Therapeutic Strategies for Overcoming Immunotherapy Resistance Mediated by Immunosuppressive Factors of the Glioblastoma Microenvironment
Abstract
1. Introduction—Glioblastoma and Its Epidemiological Features
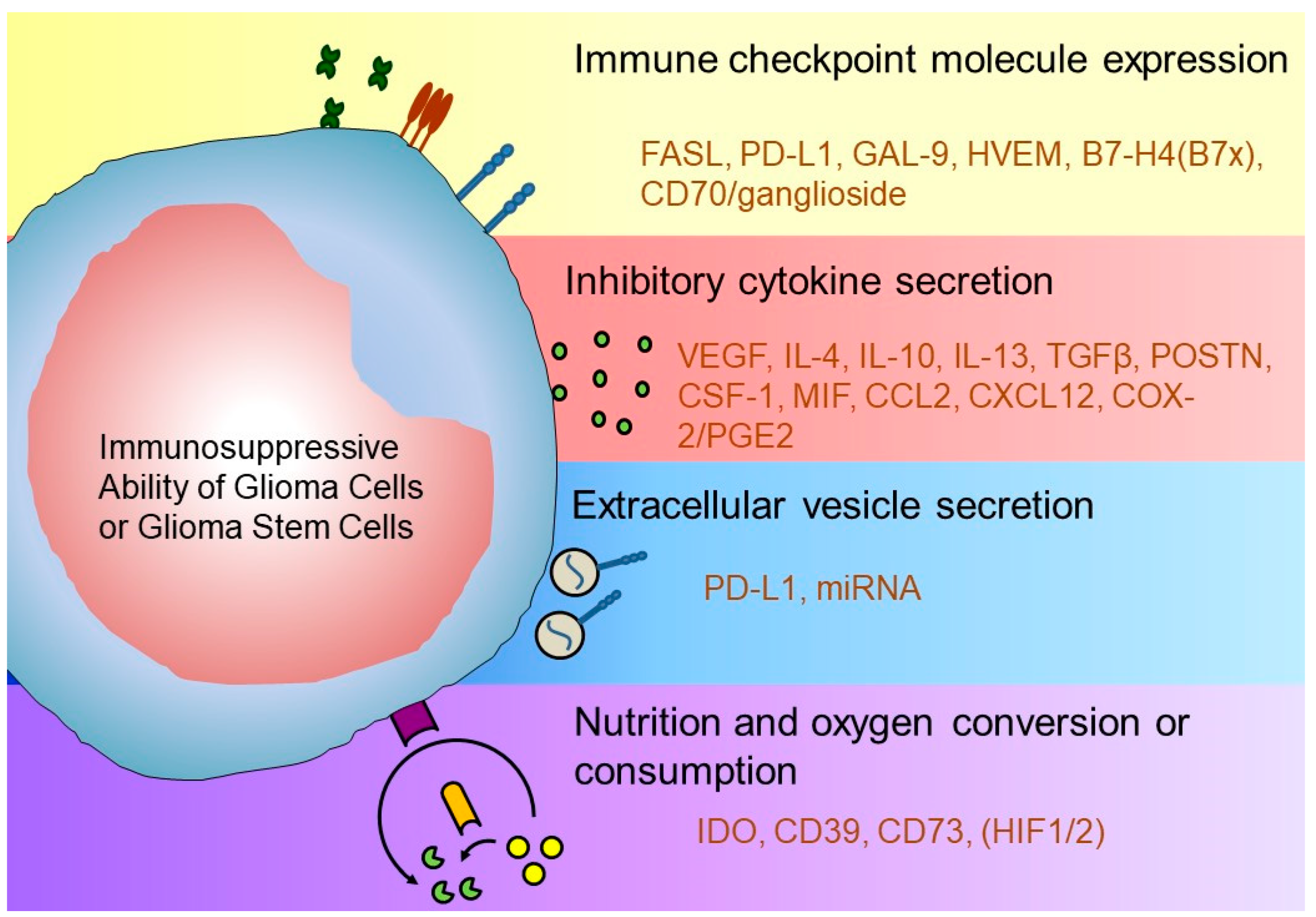
2. Glioblastoma Immune Microenvironment
3. Association between Expression of ICMs and Prognosis in GBM
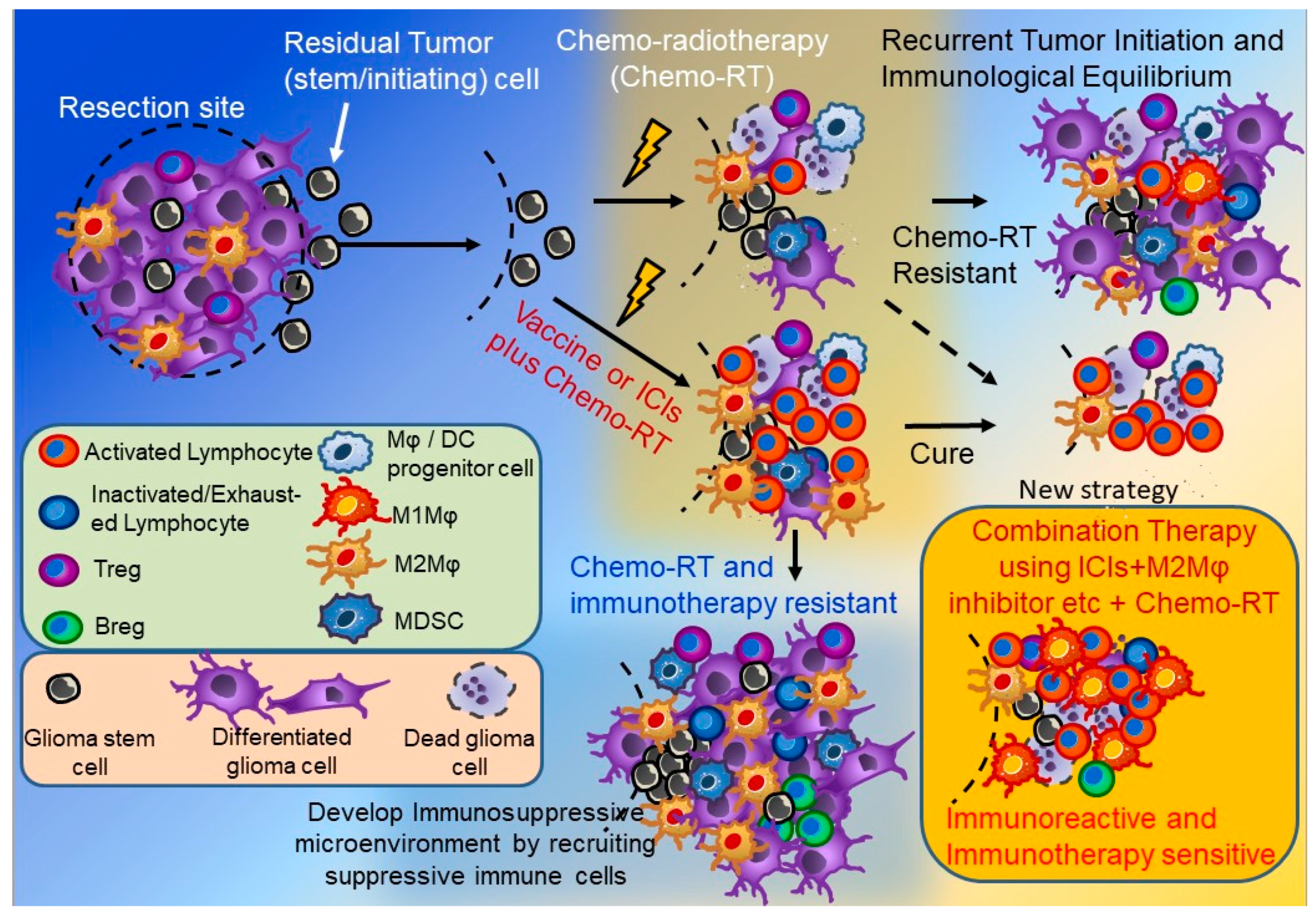
4. GBM Immunotherapy and Microenvironmental Changes after Recurrence
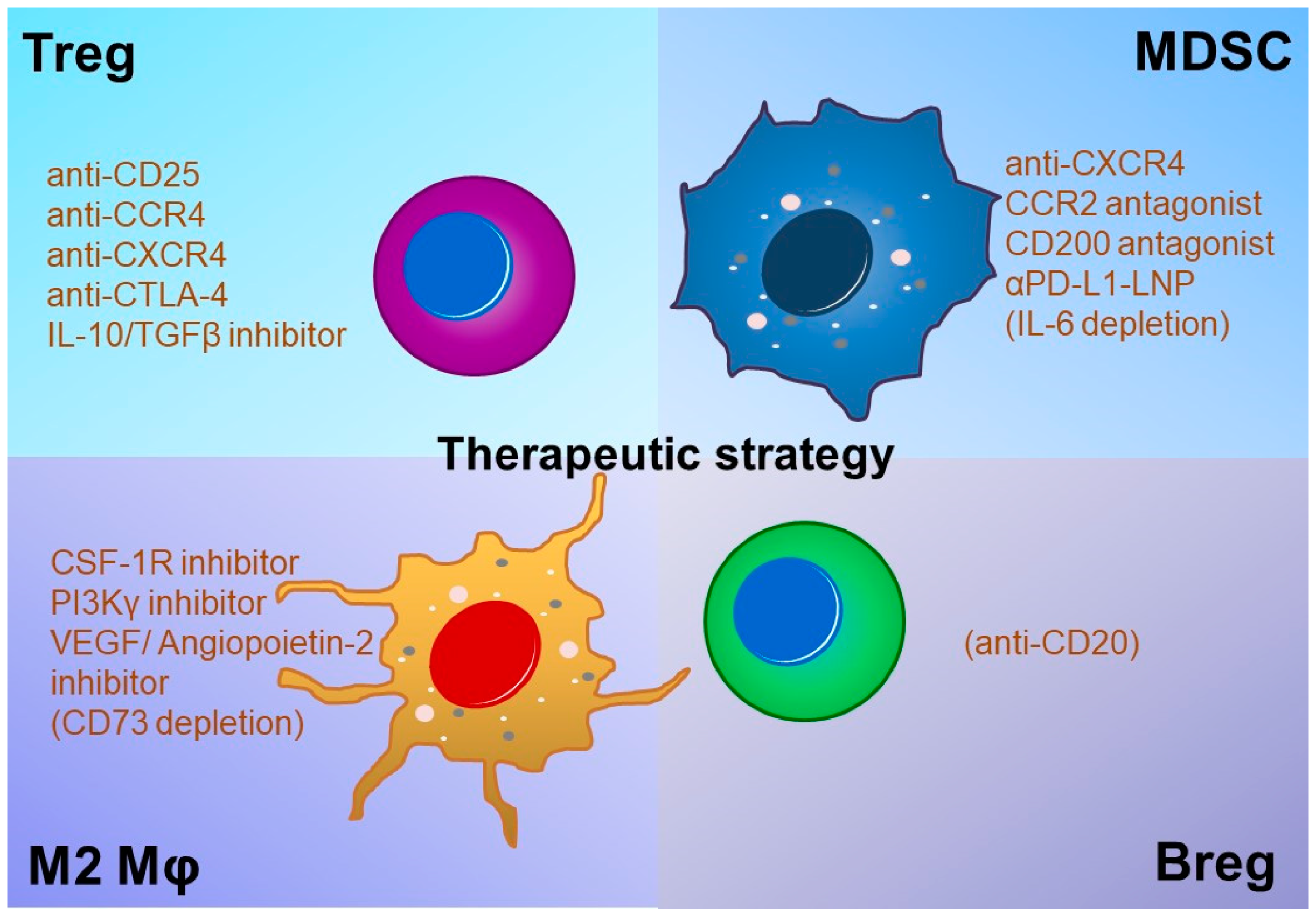
5. Regulatory T Cells (Tregs) as a Therapeutic Target of GBM
6. Myeloid-Derived Suppressor Cells (MDSCs), M2 Macrophages (M2Mφs), and Regulatory B Cells (Bregs) as Therapeutic Targets of GBM
7. Ongoing Clinical Trials
8. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFTV | autologous formalin-fixed tumor vaccine |
| AHR | aryl hydrocarbon receptor |
| ATRX | α-thalassemia/mental retardation X-linked |
| BRAF | v-raf murine sarcoma viral oncogene homolog B1 |
| BRM | biological response modifier |
| CAR | chimeric antigen receptor |
| CMV | cytomegalovirus |
| CSF-1 | colony-stimulating factor-1 |
| CTLA-4 | cytotoxic T lymphocyte antigen-4 |
| DC | dendritic cell |
| EGFR | epidermal growth factor receptor |
| FOXP3 | forkhead box protein P3 |
| GARP | glycoprotein A repetitions predominant |
| GBM | glioblastoma |
| GSC | glioma stem cell |
| ICI | immune checkpoint inhibitor |
| ICM | immune checkpoint molecule |
| IDH | isocitrate dehydrogenase |
| IDO | indoleamine 2,3 dioxygenase |
| IL | interleukin |
| IL-7-hyFc | IL-7-hybrid-Fc recombinant protein |
| KPS | Karnofski performance status |
| LNP | lipid nanoparticle |
| M2Mφ | M2-type macrophage |
| MDSC | myeloid-derived suppressor cell |
| MGMT | O6-methylguanine-DNA methyltransferase |
| MIF | macrophage migration inhibitory factor |
| oHSV | oncolytic herpes simplex viruses |
| PD-1 | programmed cell death-1; PD-L1/2 programmed cell death ligand 1/2 |
| PDPN | podoplanin |
| PI3Kγ | phosphatidylinositol 3-kinase γ |
| PRMT 5 | protein arginine methyltransferase 5 |
| PMN | polymorphonuclear |
| PTEN | phosphatase and tensin homolog |
| RT | radiation therapy |
| TAM | tumor-associated macrophage |
| TAMC | tumor-associated bone marrow cell |
| TERT | telomerase reverse transcriptase |
| TIL | tumor-infiltrated lymphocyte |
| TIM-3 | T-cell immunoglobulin and mucin domain 3 |
| TMZ | temozolomide |
| Treg | regulatory T cell |
| VEGF | vascular endothelial growth factor |
| WHO | World Health Organization |
References
- Committee of Brain Tumor Registry of Japan. Brain Tumor Registry of Japan (2005–2008). Neurol. Med. Chir. 2017, 57 (Suppl. 1), 9–102. [Google Scholar] [CrossRef] [PubMed]
- Crocetti, E.; Trama, A.; Stiller, C.; Caldarella, A.; Soffietti, R.; Jaal, J.; Weber, D.C.; Ricardi, U.; Slowinski, J.; Brandes, A. RARECARE working group. Epidemiology of glial and non-glial brain tumours in Europe. Eur. J. Cancer 2012, 48, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro Oncol. 2015, 20 (Suppl. 4), iv1–iv86. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Gorlia, T.; Stupp, R.; Brandes, A.A.; Rampling, R.R.; Fumoleau, P.; Dittrich, C.; Campone, M.M.; Twelves, C.C.; Raymond, E.; Hegi, M.E.; et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: A pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur. J. Cancer 2012, 48, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Hodges, T.; Arko, L.; Shen, M.; Dello Iacono, D.; Nabb, A.M.; Bailey, N.O.; Kreisl, T.N.; Iwamoto, F.M.; Sul, J.; et al. Scale to predict survival after surgery for recurrent glioblastoma multiforme. J. Clin. Oncol. 2010, 28, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Vredenburgh, J.J.; Desjardins, A.; Herndon, J.E., 2nd; Marcello, J.; Reardon, D.A.; Quinn, J.A.; Rich, J.N.; Sathornsumetee, S.; Gururangan, S.; Sampson, J.; et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J. Clin. Oncol. 2007, 25, 4722–4729. [Google Scholar] [CrossRef]
- Westphal, M.; Hilt, D.C.; Bortey, E.; Delavault, P.; Olivares, R.; Warnke, P.C.; Whittle, I.R.; Jääskeläinen, J.; Ram, Z. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003, 5, 79–88. [Google Scholar] [CrossRef]
- Sandmann, T.; Bourgon, R.; Garcia, J.; Li, C.; Cloughesy, T.; Chinot, O.L.; Wick, W.; Nishikawa, R.; Mason, W.; Henriksson, R.; et al. Patients With Proneural Glioblastoma May Derive Overall Survival Benefit From the Addition of Bevacizumab to First-Line Radiotherapy and Temozolomide: Retrospective Analysis of the AVAglio Trial. J. Clin. Oncol. 2015, 33, 2735–2744. [Google Scholar] [CrossRef]
- Cohen, A.L.; Colman, H. Glioma biology and molecular markers. Cancer Treat Res. 2015, 163, 15–30. [Google Scholar] [CrossRef]
- Ludwig, K.; Kornblum, H.I. Molecular markers in glioma. J. Neurooncol. 2017, 134, 505–512. [Google Scholar] [CrossRef]
- Karsy, M.; Neil, J.A.; Guan, J.; Mahan, M.A.; Colman, H.; Jensen, R.L. A practical review of prognostic correlations of molecular biomarkers in glioblastoma. Neurosurg. Focus 2015, 38, E4. [Google Scholar] [CrossRef] [PubMed]
