbres, M.; Guzmán, M. Cannabinoid receptors as novel targets for the treatment of melanoma. FASEB J. 2006, 20, 2633–2635. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Offidani, M.; Amantini, C.; Gentili, S.; Soriani, A.; Cardinali, C.; Leoni, P.; Santoni, G. Cannabinoids synergize with carfilzomib, reducing multiple myeloma cells viability and migration. Oncotarget 2016, 7, 77543–77557. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.L.; Hill, D.S.; McKee, C.S.; Hernandez-Tiedra, S.; Lorente, M.; Lopez-Valero, I.; Eleni Anagnostou, M.; Babatunde, F.; Corazzari, M.; Redfern, C.P.F.; et al. Exploiting cannabinoid-induced cytotoxic autophagy to drive melanoma cell death. J. Investig. Dermatol. 2015, 135, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Glodde, N.; Jakobs, M.; Bald, T.; Tüting, T.; Gaffal, E. Differential role of cannabinoids in the pathogenesis of skin cancer. Life Sci. 2015, 138, 35–40. [Google Scholar] [CrossRef]
- Verykiou, S.; Alexander, M.; Edwards, N.; Plummer, R.; Chaudhry, B.; Lovat, P.E.; Hill, D.S. Harnessing autophagy to overcome mitogen-activated protein kinase kinase inhibitor-induced resistance in metastatic melanoma. Br. J. Dermatol. 2019, 180, 346–356. [Google Scholar] [CrossRef]
- Sido, J.M.; Yang, X.; Nagarkatti, P.S.; Nagarkatti, M. Δ9-Tetrahydrocannabinol-mediated epigenetic modifications elicit myeloid-derived suppressor cell activation via STAT3/S100A8. J. Leukoc. Biol. 2015, 97, 677–688. [Google Scholar] [CrossRef]
- Vara, D.; Salazar, M.; Olea-Herrero, N.; Guzmán, M.; Velasco, G.; Díaz-Laviada, I. Anti-tumoral action of cannabinoids on hepatocellular carcinoma: Role of AMPK-dependent activation of autophagy. Cell Death Differ. 2011, 18, 1099–1111. [Google Scholar] [CrossRef]
- Vara, D.; Morell, C.; Rodríguez-Henche, N.; Diaz-Laviada, I. Involvement of PPARγ in the antitumoral action of cannabinoids on hepatocellular carcinoma. Cell Death Dis. 2013, 4, e618. [Google Scholar] [CrossRef] [PubMed]
- Leelawat, S.; Leelawat, K.; Narong, S.; Matangkasombut, O. The dual effects of delta(9)-tetrahydrocannabinol on cholangiocarcinoma cells: Anti-invasion activity at low concentration and apoptosis induction at high concentration. Cancer Investig. 2010, 28, 357–363. [Google Scholar] [CrossRef]
- Prester, L.; Mikolić, A.; Jurič, A.; Fuchs, N.; Neuberg, M.; Lucić Vrdoljak, A.; Brčić Karačonji, I. Effects of Δ9-tetrahydrocannabinol on irinotecan-induced clinical effects in rats. Chem. Biol. Interact. 2018, 294, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, A.; Gironella, M.; Lorente, M.; Garcia, S.; Guzmán, M.; Velasco, G.; Iovanna, J.L. Cannabinoids induce apoptosis of pancreatic tumor cells via endoplasmic reticulum stress-related genes. Cancer Res 2006, 66, 6748–6755. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Schiano Moriello, A.; Iappelli, M.; Verde, R.; Stott, C.G.; Cristino, L.; Orlando, P.; Di Marzo, V. Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: Pro-apoptotic effects and underlying mechanisms. Br. J. Pharmacol. 2013, 168, 79–102. [Google Scholar] [CrossRef]
- Ruiz, L.; Miguel, A.; Díaz-Laviada, I. Delta9-tetrahydrocannabinol induces apoptosis in human prostate PC-3 cells via a receptor-independent mechanism. FEBS Lett. 1999, 458, 400–404. [Google Scholar] [CrossRef]
- Greenhough, A.; Patsos, H.A.; Williams, A.C.; Paraskeva, C. The cannabinoid delta(9)-tetrahydrocannabinol inhibits RAS-MAPK and PI3K-AKT survival signalling and induces BAD-mediated apoptosis in colorectal cancer cells. Int. J. Cancer 2007, 121, 2172–2180. [Google Scholar] [CrossRef]
- Hernán Pérez de la Ossa, D.; Gil-Alegre, M.E.; Ligresti, A.; Aberturas, M.D.R.; Molpeceres, J.; Torres, A.I.; Di Marzo, V. Preparation and characterization of Δ9-tetrahydrocannabinol-loaded biodegradable polymeric microparticles and their antitumoral efficacy on cancer cell lines. J. Drug Target. 2013, 21, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.L.; Allen, J.D.; Arnold, J.C. Interaction of plant cannabinoids with the multidrug transporter ABCC1 (MRP1). Eur. J. Pharmacol. 2008, 591, 128–131. [Google Scholar] [CrossRef]
- Holland, M.L.; Lau, D.T.; Allen, J.D.; Arnold, J.C. The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Br. J. Pharmacol. 2007, 152, 815–824. [Google Scholar] [CrossRef]
- Ramer, R.; Hinz, B. Inhibition of cancer cell invasion by cannabinoids via increased expression of tissue inhibitor of matrix metalloproteinases-1. J. Natl. Cancer Inst. 2008, 100, 59–69. [Google Scholar] [CrossRef]
- Whyte, D.A.; Al-Hammadi, S.; Balhaj, G.; Brown, O.M.; Penefsky, H.S.; Souid, A.-K. Cannabinoids inhibit cellular respiration of human oral cancer cells. Pharmacology 2010, 85, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef]
- McAllister, S.D.; Christian, R.T.; Horowitz, M.P.; Garcia, A.; Desprez, P.-Y. Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells. Mol. Cancer Ther. 2007, 6, 2921–2927. [Google Scholar] [CrossRef]
- Elbaz, M.; Nasser, M.W.; Ravi, J.; Wani, N.A.; Ahirwar, D.K.; Zhao, H.; Oghumu, S.; Satoskar, A.R.; Shilo, K.; Carson, W.E.; et al. Modulation of the tumor microenvironment and inhibition of EGF/EGFR pathway: Novel anti-tumor mechanisms of Cannabidiol in breast cancer. Mol. Oncol. 2015, 9, 906–919. [Google Scholar] [CrossRef]
- García-Morales, L.; Castillo, A.M.; Ramírez, J.T.; Zamudio-Meza, H.; Domínguez-Robles, M.d.; Meza, I. CBD reverts the mesenchymal invasive phenotype of breast cancer cells induced by the inflammatory cytokine IL-1β. Int. J. Mol. Sci. 2020, 21, 2429. [Google Scholar] [CrossRef] [PubMed]
- Fraguas-Sánchez, A.I.; Fernández-Carballido, A.; Simancas-Herbada, R.; Martin-Sabroso, C.; Torres-Suárez, A.I. CBD loaded microparticles as a potential formulation to improve paclitaxel and doxorubicin-based chemotherapy in breast cancer. Int. J. Pharm. 2020, 574, 118916. [Google Scholar] [CrossRef]
- Murase, R.; Kawamura, R.; Singer, E.; Pakdel, A.; Sarma, P.; Judkins, J.; Elwakeel, E.; Dayal, S.; Martinez-Martinez, E.; Amere, M.; et al. Targeting multiple cannabinoid anti-tumour pathways with a resorcinol derivative leads to inhibition of advanced stages of breast cancer. Br. J. Pharmacol. 2014, 171, 4464–4477. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Heinemann, K.; Merkord, J.; Rohde, H.; Salamon, A.; Linnebacher, M.; Hinz, B. COX-2 and PPAR-confer cannabidiol-induced apoptosis of human lung cancer cells. Mol. Cancer Ther. 2013, 12, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Rohde, A.; Merkord, J.; Rohde, H.; Hinz, B. Decrease of plasminogen activator inhibitor-1 may contribute to the anti-invasive action of cannabidiol on human lung cancer cells. Pharm. Res. 2010, 27, 2162–2174. [Google Scholar] [CrossRef] [PubMed]
- Ramer, R.; Merkord, J.; Rohde, H.; Hinz, B. Cannabidiol inhibits cancer cell invasion via upregulation of tissue inhibitor of matrix metalloproteinases-1. Biochem. Pharmacol. 2010, 79, 955–966. [Google Scholar] [CrossRef] [PubMed]
- McMahon, G.A.; Petitclerc, E.; Stefansson, S.; Smith, E.; Wong, M.K.; Westrick, R.J.; Ginsburg, D.; Brooks, P.C.; Lawrence, D.A. Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J. Biol. Chem. 2001, 276, 33964–33968. [Google Scholar] [CrossRef] [PubMed]
- Haustein, M.; Ramer, R.; Linnebacher, M.; Manda, K.; Hinz, B. Cannabinoids increase lung cancer cell lysis by lymphokine-activated killer cells via upregulation of ICAM-1. Biochem. Pharmacol. 2014, 92, 312–325. [Google Scholar] [CrossRef]
- Scott, K.A.; Dalgleish, A.G.; Liu, W.M. The combination of cannabidiol and Δ9-tetrahydrocannabinol enhances the anticancer effects of radiation in an orthotopic murine glioma model. Mol. Cancer Ther. 2014, 13, 2955–2967. [Google Scholar] [CrossRef] [PubMed]
- Nabissi, M.; Morelli, M.B.; Santoni, M.; Santoni, G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 2013, 34, 48–57. [Google Scholar] [CrossRef]
- Massi, P.; Vaccani, A.; Ceruti, S.; Colombo, A.; Abbracchio, M.P.; Parolaro, D. Antitumor effects of cannabidiol, a nonpsychoactive cannabinoid, on human glioma cell lines. J. Pharmacol. Exp. Ther. 2004, 308, 838–845. [Google Scholar] [CrossRef]
- Alharris, E.; Singh, N.P.; Nagarkatti, P.S.; Nagarkatti, M. Role of miRNA in the regulation of cannabidiol-mediated apoptosis in neuroblastoma cells. Oncotarget 2019, 10, 45–59. [Google Scholar] [CrossRef]
- Singer, E.; Judkins, J.; Salomonis, N.; Matlaf, L.; Soteropoulos, P.; McAllister, S.; Soroceanu, L. Reactive oxygen species-mediated therapeutic response and resistance in glioblastoma. Cell Death Dis. 2015, 6, e1601. [Google Scholar] [CrossRef]
- Fisher, T.; Golan, H.; Schiby, G.; PriChen, S.; Smoum, R.; Moshe, I.; Peshes-Yaloz, N.; Castiel, A.; Waldman, D.; Gallily, R.; et al. In vitro and in vivo efficacy of non-psychoactive cannabidiol in neuroblastoma. Curr. Oncol. Tor. Ont 2016, 23, S15–S22. [Google Scholar] [CrossRef]
- Scott, K.A.; Dennis, J.L.; Dalgleish, A.G.; Liu, W.M. Inhibiting heat shock proteins can potentiate the cytotoxic effect of cannabidiol in human glioma cells. Anticancer Res. 2015, 35, 5827–5837. [Google Scholar] [PubMed]
- Aviello, G.; Romano, B.; Borrelli, F.; Capasso, R.; Gallo, L.; Piscitelli, F.; Di Marzo, V.; Izzo, A.A. Chemopreventive effect of the non-psychotropic phytocannabinoid cannabidiol on experimental colon cancer. J. Mol. Med. 2012, 90, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Honarmand, M.; Namazi, F.; Mohammadi, A.; Nazifi, S. Can cannabidiol inhibit angiogenesis in colon cancer? Comp. Clin. Pathol. 2019, 28, 165–172. [Google Scholar] [CrossRef]
- Kosgodage, U.S.; Mould, R.; Henley, A.B.; Nunn, A.V.; Guy, G.W.; Thomas, E.L.; Inal, J.M.; Bell, J.D.; Lange, S. Cannabidiol (CBD) is a novel inhibitor for exosome and microvesicle (EMV) release in cancer. Front. Pharmacol. 2018, 9, 889. [Google Scholar] [CrossRef] [PubMed]
- Simmerman, E.; Qin, X.; Yu, J.C.; Baban, B. Cannabinoids as a potential new and novel treatment for melanoma: A pilot study in a murine model. J. Surg. Res. 2019, 235, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kargl, J.; Andersen, L.; Hasenöhrl, C.; Feuersinger, D.; Stančić, A.; Fauland, A.; Magnes, C.; El-Heliebi, A.; Lax, S.; Uranitsch, S.; et al. GPR55 promotes migration and adhesion of colon cancer cells indicating a role in metastasis. Br. J. Pharmacol. 2016, 173, 142–154. [Google Scholar] [CrossRef]
- Jeong, S.; Yun, H.K.; Jeong, Y.A.; Jo, M.J.; Kang, S.H.; Kim, J.L.; Kim, D.Y.; Park, S.H.; Kim, B.R.; Na, Y.J.; et al. Cannabidiol-induced apoptosis is mediated by activation of Noxa in human colorectal cancer cells. Cancer Lett. 2019, 447, 12–23. [Google Scholar] [CrossRef]
- Morelli, M.B.; Offidani, M.; Alesiani, F.; Discepoli, G.; Liberati, S.; Olivieri, A.; Santoni, M.; Santoni, G.; Leoni, P.; Nabissi, M. The effects of cannabidiol and its synergism with bortezomib in multiple myeloma cell lines. A role for transient receptor potential vanilloid type-2. Int. J. Cancer 2014, 134, 2534–2546. [Google Scholar] [CrossRef]
- McKallip, R.J.; Jia, W.; Schlomer, J.; Warren, J.W.; Nagarkatti, P.S.; Nagarkatti, M. Cannabidiol-induced apoptosis in human leukemia cells: A novel role of cannabidiol in the regulation of p22phox and Nox4 expression. Mol. Pharmacol. 2006, 70, 897–908. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. Cannabinoid-induced cell death in endometrial cancer cells: Involvement of TRPV1 receptors in apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef]
- Lukhele, S.T.; Motadi, L.R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Altern. Med. 2016, 16, 335. [Google Scholar] [CrossRef]
- Borrelli, F.; Pagano, E.; Romano, B.; Panzera, S.; Maiello, F.; Coppola, D.; De Petrocellis, L.; Buono, L.; Orlando, P.; Izzo, A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis 2014, 35, 2787–2797. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.-H.; Han, D.S.; Yook, C.N.; Kim, Y.C.; Kwak, J.S. Synthesis and antitumor activity of cannabigerol. Arch. Pharm. Res. 1996, 19, 228–230. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, Y.O.; Kwag, J.S.; Choi, K.E.; Jung, W.Y.; Han, D.S. Boron trifluoride etherate on silica-A modified lewis acid reagent (VII). Antitumor activity of cannabigerol against human oral epitheloid carcinoma cells. Arch. Pharm. Res. 1998, 21, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, M.B.; Abazia, D.T. Medicinal cannabis: History, pharmacology, and implications for the acute care setting. P T Peer Rev. J. Formul. Manag. 2017, 42, 180–188. [Google Scholar]
- Takeda, S.; Ikeda, E.; Su, S.; Harada, M.; Okazaki, H.; Yoshioka, Y.; Nishimura, H.; Ishii, H.; Kakizoe, K.; Taniguchi, A.; et al. Δ9-THC modulation of fatty acid 2-hydroxylase (FA2H) gene expression: Possible involvement of induced levels of PPARα in MDA-MB-231 breast cancer cells. Toxicology 2014, 326, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Widmer, M.; Hanemann, C.O.; Zajicek, J. High concentrations of cannabinoids activate apoptosis in human U373MG glioma cells. J. Neurosci. Res. 2008, 86, 3212–3220. [Google Scholar] [CrossRef]
- Carracedo, A.; Geelen, M.J.H.; Diez, M.; Hanada, K.; Guzmán, M.; Velasco, G. Ceramide sensitizes astrocytes to oxidative stress: Protective role of cannabinoids. Biochem. J. 2004, 380, 435–440. [Google Scholar] [CrossRef]
- Görgün, G.T.; Whitehill, G.; Anderson, J.L.; Hideshima, T.; Maguire, C.; Laubach, J.; Raje, N.; Munshi, N.C.; Richardson, P.G.; Anderson, K.C. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood 2013, 121, 2975–2987. [Google Scholar] [CrossRef]
- Salazar, M.; Lorente, M.; García-Taboada, E.; Hernández-Tiedra, S.; Davila, D.; Francis, S.E.; Guzmán, M.; Kiss-Toth, E.; Velasco, G. The pseudokinase tribbles homologue-3 plays a crucial role in cannabinoid anticancer action. Biochim. Biophys. Acta 2013, 1831, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, W.; Shen, K.; Shen, W. ∆9-tetrahydrocannabinol inhibits epithelial-mesenchymal transition and metastasis by targeting matrix metalloproteinase-9 in endometrial cancer. Oncol. Lett. 2018, 15, 8527–8535. [Google Scholar] [CrossRef] [PubMed]
- Inglet, S.; Winter, B.; Yost, S.E.; Entringer, S.; Lian, A.; Biksacky, M.; Pitt, R.D.; Mortensen, W. Clinical data for the use of cannabis-based treatments: A comprehensive review of the literature. Ann. Pharmacother. 2020. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.J.; Knoderer, H.M. Characterization of dronabinol usage in a pediatric oncology population. J. Pediatr. Pharmacol. Ther. JPPT Off. J. PPAG 2015, 20, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Good, P.D.; Greer, R.M.; Huggett, G.E.; Hardy, J.R. An open-label pilot study testing the feasibility of assessing total symptom burden in trials of cannabinoid medications in palliative care. J. Palliat. Med. 2020, 23, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Kis, B.; Ifrim, F.C.; Buda, V.; Avram, S.; Pavel, I.Z.; Antal, D.; Paunescu, V.; Dehelean, C.A.; Ardelean, F.; Diaconeasa, Z.; et al. Cannabidiol-from plant to human body: A promising bioactive molecule with multi-target effects in cancer. Int. J. Mol. Sci. 2019, 20, 5905. [Google Scholar] [CrossRef] [PubMed]
- Kalenderoglou, N.; Macpherson, T.; Wright, K.L. Cannabidiol reduces leukemic cell size—But is it important? Front. Pharmacol. 2017, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Weedmaps Cannabis Dictionary. Available online: https://weedmaps.com/learn/dictionary/ (accessed on 21 May 2020).
- Navarro, G.; Varani, K.; Reyes-Resina, I.; Sánchez de Medina, V.; Rivas-Santisteban, R.; Sánchez-Carnerero Callado, C.; Vincenzi, F.; Casano, S.; Ferreiro-Vera, C.; Canela, E.I.; et al. Cannabigerol action at cannabinoid CB1 and CB2 receptors and at CB1-CB2 heteroreceptor complexes. Front. Pharmacol. 2018, 9, 632. [Google Scholar] [CrossRef]
- Izzo, A.A.; Capasso, R.; Aviello, G.; Borrelli, F.; Romano, B.; Piscitelli, F.; Gallo, L.; Capasso, F.; Orlando, P.; Di Marzo, V. Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br. J. Pharmacol. 2012, 166, 1444–1460. [Google Scholar] [CrossRef]
- Huestis, M.A. Pharmacokinetics and metabolism of the plant cannabinoids, Δ9-tetrahydrocannibinol, cannabidiol and cannabinol. In Cannabinoids; Pertwee, R.G., Ed.; Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2005; Volume 168, pp. 657–690. ISBN 978-3-540-22565-2. [Google Scholar]
- Giese, M.W.; Lewis, M.A.; Giese, L.; Smith, K.M. Method for the analysis of cannabinoids and terpenes in cannabis. J. AOAC Int. 2015, 98, 1503–1522. [Google Scholar] [CrossRef]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa—From plant genome to humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- Casano, S.; Grassi, G.; Martini, V.; Michelozzi, M. Variations in terpene profiles of different strains of Cannabis sativa L. Acta Hortic. 2011, 115–121. [Google Scholar] [CrossRef]
- Saleh, M.M.; Hashem, F.A.; Glombitza, K.W. Cytotoxicity and in vitro effects on human cancer cell lines of volatiles of Apium graveolens var filicinum. Pharm. Pharmacol. Lett. 1998, 8, 97–99. [Google Scholar]
- Ferraz, R.P.C.; Bomfim, D.S.; Carvalho, N.C.; Soares, M.B.P.; Da Silva, T.B.; Machado, W.J.; Prata, A.P.N.; Costa, E.V.; Moraes, V.R.S.; Nogueira, P.C.L.; et al. Cytotoxic effect of leaf essential oil of Lippia gracilis Schauer (Verbenaceae). Phytomedicine 2013, 20, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.L.; Figueiredo, P.M.; Yano, T. Cytotoxic evaluation of essential oil from Zanthoxylum rhoifolium Lam. leaves. Acta Amaz. 2007, 37, 281–286. [Google Scholar] [CrossRef]
- Sobral, M.V.; Xavier, A.L.; Lima, T.C.; De Sousa, D.P. Antitumor activity of monoterpenes found in essential oils. Sci. World J. 2014, 2014, 953451. [Google Scholar] [CrossRef] [PubMed]
- Mitić-Ćulafić, D.; Žegura, B.; Nikolić, B.; Vuković-Gačić, B.; Knežević-Vukčević, J.; Filipič, M. Protective effect of linalool, myrcene and eucalyptol against t-butyl hydroperoxide induced genotoxicity in bacteria and cultured human cells. Food Chem. Toxicol. 2009, 47, 260–266. [Google Scholar] [CrossRef]
- National Toxicology Program. NTP technical report on the toxicology and carcinogenesis studies of beta-myrcene (CAS No. 123-35-3) in F344/N rats and B6C3F1 mice (Gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 2010, 557, 1–163. [Google Scholar]
- Chung, K.-S.; Hong, J.Y.; Lee, J.-H.; Lee, H.-J.; Park, J.Y.; Choi, J.-H.; Park, H.-J.; Hong, J.; Lee, K.-T. β-caryophyllene in the essential oil from chrysanthemum boreale induces G1 phase cell cycle arrest in human lung cancer cells. Molecules 2019, 24, 3754. [Google Scholar] [CrossRef]
- Arul, S.; Rajagopalan, H.; Ravi, J.; Dayalan, H. Beta-caryophyllene suppresses ovarian cancer proliferation by inducing cell cycle arrest and apoptosis. Anticancer Agents Med. Chem. 2020, 20. [Google Scholar] [CrossRef]
- Annamalai, V.; Kotakonda, M.; Periyannan, V. JAK1/STAT3 regulatory effect of β-caryophyllene on MG-63 osteosarcoma cells via ROS-induced apoptotic mitochondrial pathway by DNA fragmentation. J. Biochem. Mol. Toxicol. 2020. [Google Scholar] [CrossRef]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Synergistic interaction of β-caryophyllene with aromadendrene oxide 2 and phytol induces apoptosis on skin epidermoid cancer cells. Phytomedicine 2018, 47, 121–134. [Google Scholar] [CrossRef]
- Di Sotto, A.; Di Giacomo, S.; Rubini, E.; Macone, A.; Gulli, M.; Mammola, C.L.; Eufemi, M.; Mancinelli, R.; Mazzanti, G. Modulation of STAT3 Signaling, cell redox defenses and cell cycle checkpoints by β-caryophyllene in cholangiocarcinoma cells: Possible mechanisms accounting for doxorubicin chemosensitization and chemoprevention. Cells 2020, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Di Sotto, A.; Mazzanti, G.; Wink, M. Chemosensitizing properties of β-caryophyllene and β-caryophyllene oxide in combination with doxorubicin in human cancer cells. Anticancer Res. 2017, 37, 1191–1196. [Google Scholar] [CrossRef]
- Di Sotto, A.; Irannejad, H.; Eufemi, M.; Mancinelli, R.; Abete, L.; Mammola, C.L.; Altieri, F.; Mazzanti, G.; Di Giacomo, S. Potentiation of low-dose doxorubicin cytotoxicity by affecting P-glycoprotein through caryophyllane sesquiterpenes in HepG2 cells: An in vitro and in silico study. Int. J. Mol. Sci. 2020, 21, 633. [Google Scholar] [CrossRef] [PubMed]
- Ambrož, M.; Matoušková, P.; Skarka, A.; Zajdlová, M.; Žáková, K.; Skálová, L. The effects of selected sesquiterpenes from Myrica rubra essential oil on the efficacy of doxorubicin in sensitive and resistant cancer cell lines. Molecules 2017, 22, 1021. [Google Scholar] [CrossRef] [PubMed]
- Hanušová, V.; Caltová, K.; Svobodová, H.; Ambrož, M.; Skarka, A.; Murínová, N.; Králová, V.; Tomšík, P.; Skálová, L. The effects of β-caryophyllene oxide and trans-nerolidol on the efficacy of doxorubicin in breast cancer cells and breast tumor-bearing mice. Biomed. Pharmacother. 2017, 95, 828–836. [Google Scholar] [CrossRef]
- Ambrož, M.; Šmatová, M.; Šadibolová, M.; Pospíšilová, E.; Hadravská, P.; Kašparová, M.; Skarková, V.H.; Králová, V.; Skálová, L. Sesquiterpenes α-humulene and β-caryophyllene oxide enhance the efficacy of 5-fluorouracil and oxaliplatin in colon cancer cells. Acta Pharm. 2019, 69, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, S.; Briz, O.; Monte, M.J.; Sanchez-Vicente, L.; Abete, L.; Lozano, E.; Mazzanti, G.; Di Sotto, A.; Marin, J.J.G. Chemosensitization of hepatocellular carcinoma cells to sorafenib by β-caryophyllene oxide-induced inhibition of ABC export pumps. Arch. Toxicol. 2019, 93, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.F.N.; Al Taee, H.; Azimullah, S.; Tariq, S.; Adeghate, E.; Ojha, S. β-Caryophyllene, a natural bicyclic sesquiterpene attenuates doxorubicin-induced chronic cardiotoxicity via activation of myocardial cannabinoid type-2 (CB2) receptors in rats. Chem. Biol. Interact. 2019, 304, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Bonesi, M.; Menichini, F.; Dodaro, D.; Passalacqua, N.G.; Statti, G.; Menichini, F. In vitro cytotoxic effects of Senecio stabianus Lacaita (Asteraceae) on human cancer cell lines. Nat. Prod. Res. 2009, 23, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yuan, J.; Hao, J.; Wen, Y.; Lv, Y.; Chen, L.; Yang, X. α-Humulene inhibits hepatocellular carcinoma cell proliferation and induces apoptosis through the inhibition of Akt signaling. Food Chem. Toxicol. 2019, 134, 110830. [Google Scholar] [CrossRef] [PubMed]
- Legault, J.; Pichette, A. Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol. 2007, 59, 1643–1647. [Google Scholar] [CrossRef]
- Ye, Z.; Liang, Z.; Mi, Q.; Guo, Y. Limonene terpenoid obstructs human bladder cancer cell (T24 cell line) growth by inducing cellular apoptosis, caspase activation, G2/M phase cell cycle arrest and stops cancer metastasis. J. BUON Off. J. Balk. Union Oncol. 2020, 25, 280–285. [Google Scholar]
- Jia, S.-S.; Xi, G.-P.; Zhang, M.; Chen, Y.-B.; Lei, B.; Dong, X.-S.; Yang, Y.-M. Induction of apoptosis by d-limonene is mediated by inactivation of Akt in LS174T human colon cancer cells. Oncol. Rep. 2013, 29, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh, M.; Maleki, H.; Barani, M.; Fahmidehkar, M.; Mahmoodi, M.; Torkzadeh-Mahani, M. In vitro cytotoxicity assay of d-limonene niosomes: An efficient nano-carrier for enhancing solubility of plant-extracted agents. Res. Pharm. Sci. 2019, 14, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Hafidh, R.R.; Hussein, S.Z.; MalAllah, M.Q.; Abdulamir, A.S.; Abu Bakar, F. A high-throughput quantitative expression analysis of cancer-related genes in human HepG2 cells in response to limonene, a potential anticancer agent. Curr. Cancer Drug Targets 2018, 18, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Z.; Wang, L.; Liu, D.-W.; Tang, G.-Y.; Zhang, H.-Y. Synergistic inhibitory effect of berberine and d-limonene on human gastric carcinoma cell line MGC803. J. Med. Food 2014, 17, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; Rabi, T. d-Limonene sensitizes docetaxel-induced cytotoxicity in human prostate cancer cells: Generation of reactive oxygen species and induction of apoptosis. J. Carcinog. 2009, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Berliocchi, L.; Chiappini, C.; Adornetto, A.; Gentile, D.; Cerri, S.; Russo, R.; Bagetta, G.; Corasaniti, M.T. Early LC3 lipidation induced by d-limonene does not rely on mTOR inhibition, ERK activation and ROS production and it is associated with reduced clonogenic capacity of SH-SY5Y neuroblastoma cells. Phytomedicine 2018, 40, 98–105. [Google Scholar] [CrossRef]
- Russo, R.; Cassiano, M.G.V.; Ciociaro, A.; Adornetto, A.; Varano, G.P.; Chiappini, C.; Berliocchi, L.; Tassorelli, C.; Bagetta, G.; Corasaniti, M.T. Role of d-limonene in autophagy induced by bergamot essential oil in SH-SY5Y neuroblastoma cells. PLoS ONE 2014, 9, e113682. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Lin, H.; Wang, Y.; Lv, W.; Zhang, S.; Qian, Y.; Deng, X.; Feng, N.; Yu, H.; Qian, B. d-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. Onco Targets Ther. 2018, 11, 1833–1847. [Google Scholar] [CrossRef]
- Haag, J.D.; Lindstrom, M.J.; Gould, M.N. Limonene-induced regression of mammary carcinomas. Cancer Res. 1992, 52, 4021–4026. [Google Scholar]
- Gould, M.N.; Moore, C.J.; Zhang, R.; Wang, B.; Kennan, W.S.; Haag, J.D. Limonene chemoprevention of mammary carcinoma induction following direct in situ transfer of v-Ha-ras. Cancer Res. 1994, 54, 3540–3543. [Google Scholar] [PubMed]
- Elegbede, J.A.; Elson, C.E.; Tanner, M.A.; Qureshi, A.; Gould, M.N. Regression of rat primary mammary tumors following dietary d-limonene. J. Natl. Cancer Inst. 1986, 76, 323–325. [Google Scholar] [PubMed]
- Nakaizumi, A.; Baba, M.; Uehara, H.; Iishi, H.; Tatsuta, M. d-Limonene inhibits N-nitrosobis(2-oxopropyl)amine induced hamster pancreatic carcinogenesis. Cancer Lett. 1997, 117, 99–103. [Google Scholar] [CrossRef]
- Manuele, M.G.; Barreiro Arcos, M.L.; Davicino, R.; Ferraro, G.; Cremaschi, G.; Anesini, C. Limonene exerts antiproliferative effects and increases nitric oxide levels on a lymphoma cell line by dual mechanism of the ERK pathway: Relationship with oxidative stress. Cancer Investig. 2009, 28, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.-G.; Zhan, L.-B.; Feng, B.-A.; Qu, M.-Y.; Yu, L.-H.; Xie, J.-H. Inhibition of growth and metastasis of human gastric cancer implanted in nude mice by d-limonene. World J. Gastroenterol. 2004, 10, 2140–2144. [Google Scholar] [CrossRef]
- Uedo, N.; Tatsuta, M.; Iishi, H.; Baba, M.; Sakai, N.; Yano, H.; Otani, T. Inhibition by d-limonene of gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Cancer Lett. 1999, 137, 131–136. [Google Scholar] [CrossRef]
- Wattenberg, L.W.; Coccia, J.B. Inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone carcinogenesis in mice by d-limonene and citrus fruit oils. Carcinogenesis 1991, 12, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Siddiqui, M.; Athar, M.; Alam, M.S. Limonene modulates inflammation, oxidative stress and Ras-ERK pathway to inhibit murine skin tumorigenesis. Hum. Exp. Toxicol. 2012, 31, 798–811. [Google Scholar] [CrossRef]
- Giri, R.K.; Parija, T.; Das, B.R. d-limonene chemoprevention of hepatocarcinogenesis in AKR mice: Inhibition of c-jun and c-myc. Oncol. Rep. 1999, 6, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Raphael, T.J.; Kuttan, G. Effect of naturally occurring monoterpenes carvone, limonene and perillic acid in the inhibition of experimental lung metastasis induced by B16F-10 melanoma cells. J. Exp. Clin. Cancer Res. CR 2003, 22, 419–424. [Google Scholar] [PubMed]
- Miller, J.A.; Pappan, K.; Thompson, P.A.; Want, E.J.; Siskos, A.P.; Keun, H.C.; Wulff, J.; Hu, C.; Lang, J.E.; Chow, H.-H.S. Plasma metabolomic profiles of breast cancer patients after short-term limonene intervention. Cancer Prev. Res. (Phila. Pa) 2015, 8, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhang, Y.; Zhu, Y.; Zhou, B.; Ren, C.; Liang, S.; Guo, Y. α-pinene induces apoptotic cell death via caspase activation in human ovarian cancer cells. Med. Sci. Monit. 2019, 25, 6631–6638. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, M.; Yang, M.; Yang, J.; Xie, J.; Lu, X.; Wang, F.; Chen, W. α-pinene regulates miR-221 and induces G2/M phase cell cycle arrest in human hepatocellular carcinoma cells. Biosci. Rep. 2018, 38, BSR20180980. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, R.; Wang, Y.; Yang, Y. α-pinene inhibits human prostate cancer growth in a mouse xenograft model. Chemotherapy 2018, 63, 1–7. [Google Scholar] [CrossRef]
- Li, Y.-L.; Yeung, C.-M.; Chiu, L.C.M.; Cen, Y.-Z.; Ooi, V.E.C. Chemical composition and antiproliferative activity of essential oil from the leaves of a medicinal herb, Schefflera heptaphylla. Phytother. Res. 2009, 23, 140–142. [Google Scholar] [CrossRef]
- Matsuo, A.L.; Figueiredo, C.R.; Arruda, D.C.; Pereira, F.V.; Borin Scutti, J.A.; Massaoka, M.H.; Travassos, L.R.; Sartorelli, P.; Lago, J.H.G. α-Pinene isolated from Schinus terebinthifolius Raddi (Anacardiaceae) induces apoptosis and confers antimetastatic protection in a melanoma model. Biochem. Biophys. Res. Commun. 2011, 411, 449–454. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, S.; Liu, X.; Gao, X. Synergistic antitumor effect of α-pinene and β-pinene with paclitaxel against non-small-cell lung carcinoma (NSCLC). Drug Res. 2014, 65, 214–218. [Google Scholar] [CrossRef]
- Chen, W.-Q.; Xu, B.; Mao, J.-W.; Wei, F.-X.; Li, M.; Liu, T.; Jin, X.-B.; Zhang, L.-R. Inhibitory effects of α-pinene on hepatoma carcinoma cell proliferation. Asian Pac. J. Cancer Prev. 2014, 15, 3293–3297. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Li, M.; Mao, J.; Zhang, L.; Huang, R.; Jin, X.; Ye, L. Anti-tumor effect of α-pinene on human hepatoma cell lines through inducing G2/M cell cycle arrest. J. Pharmacol. Sci. 2015, 127, 332–338. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, G. Linalool monoterpene exerts potent antitumor effects in OECM 1 human oral cancer cells by inducing sub-G1 cell cycle arrest, loss of mitochondrial membrane potential and inhibition of PI3K/AKT biochemical pathway. J. BUON Off. J. Balk. Union Oncol. 2019, 24, 323–328. [Google Scholar]