- Minniti, G.; Scaringi, C.; Arcella, A.; Lanzetta, G.; Di Stefano, D.; Scarpino, S.; Bozzao, A.; Pace, A.; Villani, V.; Salvati, M.; et al. IDH1 mutation and MGMT methylation status predict survival in patients with anaplastic astrocytoma treated with temozolomide-based chemoradiotherapy. J. Neurooncol. 2014, 118, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Van den Bent, M.J.; Brandes, A.A.; Taphoorn, M.J.; Kros, J.M.; Kouwenhoven, M.C.; Delattre, J.Y.; Bernsen, H.J.; Frenay, M.; Tijssen, C.C.; Grisold, W.; et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long-term follow-up of EORTC brain tumor group study 26951. J. Clin. Oncol. 2013, 31, 344–350. [Google Scholar] [CrossRef]
- Stark, A.M.; Witzel, P.; Strege, R.J.; Hugo, H.-H.; Mehdorn, H.M. p53, mdm2, EGFR, and msh2 expression in paired initial and recurrent glioblastoma multiforme. J. Neurol. Neurosurg. Psychiatry 2003, 74, 779–783. [Google Scholar] [CrossRef]
- Van den Bent, M.J.; Gao, Y.; Kerkhof, M.; Kros, J.M.; Gorlia, T.; van Zwieten, K.; Prince, J.; van Duinen, S.; Sillevis Smitt, P.A.; Taphoorn, M.; et al. Changes in the EGFR amplification and EGFRvIII expression between paired primary and recurrent glioblastomas. Neuro Oncol. 2015, 17, 935–941. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.R.; Wimberly, H.; Lannin, D.R.; Nixon, C.; Rimm, D.L.; Bossuyt, V. Multiplexed quantitative analysis of CD3, CD8, and CD20 predicts response to neoadjuvant chemotherapy in breast cancer. Clin. Cancer Res. 2014, 20, 5995–6005. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, A.M.; Henriksson, M.L.; Van Guelpen, B.; Stenling, R.; Oberg, A.; Rutegård, J.; Palmqvist, R. Colorectal cancer prognosis depends on T-cell infiltration and molecular characteristics of the tumor. Mod. Pathol. 2011, 24, 671–682. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Gratas, C.; Tohma, Y.; Van Meir, E.G.; Klein, M.; Tenan, M.; Ishii, N.; Tachibana, O.; Kleihues, P.; Ohgaki, H. Fas ligand expression in glioblastoma cell lines and primary astrocytic brain tumors. Brain Pathol. 1997, 7, 863–869. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Kiesel, B.; Widhalm, G.; Rajky, O.; Ricken, G.; Wöhrer, A.; Dieckmann, K.; Filipits, M.; Brandstetter, A.; Weller, M.; et al. Programmed death ligand 1 expression and tumor-infiltrating lymphocytes in glioblastoma. Neuro Oncol. 2015, 17, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Lailler, C.; Louandre, C.; Morisse, M.C.; Lhossein, T.; Godin, C.; Lottin, M.; Constans, J.M.; Chauffert, B.; Galmiche, A.; Saidak, Z. ERK1/2 signaling regulates the immune microenvironment and macrophage recruitment in glioblastoma. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Carlsson, R.; Ambjørn, M.; Hasan, M.; Badn, W.; Darabi, A.; Siesjö, P.; Issazadeh-Navikas, S. PD-L1 expression by neurons nearby tumors indicates better prognosis in glioblastoma patients. J. Neurosci. 2013, 33, 14231–14245. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Ishikawa, E.; Matsuda, M.; Akutsu, H.; Osuka, S.; Sakamoto, N.; Takano, S.; Yamamoto, T.; Tsuboi, K.; Matsumura, A. Assessment of PD-1 positive cells on initial and secondary resected tumor specimens of newly diagnosed glioblastoma and its implications on patient outcome. J. Neurooncol. 2017, 133, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Nduom, E.K.; Wei, J.; Yaghi, N.K.; Huang, N.; Kong, L.Y.; Gabrusiewicz, K.; Ling, X.; Zhou, S.; Ivan, C.; Chen, J.Q.; et al. PD-L1 expression and prognostic impact in glioblastoma. Neuro Oncol. 2016, 18, 195–205. [Google Scholar] [CrossRef]
- Chen, Q.; Han, B.; Meng, X.; Duan, C.; Yang, C.; Wu, Z.; Magafurov, D.; Zhao, S.; Safin, S.; Jiang, C.; et al. Immunogenomic analysis reveals LGALS1 contributes to the immune heterogeneity and immunosuppression in glioma. Int. J. Cancer 2019, 145, 517–530. [Google Scholar] [CrossRef]
- Yuan, F.; Ming, H.; Wang, Y.; Yang, Y.; Yi, L.; Li, T.; Ma, H.; Tong, L.; Zhang, L.; Liu, P.; et al. Molecular and clinical characterization of Galectin-9 in glioma through 1027 samples. J. Cell Physiol. 2020, 235, 4326–4334. [Google Scholar] [CrossRef]
- Han, M.Z.; Wang, S.; Zhao, W.B.; Ni, S.L.; Yang, N.; Kong, Y.; Huang, B.; Chen, A.J.; Li, X.G.; Wang, J.; et al. Immune checkpoint molecule herpes virus entry mediator is overexpressed and associated with poor prognosis in human glioblastoma. EBioMedicine 2019, 43, 159–170. [Google Scholar] [CrossRef]
- Yao, Y.; Ye, H.; Qi, Z.; Mo, L.; Yue, Q.; Baral, A.; Hoon, D.S.B.; Vera, J.C.; Heiss, J.D.; Chen, C.C.; et al. B7-H4(B7x)-Mediated Cross-talk between Glioma-Initiating Cells and Macrophages via the IL6/JAK/STAT3 Pathway Lead to Poor Prognosis in Glioma Patients. Clin. Cancer Res. 2016, 22, 2778–2790. [Google Scholar] [CrossRef]
- Chahlavi, A.; Rayman, P.; Richmond, A.L.; Biswas, K.; Zhang, R.; Vogelbaum, M.; Tannenbaum, C.; Barnett, G.; Finke, J.H. Glioblastomas induce T-lymphocyte death by two distinct pathways involving gangliosides and CD70. Cancer Res. 2005, 65, 5428–5438. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Gabriely, G.; Rothhammer, V.; Mascanfroni, I.D.; Wheeler, M.A.; Chao, C.C.; Gutiérrez-Vázquez, C.; Kenison, J.; Tjon, E.C.; Barroso, A.; et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 2019, 22, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Eisemann, T.; Costa, B.; Peterziel, H.; Angel, P. Podoplanin Positive Myeloid Cells Promote Glioma Development by Immune Suppression. Front. Oncol. 2019, 9, 187. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, N.; Kim, E.; Sprang, B.; Leukel, P.; Khafaji, F.; Ringel, F.; Sommer, C.; Tuettenberg, J.; Tuettenberg, A. GARP as an Immune Regulatory Molecule in the Tumor Microenvironment of Glioblastoma Multiforme. Int. J. Mol. Sci. 2019, 20, 3676. [Google Scholar] [CrossRef]