- Rodenak-Kladniew, B.; Castro, A.; Stärkel, P.; De Saeger, C.; García de Bravo, M.; Crespo, R. Linalool induces cell cycle arrest and apoptosis in HepG2 cells through oxidative stress generation and modulation of Ras/MAPK and Akt/mTOR pathways. Life Sci. 2018, 199, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, C.; Zhang, J. SIRT3-SOD2-ROS pathway is involved in Linalool-induced glioma cell apoptotic death. Acta Biochim. Pol. 2017, 64. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-Y.; Shen, Y.-L. Linalool exhibits cytotoxic effects by activating antitumor immunity. Molecules 2014, 19, 6694–6706. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.-Y.; Shieh, D.-E.; Chen, C.-C.; Yeh, C.-S.; Dong, H.-P. Linalool induces cell cycle arrest and apoptosis in leukemia cells and cervical cancer cells through CDKIs. Int. J. Mol. Sci. 2015, 16, 28169–28179. [Google Scholar] [CrossRef]
- Iwasaki, K.; Zheng, Y.-W.; Murata, S.; Ito, H.; Nakayama, K.; Kurokawa, T.; Sano, N.; Nowatari, T.; Villareal, M.O.; Nagano, Y.N.; et al. Anticancer effect of linalool via cancer-specific hydroxyl radical generation in human colon cancer. World J. Gastroenterol. 2016, 22, 9765–9774. [Google Scholar] [CrossRef]
- Jana, S.; Patra, K.; Sarkar, S.; Jana, J.; Mukherjee, G.; Bhattacharjee, S.; Mandal, D.P. Antitumorigenic potential of linalool is accompanied by modulation of oxidative stress: An in vivo study in sarcoma-180 solid tumor model. Nutr. Cancer 2014, 66, 835–848. [Google Scholar] [CrossRef]
- Miyashita, M.; Sadzuka, Y. Effect of linalool as a component of Humulus lupulus on doxorubicin-induced antitumor activity. Food Chem. Toxicol. 2013, 53, 174–179. [Google Scholar] [CrossRef]
- Ravizza, R.; Gariboldi, M.B.; Molteni, R.; Monti, E. Linalool, a plant-derived monoterpene alcohol, reverses doxorubicin resistance in human breast adenocarcinoma cells. Oncol. Rep. 2008, 20, 625–630. [Google Scholar] [CrossRef]
- Gunaseelan, S.; Balupillai, A.; Govindasamy, K.; Muthusamy, G.; Ramasamy, K.; Shanmugam, M.; Prasad, N.R. The preventive effect of linalool on acute and chronic UVB-mediated skin carcinogenesis in Swiss albino mice. Photochem. Photobiol. Sci. 2016, 15, 851–860. [Google Scholar] [CrossRef]
- Rigo, A.; Ferrarini, I.; Lorenzetto, E.; Darra, E.; Liparulo, I.; Bergamini, C.; Sissa, C.; Cavalieri, E.; Vinante, F. BID and the α-bisabolol-triggered cell death program: Converging on mitochondria and lysosomes. Cell Death Dis. 2019, 10, 889. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Peng, L.; Sang, H.; Ping Li, Q.; Cheng, S. Anticancer effects of α-Bisabolol in human non-small cell lung carcinoma cells are mediated via apoptosis induction, cell cycle arrest, inhibition of cell migration and invasion and upregulation of P13K/AKT signalling pathway. J. BUON Off. J. Balk. Union Oncol. 2018, 23, 1407–1412. [Google Scholar]
- Cavalieri, E.; Mariotto, S.; Fabrizi, C.; De Prati, A.C.; Gottardo, R.; Leone, S.; Berra, L.V.; Lauro, G.M.; Ciampa, A.R.; Suzuki, H. α-Bisabolol, a nontoxic natural compound, strongly induces apoptosis in glioma cells. Biochem. Biophys. Res. Commun. 2004, 315, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.; Bergamin, L.; Dos Santos Stuepp, C.; Braganhol, E.; Terroso, T.; Pohlmann, A.; Guterres, S.; Battastini, A. Alpha-bisabolol promotes glioma cell death by modulating the adenosinergic system. Anticancer Res. 2017, 37, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Rigo, A.; Ferrarini, I.; Bonalumi, A.; Tecchio, C.; Montresor, A.; Laudanna, C.; Vinante, F. Efficient lysis of B-chronic lymphocytic leukemia cells by the plant-derived sesquiterpene alcohol α-bisabolol, a dual proapoptotic and antiautophagic agent. Oncotarget 2018, 9, 25877–25890. [Google Scholar] [CrossRef][Green Version]
- Cavalieri, E.; Rigo, A.; Bonifacio, M.; De Prati, A.; Guardalben, E.; Bergamini, C.; Fato, R.; Pizzolo, G.; Suzuki, H.; Vinante, F. Pro-apoptotic activity of α-bisabolol in preclinical models of primary human acute leukemia cells. J. Transl. Med. 2011, 9, 45. [Google Scholar] [CrossRef]
- Chen, W.; Hou, J.; Yin, Y.; Jang, J.; Zheng, Z.; Fan, H.; Zou, G. α-Bisabolol induces dose- and time-dependent apoptosis in HepG2 cells via a Fas- and mitochondrial-related pathway, involves p53 and NFκB. Biochem. Pharmacol. 2010, 80, 247–254. [Google Scholar] [CrossRef]
- Seki, T.; Kokuryo, T.; Yokoyama, Y.; Suzuki, H.; Itatsu, K.; Nakagawa, A.; Mizutani, T.; Miyake, T.; Uno, M.; Yamauchi, K.; et al. Antitumor effects of α-bisabolol against pancreatic cancer. Cancer Sci. 2011, 102, 2199–2205. [Google Scholar] [CrossRef]
- Fang, D.; Wang, H.; Li, M.; Wei, W. α-bisabolol enhances radiotherapy-induced apoptosis in endometrial cancer cells by reducing the effect of XIAP on inhibiting caspase-3. Biosci. Rep. 2019, 39, BSR20190696. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, J.K.; Gupta, S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J. Agric. Food Chem. 2007, 55, 9470–9478. [Google Scholar] [CrossRef]
- Srivastava, J.K.; Shankar, E.; Gupta, S. Chamomile: A herbal medicine of the past with a bright future (review). Mol. Med. Rep. 2010, 3. [Google Scholar] [CrossRef]
- Yao, Y.-Q.; Ding, X.; Jia, Y.-C.; Huang, C.-X.; Wang, Y.-Z.; Xu, Y.-H. Anti-tumor effect of beta-elemene in glioblastoma cells depends on p38 MAPK activation. Cancer Lett. 2008, 264, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Rigo, A.; Vinante, F. The antineoplastic agent α-bisabolol promotes cell death by inducing pores in mitochondria and lysosomes. Apoptosis 2016, 21, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Costarelli, L.; Malavolta, M.; Giacconi, R.; Cipriano, C.; Gasparini, N.; Tesei, S.; Pierpaoli, S.; Orlando, F.; Suzuki, H.; Perbellini, L.; et al. In vivo effect of alpha-bisabolol, a nontoxic sesquiterpene alcohol, on the induction of spontaneous mammary tumors in HER-2/neu transgenic mice. Oncol. Res. 2010, 18, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Kokuryo, T.; Yokoyama, Y.; Yamaguchi, J.; Miwa, T.; Shibuya, M.; Yamamoto, Y.; Nagino, M. The anticancer effects of novel α-bisabolol derivatives against pancreatic cancer. Anticancer Res. 2017, 37, 589–598. [Google Scholar] [CrossRef]
- Quintanilha, N.P.; Dos Santos Miranda Costa, I.; Freiman de Souza Ramos, M.; Campos de Oliveira Miguel, N.; Riemma Pierre, M.B. α-Bisabolol improves 5-aminolevulinic acid retention in buccal tissues: Potential application in the photodynamic therapy of oral cancer. J. Photochem. Photobiol. B 2017, 174, 298–305. [Google Scholar] [CrossRef]
- Wang, G.; Li, X.; Huang, F.; Zhao, J.; Ding, H.; Cunningham, C.; Coad, J.E.; Flynn, D.C.; Reed, E.; Li, Q.Q. Antitumor effect of beta-elemene in non-small-cell lung cancer cells is mediated via induction of cell cycle arrest and apoptotic cell death. Cell. Mol. Life Sci. CMLS 2005, 62, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, G.; Zhao, J.; Ding, H.; Cunningham, C.; Chen, F.; Flynn, D.C.; Reed, E.; Li, Q.Q. Antiproliferative effect of beta-elemene in chemoresistant ovarian carcinoma cells is mediated through arrest of the cell cycle at the G2-M phase. Cell. Mol. Life Sci. CMLS 2005, 62, 894–904. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, R.; Xu, L.; Xie, S.; Dong, J.; Jing, Y. β-Elemene piperazine derivatives induce apoptosis in human leukemia cells through downregulation of c-FLIP and generation of ROS. PLoS ONE 2011, 6, e15843. [Google Scholar] [CrossRef]
- Li, Q.Q.; Wang, G.; Huang, F.; Banda, M.; Reed, E. Antineoplastic effect of beta-elemene on prostate cancer cells and other types of solid tumour cells. J. Pharm. Pharmacol. 2010, 62, 1018–1027. [Google Scholar] [CrossRef]
- Chen, W.; Lu, Y.; Wu, J.; Gao, M.; Wang, A.; Xu, B. Beta-elemene inhibits melanoma growth and metastasis via suppressing vascular endothelial growth factor-mediated angiogenesis. Cancer Chemother. Pharmacol. 2011, 67, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Ling, C.; Li, W.; Jiang, H.; Zhi, Q.; Jiang, M. Molecular mechanisms of anti-cancer activities of β-elemene: Targeting hallmarks of cancer. Anticancer Agents Med. Chem. 2016, 16, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Xu, Y.; Dong, B.; Zhang, J.; Wei, Z.; Xu, Y.; Yao, Y. β-elemene inhibits proliferation of human glioblastoma cells through the activation of glia maturation factor β and induces sensitization to cisplatin. Oncol. Rep. 2011, 26, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, Z.; Zhang, B.; Guo, L.; Liu, S.; Li, H.; Zhang, J.; Ye, Q. β-elemene sensitizes hepatocellular carcinoma cells to oxaliplatin by preventing oxaliplatin-induced degradation of copper transporter 1. Sci. Rep. 2016, 6, 21010. [Google Scholar] [CrossRef]
- Balavandi, Z.; Neshasteh-Riz, A.; Koosha, F.; Eynali, S.; Hoormand, M.; Shahidi, M. The use of β-elemene to enhance radio sensitization of A375 human melanoma cells. Cell J. Yakhteh 2019, 21. [Google Scholar] [CrossRef]
- Huang, C.; Yu, Y. Synergistic cytotoxicity of β-elemene and cisplatin in gingival squamous cell carcinoma by inhibition of STAT3 signaling pathway. Med. Sci. Monit. 2017, 23, 1507–1513. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Liu, Y.; Jiang, L.; Wang, J. Multi-targeting by β-elemene and its anticancer properties: A good choice for oncotherapy and radiochemotherapy sensitization. Nutr. Cancer 2020, 72, 554–567. [Google Scholar] [CrossRef]
- Zhang, G.-N.; Ashby, C.R.; Zhang, Y.-K.; Chen, Z.-S.; Guo, H. The reversal of antineoplastic drug resistance in cancer cells by β-elemene. Chin. J. Cancer 2015, 34, 45. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, L.; Wang, L.; Zhou, T.; Liu, J.W.; Gao, Y.J. Preliminary study of the effects of β-elemene on MCF-7/ADM breast cancer stem cells. Genet. Mol. Res. 2015, 14, 2347–2355. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Sui, X.; Wu, Q.; Wang, J.; Xu, C. Elemene injection as adjunctive treatment to platinum-based chemotherapy in patients with stage III/IV non-small cell lung cancer: A meta-analysis following the PRISMA guidelines. Phytomedicine 2019, 59, 152787. [Google Scholar] [CrossRef]
- Xu, H.-B.; Zheng, L.-P.; Li, L.; Xu, L.; Fu, J. Elemene, one ingredient of a Chinese herb, against malignant tumors: A literature-based meta-analysis. Cancer Investig. 2013, 31, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, D.S.; Ferraz, R.P.C.; Carvalho, N.C.; Soares, M.B.P.; Pinheiro, M.L.B.; Costa, E.V.; Bezerra, D.P. Eudesmol isomers induce caspase-mediated apoptosis in human hepatocellular carcinoma HepG2 cells. Basic Clin. Pharmacol. Toxicol. 2013, 113, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Ma, E.-L.; Li, Y.-C.; Tsuneki, H.; Xiao, J.-F.; Xia, M.-Y.; Wang, M.-W.; Kimura, I. β-Eudesmol suppresses tumour growth through inhibition of tumour neovascularisation and tumour cell proliferation. J. Asian Nat. Prod. Res. 2008, 10, 159–167. [Google Scholar] [CrossRef]
- Ben Sghaier, M.; Mousslim, M.; Pagano, A.; Ammari, Y.; Luis, J.; Kovacic, H. β-eudesmol, a sesquiterpene from Teucrium ramosissimum, inhibits superoxide production, proliferation, adhesion and migration of human tumor cell. Environ. Toxicol. Pharmacol. 2016, 46, 227–233. [Google Scholar] [CrossRef]
- Kotawong, K.; Chaijaroenkul, W.; Muhamad, P.; Na-Bangchang, K. Cytotoxic activities and effects of atractylodin and β-eudesmol on the cell cycle arrest and apoptosis on cholangiocarcinoma cell line. J. Pharmacol. Sci. 2018, 136, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Mathema, V.B.; Chaijaroenkul, W.; Karbwang, J.; Na-Bangchang, K. Growth inhibitory effect of β-eudesmol on cholangiocarcinoma cells and its potential suppressive effect on heme oxygenase-1 production, STAT1/3 activation, and NF-κB downregulation. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Srijiwangsa, P.; Ponnikorn, S.; Na-Bangchang, K. Effect of β-Eudesmol on NQO1 suppression-enhanced sensitivity of cholangiocarcinoma cells to chemotherapeutic agents. BMC Pharmacol. Toxicol. 2018, 19, 32. [Google Scholar] [CrossRef]
- Plengsuriyakarn, T.; Karbwang, J.; Na-Bangchang, K. Anticancer activity using positron emission tomography-computed tomography and pharmacokinetics of β-eudesmol in human cholangiocarcinoma xenografted nude mouse model. Clin. Exp. Pharmacol. Physiol. 2015, 42, 293–304. [Google Scholar] [CrossRef]
- Leighton, X.; Bera, A.; Eidelman, O.; Eklund, M.; Puthillathu, N.; Pollard, H.B.; Srivastava, M. High ANXA7 potentiates eucalyptol toxicity in hormone-refractory prostate cancer. Anticancer Res. 2018, 38, 3831–3842. [Google Scholar] [CrossRef]
- Moteki, H.; Hibasami, H.; Yamada, Y.; Katsuzaki, H.; Imai, K.; Komiya, T. Specific induction of apoptosis by 1,8-cineole in two human leukemia cell lines, but not a in human stomach cancer cell line. Oncol. Rep. 2002, 9, 757–760. [Google Scholar] [CrossRef]
- Abdalla, A.N.; Shaheen, U.; Abdallah, Q.M.A.; Flamini, G.; Bkhaitan, M.M.; Abdelhady, M.I.S.; Ascrizzi, R.; Bader, A. Proapoptotic activity of Achillea membranacea essential oil and its major constituent 1,8-cineole against A2780 ovarian cancer cells. Molecules 2020, 25, 1582. [Google Scholar] [CrossRef]
- Murata, S.; Shiragami, R.; Kosugi, C.; Tezuka, T.; Yamazaki, M.; Hirano, A.; Yoshimura, Y.; Suzuki, M.; Shuto, K.; Ohkohchi, N.; et al. Antitumor effect of 1, 8-cineole against colon cancer. Oncol. Rep. 2013, 30, 2647–2652. [Google Scholar] [CrossRef] [PubMed]
- Sampath, S.; Subramani, S.; Janardhanam, S.; Subramani, P.; Yuvaraj, A.; Chellan, R. Bioactive compound 1,8-Cineole selectively induces G2/M arrest in A431 cells through the upregulation of the p53 signaling pathway and molecular docking studies. Phytomedicine 2018, 46, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Li, Y.; Hou, Y.; Yang, M.; Fu, X.; Zhao, B.; Jiang, H.; Fu, X. Enhanced anticancer efficiency of doxorubicin against human glioma by natural borneol through triggering ROS-mediated signal. Biomed. Pharmacother. 2019, 118, 109261. [Google Scholar] [CrossRef]
- Meng, X.; Dong, X.; Wang, W.; Yang, L.; Zhang, X.; Li, Y.; Chen, T.; Ma, H.; Qi, D.; Su, J. Natural borneol enhances paclitaxel-induced apoptosis of ESCC cells by inactivation of the PI3K/AKT. J. Food Sci. 2018, 83, 1436–1443. [Google Scholar] [CrossRef]
- Su, J.; Lai, H.; Chen, J.; Li, L.; Wong, Y.-S.; Chen, T.; Li, X. Natural borneol, a monoterpenoid compound, potentiates selenocystine-induced apoptosis in human hepatocellular carcinoma cells by enhancement of cellular uptake and activation of ROS-mediated DNA damage. PLoS ONE 2013, 8, e63502. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, L.; Su, J.; Li, B.; Chen, T.; Wong, Y.-S. Synergistic apoptosis-inducing effects on A375 human melanoma cells of natural borneol and curcumin. PLoS ONE 2014, 9, e101277. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Su, J.; Chen, T. Natural borneol enhances bisdemethoxycurcumin-induced cell cycle arrest in the G2/M phase through up-regulation of intracellular ROS in HepG2 cells. Food Funct. 2015, 6, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-J.; Yin, Y.-B.; Sun, J.-Y.; Feng, S.; Ma, J.-K.; Fu, X.-Y.; Hou, Y.-J.; Yang, M.-F.; Sun, B.-L.; Fan, C.-D. Natural borneol is a novel chemosensitizer that enhances temozolomide-induced anticancer efficiency against human glioma by triggering mitochondrial dysfunction and reactive oxide species-mediated oxidative damage. Onco Targets Ther. 2018, 11, 5429–5439. [Google Scholar] [CrossRef]
- Meng, L.; Chu, X.; Xing, H.; Liu, X.; Xin, X.; Chen, L.; Jin, M.; Guan, Y.; Huang, W.; Gao, Z. Improving glioblastoma therapeutic outcomes via doxorubicin-loaded nanomicelles modified with borneol. Int. J. Pharm. 2019, 567, 118485. [Google Scholar] [CrossRef]
- Zou, L.; Wang, D.; Hu, Y.; Fu, C.; Li, W.; Dai, L.; Yang, L.; Zhang, J. Drug resistance reversal in ovarian cancer cells of paclitaxel and borneol combination therapy mediated by PEG-PAMAM nanoparticles. Oncotarget 2017, 8, 60453–60468. [Google Scholar] [CrossRef]
- Han, S.; Zheng, H.; Lu, Y.; Sun, Y.; Huang, A.; Fei, W.; Shi, X.; Xu, X.; Li, J.; Li, F. A novel synergetic targeting strategy for glioma therapy employing borneol combination with angiopep-2-modified, DOX-loaded PAMAM dendrimer. J. Drug Target. 2018, 26, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, J.; Han, S.; Tao, C.; Fang, L.; Sun, Y.; Zhu, J.; Liang, Z.; Li, F. A novel doxorubicin loaded folic acid conjugated PAMAM modified with borneol, a nature dual-functional product of reducing PAMAM toxicity and boosting BBB penetration. Eur. J. Pharm. Sci. 2016, 88, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Chen, J.; Ji, S.; Chan, L.; Zheng, W.; Chen, T. Construction of a cancer-targeted nanosystem as a payload of iron complexes to reverse cancer multidrug resistance. J. Mater. Chem. B 2015, 3, 4345–4354. [Google Scholar] [CrossRef]
- Yin, Y.; Cao, L.; Ge, H.; Duanmu, W.; Tan, L.; Yuan, J.; Tunan, C.; Li, F.; Hu, R.; Gao, F.; et al. L-Borneol induces transient opening of the blood–brain barrier and enhances the therapeutic effect of cisplatin. NeuroReport 2017, 28, 506–513. [Google Scholar] [CrossRef]
- Bayala, B.; Bassole, I.H.N.; Gnoula, C.; Nebie, R.; Yonli, A.; Morel, L.; Figueredo, G.; Nikiema, J.-B.; Lobaccaro, J.-M.A.; Simpore, J. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from burkina faso. PLoS ONE 2014, 9, e92122. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, Y.; Cui, H.-F.; Cao, C.-Y.; Zhang, Y.-B. 4-Terpineol exhibits potent in vitro and in vivo anticancer effects in Hep-G2 hepatocellular carcinoma cells by suppressing cell migration and inducing apoptosis and sub-G1 cell cycle arrest. J. BUON Off. J. Balk. Union Oncol. 2016, 21, 1195–1202. [Google Scholar]
- Lampronti, I.; Saab, A.M.; Gambari, R. Antiproliferative activity of essential oils derived from plants belonging to the Magnoliophyta division. Int. J. Oncol. 2006, 29, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-L.; Yin, Z.-Q.; Du, Y.-H.; Feng, R.-Z.; Ye, K.-C.; Wei, Q.; Hu, Y.; He, L.; Liao, L.; Wang, Y. γ-terpineol inhibits cell growth and induces apoptosis in human liver cancer BEL-7402 cells in vitro. Int. J. Clin. Exp. Pathol. 2014, 7, 6524–6533. [Google Scholar] [PubMed]
- Deeb, S.J.; El-Baba, C.O.; Hassan, S.B.; Larsson, R.L.; Gali-Muhtasib, H.U. Sage components enhance cell death through nuclear factor kappa-B signaling. Front. Biosci. Elite Ed. 2011, 3, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Assmann, C.E.; Cadoná, F.C.; Bonadiman, B.D.S.R.; Dornelles, E.B.; Trevisan, G.; Da Cruz, I.B.M. Tea tree oil presents in vitro antitumor activity on breast cancer cells without cytotoxic effects on fibroblasts and on peripheral blood mononuclear cells. Biomed. Pharmacother. 2018, 103, 1253–1261. [Google Scholar] [CrossRef] [PubMed]
- Jamali, T.; Kavoosi, G.; Ardestani, S.K. In-vitro and in-vivo anti-breast cancer activity of OEO (Oliveria decumbens vent essential oil) through promoting the apoptosis and immunomodulatory effects. J. Ethnopharmacol. 2020, 248, 112313. [Google Scholar] [CrossRef] [PubMed]
- Döll-Boscardin, P.M.; Sartoratto, A.; Sales Maia, B.H.L.D.N.; Padilha de Paula, J.; Nakashima, T.; Farago, P.V.; Kanunfre, C.C. In vitro cytotoxic potential of essential oils of Eucalyptus benthamii and its related terpenes on tumor cell lines. Evid. Based Complement. Alternat. Med. 2012, 2012, 342652. [Google Scholar] [CrossRef] [PubMed]
- Marrelli, M.; Conforti, F.; Formisano, C.; Rigano, D.; Arnold, N.A.; Menichini, F.; Senatore, F. Composition, antibacterial, antioxidant and antiproliferative activities of essential oils from three Origanum species growing wild in Lebanon and Greece. Nat. Prod. Res. 2016, 30, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Ambrož, M.; Boušová, I.; Skarka, A.; Hanušová, V.; Králová, V.; Matoušková, P.; Szotáková, B.; Skálová, L. The influence of sesquiterpenes from Myrica rubra on the antiproliferative and pro-oxidative effects of doxorubicin and its accumulation in cancer cells. Molecules 2015, 20, 15343–15358. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Cui, X.; Cui, H.; Jin, Y.; Jin, W.; Sun, H. Geraniol and lupeol inhibit growth and promote apoptosis in human hepatocarcinoma cells through the MAPK signaling pathway. J. Cell. Biochem. 2019, 120, 5033–5041. [Google Scholar] [CrossRef] [PubMed]
- Qi, F.; Yan, Q.; Zheng, Z.; Liu, J.; Chen, Y.; Zhang, G. Geraniol and geranyl acetate induce potent anticancer effects in colon cancer Colo-205 cells by inducing apoptosis, DNA damage and cell cycle arrest. J. BUON Off. J. Balk. Union Oncol. 2018, 23, 346–352. [Google Scholar]
- Lee, S.; Park, Y.R.; Kim, S.-H.; Park, E.-J.; Kang, M.J.; So, I.; Chun, J.N.; Jeon, J.-H. Geraniol suppresses prostate cancer growth through down-regulation of E2F8. Cancer Med. 2016, 5, 2899–2908. [Google Scholar] [CrossRef] [PubMed]
- Ryabchenko, B.; Tulupova, E.; Schmidt, E.; Wlcek, K.; Buchbauer, G.; Jirovetz, L. Investigation of anticancer and antiviral properties of selected aroma samples. Nat. Prod. Commun. 2008, 3. [Google Scholar] [CrossRef]
- Kubo, I.; Morimitsu, Y. Cytotoxicity of green tea flavor compounds against two solid tumor cells. J. Agric. Food Chem. 1995, 43, 1626–1628. [Google Scholar] [CrossRef]
- Boris, R.; Elena, T.; Erich, S.; Walter, J.; Gerhard, B.; Leopold, J. Cytotoxic properties of selected sesquiterpene alcohols on human cervix carcinoma cell lines. J. Essent. Oil Bear. Plants 2013, 14, 316–319. [Google Scholar] [CrossRef]
- Tatman, D.; Mo, H. Volatile isoprenoid constituents of fruits, vegetables and herbs cumulatively suppress the proliferation of murine B16 melanoma and human HL-60 leukemia cells. Cancer Lett. 2002, 175, 129–139. [Google Scholar] [CrossRef]
- Wattenberg, L.W. Inhibition of azoxymethane-induced neoplasia of the large bowel by 3-hydroxy-3,7,11-trimethyl-1,6,10-dodecatriene (nerolidol). Carcinogenesis 1991, 12, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-L.; Chang, J.-C.; Fang, L.-W.; Hsu, H.-F.; Lee, L.-C.; Yang, J.-F.; Liang, M.-T.; Hsiao, P.-C.; Wang, C.-P.; Wang, S.-W.; et al. Bulnesia sarmientoi supercritical fluid extract exhibits necroptotic effects and anti-metastatic activity on lung cancer cells. Molecules 2018, 23, 3304. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, J.; Luo, Y.; Huang, N.; Zhen, N.; Zhou, Y.; Sun, F.; Li, Z.; Pan, Q.; Li, Y. (−)-Guaiol regulates RAD51 stability via autophagy to induce cell apoptosis in non-small cell lung cancer. Oncotarget 2016, 7, 62585–62597. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Wu, J.; Huang, N.; Cui, Z.; Luo, Y.; Sun, F.; Pan, Q.; Li, Y.; Yang, Q. (−)-Guaiol regulates autophagic cell death depending on mTOR signaling in NSCLC. Cancer Biol. Ther. 2018, 19, 706–714. [Google Scholar] [CrossRef]
- Girola, N.; Figueiredo, C.R.; Farias, C.F.; Azevedo, R.A.; Ferreira, A.K.; Teixeira, S.F.; Capello, T.M.; Martins, E.G.A.; Matsuo, A.L.; Travassos, L.R.; et al. Camphene isolated from essential oil of Piper cernuum (Piperaceae) induces intrinsic apoptosis in melanoma cells and displays antitumor activity in vivo. Biochem. Biophys. Res. Commun. 2015, 467, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-L.; Li, Y.-C.; Chang, W.-C.; Chung, J.-G.; Hsieh, L.-C.; Wu, C.-C. Induction of necrosis in human liver tumor cells by α-phellandrene. Nutr. Cancer 2014, 66, 970–979. [Google Scholar] [CrossRef]
- Lin, J.-J.; Yu, C.-C.; Lu, K.-W.; Chang, S.-J.; Yu, F.-S.; Liao, C.-L.; Lin, J.-G.; Chung, J.-G. α-Phellandrene alters expression of genes associated with DNA damage, cell cycle, and apoptosis in murine leukemia WEHI-3 cells. Anticancer Res. 2014, 34, 4161–4180. [Google Scholar]
- Lin, J.-J.; Wu, C.-C.; Hsu, S.-C.; Weng, S.-W.; Ma, Y.-S.; Huang, Y.-P.; Lin, J.-G.; Chung, J.-G. Alpha-phellandrene-induced DNA damage and affect DNA repair protein expression in WEHI-3 murine leukemia cells in vitro: α-phellandrene induced DNA damage and affect DNA repair in Wehi-3 cells. Environ. Toxicol. 2015, 30, 1322–1330. [Google Scholar] [CrossRef]
- Hsieh, L.-C.; Hsieh, S.-L.; Chen, C.-T.; Chung, J.-G.; Wang, J.-J.; Wu, C.-C. Induction of α-phellandrene on autophagy in human liver tumor cells. Am. J. Chin. Med. 2015, 43, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-J.; Hsu, S.-C.; Lu, K.-W.; Ma, Y.-S.; Wu, C.-C.; Lu, H.-F.; Chen, J.-C.; Lin, J.-G.; Wu, P.-P.; Chung, J.-G. Alpha-phellandrene-induced apoptosis in mice leukemia WEHI-3 cells in vitro: A-phellandrene induced apoptosis in mice leukemia Wehi-3 cells. Environ. Toxicol. 2016, 31, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical composition, anti-inflammatory activity and cytotoxic effects of essential oils from three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Kohoude, M.J.; Gbaguidi, F.; Agbani, P.; Ayedoun, M.-A.; Cazaux, S.; Bouajila, J. Chemical composition and biological activities of extracts and essential oil of Boswellia dalzielii leaves. Pharm. Biol. 2017, 55, 33–42. [Google Scholar] [CrossRef]
- Mulyaningsih, S.; Youns, M.; El-Readi, M.Z.; Ashour, M.L.; Nibret, E.; Sporer, F.; Herrmann, F.; Reichling, J.; Wink, M. Biological activity of the essential oil of Kadsura longipedunculata (Schisandraceae) and its major components: Activity of Kadsura longipedunculata oil. J. Pharm. Pharmacol. 2010, 62, 1037–1044. [Google Scholar] [CrossRef]
- Al-Qudah, M.A.; Saleh, A.M.; Alhawsawi, N.L.; Al-Jaber, H.I.; Rizvi, S.A.; Afifi, F.U. Composition, antioxidant, and cytotoxic activities of the essential oils from fresh and air-dried aerial parts of Pallenis spinosa. Chem. Biodivers. 2017, 14, e1700146. [Google Scholar] [CrossRef]
- Ornano, L.; Venditti, A.; Sanna, C.; Ballero, M.; Maggi, F.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Bianco, A. Chemical composition and biological activity of the essential oil from Helichrysum microphyllum Cambess. ssp. tyrrhenicum Bacch., Brullo e Giusso growing in La Maddalena Archipelago, Sardinia. J. Oleo Sci. 2015, 64, 19–26. [Google Scholar] [CrossRef]
- Hui, L.-M.; Zhao, G.-D.; Zhao, J.-J. δ-Cadinene inhibits the growth of ovarian cancer cells via caspase-dependent apoptosis and cell cycle arrest. Int. J. Clin. Exp. Pathol. 2015, 8, 6046–6056. [Google Scholar]
- Biswas, R.; Mandal, S.K.; Dutta, S.; Bhattacharyya, S.S.; Boujedaini, N.; Khuda-Bukhsh, A.R. Thujone-rich fraction of Thuja occidentalis demonstrates major anti-cancer potentials: Evidences from in vitro studies on A375 cells. Evid. Based Complement. Alternat. Med. 2011, 2011, 568148. [Google Scholar] [CrossRef]
- Pelkonen, O.; Abass, K.; Wiesner, J. Thujone and thujone-containing herbal medicinal and botanical products: Toxicological assessment. Regul. Toxicol. Pharmacol. RTP 2013, 65, 100–107. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Afshari, A.R.; Hosseinzadeh, H. Review on the potential therapeutic roles of Nigella sativa in the treatment of patients with cancer: Involvement of apoptosis—Black cumin and cancer. J. Pharmacopunct. 2017, 20, 158–172. [Google Scholar] [CrossRef]
- Lenis-Rojas, O.A.; Robalo, M.P.; Tomaz, A.I.; Carvalho, A.; Fernandes, A.R.; Marques, F.; Folgueira, M.; Yáñez, J.; Vázquez-García, D.; López Torres, M.; et al. RuII (p-cymene) compounds as effective and selective anticancer candidates with no toxicity in vivo. Inorg. Chem. 2018, 57, 13150–13166. [Google Scholar] [CrossRef]
- Corrales Sánchez, V.; Nieto-Jiménez, C.; Castro-Osma, J.A.; de Andrés, F.; Pacheco-Liñán, P.J.; Bravo, I.; Rodríguez Fariñas, N.; Niza, E.; Domínguez-Jurado, E.; Lara-Sánchez, A.; et al. Screening and preliminary biochemical and biological studies of [RuCl(p-cymene)(N, N-bis(diphenylphosphino)-isopropylamine)][BF4] in breast cancer models. ACS Omega 2019, 4, 13005–13014. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, M.Z.; Ali, A.; Duong, H.-Q.; Chen, J.; Rahman, F.-U. Anticancer activity and mechanism of bis-pyrimidine based dimetallic Ru(II)(η6-p-cymene) complex in human non-small cell lung cancer via p53-dependent pathway. J. Inorg. Biochem. 2019, 194, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Formagio, A.S.N.; Vieira, M.D.C.; Dos Santos, L.A.C.; Cardoso, C.A.L.; Foglio, M.A.; De Carvalho, J.E.; Andrade-Silva, M.; Kassuya, C.A.L. Composition and evaluation of the anti-inflammatory and anticancer activities of the essential oil from Annona sylvatica A. St.-Hil. J. Med. Food 2013, 16, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Yongram, C.; Sungthong, B.; Puthongking, P.; Weerapreeyakul, N. Chemical composition, antioxidant and cytotoxicity activities of leaves, bark, twigs and oleo-resin of Dipterocarpus alatus. Molecules 2019, 24, 3083. [Google Scholar] [CrossRef]
- Šobotník, J.; Hanus, R.; Kalinová, B.; Piskorski, R.; Cvačka, J.; Bourguignon, T.; Roisin, Y. (E,E)-α-farnesene, an alarm pheromone of the termite Prorhinotermes canalifrons. J. Chem. Ecol. 2008, 34, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.-N.; Lim, J.-Q.; Afiqah, F.; Nik Mohamed Kamal, N.N.S.; Abdul Aziz, F.A.; Tong, W.-Y.; Leong, C.-R.; Lim, J.-W. Chemical composition and cytotoxic activity of Garcinia atroviridis Griff. ex T. Anders. essential oils in combination with tamoxifen. Nat. Prod. Res. 2018, 32, 854–858. [Google Scholar] [CrossRef]