- Tamura, R.; Tanaka, T.; Ohara, K.; Miyake, K.; Morimoto, Y.; Yamamoto, Y.; Kanai, R.; Akasaki, Y.; Murayama, Y.; Tamiya, T.; et al. Persistent restoration to the immunosupportive tumor microenvironment in glioblastoma by bevacizumab. Cancer Sci. 2019, 110, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Otvos, B.; Silver, D.J.; Mulkearns-Hubert, E.E.; Alvarado, A.G.; Turaga, S.M.; Sorensen, M.D.; Rayman, P.; Flavahan, W.A.; Hale, J.S.; Stoltz, K.; et al. Cancer Stem Cell-Secreted Macrophage Migration Inhibitory Factor Stimulates Myeloid Derived Suppressor Cell Function and Facilitates Glioblastoma Immune Evasion. Stem. Cells 2016, 34, 2026–2039. [Google Scholar] [CrossRef] [PubMed]
- Zisakis, A.; Piperi, C.; Themistocleous, M.S.; Korkolopoulou, P.; Boviatsis, E.I.; Sakas, D.E.; Patsouris, E.; Lea, R.W.; Kalofoutis, A. Comparative analysis of peripheral and localised cytokine secretion in glioblastoma patients. Cytokine 2007, 39, 99–105. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Suzuki, A.; Leland, P.; Joshi, B.H.; Puri, R.K. Identification of a novel role of IL-13Rα2 in human Glioblastoma multiforme: Interleukin-13 mediates signal transduction through AP-1 pathway. J. Transl. Med. 2018, 16, 369. [Google Scholar] [CrossRef]
- Qiu, B.; Zhang, D.; Wang, C.; Tao, J.; Tie, X.; Qiao, Y.; Xu, K.; Wang, Y.; Wu, A. IL-10 and TGF-β2 are overexpressed in tumor spheres cultured from human gliomas. Mol. Biol. Rep. 2011, 38, 3585–3591. [Google Scholar] [CrossRef]
- Zhou, W.; Ke, S.Q.; Huang, Z.; Flavahan, W.; Fang, X.; Paul, J.; Wu, L.; Sloan, A.E.; McLendon, R.E.; Li, X.; et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 2015, 17, 170–182. [Google Scholar] [CrossRef]
- Kast, R.E.; Hill, Q.A.; Wion, D.; Mellstedt, H.; Focosi, D.; Karpel-Massler, G.; Heiland, T.; Halatsch, M.E. Glioblastoma-synthesized G-CSF and GM-CSF contribute to growth and immunosuppression: Potential therapeutic benefit from dapsone, fenofibrate, and ribavirin. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef]
- Feng, X.; Szulzewsky, F.; Yerevanian, A.; Chen, Z.; Heinzmann, D.; Rasmussen, R.D.; Alvarez-Garcia, V.; Kim, Y.; Wang, B.; Tamagno, I.; et al. Loss of CX3CR1 increases accumulation of inflammatory monocytes and promotes gliomagenesis. Oncotarget 2015, 6, 15077–15094. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.A.; Link, B.; Glas, M.; Herrlinger, U.; Wenz, F.; Umansky, V.; Brown, J.M.; Herskind, C. Targeting the Post-Irradiation Tumor Microenvironment in Glioblastoma via Inhibition of CXCL12. Cancers 2019, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Shi, Z.; Jiang, J. Cyclooxygenase-2 in glioblastoma multiforme. Drug Discov. Today 2017, 22, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, Y.; Wang, S.; Shen, Q.; Zhou, X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018, 415, 117–128. [Google Scholar] [CrossRef]
- Ricklefs, F.L.; Alayo, Q.; Krenzlin, H.; Mahmoud, A.B.; Speranza, M.C.; Nakashima, H.; Hayes, J.L.; Lee, K.; Balaj, L.; Passaro, C.; et al. Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci. Adv. 2018, 4, eaar2766. [Google Scholar] [CrossRef]
- Rooj, A.K.; Mineo, M.; Godlewski, J. MicroRNA and extracellular vesicles in glioblastoma: Small but powerful. Brain Tumor. Pathol. 2016, 33, 77–88. [Google Scholar] [CrossRef]
- Kesarwani, P.; Prabhu, A.; Kant, S.; Chinnaiyan, P. Metabolic remodeling contributes towards an immune-suppressive phenotype in glioblastoma. Cancer Immunol. Immunother. 2019, 68, 1107–1120. [Google Scholar] [CrossRef]
- Wainwright, D.A.; Balyasnikova, I.V.; Chang, A.L.; Ahmed, A.U.; Moon, K.S.; Auffinger, B.; Tobias, A.L.; Han, Y.; Lesniak, M.S. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin. Cancer Res. 2012, 18, 6110–6121. [Google Scholar] [CrossRef]
- Zhai, L.; Ladomersky, E.; Lauing, K.L.; Wu, M.; Genet, M.; Gritsina, G.; Győrffy, B.; Brastianos, P.K.; Binder, D.C.; Sosman, J.A.; et al. Infiltrating T Cells Increase IDO1 Expression in Glioblastoma and Contribute to Decreased Patient Survival. Clin. Cancer Res. 2017, 23, 6650–6660. [Google Scholar] [CrossRef]
- Xu, S.; Shao, Q.Q.; Sun, J.T.; Yang, N.; Xie, Q.; Wang, D.H.; Huang, Q.B.; Huang, B.; Wang, X.Y.; Li, X.G.; et al. Synergy between the ectoenzymes CD39 and CD73 contributes to adenosinergic immunosuppression in human malignant gliomas. Neuro Oncol. 2013, 15, 1160–1172. [Google Scholar] [CrossRef]
- Banasavadi-Siddegowda, Y.K.; Welker, A.M.; An, M.; Yang, X.; Zhou, W.; Shi, G.; Imitola, J.; Li, C.; Hsu, S.; Wang, J.; et al. PRMT5 as a druggable target for glioblastoma therapy. Neuro Oncol. 2018, 20, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Miska, J.; Lee-Chang, C.; Rashidi, A.; Muroski, M.E.; Chang, A.L.; Lopez-Rosas, A.; Zhang, P.; Panek, W.K.; Cordero, A.; Han, Y.; et al. HIF-1α Is a Metabolic Switch between Glycolytic-Driven Migration and Oxidative Phosphorylation-Driven Immunosuppression of Tregs in Glioblastoma. Cell Rep. 2019, 27, 226–237. [Google Scholar] [CrossRef] [PubMed]