- Afoulous, S.; Ferhout, H.; Raoelison, E.G.; Valentin, A.; Moukarzel, B.; Couderc, F.; Bouajila, J. Chemical composition and anticancer, antiinflammatory, antioxidant and antimalarial activities of leaves essential oil of Cedrelopsis grevei. Food Chem. Toxicol. 2013, 56, 352–362. [Google Scholar] [CrossRef]
- Jiang, R.; Sun, L.; Wang, Y.; Liu, J.; Liu, X.; Feng, H.; Zhao, D. Chemical composition, and cytotoxic, antioxidant and antibacterial activities of the essential oil from ginseng leaves. Nat. Prod. Commun. 2014, 9, 865–868. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mannino, F.; Arcoraci, V.; Rottura, M.; Ieni, A.; Minutoli, L.; Metro, D.; et al. β-caryophyllene inhibits cell proliferation through a direct modulation of CB2 receptors in glioblastoma cells. Cancers 2020, 12, 1038. [Google Scholar] [CrossRef]
- Legault, J.; Dahl, W.; Debiton, E.; Pichette, A.; Madelmont, J. Antitumor activity of balsam fir oil: Production of reactive oxygen species induced by α-humulene as possible mechanism of action. Planta Med. 2003, 69, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.; Dizeyi, N.; Abrahamsson, P.-A.; Persson, J. The effect of a novel botanical agent TBS-101 on invasive prostate cancer in animal models. Anticancer Res. 2009, 29, 3917–3924. [Google Scholar]
- Boch, R.; Shearer, D. Production of geraniol by honey bees of various ages. J. Insect Physiol. 1963, 9, 431–434. [Google Scholar] [CrossRef]
- Cho, M.; So, I.; Chun, J.N.; Jeon, J.-H. The antitumor effects of geraniol: Modulation of cancer hallmark pathways (Review). Int. J. Oncol. 2016, 48, 1772–1782. [Google Scholar] [CrossRef]
- Shanmugapriya, S.; Subramanian, P.; Kanimozhi, S. Geraniol inhibits endometrial carcinoma via downregulating oncogenes and upregulating tumour suppressor genes. Indian J. Clin. Biochem. 2017, 32, 214–219. [Google Scholar] [CrossRef]
- Kaiser, R. The Scent of Orchids: Olfactory and Chemical Investigations; Elsevier Science Publishers: New York, NY, USA, 1993; ISBN 978-0-444-89841-8. [Google Scholar]
- Lawless, J. The Illustrated Encyclopedia of Essential Oils: The Complete Guide to the Use of Oils in Aromatherapy and Herbalism; Element: Shaftesbury, UK; Rockport, MA, USA, 1995; ISBN 978-1-85230-661-8. [Google Scholar]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals, 2nd ed.; Elsevier Ltd.: Edinburgh, UK, 2013; ISBN 978-0-443-06241-4. [Google Scholar]
- Billen, J.; Morgan, E.D. Pheromone Communication in Social Insects: Ants, Wasps, Bees, and Termites; Vander Meer, R., Breed, M., Espelie, K., Winston, M., Eds.; Westview Press: Boulder, CO, USA, 1998; ISBN 978-0-8133-8976-9. [Google Scholar]
- Pudełek, M.; Catapano, J.; Kochanowski, P.; Mrowiec, K.; Janik-Olchawa, N.; Czyż, J.; Ryszawy, D. Therapeutic potential of monoterpene α-thujone, the main compound of Thuja occidentalis L. essential oil, against malignant glioblastoma multiforme cells in vitro. Fitoterapia 2019, 134, 172–181. [Google Scholar] [CrossRef]
- Torres, A.; Vargas, Y.; Uribe, D.; Carrasco, C.; Torres, C.; Rocha, R.; Oyarzún, C.; San Martín, R.; Quezada, C. Pro-apoptotic and anti-angiogenic properties of the α/β-thujone fraction from Thuja occidentalis on glioblastoma cells. J. Neurooncol. 2016, 128, 9–19. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Park, H.; Lim, W.; Song, G. α,β-Thujone suppresses human placental choriocarcinoma cells via metabolic disruption. Reproduction 2020, 159, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Kuttan, G. Thujone inhibits lung metastasis induced by B16F-10 melanoma cells in C57BL/6 mice. Can. J. Physiol. Pharmacol. 2011, 89, 691–703. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Toxicology and carcinogenesis studies of alpha,beta-thujone (CAS No. 76231-76-0) in F344/N rats and B6C3F1 mice (gavage studies). Natl. Toxicol. Program Tech. Rep. Ser. 2011, 570, 1–260. [Google Scholar]
- Hazekamp, A.; Tejkalová, K.; Papadimitriou, S. Cannabis: From cultivar to chemovar II—A metabolomics approach to cannabis classification. Cannabis Cannabinoid Res. 2016, 1, 202–215. [Google Scholar] [CrossRef]
- George, S.; Nair, S.A.; Venkataraman, R.; Baby, S. Chemical composition, antibacterial and anticancer activities of volatile oil of Melicope denhamii leaves. Nat. Prod. Res. 2015, 29, 1959–1962. [Google Scholar] [CrossRef]
- Gibson, R.W.; Pickett, J.A. Wild potato repels aphids by release of aphid alarm pheromone. Nature 1983, 302, 608–609. [Google Scholar] [CrossRef]
- Wen, W.; Alseekh, S.; Fernie, A.R. Conservation and diversification of flavonoid metabolism in the plant kingdom. Curr. Opin. Plant Biol. 2020, 55, 100–108. [Google Scholar] [CrossRef]
- Flores-Sanchez, I.J.; Verpoorte, R. PKS activities and biosynthesis of cannabinoids and flavonoids in Cannabis sativa L. plants. Plant Cell Physiol. 2008, 49, 1767–1782. [Google Scholar] [CrossRef]
- Kashyap, D.; Sharma, A.; Tuli, H.S.; Sak, K.; Punia, S.; Mukherjee, T.K. Kaempferol—A dietary anticancer molecule with multiple mechanisms of action: Recent trends and advancements. J. Funct. Foods 2017, 30, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Xue, L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol. Res. 2019, 27, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Ma, C. Kaempferol suppresses human gastric cancer SNU-216 cell proliferation, promotes cell autophagy, but has no influence on cell apoptosis. Braz. J. Med. Biol. Res. Rev. Bras. Pesqui. Medicas E Biol. 2019, 52, e7843. [Google Scholar] [CrossRef] [PubMed]
- Da, J.; Xu, M.; Wang, Y.; Li, W.; Lu, M.; Wang, Z. Kaempferol promotes apoptosis while inhibiting cell proliferation via androgen-dependent pathway and suppressing vasculogenic mimicry and invasion in prostate cancer. Anal. Cell. Pathol. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Budisan, L.; Gulei, D.; Jurj, A.; Braicu, C.; Zanoaga, O.; Cojocneanu, R.; Pop, L.; Raduly, L.; Barbat, A.; Moldovan, A.; et al. Inhibitory effect of CAPE and kaempferol in colon cancer cell lines-possible implications in new therapeutic strategies. Int. J. Mol. Sci. 2019, 20, 1199. [Google Scholar] [CrossRef] [PubMed]
- Sezer, E.D.; Oktay, L.M.; Karadadaş, E.; Memmedov, H.; Selvi Gunel, N.; Sözmen, E. assessing anticancer potential of blueberry flavonoids, quercetin, kaempferol, and gentisic acid, through oxidative stress and apoptosis parameters on HCT-116 cells. J. Med. Food 2019, 22, 1118–1126. [Google Scholar] [CrossRef]
- Abdullah, A.; Talwar, P.; D’HELLENCOURT, C.L.; Ravanan, P. IRE1α is critical for Kaempferol-induced neuroblastoma differentiation. FEBS J. 2019, 286, 1375–1392. [Google Scholar] [CrossRef]
- Yang, S.; Si, L.; Jia, Y.; Jian, W.; Yu, Q.; Wang, M.; Lin, R. Kaempferol exerts anti-proliferative effects on human ovarian cancer cells by inducing apoptosis, G0/G1 cell cycle arrest and modulation of MEK/ERK and STAT3 pathways. J. BUON Off. J. Balk. Union Oncol. 2019, 24, 975–981. [Google Scholar]
- Rusak, G.; Gutzeit, H.O.; Müller, J.L. Structurally related flavonoids with antioxidative properties differentially affect cell cycle progression and apoptosis of human acute leukemia cells. Nutr. Res. 2005, 25, 143–155. [Google Scholar] [CrossRef]
- Song, H.; Bao, J.; Wei, Y.; Chen, Y.; Mao, X.; Li, J.; Yang, Z.; Xue, Y. Kaempferol inhibits gastric cancer tumor growth: An in vitro and in vivo study. Oncol. Rep. 2015, 33, 868–874. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, J.; Rankin, G.O.; Chen, Y.C. Kaempferol induces G2/M cell cycle arrest via checkpoint kinase 2 and promotes apoptosis via death receptors in human ovarian carcinoma A2780/CP70 cells. Molecules 2018, 23, 1095. [Google Scholar] [CrossRef]
- Yao, S.; Wang, X.; Li, C.; Zhao, T.; Jin, H.; Fang, W. Kaempferol inhibits cell proliferation and glycolysis in esophagus squamous cell carcinoma via targeting EGFR signaling pathway. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2016, 37, 10247–10256. [Google Scholar] [CrossRef]
- Lei, X.; Guo, J.; Wang, Y.; Cui, J.; Feng, B.; Su, Y.; Zhao, H.; Yang, W.; Hu, Y. Inhibition of endometrial carcinoma by Kaempferol is interceded through apoptosis induction, G2/M phase cell cycle arrest, suppression of cell invasion and upregulation of m-TOR/PI3K signalling pathway. J. BUON Off. J. Balk. Union Oncol. 2019, 24, 1555–1561. [Google Scholar]
- Riahi-Chebbi, I.; Souid, S.; Othman, H.; Haoues, M.; Karoui, H.; Morel, A.; Srairi-Abid, N.; Essafi, M.; Essafi-Benkhadir, K. The Phenolic compound Kaempferol overcomes 5-fluorouracil resistance in human resistant LS174 colon cancer cells. Sci. Rep. 2019, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.H. Kaempferol inhibits pancreatic cancer cell growth and migration through the blockade of EGFR-related pathway in vitro. PLoS ONE 2016, 11, e0155264. [Google Scholar] [CrossRef] [PubMed]
- Jo, E.; Park, S.J.; Choi, Y.S.; Jeon, W.-K.; Kim, B.-C. Kaempferol suppresses transforming growth factor-β1-induced epithelial-to-mesenchymal transition and migration of A549 lung cancer cells by inhibiting Akt1-mediated phosphorylation of Smad3 at threonine-179. Neoplasia N. Y. 2015, 17, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, T.; Deng, R.; Jiang, X.; Xiong, H.; Wang, Y.; Yu, Q.; Wang, X.; Chen, C.; Zhu, Y. Low dose of kaempferol suppresses the migration and invasion of triple-negative breast cancer cells by downregulating the activities of RhoA and Rac1. Onco Targets Ther. 2017, 10, 4809–4819. [Google Scholar] [CrossRef]
- Hung, T.-W.; Chen, P.-N.; Wu, H.-C.; Wu, S.-W.; Tsai, P.-Y.; Hsieh, Y.-S.; Chang, H.-R. Kaempferol inhibits the invasion and migration of renal cancer cells through the downregulation of AKT and FAK pathways. Int. J. Med. Sci. 2017, 14, 984–993. [Google Scholar] [CrossRef]
- Chien, H.-W.; Wang, K.; Chang, Y.-Y.; Hsieh, Y.-H.; Yu, N.-Y.; Yang, S.-F.; Lin, H.-W. Kaempferol suppresses cell migration through the activation of the ERK signaling pathways in ARPE-19 cells. Environ. Toxicol. 2019, 34, 312–318. [Google Scholar] [CrossRef]
- Lee, G.-A.; Choi, K.-C.; Hwang, K.-A. Kaempferol, a phytoestrogen, suppressed triclosan-induced epithelial-mesenchymal transition and metastatic-related behaviors of MCF-7 breast cancer cells. Environ. Toxicol. Pharmacol. 2017, 49, 48–57. [Google Scholar] [CrossRef]
- Luo, H.; Jiang, B.-H.; King, S.M.; Chen, Y.C. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr. Cancer 2008, 60, 800–809. [Google Scholar] [CrossRef]
- Seydi, E.; Salimi, A.; Rasekh, H.R.; Mohsenifar, Z.; Pourahmad, J. Selective cytotoxicity of luteolin and kaempferol on cancerous hepatocytes obtained from rat model of hepatocellular carcinoma: Involvement of ROS-mediated mitochondrial targeting. Nutr. Cancer 2018, 70, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, A.; Naimi, A.; Hagh, M.F.; Saraei, R.; Marofi, F.; Solali, S. Kaempferol improves TRAIL-mediated apoptosis in leukemia MOLT-4 cells by the inhibition of anti-apoptotic proteins and promotion of death receptors expression. Anticancer Agents Med. Chem. 2019, 19, 1835–1845. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wei, L.; Lin, S.; Chen, Y.; Lin, J.; Peng, J. Synergistic effect of kaempferol and 5-fluorouracil on the growth of colorectal cancer cells by regulating the PI3K/Akt signaling pathway. Mol. Med. Rep. 2019, 20, 728–734. [Google Scholar] [CrossRef]
- Dang, Q.; Song, W.; Xu, D.; Ma, Y.; Li, F.; Zeng, J.; Zhu, G.; Wang, X.; Chang, L.S.; He, D.; et al. Kaempferol suppresses bladder cancer tumor growth by inhibiting cell proliferation and inducing apoptosis. Mol. Carcinog. 2015, 54, 831–840. [Google Scholar] [CrossRef]
- Mamouni, K.; Zhang, S.; Li, X.; Chen, Y.; Yang, Y.; Kim, J.; Bartlett, M.G.; Coleman, I.M.; Nelson, P.S.; Kucuk, O.; et al. A Novel Flavonoid composition targets androgen receptor signaling and inhibits prostate cancer growth in preclinical models. Neoplasia N. Y. 2018, 20, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Cui, W.; Yang, X.; Tong, B. Kaempferol inhibits the growth and metastasis of cholangiocarcinoma in vitro and in vivo. Acta Biochim. Biophys. Sin. 2016, 48, 238–245. [Google Scholar] [CrossRef]
- Huang, W.-W.; Chiu, Y.-J.; Fan, M.-J.; Lu, H.-F.; Yeh, H.-F.; Li, K.-H.; Chen, P.-Y.; Chung, J.-G.; Yang, J.-S. Kaempferol induced apoptosis via endoplasmic reticulum stress and mitochondria-dependent pathway in human osteosarcoma U-2 OS cells. Mol. Nutr. Food Res. 2010, 54, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Xu, H.; Fan, P.-Z.; Xie, J.; He, J.; Yu, J.; Gu, X.; Zhang, C.-J. Kaempferol blocks neutrophil extracellular traps formation and reduces tumour metastasis by inhibiting ROS-PAD4 pathway. J. Cell. Mol. Med. 2020. [Google Scholar] [CrossRef]
- Xu, F.; Matsuda, H.; Hata, H.; Sugawara, K.; Nakamura, S.; Yoshikawa, M. Structures of new flavonoids and benzofuran-type stilbene and degranulation inhibitors of rat basophilic leukemia cells from the Brazilian herbal medicine Cissus sicyoides. Chem. Pharm. Bull. (Tokyo) 2009, 57, 1089–1095. [Google Scholar] [CrossRef][Green Version]
- Alexandrakis, M.; Letourneau, R.; Kempuraj, D.; Kandere-Grzybowska, K.; Huang, M.; Christodoulou, S.; Boucher, W.; Seretakis, D.; Theoharides, T.C. Flavones inhibit proliferation and increase mediator content in human leukemic mast cells (HMC-1). Eur. J. Haematol. 2003, 71, 448–454. [Google Scholar] [CrossRef]
- Magura, J.; Moodley, R.; Maduray, K.; Mackraj, I. Phytochemical constituents and in vitro anticancer screening of isolated compounds from Eriocephalus africanus. Nat. Prod. Res. 2020, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yu, M.; Shi, N.; Zhou, Y.; Li, F.; Li, X.; Huang, X.; Jin, J. Apigenin and Abivertinib, a novel BTK inhibitor synergize to inhibit diffuse large B-cell lymphoma in vivo and vitro. J. Cancer 2020, 11, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.-G.; Wang, L.; Liu, W.-J.; Wang, J.-F.; Zhao, E.-J.; Zhou, F.-M.; Ji, X.-B.; Wang, L.-H.; Xia, Z.-K.; Wang, W.; et al. Apigenin inhibits IL-6 transcription and suppresses esophageal carcinogenesis. Front. Pharmacol. 2019, 10, 1002. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, X.; Chen, C.; Huijuan, W.; Zhao, H.; Liu, W.; Xiang, Z.; Wang, Q. Apigenin, a flavonoid constituent derived from P. villosa, inhibits hepatocellular carcinoma cell growth by CyclinD1/CDK4 regulation via p38 MAPK-p21 signaling. Pathol. Res. Pract. 2020, 216, 152701. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.L.C.; Amparo, J.A.O.; Silva, A.B.; Silva, K.C.; Braga-de-Souza, S.; Barbosa, P.R.; Lopes, G.P.D.F.; Costa, S.L. Apigenin from Croton betulaster Müll restores the immune profile of microglia against glioma cells. Phytother. Res. 2019, 33, 3191–3202. [Google Scholar] [CrossRef]
- Şirin, N.; Elmas, L.; Seçme, M.; Dodurga, Y. Investigation of possible effects of apigenin, sorafenib and combined applications on apoptosis and cell cycle in hepatocellular cancer cells. Gene 2020, 737, 144428. [Google Scholar] [CrossRef]
- Zhang, E.; Zhang, Y.; Fan, Z.; Cheng, L.; Han, S.; Che, H. Apigenin inhibits histamine-induced cervical cancer tumor growth by regulating estrogen receptor expression. Molecules 2020, 25, 1960. [Google Scholar] [CrossRef]
- Moreau, M.; Ibeh, U.; Decosmo, K.; Bih, N.; Yasmin-Karim, S.; Toyang, N.; Lowe, H.; Ngwa, W. Flavonoid derivative of cannabis demonstrates therapeutic potential in preclinical models of metastatic pancreatic cancer. Front. Oncol. 2019, 9, 660. [Google Scholar] [CrossRef]
- Ranjbar, N.; Saravani, R.; Faezizadeh, Z. Silymarin inhibits Toll-like receptor 8 gene expression and apoptosis in Ramos cancer cell line. Avicenna J. Phytomed. 2020, 10, 161–169. [Google Scholar]
- Czarnik-Kwaśniak, J.; Kwaśniak, K.; Kwasek, P.; Świerzowska, E.; Strojewska, A.; Tabarkiewicz, J. The influence of lycopene, [6]-gingerol, and silymarin on the apoptosis on U-118MG glioblastoma cells in vitro model. Nutrients 2019, 12, 96. [Google Scholar] [CrossRef]
- Kim, S.; Choo, G.; Yoo, E.; Woo, J.; Han, S.; Lee, J.; Jung, J. Silymarin induces inhibition of growth and apoptosis through modulation of the MAPK signaling pathway in AGS human gastric cancer cells. Oncol. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bektur Aykanat, N.E.; Kacar, S.; Karakaya, S.; Sahinturk, V. Silymarin suppresses HepG2 hepatocarcinoma cell progression through downregulation of Slit-2/Robo-1 pathway. Pharmacol. Rep. 2020, 72, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, M.; Adhikari, B.; Ghimire, B.; Baboota, S.; Choi, E.H. Cold atmospheric plasma and silymarin nanoemulsion activate autophagy in human melanoma cells. Int. J. Mol. Sci. 2020, 21, 1939. [Google Scholar] [CrossRef]
- Si, L.; Fu, J.; Liu, W.; Hayashi, T.; Nie, Y.; Mizuno, K.; Hattori, S.; Fujisaki, H.; Onodera, S.; Ikejima, T. Silibinin inhibits migration and invasion of breast cancer MDA-MB-231 cells through induction of mitochondrial fusion. Mol. Cell. Biochem. 2020, 463, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Flaig, T.W.; Gustafson, D.L.; Su, L.-J.; Zirrolli, J.A.; Crighton, F.; Harrison, G.S.; Pierson, A.S.; Agarwal, R.; Glodé, L.M. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Investig. New Drugs 2006, 25, 139–146. [Google Scholar] [CrossRef]
- Gao, G.; Ge, R.; Li, Y.; Liu, S. Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3265–3271. [Google Scholar] [CrossRef]
- Tavsan, Z.; Kayali, H.A. Flavonoids showed anticancer effects on the ovarian cancer cells: Involvement of reactive oxygen species, apoptosis, cell cycle and invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, M.; Ying, Q.; Xie, X.; Yue, S.; Tong, B.; Wei, Q.; Bai, Z.; Ma, L. Decrease of AIM2 mediated by luteolin contributes to non-small cell lung cancer treatment. Cell Death Dis. 2019, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Iida, K.; Naiki, T.; Naiki-Ito, A.; Suzuki, S.; Kato, H.; Nozaki, S.; Nagai, T.; Etani, T.; Nagayasu, Y.; Ando, R.; et al. Luteolin suppresses bladder cancer growth via regulation of mechanistic target of rapamycin pathway. Cancer Sci. 2020, 111, 1165–1179. [Google Scholar] [CrossRef] [PubMed]
- Schomberg, J.; Wang, Z.; Farhat, A.; Guo, K.L.; Xie, J.; Zhou, Z.; Liu, J.; Kovacs, B.; Liu-Smith, F. Luteolin inhibits melanoma growth in vitro and in vivo via regulating ECM and oncogenic pathways but not ROS. Biochem. Pharmacol. 2020, 177, 114025. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, Q.; Shen, S.; Wei, X.; Li, G. HIF-1α/VEGF signaling-mediated epithelial-mesenchymal transition and angiogenesis is critically involved in anti-metastasis effect of luteolin in melanoma cells: Luteolin inhibt melanoma metastasis by targeting HIF-1α/VEGF signaling. Phytother. Res. 2019, 33, 798–807. [Google Scholar] [CrossRef]
- Moeng, S.; Son, S.W.; Seo, H.A.; Lee, J.S.; Kim, C.K.; Kuh, H.J.; Park, J.K. Luteolin-regulated microRNA-301-3p targets caspase-8 and modulates TRAIL sensitivity in PANC-1 cells. Anticancer Res. 2020, 40, 723–731. [Google Scholar] [CrossRef]
- Potočnjak, I.; Šimić, L.; Gobin, I.; Vukelić, I.; Domitrović, R. Antitumor activity of luteolin in human colon cancer SW620 cells is mediated by the ERK/FOXO3a signaling pathway. Toxicol. Vitr. 2020, 66, 104852. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Banaszczak, E.; Krajka-Kuźniak, V.; Papierska, K. The effect of luteolin 7-glucoside, apigenin 7-glucoside and Succisa pratensis extracts on NF-κB activation and α-amylase activity in HepG2 cells. Acta Biochim. Pol. 2020. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; Assanga, S.I.; Lujan, L.L. Flavones and flavonols may have clinical potential as CK2 inhibitors in cancer therapy. Med. Hypotheses 2020, 141, 109723. [Google Scholar] [CrossRef]
- Fan, J.-J.; Hsu, W.-H.; Lee, K.-H.; Chen, K.-C.; Lin, C.-W.; Lee, Y.-L.; Ko, T.-P.; Lee, L.-T.; Lee, M.-T.; Chang, M.-S.; et al. Dietary flavonoids luteolin and quercetin inhibit migration and invasion of squamous carcinoma through reduction of Src/Stat3/S100A7 signaling. Antioxidants 2019, 8, 557. [Google Scholar] [CrossRef]
- Xiang, C.; Wu, X.; Zhao, Z.; Feng, X.; Bai, X.; Liu, X.; Zhao, J.; Takeda, S.; Qing, Y. Nonhomologous end joining and homologous recombination involved in luteolin-induced DNA damage in DT40 cells. Toxicol. Vitr. 2020, 65, 104825. [Google Scholar] [CrossRef] [PubMed]
- Jang, C.; Moon, N.; Oh, J.; Kim, J.-S. Luteolin shifts oxaliplatin-induced cell cycle arrest at G0/G1 to apoptosis in HCT116 human colorectal carcinoma cells. Nutrients 2019, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Naiki-Ito, A.; Naiki, T.; Kato, H.; Iida, K.; Etani, T.; Nagayasu, Y.; Suzuki, S.; Yamashita, Y.; Inaguma, S.; Onishi, M.; et al. Recruitment of miR-8080 by luteolin inhibits androgen receptor splice variant 7 expression in castration-resistant prostate cancer. Carcinogenesis 2019, bgz193. [Google Scholar] [CrossRef] [PubMed]
- Franco, Y.E.M.; de Lima, C.A.; Rosa, M.N.; Silva, V.A.O.; Reis, R.M.; Priolli, D.G.; Carvalho, P.O.; Do Nascimento, J.R.; Da Rocha, C.Q.; Longato, G.B. Investigation of U-251 cell death triggered by flavonoid luteolin: Towards a better understanding on its anticancer property against glioblastomas. Nat. Prod. Res. 2020, 1–7. [Google Scholar] [CrossRef]
- Masraksa, W.; Tanasawet, S.; Hutamekalin, P.; Wongtawatchai, T.; Sukketsiri, W. Luteolin attenuates migration and invasion of lung cancer cells via suppressing focal adhesion kinase and non-receptor tyrosine kinase signaling pathway. Nutr. Res. Pract. 2020, 14, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Rao, C.; Zheng, G.; Wang, S. Luteolin suppresses colorectal cancer cell metastasis via regulation of the miR-384/pleiotrophin axis. Oncol. Rep. 2019, 42, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.-Q.; Li, Q.; Zhang, Y. Luteolin suppresses the proliferation of gastric cancer cells and acts in synergy with oxaliplatin. BioMed Res. Int. 2020, 2020, 9396512. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Pham, T.-H.; Bak, Y.; Ryu, H.-W.; Oh, S.-R.; Yoon, D.-Y. Orientin inhibits invasion by suppressing MMP-9 and IL-8 expression via the PKCα/ERK/AP-1/STAT3-mediated signaling pathways in TPA-treated MCF-7 breast cancer cells. Phytomedicine 2018, 50, 35–42. [Google Scholar] [CrossRef] [PubMed]
- An, F.; Wang, S.; Tian, Q.; Zhu, D. Effects of orientin and vitexin from Trollius chinensis on the growth and apoptosis of esophageal cancer EC-109 cells. Oncol. Lett. 2015, 10, 2627–2633. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, K.; Balasubramanian, B.; Park, S.; Natesan, K.; Liu, W.; Manju, V. Orientin induces G0/G1 Cell cycle arrest and mitochondria mediated intrinsic apoptosis in human colorectal carcinoma HT29 cells. Biomolecules 2019, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Tong, M.; Li, Z.; Huang, W.; Jin, Y.; Cao, Q.; Zhou, X.; Tong, G. The effects of orientin on proliferation and apoptosis of T24 human bladder carcinoma cells occurs through the inhibition of nuclear factor-kappaB and the hedgehog signaling pathway. Med. Sci. Monit. 2019, 25, 9547–9554. [Google Scholar] [CrossRef]
- Thangaraj, K.; Vaiyapuri, M. Orientin, a C-glycosyl dietary flavone, suppresses colonic cell proliferation and mitigates NF-κB mediated inflammatory response in 1,2-dimethylhydrazine induced colorectal carcinogenesis. Biomed. Pharmacother. 2017, 96, 1253–1266. [Google Scholar] [CrossRef]
- Thangaraj, K.; Natesan, K.; Palani, M.; Vaiyapuri, M. Orientin, a flavanoid, mitigates 1, 2 dimethylhydrazine-induced colorectal lesions in Wistar rats fed a high-fat diet. Toxicol. Rep. 2018, 5, 977–987. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Q.; Liu, H.; Luo, S. Vitexin induces apoptosis through mitochondrial pathway and PI3K/Akt/mTOR signaling in human non-small cell lung cancer A549 cells. Biol. Res. 2019, 52, 7. [Google Scholar] [CrossRef]
- Liu, N.; Wang, K.S.; Qi, M.; Zhou, Y.J.; Zeng, G.Y.; Tao, J.; Zhou, J.D.; Zhang, J.L.; Chen, X.; Peng, C. Vitexin compound 1, a novel extraction from a Chinese herb, suppresses melanoma cell growth through DNA damage by increasing ROS levels. J. Exp. Clin. Cancer Res. 2018, 37, 269. [Google Scholar] [CrossRef]
- Hanafi, M.M.M.; Afzan, A.; Yaakob, H.; Aziz, R.; Sarmidi, M.R.; Wolfender, J.-L.; Prieto, J.M. In vitro pro-apoptotic and anti-migratory effects of Ficus deltoidea L. Plant extracts on the human prostate cancer cell lines PC3. Front. Pharmacol. 2017, 8, 895. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xu, C.; Cao, X.; Wang, W. Isovitexin suppresses cancer stemness property and induces apoptosis of osteosarcoma cells by disruption of the DNMT1/miR-34a/Bcl-2 axis. Cancer Manag. Res. 2019, 11, 8923–8936. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhong, J.; Liu, Y.; Huang, Y.; Luo, F.; Zhou, Y.; Pan, X.; Cao, S.; Zhang, L.; Zhang, Y.; et al. Purified vitexin compound 1, a new neolignan isolated compound, promotes PUMA-dependent apoptosis in colorectal cancer. Cancer Med. 2018, 7, 6158–6169. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Cho, H.J.; Paul, S.; Jakhar, R.; Khan, I.; Lee, S.-J.; Kim, B.-Y.; Krishnan, M.; Khaket, T.P.; Lee, H.G.; et al. Vitexin induces apoptosis by suppressing autophagy in multi-drug resistant colorectal cancer cells. Oncotarget 2018, 9, 3278–3291. [Google Scholar] [CrossRef]
- Xu, C.; Cao, X.; Cao, X.; Liu, L.; Qiu, Y.; Li, X.; Zhou, L.; Ning, Y.; Ren, K.; Cao, J. Isovitexin inhibits stemness and induces apoptosis in hepatocellular carcinoma SK-Hep-1 spheroids by upregulating miR-34a expression. Anticancer Agents Med. Chem. 2020, 20. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Mohan, C.D.; Shanmugam, M.K.; Rangappa, S.; Sethi, G.; Siveen, K.S.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Basappa, S.; et al. Vitexin abrogates invasion and survival of hepatocellular carcinoma cells through targeting STAT3 signaling pathway. Biochimie 2020. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liu, L.; Yuan, Q.; Li, X.; Cui, Y.; Ren, K.; Zou, C.; Chen, A.; Xu, C.; Qiu, Y.; et al. Isovitexin reduces carcinogenicity and stemness in hepatic carcinoma stem-like cells by modulating MnSOD and FoxM1. J. Exp. Clin. Cancer Res. 2019, 38, 264. [Google Scholar] [CrossRef] [PubMed]
- Özsoy, S.; Becer, E.; Kabadayı, H.; Vatansever, H.S.; Yücecan, S. Quercetin—Mediated apoptosis and cellular senescence in human colon cancer. Anticancer Agents Med. Chem. 2020, 20. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, J.; Yang, L.; Li, P. Quercetin inhibits the proliferation and metastasis of human non-small cell lung cancer cell line: The key role of src-mediated fibroblast growth factor-inducible 14 (Fn14)/nuclear factor kappa B (NF-κB) pathway. Med. Sci. Monit. 2020, 26. [Google Scholar] [CrossRef]
- Peng, D.; Chen, L.; Sun, Y.; Sun, L.; Yin, Q.; Deng, S.; Niu, L.; Lou, F.; Wang, Z.; Xu, Z.; et al. Melanoma suppression by quercein is correlated with RIG-I and type I interferon signaling. Biomed. Pharmacother. 2020, 125, 109984. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Singh, R.; Kujur, P.K.; Singh, R.P. Combination of resveratrol and quercetin causes cell growth inhibition, DNA damage, cell cycle arrest, and apoptosis in oral cancer cells. ASSAY Drug Dev. Technol. 2020. [Google Scholar] [CrossRef]
- Lu, X.; Yang, F.; Chen, D.; Zhao, Q.; Chen, D.; Ping, H.; Xing, N. Quercetin reverses docetaxel resistance in prostate cancer via androgen receptor and PI3K/Akt signaling pathways. Int. J. Biol. Sci. 2020, 16, 1121–1134. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, D.; Yang, F.; Xing, N. Quercetin inhibits epithelial-to-mesenchymal transition (EMT) process and promotes apoptosis in prostate cancer via downregulating lncRNA MALAT1. Cancer Manag. Res. 2020, 12, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Yamada, N.; Matsushima-Nishiwaki, R.; Kozawa, O. Quercetin suppresses the migration of hepatocellular carcinoma cells stimulated by hepatocyte growth factor or transforming growth factor-α: Attenuation of AKT signaling pathway. Arch. Biochem. Biophys. 2020, 682, 108296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Y.; Lu, H.; Wang, H.; Feng, H.; Xu, J.; Zhang, B. Quercetin radiosensitizes non-small cell lung cancer cells through the regulation of miR-16-5p/WEE1 axis. IUBMB Life 2020, 72, 1012–1022. [Google Scholar] [CrossRef]
- Prieto-Vila, M.; Shimomura, I.; Kogure, A.; Usuba, W.; Takahashi, R.; Ochiya, T.; Yamamoto, Y. Quercetin inhibits Lef1 and resensitizes docetaxel-resistant breast cancer cells. Molecules 2020, 25, 2576. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Huang, J.; Yu, C.; Xiang, L.; Li, L.; Shi, D.; Lin, F. Quercetin enhanced paclitaxel therapeutic effects towards PC-3 prostate cancer through ER stress induction and ROS production. Onco Targets Ther. 2020, 13, 513–523. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, J.; Wang, K.; Han, W.; Wang, X.; Gao, M.; Wang, Z.; Sun, Y.; Yan, H.; Zhang, H.; et al. Quercetin overcomes colon cancer cells resistance to chemotherapy by inhibiting solute carrier family 1, member 5 transporter. Eur. J. Pharmacol. 2020, 173185. [Google Scholar] [CrossRef]