- Lee-Chang, C.; Rashidi, A.; Miska, J.; Zhang, P.; Pituch, K.C.; Hou, D.; Xiao, T.; Fischietti, M.; Kang, S.J.; Appin, C.L.; et al. Myeloid-Derived Suppressive Cells Promote B cell-Mediated Immunosuppression via Transfer of PD-L1 in Glioblastoma. Cancer Immunol. Res. 2019, 7, 1928–1943. [Google Scholar] [CrossRef] [PubMed]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Lu, H.; Li, J.; Yan, X.; Xiao, M.; Hao, J.; Alekseev, A.; Khong, H.; Chen, T.; et al. Genome-wide analysis identifies NR4A1 as a key mediator of T cell dysfunction. Nature 2019, 567, 525–529. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Robert, C.; Long, G.V.; Brady, B.; Dutriaux, C.; Maio, M.; Mortier, L.; Hassel, J.C.; Rutkowski, P.; McNeil, C.; Kalinka-Warzocha, E.; et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 2015, 372, 320–330. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, H.; Zhao, S.; Wang, Y.; Pu, H.; Wang, Y.; Zhang, Q. Expression of PD-L1 and prognosis in breast cancer: A meta-analysis. Oncotarget 2017, 8, 31347–31354. [Google Scholar] [CrossRef]
- Duechler, M.; Peczek, L.; Zuk, K.; Zalesna, I.; Jeziorski, A.; Czyz, M. The heterogeneous immune microenvironment in breast cancer is affected by hypoxia-related genes. Immunobiology 2014, 219, 158–165. [Google Scholar] [CrossRef]
- Platten, M.; Ochs, K.; Lemke, D.; Opitz, C.; Wick, W. Microenvironmental clues for glioma immunotherapy. Curr. Neurol. Neurosci. Rep. 2014, 14, 440. [Google Scholar] [CrossRef] [PubMed]
- Tamura, R.; Tanaka, T.; Akasaki, Y.; Murayama, Y.; Yoshida, K.; Sasaki, H. The role of vascular endothelial growth factor in the hypoxic and immunosuppressive tumor microenvironment: Perspectives for therapeutic implications. Med. Oncol. 2019, 37, 2. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, P.; Dzieciątkowski, T. The Intriguing History of Cancer Immunotherapy. Front. Immunol. 2019, 10, 2965. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, E.; Tsuboi, K.; Yamamoto, T.; Muroi, A.; Takano, S.; Enomoto, T.; Matsumura, A.; Ohno, T. Clinical trial of autologous formalin-fixed tumor vaccine for glioblastoma multiforme patients. Cancer Sci. 2007, 98, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, K.; Saijo, K.; Ishikawa, E.; Tsurushima, H.; Takano, S.; Morishita, Y.; Ohno, T. Effects of local injection of ex vivo expanded autologous tumor-specific T lymphocytes in cases with recurrent malignant gliomas. Clin. Cancer Res. 2003, 9, 3294–3302. [Google Scholar] [PubMed]
- Ishikawa, E.; Tsuboi, K.; Saijo, K.; Harada, H.; Takano, S.; Nose, T.; Ohno, T. Autologous natural killer cell therapy for human recurrent malignant glioma. Anticancer Res. 2004, 24, 1861–1871. [Google Scholar]
- Muragaki, Y.; Maruyama, T.; Iseki, H.; Tanaka, M.; Shinohara, C.; Takakura, K.; Tsuboi, K.; Yamamoto, T.; Matsumura, A.; Matsutani, M.; et al. Phase I/IIa trial of autologous formalin-fixed tumor vaccine concomitant with fractionated radiotherapy for newly diagnosed glioblastoma. Clin. Artic. J. Neurosurg. 2011, 115, 248–255. [Google Scholar] [CrossRef]
- Ishikawa, E.; Muragaki, Y.; Yamamoto, T.; Maruyama, T.; Tsuboi, K.; Ikuta, S.; Hashimoto, K.; Uemae, Y.; Ishihara, T.; Matsuda, M.; et al. Phase I/IIa trial of fractionated radiotherapy, temozolomide, and autologous formalin-fixed tumor vaccine for newly diagnosed glioblastoma. J. Neurosurg. 2014, 121, 543–553. [Google Scholar] [CrossRef]
- Ishikawa, E.; Yamamoto, T.; Matsumura, A. Prospect of Immunotherapy for Glioblastoma: Tumor Vaccine, Immune Checkpoint Inhibitors and Combination Therapy. Neurol. Med. Chir. 2017, 57, 321–330. [Google Scholar] [CrossRef]
- Sakamoto, N.; Ishikawa, E.; Yamamoto, T.; Satomi, K.; Nakai, K.; Sato, M.; Enomoto, T.; Morishita, Y.; Takano, S.; Ohno, T.; et al. Pathological changes after autologous formalin-fixed tumor vaccine therapy combined with temozolomide for glioblastoma-three case reports. Neurol. Med. Chir. 2011, 51, 319–325. [Google Scholar] [CrossRef]
- Omuro, A.; Vlahovic, G.; Lim, M.; Sahebjam, S.; Baehring, J.; Cloughesy, T.; Voloschin, A.; Ramkissoon, S.H.; Ligon, K.L.; Latek, R.; et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: Results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018, 20, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Cloughesy, T.F.; Mochizuki, A.Y.; Orpilla, J.R.; Hugo, W.; Lee, A.H.; Davidson, T.B.; Wang, A.C.; Ellingson, B.M.; Rytlewski, J.A.; Sanders, C.M.; et al. Neoadjuvant anti-PD-1 immunotherapy promotes a survival benefit with intratumoral and systemic immune responses in recurrent glioblastoma. Nat. Med. 2019, 25, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, A.X.; Gartrell, R.D.; Silverman, A.M.; Aparicio, L.; Chu, T.; Bordbar, D.; Shan, D.; Samanamud, J.; Mahajan, A.; et al. Immune and genomic correlates of response to anti-PD-1 immunotherapy in glioblastoma. Nat. Med. 2019, 25, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef]
- Zhou, J.; Ding, T.; Pan, W.; Zhu, L.Y.; Li, L.; Zheng, L. Increased intratumoral regulatory T cells are related to intratumoral macrophages and poor prognosis in hepatocellular carcinoma patients. Int. J. Cancer 2009, 125, 1640–1648. [Google Scholar] [CrossRef]
- Davidsson, S.; Ohlson, A.L.; Andersson, S.O.; Fall, K.; Meisner, A.; Fiorentino, M.; Andrén, O.; Rider, J.R. CD4 helper T cells, CD8 cytotoxic T cells, and FOXP3(+) regulatory T cells with respect to lethal prostate cancer. Mod. Pathol. 2013, 26, 448–455. [Google Scholar] [CrossRef]