- Liu, S.; Li, R.; Qian, J.; Sun, J.; Li, G.; Shen, J.; Xie, Y. Combination therapy of doxorubicin and quercetin on multidrug-resistant breast cancer and their sequential delivery by reduction-sensitive hyaluronic acid-based conjugate/d-α-tocopheryl poly(ethylene glycol) 1000 succinate mixed micelles. Mol. Pharm. 2020, 17, 1415–1427. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, W.; Han, J.; Liu, Q.; Gao, L.; Wang, X.; Li, X. Quercetin induces apoptosis and enhances gemcitabine therapeutic efficacy against gemcitabine-resistant cancer cells. Anticancer Drugs 2020. [Google Scholar] [CrossRef]
- Albrecht, C.; Cittadini, M.C.; Soria, E.A. Pharmacological activity of quercetin and 5 caffeoylquinic acid oral intake in male Balb/c mice with lung adenocarcinoma. Arch. Med. Res. 2020, 51, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.-K.; Yoon, J.-H.; Woo, J.K.; Kang, J.-H.; Lee, K.-R.; Oh, S.H.; Chung, S.-J.; Maeng, H.-J. Quercetin is a flavonoid breast cancer resistance protein inhibitor with an impact on the oral pharmacokinetics of sulfasalazine in rats. Pharmaceutics 2020, 12, 397. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Y.; An, Y.; Fang, G. The mechanism of anticancer action and potential clinical use of kaempferol in the treatment of breast cancer. Biomed. Pharmacother. 2019, 117, 109086. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Shah, Z.A.; Saeed, F.; Imran, A.; Arshad, M.U.; Ahmad, B.; Bawazeer, S.; Atif, M.; Peters, D.G.; et al. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytother. Res. PTR 2019, 33, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, S.; Roshini, A.; Lee, M.-H.; Yun, K. Kaempferol conjugated gold nanoclusters enabled efficient for anticancer therapeutics to A549 lung cancer cells. Int. J. Nanomed. 2019, 14, 5147–5157. [Google Scholar] [CrossRef]
- Imran, M.; Aslam Gondal, T.; Atif, M.; Shahbaz, M.; Batool Qaisarani, T.; Hanif Mughal, M.; Salehi, B.; Martorell, M.; Sharifi-Rad, J. Apigenin as an anticancer agent. Phytother. Res. 2020, ptr.6647. [Google Scholar] [CrossRef]
- Berenda, J.; Smöch, C.; Stadlbauer, C.; Mittermair, E.; Taxauer, K.; Huttary, N.; Krupitza, G.; Krenn, L. Flavonoids distinctly stabilize lymph endothelial- or blood endothelial disintegration induced by colon cancer spheroids SW620. Molecules 2020, 25, 2066. [Google Scholar] [CrossRef]
- Rea, K.A.; Casaretto, J.A.; Al-Abdul-Wahid, M.S.; Sukumaran, A.; Geddes-McAlister, J.; Rothstein, S.J.; Akhtar, T.A. Biosynthesis of cannflavins A and B from Cannabis sativa L. Phytochemistry 2019, 164, 162–171. [Google Scholar] [CrossRef]
- Delmas, D.; Xiao, J.; Vejux, A.; Aires, V. Silymarin and cancer: A dual strategy in both in chemoprevention and chemosensitivity. Molecules 2020, 25, 2009. [Google Scholar] [CrossRef]
- Kacar, S.; Bektur Aykanat, N.E.; Sahinturk, V. Silymarin inhibited DU145 cells by activating SLIT2 protein and suppressing expression of CXCR4. Med. Oncol. 2020, 37, 18. [Google Scholar] [CrossRef] [PubMed]
- Diukendjieva, A.; Zaharieva, M.M.; Mori, M.; Alov, P.; Tsakovska, I.; Pencheva, T.; Najdenski, H.; Křen, V.; Felici, C.; Bufalieri, F.; et al. Dual SMO/BRAF Inhibition by Flavonolignans from Silybum marianum. Antioxidants 2020, 9, 384. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M.; et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Huang, L.; Jin, K.; Lan, H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol. Lett. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin promotes apoptotic cell death via upregulation of Nrf2 expression by DNA demethylase and the interaction of Nrf2 with p53 in human colon cancer cells. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. Molecular targets of vitexin and isovitexin in cancer therapy: A critical review: Anticancer mechanisms of vitexin and isovitexin. Ann. N. Y. Acad. Sci. 2017, 1401, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Paul, S.; Jakhar, R.; Khan, I.; Kang, J.I.; Kim, H.M.; Yun, J.W.; Lee, S.-J.; Cho, H.J.; Lee, H.G.; et al. Vitexin confers HSF-1 mediated autophagic cell death by activating JNK and ApoL1 in colorectal carcinoma cells. Oncotarget 2017, 8, 112426–112441. [Google Scholar] [CrossRef] [PubMed]
- Formica, J.V.; Regelson, W. Review of the biology of quercetin and related bioflavonoids. Food Chem. Toxicol. 1995, 33, 1061–1080. [Google Scholar] [CrossRef]
- Tang, S.-M.; Deng, X.-T.; Zhou, J.; Li, Q.-P.; Ge, X.-X.; Miao, L. Pharmacological basis and new insights of quercetin action in respect to its anti-cancer effects. Biomed. Pharmacother. 2020, 121, 109604. [Google Scholar] [CrossRef] [PubMed]
- Mutlu Altundağ, E.; Yılmaz, A.M.; Serdar, B.S.; Jannuzzi, A.T.; Koçtürk, S.; Yalçın, A.S. Synergistic induction of apoptosis by quercetin and curcumin in chronic myeloid leukemia (K562) cells: II. Signal transduction pathways involved. Nutr. Cancer 2020, 1–10. [Google Scholar] [CrossRef]
- Mansourizadeh, F.; Alberti, D.; Bitonto, V.; Tripepi, M.; Sepehri, H.; Khoee, S.; Geninatti Crich, S. Efficient synergistic combination effect of Quercetin with Curcumin on breast cancer cell apoptosis through their loading into Apo ferritin cavity. Colloids Surf. B Biointerfaces 2020, 191, 110982. [Google Scholar] [CrossRef]
- Banerjee, A.; Pathak, S.; Jothimani, G.; Roy, S. Antiproliferative effects of combinational therapy of Lycopodium clavatum and quercetin in colon cancer cells. J. Basic Clin. Physiol. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Maruszewska, A.; Tarasiuk, J. Quercetin triggers induction of apoptotic and lysosomal death of sensitive and multidrug resistant leukaemia HL60 cells. Nutr. Cancer 2020, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shabat, S.; Fride, E.; Sheskin, T.; Tamiri, T.; Rhee, M.-H.; Vogel, Z.; Bisogno, T.; De Petrocellis, L.; Di Marzo, V.; Mechoulam, R. An entourage effect: Inactive endogenous fatty acid glycerol esters enhance 2-arachidonoyl-glycerol cannabinoid activity. Eur. J. Pharmacol. 1998, 353, 23–31. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S. From gan-zi-gun-nu to anandamide and 2-arachidonoylglycerol: The ongoing story of cannabis. Nat. Prod. Rep. 1999, 16, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ramos, J. The entourage effect of the phytocannabinoids: Letter to the Editor. Ann. Neurol. 2015, 77, 1083. [Google Scholar] [CrossRef]
- Russo, E.B. The case for the entourage effect and conventional breeding of clinical cannabis: No “strain,” no gain. Front. Plant Sci. 2019, 9, 1969. [Google Scholar] [CrossRef]
- Pacher, P.; Kogan, N.M.; Mechoulam, R. Beyond THC and endocannabinoids. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 637–659. [Google Scholar] [CrossRef]
- Cogan, P.S. The ‘entourage effect’ or ‘hodge-podge hashish’: The questionable rebranding, marketing, and expectations of cannabis polypharmacy. Expert Rev. Clin. Pharmacol. 2020, 1–11. [Google Scholar] [CrossRef]
- Finlay, D.B.; Sircombe, K.J.; Nimick, M.; Jones, C.; Glass, M. Terpenoids from cannabis do not mediate an entourage effect by acting at cannabinoid receptors. Front. Pharmacol. 2020, 11, 359. [Google Scholar] [CrossRef]
- Santiago, M.; Sachdev, S.; Arnold, J.C.; McGregor, I.S.; Connor, M. Absence of entourage: Terpenoids commonly found in Cannabis sativa do not modulate the functional activity of Δ9-THC at human CB1 and CB2 receptors. Cannabis Cannabinoid Res. 2019, 4, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Mudge, E.M.; Brown, P.N.; Murch, S.J. The terroir of cannabis: Terpene metabolomics as a tool to understand Cannabis sativa selections. Planta Med. 2019, 85, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Namdar, D.; Voet, H.; Ajjampura, V.; Nadarajan, S.; Mayzlish-Gati, E.; Mazuz, M.; Shalev, N.; Koltai, H. Terpenoids and phytocannabinoids co-produced in Cannabis sativa strains show specific interaction for cell cytotoxic activity. Molecules 2019, 24, 3031. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, S.; Fischedick, J.; Podkolinski, R.; Raber, J.C. Cannabinoids and terpenes as chemotaxonomic markers in cannabis. Nat. Prod. Chem. Res. 2015, 3. [Google Scholar] [CrossRef]
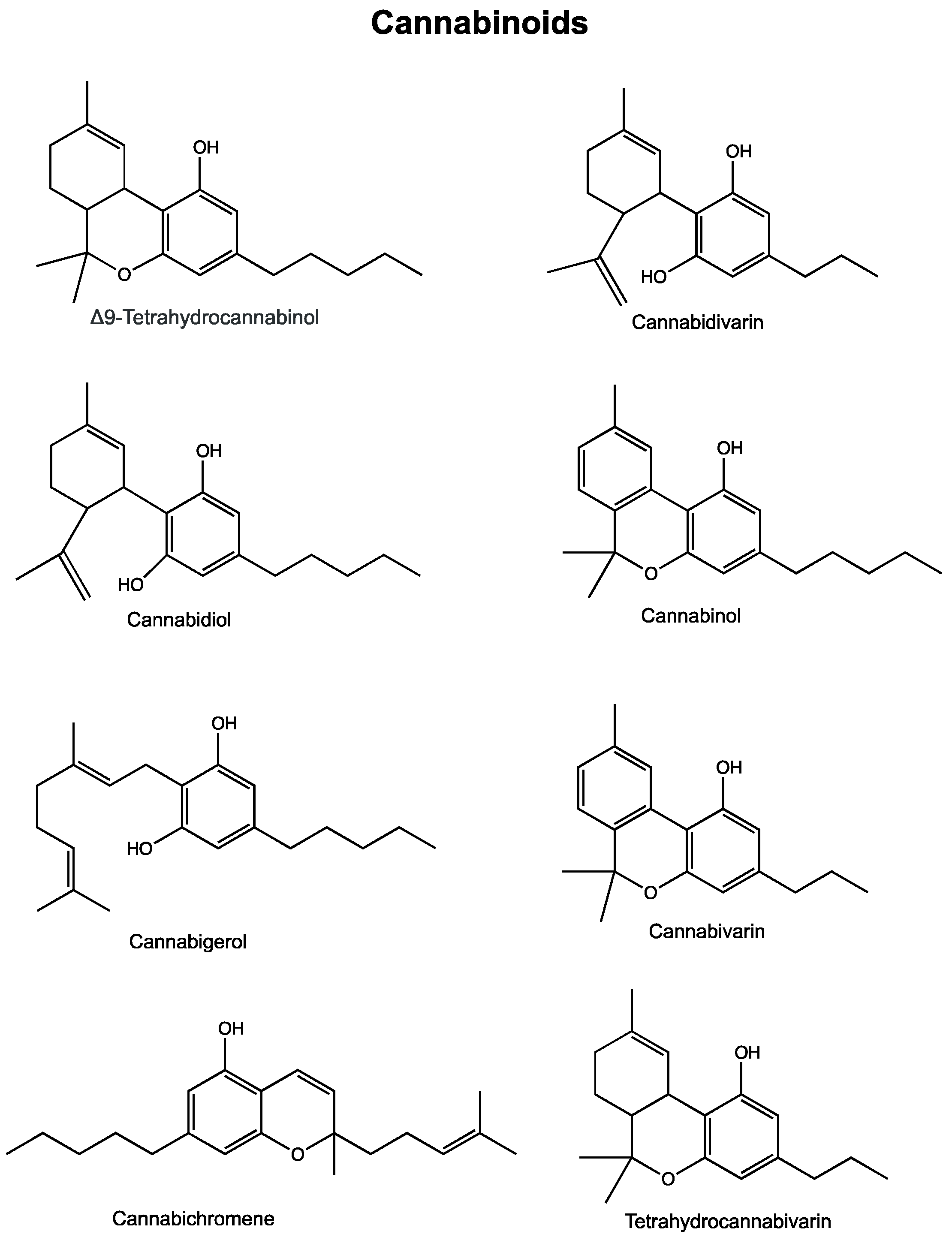
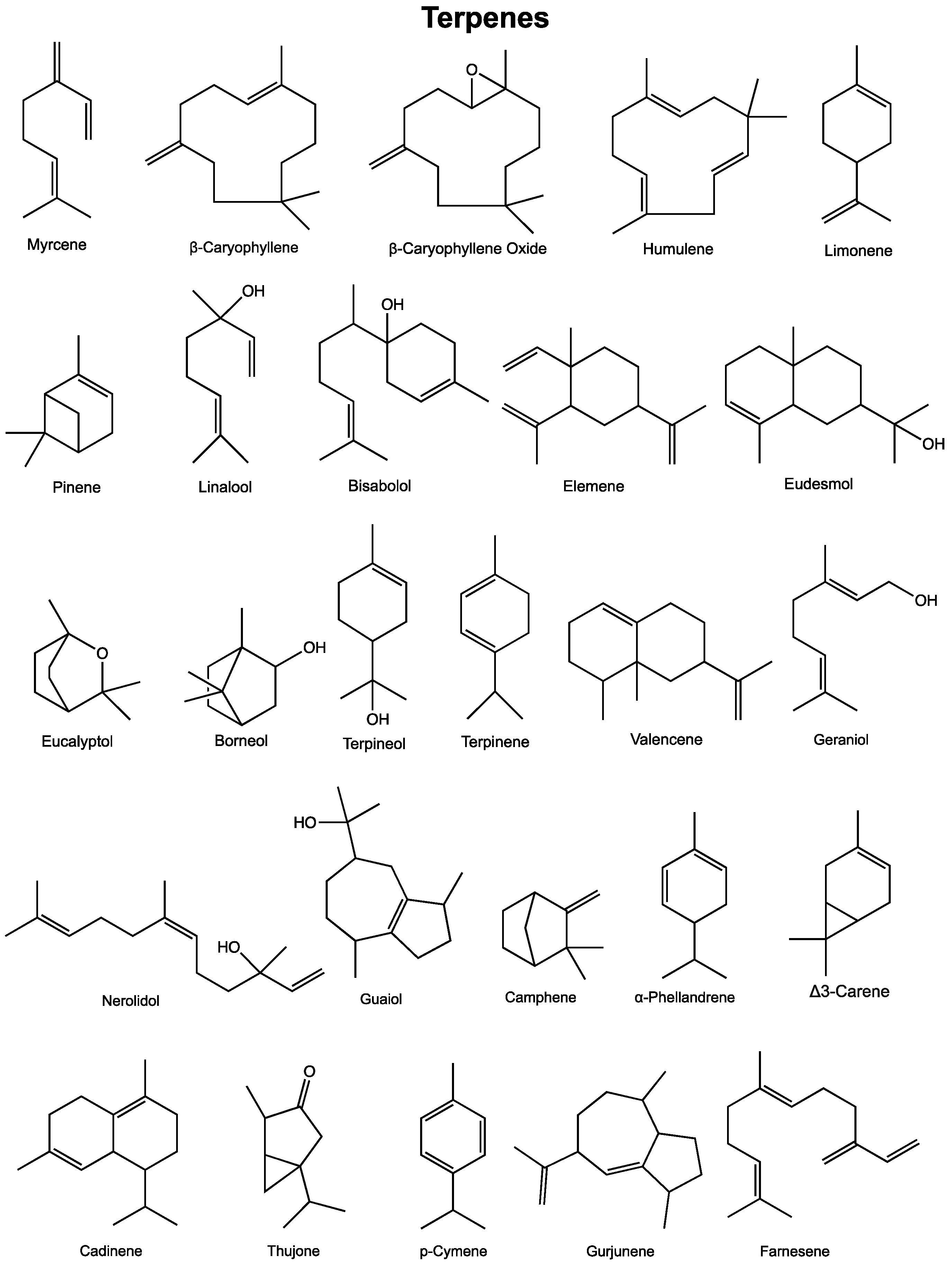
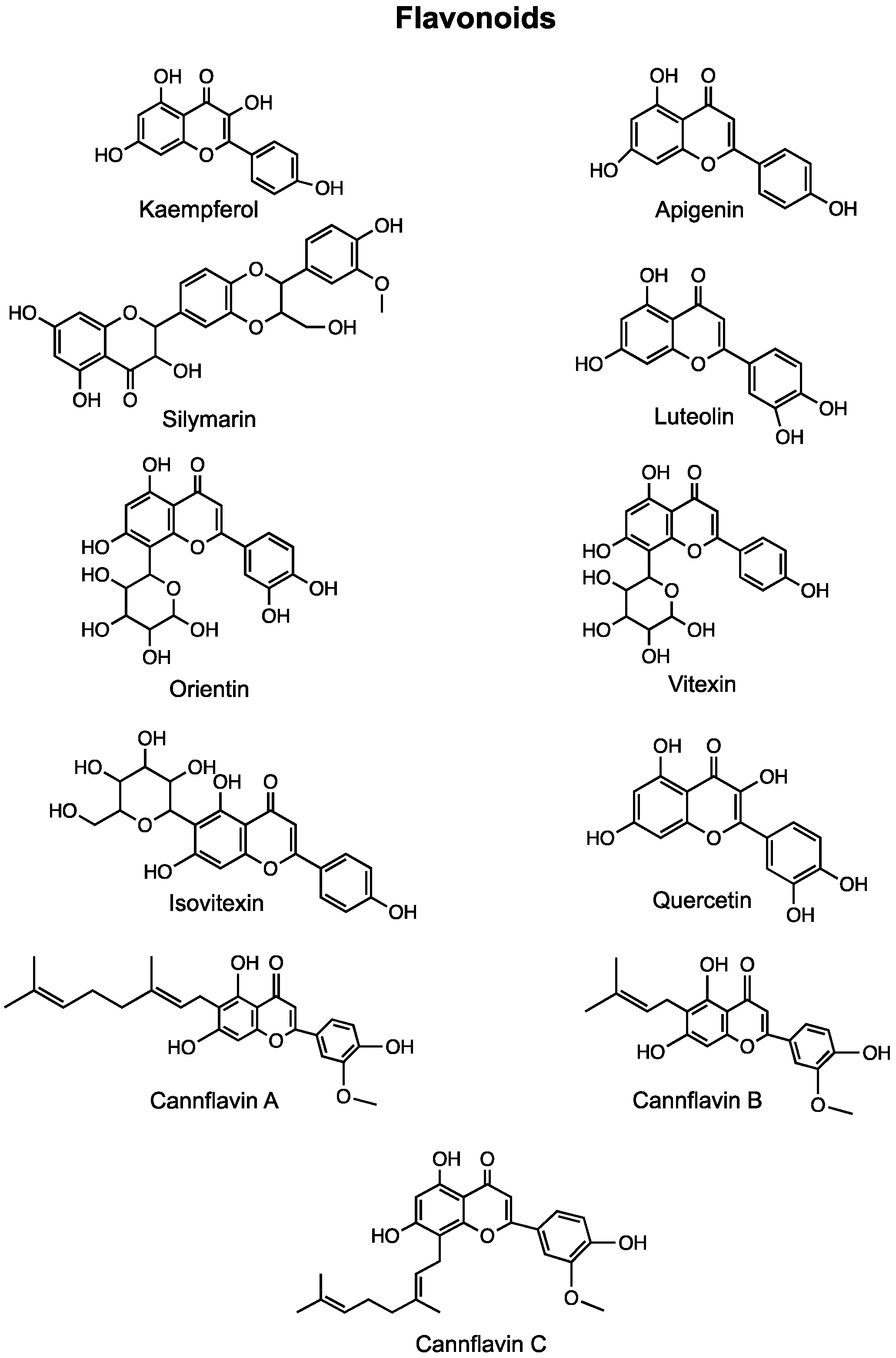
| Compound | In Vitro Effects | In Vivo Effects | Clinical Trials |
|---|---|---|---|
| Δ9-Tetrahydrocannabinol | |||
| Breast Cancer | Inhibited cell growth and proliferation [30,31,32]. Inhibited estradiol-induced proliferation [31,33]. Increased proliferation and tumor growth [34,35]. Activated transcription factor JunD [32]. Induced apoptosis and cell cycle arrest at G2/M phase [36]. Induced fatty acid 2-hydroxylase [37]. Increased production of reactive oxygen species [38]. Inhibited human P-glycoprotein and breast cancer resistance protein [39]. | Increased tumor growth and metastasis [35]. Reduced tumor growth [37,38,40,41]. Inhibited tumor angiogenesis [40]. | N/A |
| Brain Cancer | Inhibited cell viability and proliferation dose-dependently [42,43,44,45]. Induced apoptosis [46,47,48,49]. Stimulated glioma cell growth [50]. Induced autophagy via ceramide accumulation and ER stress [47,51,52]. Down-regulated expression of matrix metalloproteinase-2 [53]. THC + CBD pre-exposure increased sensitivity to radiation therapy [53]. | Reduced tumor growth [47,48,51,52]. Upregulated stress protein p8 and induced apoptosis [47]. Induced autophagy [48]. THC + Temozolomide synergistically reduced growth of xenograft tumors [54,55,56]. Down-regulated expression of metalloproteinase-2 [53]. THC-loaded nanoparticles reduced cell proliferation, angiogenesis and increased apoptosis [57]. | Pilocytic astrocytoma tumors regressed over a period of 3 years following the inhalation of cannabis over the same period [58]. Temozolomide + Sativex increased 1-year survival rate in GBM patients [NCT01812603 and NCT01812616]. Reduced tumor cell Ki67 staining in patients suffering from recurrent GBM [59]. Reduced VEGF and VEGFR-2 activation in GBM patients [60]. Dronabinol treatment did not lead to severe adverse effects in patients with primary brain tumors [61]. |
| Leukemia | Reduced proliferation and exhibited cytotoxicity [62]. Sensitized leukemia cells to anti-cancer agents [62,63,64]. Inhibited the differentiation blockage (Dronabinol) [65]. Induced apoptosis [66,67,68]. Induced apoptosis in patient-derived leukemia cells [66] | N/A | Remission achieved following the consumption of Cannabis sativa oil in a patient with terminal acute lymphoblastic leukemia [69]. Dronabinol inhibited the differentiation blockage in leukemia patients [66]. |
| Lung Cancer | Low levels induced cell proliferation or did not decrease cell survival [50,70]. Inhibited cell proliferation, chemotaxis and invasion [71,72]. Reduced migration [72]. Inhibited host immune response and killing of tumor cells [73]. Suppressed EMT of NSCLC cells [72]. THC-loaded nanoparticles exhibited cytotoxicity [74]. | Increased tumor growth and reduced tumor immunogenicity [75]. Inhibited tumor growth and metastases [71]. THC-loaded nanoparticles exhibited significant cytotoxicity [74]. | N/A |
| Melanoma & Myeloma | Inhibited growth and proliferation [76,77]. Induced apoptosis and autophagy [78]. Induced autophagic-dependent necrosis [77]. THC + CBD had synergistic effects with carfilzomib [77]. Increased cell death and decreased migration [77]. | Reduced proliferation, metastasis, angiogenesis, tumor growth and increased apoptosis [76,79]. THC:CBD in a 1:1 ratio decreased tumor growth and increased autophagy and apoptosis [78]. THC + Trametinib reduced viability, invasion and metastasis of MEKi-resistant melanoma cells [80]. Induced myeloid-derived suppressor cell function and differentiation [81]. | N/A |
| Hepatocellular Carcinoma | Decreased cell viability and induced autophagy [82]. Increased activity of PPARγ [83]. Reduced proliferation, migration, invasion, and induced apoptosis [84]. | Reduced tumor growth [82]. Increased the activity of PPARγ [83]. THC + Irinotecan reduced hepatic toxicity during acute treatment [85]. | N/A |
| Pancreatic, Prostate, Colon Cancer | Decreased cell viability [30,86,87]. Induced apoptosis [86,88,89]. THC-loaded microspheres inhibited proliferation [90]. | Reduced the growth of tumors [86]. | N/A |
| Endometrial, Cervival, Oral Cancer | Increased accumulation of anti-cancer agents in cells expressing multi-drug transporters [91,92]. Reduced invasion via increased TIMP-1 expression [93]. Inhibited mitochondrial oxygen consumption and exhibited strong toxicity [94]. | N/A | N/A |
| Cannabidiol | |||
| Breast Cancer | Induced apoptosis and autophagy [30,95]. Enhanced production of reactive oxygen species and subsequent ER stress [95]. Inhibited proliferation, migration and invasion [96,97,98]. Inhibited the EMT and reduced expression of malignant markers [98]. Increased sensitivity to anti-cancer agents doxorubicin and cisplatin [98]. Synergistic effects with paclitaxel and doxorubicin on antiproliferative activity [99]. | Inhibited tumor growth, migration, invasion, and metastasis [97]. Increased survival and decreased metastasis [100]. Down-regulated Id1 expression [100]. | N/A |
| Lung Cancer | Induced apoptosis [101]. Reduced invasion, metastasis, migration, and restored epithelial phenotype [72,102,103,104]. Increased susceptibility to lysis by lymphokine-activated killer cells [105]. | Reduced cell viability [101]. Decreased tumor growth [101,103]. Decreased metastasis [103]. | N/A |
| Glioma & Neuroblastoma | Inhibited cell proliferation and induced apoptosis [43,106,107,108,109,110,111]. Increased reactive oxygen species production [110,112]. Increased expression of heat shock proteins [112]. Induced cell cycle arrest [111]. Reduced invasion [109,111]. | CBD + THC + Temozolomide reduced tumor growth [54,55]. Reduced tumor growth [57,108]. Enhanced apoptosis and decreased angiogenesis [57]. Significantly prolonged mouse survival [110]. | Sativex + Temozolomide increased the rate of 1-year survival by 39 percent in GBM patients [NCT01812603 and NCT01812616]. |
| Colon & Prostate Cancer | Induced apoptosis, cell cycle arrest and ROS production [87]. Reduced cell proliferation, promoted apoptosis and elevated ROS levels [87,113,114,115,116]. Antagonistic activity at GPR55 reduced and prevented metastasis [117]. | Increased effects of anti-cancer agents bicalutamide and docetaxel [87]. Reduced aberrant crypt foci polyps and tumor growth [87,113,114,118]. Chemo-preventative on colon cancer cells due to up-regulated caspase-3 [113]. Decreased metastasis and angiogenesis [114]. | N/A |