- Ino, Y.; Yamazaki-Itoh, R.; Shimada, K.; Iwasaki, M.; Kosuge, T.; Kanai, Y.; Hiraoka, N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer 2013, 108, 914–923. [Google Scholar] [CrossRef]
- Jiang, W.; Liu, K.; Guo, Q.; Cheng, J.; Shen, L.; Cao, Y.; Wu, J.; Shi, J.; Cao, H.; Liu, B.; et al. Tumor-infiltrating immune cells and prognosis in gastric cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 62312–62329. [Google Scholar] [CrossRef]
- Märkl, B.; Paul, B.; Schaller, T.; Kretsinger, H.; Kriening, B.; Schenkirsch, G. The role of lymph node size and FOXP3+ regulatory T cells in node-negative colon cancer. J. Clin. Pathol. 2017, 70, 443–447. [Google Scholar] [CrossRef]
- Papaioannou, E.; Sakellakis, M.; Melachrinou, M.; Tzoracoleftherakis, E.; Kalofonos, H.; Kourea, E. A Standardized Evaluation Method for FOXP3+ Tregs and CD8+ T-cells in Breast Carcinoma: Association with Breast Carcinoma Subtypes, Stage and Prognosis. Anticancer Res. 2019, 39, 1217–1232. [Google Scholar] [CrossRef] [PubMed]
- Salama, P.; Phillips, M.; Grieu, F.; Morris, M.; Zeps, N.; Joseph, D.; Platell, C.; Iacopetta, B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 2009, 27, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Yeong, J.; Thike, A.A.; Lim, J.C.; Lee, B.; Li, H.; Wong, S.C.; Hue, S.S.; Tan, P.H.; Iqbal, J. Higher densities of Foxp3+ regulatory T cells are associated with better prognosis in triple-negative breast cancer. Breast Cancer Res. Treat. 2017, 163, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, C.; Li, Q.; Dong, J.; Liu, Y.; Huang, Y.; Jiang, T.; Wu, A. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br. J. Cancer 2014, 110, 2560–2568. [Google Scholar] [CrossRef]
- Pereira, M.B.; Barros, L.R.C.; Bracco, P.A.; Vigo, A.; Boroni, M.; Bonamino, M.H.; Lenz, G. Transcriptional characterization of immunological infiltrates and their relation with glioblastoma patients overall survival. Oncoimmunology 2018, 7, e1431083. [Google Scholar] [CrossRef] [PubMed]
- Sayour, E.J.; McLendon, P.; McLendon, R.; De Leon, G.; Reynolds, R.; Kresak, J.; Sampson, J.H.; Mitchell, D.A. Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol. Immunother. 2015, 64, 419–427. [Google Scholar] [CrossRef]
- Thomas, A.A.; Fisher, J.L.; Rahme, G.J.; Hampton, T.H.; Baron, U.; Olek, S.; Schwachula, T.; Rhodes, C.H.; Gui, J.; Tafe, L.J.; et al. Regulatory T Cells Are Not a Strong Predictor of Survival for Patients With Glioblastoma. Neuro Oncol. 2015, 17, 801–809. [Google Scholar] [CrossRef]
- Tamura, R.; Ohara, K.; Sasaki, H.; Morimoto, Y.; Kosugi, K.; Yoshida, K.; Toda, M. Difference in Immunosuppressive Cells Between Peritumoral Area and Tumor Core in Glioblastoma. World Neurosurg. 2018, 120, e601–e610. [Google Scholar] [CrossRef]
- Fecci, P.E.; Mitchell, D.A.; Whitesides, J.F.; Xie, W.; Friedman, A.H.; Archer, G.E.; Herndon, J.E., 2nd; Bigner, D.D.; Dranoff, G.; Sampson, J.H. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006, 66, 3294–3302. [Google Scholar] [CrossRef]
- Di Tacchio, M.; Macas, J.; Weissenberger, J.; Sommer, K.; Bähr, O.; Steinbach, J.P.; Senft, C.; Seifert, V.; Glas, M.; Herrlinger, U.; et al. Tumor Vessel Normalization, Immunostimulatory Reprogramming, and Improved Survival in Glioblastoma with Combined Inhibition of PD-1, Angiopoietin-2, and VEGF. Cancer Immunol. Res. 2019, 7, 1910–1927. [Google Scholar] [CrossRef]
- Jacobs, J.F.; Punt, C.J.; Lesterhuis, W.J.; Sutmuller, R.P.; Brouwer, H.M.; Scharenborg, N.M.; Klasen, I.S.; Hilbrands, L.B.; Figdor, C.G.; de Vries, I.J.; et al. Dendritic cell vaccination in combination with anti-CD25 monoclonal antibody treatment: A phase I/II study in metastatic melanoma patients. Clin. Cancer Res. 2010, 16, 5067–5078. [Google Scholar] [CrossRef] [PubMed]
- Kurose, K.; Ohue, Y.; Wada, H.; Iida, S.; Ishida, T.; Kojima, T.; Doi, T.; Suzuki, S.; Isobe, M.; Funakoshi, T.; et al. Phase Ia Study of FoxP3+ CD4 Treg Depletion by Infusion of a Humanized Anti-CCR4 Antibody, KW-0761, in Cancer Patients. Clin. Cancer Res. 2015, 21, 4327–4336. [Google Scholar] [CrossRef] [PubMed]
- Rech, A.J.; Mick, R.; Martin, S.; Recio, A.; Aqui, N.A.; Powell, D.J., Jr.; Colligon, T.A.; Trosko, J.A.; Leinbach, L.I.; Pletcher, C.H.; et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci. Transl. Med. 2012, 4, 134ra62. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Maxwell, R.; Xia, Y.; Cardarelli, P.; Oyasu, M.; Belcaid, Z.; Kim, E.; Hung, A.; Luksik, A.S.; Garzon-Muvdi, T.; et al. Combination anti-CXCR4 and anti-PD-1 immunotherapy provides survival benefit in glioblastoma through immune cell modulation of tumor microenvironment. J. Neurooncol. 2019, 143, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Grauer, O.M.; Nierkens, S.; Bennink, E.; Toonen, L.W.; Boon, L.; Wesseling, P.; Sutmuller, R.P.; Adema, G.J. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress anti glioma immune responses in vivo. Int. J. Cancer 2007, 121, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Sakaguchi, S. Targeting Treg cells in cancer immunotherapy. Eur. J. Immunol. 2019, 49, 1140–1146. [Google Scholar] [CrossRef]
- Overacre-Delgoffe, A.E.; Vignali, D.A.A. Treg Fragility: A Prerequisite for Effective Antitumor Immunity? Cancer Immunol. Res. 2018, 6, 882–887. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef]
- Law, A.M.K.; Valdes-Mora, F.; Gallego-Ortega, D. Myeloid-Derived Suppressor Cells as a Therapeutic Target for Cancer. Cells 2020, 9, 561. [Google Scholar] [CrossRef]
- Domenis, R.; Cesselli, D.; Toffoletto, B.; Bourkoula, E.; Caponnetto, F.; Manini, I.; Beltrami, A.P.; Ius, T.; Skrap, M.; Di Loreto, C.; et al. Systemic T Cells Immunosuppression of Glioma Stem Cell-Derived Exosomes Is Mediated by Monocytic Myeloid-Derived Suppressor Cells. PLoS ONE 2017, 12, e0169932. [Google Scholar] [CrossRef]
- Fleming, V.; Hu, X.; Weber, R.; Nagibin, V.; Groth, C.; Altevogt, P.; Utikal, J.; Umansky, V. Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Front. Immunol. 2018, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Alban, T.J.; Alvarado, A.G.; Sorensen, M.D.; Bayik, D.; Volovetz, J.; Serbinowski, E.; Mulkearns-Hubert, E.E.; Sinyuk, M.; Hale, J.S.; Onzi, G.R.; et al. Global immune fingerprinting in glioblastoma patient peripheral blood reveals immune-suppression signatures associated with prognosis. JCI Insight 2018, 3, 122264. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.R.; Jeon, H.; Park, C.K.; Park, S.H.; Choi, S.H. Radiogenomics Profiling for Glioblastoma-related Immune Cells Reveals CD49d Expression Correlation with MRI parameters and Prognosis. Sci. Rep. 2018, 8, 16022. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, M.P.; Lin, Y.; LaPlant, B.; Liwski, C.J.; Maas, M.L.; League, S.C.; Bauer, P.R.; Abraham, R.S.; Tollefson, M.K.; Kwon, E.D.; et al. Immune monitoring using the predictive power of immune profiles. J. Immunother. Cancer 2013, 1, 7. [Google Scholar] [CrossRef]
- Chang, A.L.; Miska, J.; Wainwright, D.A.; Dey, M.; Rivetta, C.V.; Yu, D.; Kanojia, D.; Pituch, K.C.; Qiao, J.; Pytel, P.; et al. CCL2 Produced by the Glioma Microenvironment Is Essential for the Recruitment of Regulatory T Cells and Myeloid-Derived Suppressor Cells. Cancer Res. 2016, 76, 5671–5682. [Google Scholar] [CrossRef]
- Flores-Toro, J.A.; Luo, D.; Gopinath, A.; Sarkisian, M.R.; Campbell, J.J.; Charo, I.F.; Singh, R.; Schall, T.J.; Datta, M.; Jain, R.K.; et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc. Natl. Acad. Sci. USA 2020, 117, 1129–1138. [Google Scholar] [CrossRef]
- Moertel, C.L.; Xia, J.; LaRue, R.; Waldron, N.N.; Andersen, B.M.; Prins, R.M.; Okada, H.; Donson, A.M.; Foreman, N.K.; Hunt, M.A.; et al. CD200 in CNS tumor-induced immunosuppression: The role for CD200 pathway blockade in targeted immunotherapy. J. Immunother. Cancer 2014, 2, 46. [Google Scholar] [CrossRef]
- Lamano, J.B.; Lamano, J.B.; Li, Y.D.; DiDomenico, J.D.; Choy, W.; Veliceasa, D.; Oyon, D.E.; Fakurnejad, S.; Ampie, L.; Kesavabhotla, K.; et al. Glioblastoma-Derived IL6 Induces Immunosuppressive Peripheral Myeloid Cell PD-L1 and Promotes Tumor Growth. Clin. Cancer Res. 2019, 25, 3643–3657. [Google Scholar] [CrossRef]
- Zhang, P.; Miska, J.; Lee-Chang, C.; Rashidi, A.; Panek, W.K.; An, S.; Zannikou, M.; Lopez-Rosas, A.; Han, Y.; Xiao, T.; et al. Therapeutic targeting of tumor-associated myeloid cells synergizes with radiation therapy for glioblastoma. Proc. Natl. Acad. Sci. USA 2019, 116, 23714–23723. [Google Scholar] [CrossRef]
- Aras, S.; Zaidi, M.R. TAMeless traitors: Macrophages in cancer progression and metastasis. Br. J. Cancer 2017, 117, 1583–1591. [Google Scholar] [CrossRef]
- Su, S.; Zhao, J.; Xing, Y.; Zhang, X.; Liu, J.; Ouyang, Q.; Chen, J.; Su, F.; Liu, Q.; Song, E. Immune Checkpoint Inhibition Overcomes ADCP-Induced Immunosuppression by Macrophages. Cell 2018, 175, 442–457.e23. [Google Scholar] [CrossRef]
- Chuang, H.Y.; Su, Y.K.; Liu, H.W.; Chen, C.H.; Chiu, S.C.; Cho, D.Y.; Lin, S.Z.; Chen, Y.S.; Lin, C.M. Preclinical Evidence of STAT3 Inhibitor Pacritinib Overcoming Temozolomide Resistance via Downregulating miR-21-Enriched Exosomes from M2 Glioblastoma-Associated Macrophages. J. Clin. Med. 2019, 8, 959. [Google Scholar] [CrossRef] [PubMed]
- Gabrusiewicz, K.; Li, X.; Wei, J.; Hashimoto, Y.; Marisetty, A.L.; Ott, M.; Wang, F.; Hawke, D.; Yu, J.; Healy, L.M.; et al. Glioblastoma stem cell-derived exosomes induce M2 macrophages and PD-L1 expression on human monocytes. Oncoimmunology 2018, 7, e1412909. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Feng, X.; Herting, C.J.; Garcia, V.A.; Nie, K.; Pong, W.W.; Rasmussen, R.; Dwivedi, B.; Seby, S.; Wolf, S.A.; et al. Cellular and Molecular Identity of Tumor-Associated Macrophages in Glioblastoma. Cancer Res. 2017, 77, 2266–2278. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hambardzumyan, D. Immune Microenvironment in Glioblastoma Subtypes. Front. Immunol. 2018, 9, 1004. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Walle, T.; Cornish, A.E.; Basu, S.; Anandhan, S.; Fernandez, I.; Vence, L.; Blando, J.; Zhao, H.; Yadav, S.S.; et al. Immune profiling of human tumors identifies CD73 as a combinatorial target in glioblastoma. Nat. Med. 2020, 26, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Antonios, J.P.; Soto, H.; Everson, R.G.; Moughon, D.; Orpilla, J.R.; Shin, N.P.; Sedighim, S.; Treger, J.; Odesa, S.; Tucker, A.; et al. Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma. Neuro Oncol. 2017, 19, 796–807. [Google Scholar] [CrossRef]