| Myeloma, Melanoma, Leukemia | Reduced cell viability [77,119]. Induced apoptosis due to ceramide accumulation [120]. Decreased P-glycoprotein expression and sensitized cells to Vinblastine [64]. Increased cytotoxicity of bortezomib and carfilzomib [77,119]. | Increased mouse survival and reduced tumor growth [78,116]. | N/A |
| Cervical, Endometrial, Ovarian Cancer | Inhibited cell growth and induced apoptosis [121,122]. Increased intracellular accumulation of multi-drug transporter substrates Fluo3, vincristine, and mitoxantrone [91,92]. | N/A | N/A |
| Cannabigerol | Significant inhibitory effects on cell proliferation [123,124,125]. Inhibited [14C]anandamide uptake and activated TRPV1 receptor [30]. Stimulated apoptosis and ROS production [123]. | Decreased tumor growth due to antagonistic activity at TRPM8 receptors [123]. | N/A |
| Cannabichromene | Inhibited cell viability and growth [30,87,123]. Significantly activated caspase 3/7 [87]. Elevated intracellular Ca2+ levels [87]. | N/A | N/A |
| Cannabidivarin | Dose-dependent inhibitory effects on cell viability [87,123]. | N/A | N/A |
| Cannabinol | Cytotoxic effects at high concentrations [87]. Antiproliferative effects [96]. Inhibited multi-drug transporter ABCG2 and promoted accumulation of mitoxantrone [92]. | N/A | N/A |
| Tetrahydrocannabivarin | Cytotoxic effects at higher concentrations [87]. | N/A | N/A |
| Compound | In Vitro effects | In Vivo effects | Clinical Trials |
|---|---|---|---|
| Myrcene | Exhibited cytotoxic effects [145,146,147,148]. Decreased DNA damage [149]. | Carcinogenic [150]. | N/A |
| β-Caryophyllene and metabolite caryophyllene oxide | Exhibited cytotoxic effects [151,152]. Induced apoptosis [151,152,153,154]. Induced cell cycle arrest [151,152,154]. Increased ROS production [153]. Activated the JAK1/STAT3 pathway [153,155]. Enhanced doxorubicin sensitivity [155,156,157,158,159]. Enhanced 5-fluoruracil sensitivity [160]. Enhanced oxaliplatin sensitivity [160]. Enhanced sorafenib sensitivity [161]. | Decreased doxorubicin-induced cardiotoxicity [162]. | N/A |
| Humulene | Exhibited cytotoxic effects [163,164,165]. Increased ROS production [163]. Inhibited Akt activation [164]. Enhanced effects of 5-fluoruracil [160]. Enhanced effects of oxaliplatin [160]. Enhanced effects of doxorubicin [158]. | Inhibited cell proliferation [164]. Increased occurrence of apoptosis [164]. | N/A |
| Limonene | Exhibited cytotoxic effects [166,167,168]. Induced cell cycle arrest [166]. Decreased migration [166]. Decreased invasion [166]. Induced apoptosis [166,167,169,170,171]. Inhibited the PI3K/Akt pathway [167]. Induced autophagy [172,173,174]. Enhanced sensitivity to docetaxel [171]. | Decreased tumor growth [174,175,176,177,178]. Induced apoptosis [179,180,181,182,183]. Increased latency period [175,176,177,183]. Decreased c-jun and c-myc expression [184]. Decreased metastasis [180,181,182,185]. | Decreased cell cycle regulatory protein expression in human tumors [186]. |
| Pinene | Reduced cell viability [187,188,189,190]. Induced apoptosis [187,188,189,191,192]. Increased ROS production [188,191]. Induced cell cycle arrest [187,188,192,193,194]. Acted synergistically with paclitaxel [192]. | Reduced tumor growth [191]. Reduced the number of tumor nodules [191]. | N/A |
| Linalool | Reduced cell viability [195,196,197,198,199]. Induced apoptosis [195,197,200,201]. Induced cell cycle arrest [195,196,198,199]. Decreased p-Akt and PI3K expression [195]. Increased expression of pro-apoptotic proteins Bax, Bak, caspase-2, caspase-9 [197]. Decreased expression of Bcl-2 and Bcl-xl [197]. Increased doxorubicin sensitivity by increasing doxorubicin influx [202,203]. | Reduced xenograft tumor volume [200,201]. Caused tumor specific lipid peroxidation [200]. Reduced tumor incidence following UVB-exposure [204]. Acted synergistically with doxorubicin to decrease tumor weight in mice [202]. | N/A |
| Bisabolol | Exhibited cytotoxic effects and inhibited cell growth [205,206,207,208,209,210,211,212,213,214,215,216]. Induced apoptosis [217]. Induced autophagy [217]. Inhibited the PIK3/Akt signalling pathway [206]. Increased sensitivity to radiotherapy [213]. | Not generally toxic in murine models [217]. Decreased number of palpable tumor masses [218]. Inhibited xenograft tumor growth [219]. Increased 5-aminolevulinic acid retention in buccal tissue [220]. | N/A |
| Elemene | Induced cell cycle arrest [216,221,222,223,224,225]. Induced apoptosis [216,221,222,223,224,225,226]. Inhibited MAPK and PI3K/Akt/mTOR signalling [226]. Reduced invasion and metastasis [226]. Inhibited angiogenesis [226]. Enhanced sensitivity to several chemotherapeutic agents [227,228,229,230,231]. Increased sensitivity to paclitaxel, colchicine, and vinblastine through ABCB1 inhibition [232,233]. | N/A | Injection shown to be effective adjective treatment to platinum-based chemotherapy [234]. Reduced toxicity of chemotherapy [234]. Positive effect in combination with chemotherapy in several cancer types [235]. |
| Eudesmols | Exhibited cytotoxic effects [236,237]. Inhibited cell proliferation [238]. Inhibited superoxide production [238]. Inhibited adhesion and migration [238]. Induced apoptosis [239,240]. Induced cell cycle arrest [239,240]. Enhanced cytotoxicity of 5-fluoruracil [241]. Enhanced anti-migratory effects of doxorubicin [241]. | Reduced tumor growth [237,242]. Increased survival [242]. Reduced metastasis [242]. | N/A |
| Eucalyptol | Exhibited cytotoxic effects [148,243]. Induced apoptosis [244,245,246,247]. Induced cell cycle arrest [245,247]. Changed gene expression of MDM4, NF-kB, and VEGF in ANXA7 expressing cells [243]. Inactivated survivin and Akt [246]. Activated p38 [246]. Interacted with Bcl-2 and PARP1 receptor [247]. | Reduced tumor progression in xenograft tumors [246]. | N/A |
| Borneol | Enhanced doxorubicin induced cell cycle arrest [248]. Increased doxorubicin-induced interference with MAPKs and PI3K/Akt pathways in vitro and in vivo [248]. Enhanced prop-apoptotic effects of paclitaxel [249]. Increased cellular uptake of selenocystine [250]. Acted synergistically with curcumin-based drugs [251,252]. Acted synergistically with temozolomide [253]. Enhanced doxorubicin delivery in vitro and in vivo using borneol modified nanomicelles [254]. Enhanced chemotherapeutic effects when loaded in nanomolecule formulations [255,256,257,258]. | Induced transient disruption of the blood-brain barrier [259]. | N/A |
| Terpineol | Exhibited cytotoxic effects [260,261]. Inhibited cell proliferation [262,263]. Induced apoptosis [263]. Induced cell cycle arrest [261,263]. Reduced cell migration [261]. Potentiated the effects of oxaliplatin and 5-fluoruracil [264]. | Reduced tumor weight and volume [261]. | N/A |
| Terpinene isomers | Reduced proliferation [265,266,267,268]. Induced apoptosis [267,268]. | N/A | N/A |
| Valencene | Reduced cellular proliferation [158,269]. Acted synergistically with doxorubicin to reduce proliferation [269]. | N/A | N/A |
| Geraniol | Reduced cellular proliferation [270,271]. Induced apoptosis [270]. Induced cell cycle arrest [271,272]. Downregulated Blc-2 and upregulated Bax [270,271]. | N/A | N/A |
| Nerolidol | Exhibited cytotoxic effects [273,274,275]. Induced apoptosis [159,276]. Induced cell cycle arrest [276]. Decreased adhesion of TNF-α induced cells [159]. Acted synergistically with doxorubicin to reduce cell viability [159,269]. | Inhibited azoxymethane induced cancer [277]. | N/A |
| Guaiol | Reduced cell proliferation [278,279]. Reduced metastasis [278]. Inhibited mTORC2-Akt signalling to induce autophagy [280]. | Reduced cell growth [279]. | N/A |
| Camphene | Inhibited cell proliferation [281]. Induced apoptosis [281]. | Reduced subcutaneous tumor growth in a syngeneic model [281]. | N/A |
| α-phellandrene | Decreased cell viability [282]. Induced cell cycle arrest [194]. Altered expression of genes involved in apoptosis, DNA damage, and cell cycle [283]. Increased expression of phosphorylated p53, phosphorylated-H2A.X, 14-3-3-σ, and MDC1 [284,285]. Decreased expression of p53, MGMT, DNA-PK, and BRCA-1 [284]. Increased ROS production [282,286]. Induced autophagy [285]. Induced necrosis [282,285]. | N/A | N/A |
| Δ-3-carene | Cytotoxic when found in essential oil extracts [287,288]. | N/A | N/A |
| Cadinenes | Cytotoxic when found in essential oil extracts [289,290,291]. Inhibited cell growth [292]. Induced apoptosis [292]. Induced cell cycle arrest [292]. | N/A | N/A |
| Thujone | Exhibited cytotoxic effects [293]. Induced apoptosis [293]. | Brain, liver, and kidney toxicity [294]. | N/A |
| p-Cymene | Anti-tumor effects when found in essential oils from Nigella sativa [295]. Ruthenium11(p-Cymene) complexes were effective and selective against several cancers [296,297,298]. | N/A | N/A |
| Gurjunene | Inhibited cell growth when found in an essential oil extract [299,300,301]. Induced apoptosis [299] | N/A | N/A |
| Farnesene | Induced cell death when found in an essential oil extract [302,303,304]. Essential oil Garcinia atroviridis acted synergistically with tamoxifen [302]. | N/A | N/A |
| Compound | In Vitro Effects | In Vivo Effects | Clinical Trials |
|---|---|---|---|
| Kaempferol | Inhibited cell viability in a dose-dependent manner [327,328,329,330,331,332,333,334,335]. Induced cell cycle arrest at the G2/M or G0/G1 phase [329,335,336,337,338,339,340]. Reduced migration and invasion [340,341,342,343,344,345,346]. Inhibited the EMT and reduced resistance to chemotherapeutic agents [343,347]. Altered expression of VEGF [348]. Induced apoptosis and autophagy [330,340]. Kaempferol + Luteolin inhibited cell proliferation, induced cell death, inhibited migration and invasion [349]. Kaempferol + TRAIL induced apoptosis [350]. Kaempferol + 5-fluorouracil had synergistic anti-proliferative effects and re-sensitized resistant cells to chemotherapeutic agents [341,351]. | Increased mouse survival [339,352,353,354,355]. Reduced tumor growth and metastasis [339,352,353,354,355,356]. Caused degranulation and accumulation of mediators in leukemia cells [357,358]. | N/A |
| Apigenin | Reduced cell viability and proliferation [359,360,361]. Induced cell cycle arrest at the G1 or G2/M phase [360,362,363]. Inhibited hypoxia-induced resistance via suppression of HIF-1α [362]. Enhanced activity of paclitaxel [362]. Apigenin + Sorafenib increased apoptosis and decreased migration and invasion [364]. Apigenin + Abivertinib had synergistic anti-cancer effects [360]. Induced apoptosis and reduced angiogenesis [361]. | Exacerbated the effects of paclitaxel [362]. Inhibited tumor growth via ER-mediated PI3K/Akt/mTOR pathway [365]. Apigenin + Abivertinib exhibited synergistic anti-cancer effects [360]. Apigenin combined with IL-6 inhibition potentiated anti-cancer effects of apigenin [365]. | N/A |
| Cannflavin B | Increased apoptosis [366]. | Delayed local and metastatic tumor progression [366]. Increased survival [366]. | N/A |
| Silymarin | Induced apoptosis [367,368,369,370]. Reduced cell viability and proliferation [368,370]. Silymarin nanoemulsion + cold atmospheric plasma reduced intracellular ATP levels and down-regulate transcriptional and survival pathways [371]. Inhibited EMT and migration [372]. | Reduced tumor volume and induced apoptosis [369]. | High dose silibinin was well tolerated in patients; common adverse event observed was asymptomatic liver toxicity [373]. |
| Luteolin | Caused cell cycle arrest [362,374,375,376,377]. Decreased cell viability and proliferation [378,379,380,381,382,383]. Inhibited the EMT [376,381,384]. Inflicted double-stranded DNA breaks and prevented nonhomologous end joining [385]. Induced apoptosis [374,375,382,386,387,388]. Reduced migration and invasion [378,379,388,389,390]. Luteolin + Oxaliplatin inhibited proliferation, induced apoptosis and altered the cell cycle [391]. | Inhibited cell growth [378]. Reduced migration, invasion and metastasis [384]. Inhibited angiogenesis [387]. Decreased tumor volume and dimension [377]. | N/A |
| Orientin | Reduced migration and invasion [392]. Induced apoptosis and altered apoptotic protein levels [393,394]. Caused cell cycle arrest [394,395]. Decreased cell proliferation [393,395]. | Antiproliferative effects [396]. Improved tumor marker levels and decreased proliferative marker levels [396]. Reduced occurrence of polyps and aberrant crypt foci [397]. Increased antioxidant defense [397]. | N/A |
| Vitexin & Isovitexin | Reduced cell viability and proliferation [398,399,400,401]. Induced apoptosis [398,399,401,402,403,404]. Caused cell cycle arrest at the G2/M phase [399]. Vitexin + 5-fluorouracil had synergistic anti-tumor effects via PUMA induction [402]. Vitexin + Doxorubicin + Sorafenib induced apoptosis [405]. | Inhibited cell/tumor growth [399,401,403,406]. Induced apoptosis [403]. Reduced overall tumor size [401]. | N/A |
| Quercetin | Decreased cell viability and proliferation [407,408,409,410]. Induced apoptosis [407,409,411,412]. Reduced migration [413]. Increased the radiosensitivity of cells [414]. Reversed docetaxel resistance [411,415]. Inhibited the EMT and downregulated expression of MALAT1 [412]. Quercetin + Paclitaxel reduced cell proliferation, migration, and induced apoptosis and cell cycle arrest [416]. Quercetin + Doxorubicin caused increased cytotoxicity and induced apoptosis [417,418]. Quercetin + Gemcitabine caused increased apoptosis in gemcitabine-resistant cancer cells [419]. | Inhibited cell proliferation and tumor growth [408,411,412]. Delayed appearance of lung adenocarcinoma [420]. Reversed docetaxel resistance [411]. Inhibited breast cancer resistance protein [421]. Quercetin + Paclitaxel increased anti-cancer effects of paclitaxel [416]. Quercetin + Doxorubicin decreased tumor growth [418]. Quercetin + Docetaxel decreased tumor growth [411]. | N/A |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomko, A.M.; Whynot, E.G.; Ellis, L.D.; Dupré, D.J. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers 2020, 12, 1985. https://doi.org/10.3390/cancers12071985
Tomko AM, Whynot EG, Ellis LD, Dupré DJ. Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers. 2020; 12(7):1985. https://doi.org/10.3390/cancers12071985
Chicago/Turabian StyleTomko, Andrea M., Erin G. Whynot, Lee D. Ellis, and Denis J. Dupré. 2020. "Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis" Cancers 12, no. 7: 1985. https://doi.org/10.3390/cancers12071985
APA StyleTomko, A. M., Whynot, E. G., Ellis, L. D., & Dupré, D. J. (2020). Anti-Cancer Potential of Cannabinoids, Terpenes, and Flavonoids Present in Cannabis. Cancers, 12(7), 1985. https://doi.org/10.3390/cancers12071985