- Evans, C.A.; Liu, T.; Lescarbeau, A.; Nair, S.J.; Grenier, L.; Pradeilles, J.A.; Glenadel, Q.; Tibbitts, T.; Rowley, A.M.; DiNitto, J.P.; et al. Discovery of a Selective Phosphoinositide-3-Kinase (PI3K)-γ Inhibitor (IPI-549) as an Immuno-Oncology Clinical Candidate. ACS Med. Chem. Lett. 2016, 7, 862–867. [Google Scholar] [CrossRef]
- Kaneda, M.M.; Messer, K.S.; Ralainirina, N.; Li, H.; Leem, C.J.; Gorjestani, S.; Woo, G.; Nguyen, A.V.; Figueiredo, C.C.; Foubert, P.; et al. PI3Kγ is a molecular switch that controls immune suppression. Nature 2016, 539, 437–442. [Google Scholar] [CrossRef]
- Miyazaki, T.; Ishikawa, E.; Matsuda, M.; Sugii, N.; Kohzuki, H.; Akutsu, H.; Sakamoto, N.; Takano, S.; Matsumura, A. Infiltration of CD163-positive macrophages in glioma tissues after treatment with anti-PD-L1 antibody and role of PI3Kγ inhibitor as a combination therapy with anti-PD-L1 antibody in in vivo model using temozolomide-resistant murine glioma-initiating cells. Brain Tumor Pathol. 2020, 37, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Martuza, R.L.; Rabkin, S.D. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell 2017, 32, 253–267.e5. [Google Scholar] [CrossRef] [PubMed]
- Keskin, D.B.; Anandappa, A.J.; Sun, J.; Tirosh, I.; Mathewson, N.D.; Li, S.; Oliveira, G.; Giobbie-Hurder, A.; Felt, K.; Gjini, E.; et al. Neoantigen vaccine generates intratumoral T cell responses in phase Ib glioblastoma trial. Nature 2019, 565, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, D.; Alizadeh, D.; Wang, D.; Weist, M.R.; Shepphird, J.K.; Brown, C.E. CAR T cells for brain tumors: Lessons learned and road ahead. Immunol. Rev. 2019, 290, 60–84. [Google Scholar] [CrossRef]
- Burger, M.C.; Zhang, C.; Harter, P.N.; Romanski, A.; Strassheimer, F.; Senft, C.; Tonn, T.; Steinbach, J.P.; Wels, W.S. CAR-Engineered NK Cells for the Treatment of Glioblastoma: Turning Innate Effectors Into Precision Tools for Cancer Immunotherapy. Front. Immunol. 2019, 10, 2683. [Google Scholar] [CrossRef]
- Harrer, D.C.; Dörrie, J.; Schaft, N. CSPG4 as Target for CAR-T-Cell Therapy of Various Tumor Entities-Merits and Challenges. Int. J. Mol. Sci. 2019, 20, 5942. [Google Scholar] [CrossRef]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef]
- Ishikawa, E.; Muragaki, Y.; Yamamoto, T.; Ohno, T.; Matsumura, A. Vaccine Therapy of High-Grade Gliomas. Prog. Neurol. Surg. 2018, 32, 101–111. [Google Scholar] [CrossRef]
- Saenz-Antoñanzas, A.; Auzmendi-Iriarte, J.; Carrasco-Garcia, E.; Moreno-Cugnon, L.; Ruiz, I.; Villanua, J.; Egaña, L.; Otaegui, D.; Samprón, N.; Matheu, A. Liquid Biopsy in Glioblastoma: Opportunities, Applications and Challenges. Cancers 2019, 11, 950. [Google Scholar] [CrossRef]

| Status/Phase | Study Title | Interventions | NCT Number |
|---|---|---|---|
| N/A/I | Nivolumab With DC Vaccines for Recurrent Brain Tumors | Nivolumab + dendritic cell vaccine | NCT02529072 |
| 1/II | Autologous Lymphoid Effector Cells Specific Against Tumour (ALECSAT) as Add on to Standard of Care in Patients with Glioblastoma | ALECSAT + radiotherapy + temozolomide | NCT02799238 |
| 1/I | Vaccine Therapy in Treating Patients with Newly Diagnosed Glioblastoma Multiforme | Tetanus toxoid + therapeutic autologous dendritic cells + therapeutic autologous lymphocytes | NCT00639639 |
| 1/II | Pembrolizumab in Treating Patients with Recurrent Glioblastoma | Pembrolizumab | NCT02337686 |
| 1/I/II | INO-5401 and INO-9012 Delivered by Electroporation (EP) in Combination with Cemiplimab (REGN2810) in Newly-Diagnosed Glioblastoma (GBM) | DNA-based cancer vaccine INO-5401(hTERT, WT1, and PSMA) + INO-9012 (IL-12) + cemiplimab + radiotherapy + temozolomide | NCT03491683 |
| 1/I | DNX-2440 Oncolytic Adenovirus for Recurrent Glioblastoma | DNX-2440 (Oncolytic Adenovirus) | NCT03714334 |
| 1/I | A Pilot Surgical Trial to Evaluate Early Immunologic Pharmacodynamic Parameters For The PD-1 Checkpoint Inhibitor, Pembrolizumab (MK-3475), In Patients With Surgically Accessible Recurrent/Progressive Glioblastoma | Pembrolizumab | NCT02852655 |
| 1/II | Avelumab in Patients with Newly Diagnosed Glioblastoma Multiforme | Avelumab | NCT03047473 |
| 1/II | Dendritic Cell Vaccine for Patients with Brain Tumors | Autologous tumor lysate-pulsed DC vaccination + adjuvant polyICLC | NCT01204684 |
| 1/II | Avelumab With Hypofractionated Radiation Therapy in Adults with Isocitrate Dehydrogenase (IDH) Mutant Glioblastoma | Avelumab + hypofractionated radiation therapy (HFRT) | NCT02968940 |
| 1/II | Convection-Enhanced Delivery (CED) of MDNA55 in Adults with Recurrent or Progressive Glioblastoma | MDNA55 (a fusion protein comprising a genetically engineered Interleukin-4 (IL-4) linked to a modified version of the Pseudomonas aeruginosa exotoxin A (PE)) | NCT02858895 |
| 1/I | Phase I Study of a Dendritic Cell Vaccine for Patients with Either Newly Diagnosed or Recurrent Glioblastoma | Dendritic cell vaccine + radiotherapy + temozolomide ± bevacizumab | NCT02010606 |
| 1/II | Tremelimumab and Durvalumab in Combination or Alone in Treating Patients with Recurrent Malignant Glioma | Durvalumab + tremelimumab | NCT02794883 |
| 1/II | Combination Adenovirus + Pembrolizumab to Trigger Immune Virus Effects | DNX-2401 (Oncolytic adenovirus) + pembrolizumab | NCT02798406 |
| 1/I | A Phase I Study of AdV-tk + Prodrug Therapy in Combination with Radiation Therapy for Pediatric Brain Tumors | AdV-tk (an adenoviral vector (disabled virus) engineered to express the Herpes thymidine kinase gene) + valacyclovir + radiation | NCT00634231 |
| 2/II | Bevacizumab with or Without Trebananib in Treating Patients With Recurrent Brain Tumors | Bevacizumab + trebananib | NCT01609790 |
| 2/II | Phase 2 Study of Durvalumab (MEDI4736) in Patients with Glioblastoma | Durvalumab + radiotherapy + temozolomide + bevacizumab | NCT02336165 |
| 3/II/III | Proteome-Based Personalized Immunotherapy of Glioblastoma | Dendritic vaccine + allogeneic hematopoietic stem cells + cytotoxic lymphocytes | NCT01759810 |
| 3/I | Immunogene-modified T (IgT) Cells Against Glioblastoma Multiforme | Antigen-specific IgT cells | NCT03170141 |
| 4/I/II | Adjuvant Dendritic Cell-immunotherapy Plus Temozolomide in Glioblastoma Patients | Dendritic cell vaccine + temozolomide | NCT02649582 |
| 4/II/III | Dendritic Cell Immunotherapy Against Cancer Stem Cells in Glioblastoma Patients Receiving Standard Therapy | Dendritic cell immunization + adjuvant temozolomide | NCT03548571 |
| 4/II | Immunotherapy Targeted Against Cytomegalovirus in Patients with Newly-Diagnosed WHO Grade IV Unmethylated Glioma | Human CMV pp65-LAMP mRNA-pulsed autologous DCs containing GM CSF + temozolomide + tetanus–diphtheria toxoid (Td) 111-Indium-labeling of Cells for in vivo Trafficking Studies | NCT03927222 |
| 4/II | V-Boost Immunotherapy in Glioblastoma Multiforme Brain Cancer | V-Boost (an oral tablet which contains specially formulated hydrolyzed GBM antigens along with alloantigens) | NCT03916757 |
| 4/II | Study of DC Vaccination Against Glioblastoma | Dendritic cell vaccine + radiotherapy + temozolomide | NCT01567202 |
| 4/I | Pembrolizumab and Vorinostat Combined with Temozolomide for Newly Diagnosed Glioblastoma | Pembrolizumab + vorinostat + temozolomide + radiotherapy | NCT03426891 |
| 4/III | An Investigational Immuno-Therapy Study of Temozolomide Plus Radiation Therapy with Nivolumab or Placebo, for Newly Diagnosed Patients with Glioblastoma (GBM, a Malignant Brain Cancer) | Nivolumab + temozolomide + radiotherapy | NCT02667587 |
| 4/III | An Investigational Immuno-Therapy Study of Nivolumab Compared to Temozolomide, Each Given with Radiation Therapy, for Newly-diagnosed Patients With Glioblastoma (GBM, a Malignant Brain Cancer) | Nivolumab + temozolomide + radiotherapy | NCT02617589 |
| 4/I | Biomarker-Driven Therapy Using Immune Activators with Nivolumab in Patients with First Recurrence of Glioblastoma | Nivolumab + anti-GITR monoclonal antibody MK-4166 + IDO1 inhibitor INCB024360 + ipilimumab | NCT03707457 |
| 4/II | Radiation Therapy Plus Temozolomide and Pembrolizumab with and without HSPPC-96 in Newly Diagnosed Glioblastoma (GBM) | Pembrolizumab + HSPPC-96 (an autologous tumor-derived heat shock protein peptide-complex) + temozolomide | NCT03018288 |
| 4/I | Nivolumab, BMS-986205, and Radiation Therapy with or without Temozolomide in Treating Patients with Newly Diagnosed Glioblastoma | IDO1 Inhibitor BMS-986205 + nivolumab + radiation therapy + temozolomide | NCT04047706 |
| 4/I | Pembrolizumab and a Vaccine (ATL-DC) for the Treatment of Surgically Accessible Recurrent Glioblastoma | Dendritic cell tumor cell lysate vaccine + pembrolizumab + poly ICLC | NCT04201873 |
| 4/I | Genetically Modified T-cells in Treating Patients with Recurrent or Refractory Malignant Glioma | IL13Rα2-specific, hinge-optimized, 41BB-costimulatory CAR/truncated CD19-expressing Autologous T lymphocytes | NCT02208362 |
| 4/I | IL13Ralpha2-Targeted Chimeric Antigen Receptor (CAR) T Cells with or without Nivolumab and Ipilimumab in Treating Patients with Recurrent or Refractory Glioblastoma | IL13Ralpha2-specific hinge-optimized 4-1BB-co-stimulatory CAR/Truncated CD19-expressing autologous TN/MEM cells + ipilimumab + nivolumab | NCT04003649 |
| 4/I/II | Atezolizumab in Combination with Temozolomide and Radiation Therapy in Treating Patients with Newly Diagnosed Glioblastoma | Atezolizumab + radiation therapy + temozolomide | NCT03174197 |
| 4/II | Immunotherapy Using Tumor Infiltrating Lymphocytes for Patients with Metastatic Cancer | Young TIL + aldesleukin + cyclophosphamide | NCT01174121 |
| 4/I/II | NCT Neuro Master Match - N²M² (NOA-20) | APG101 (a soluble CD95-Fc fusion protein) or alectinib or idasanutlin or atezolizumab or vismodegib or palbociclib | NCT03158389 |
| 4/II | Efficiency of Vaccination with Lysate-loaded Dendritic Cells in Patients with Newly Diagnosed Glioblastoma | autologous, tumor lysate-loaded, mature dendritic cells (DC) + radiation therapy + temozolomide | NCT03395587 |
| 4/I | Memory-Enriched T Cells in Treating Patients with Recurrent or Refractory Grade III-IV Glioma | CD19CAR-CD28-CD3zeta-EGFRt-expressing Tcm-enriched T-lymphocytes + CD19CAR-CD28-CD3zeta-EGFRt-expressing Tn/mem-enriched T-lymphocytes | NCT03389230 |
| 4/I/II | A Phase I/IIa Study Evaluating Temferon in Patients with Glioblastoma and Unmethylated MGMT | Temferon | NCT03866109 |
| 4/I | Phase I EGFR BATs in Newly Diagnosed Glioblastoma | EGFR BATs + radiation therapy + temozolomide | NCT03344250 |
| 4/I | Adoptive Cell Therapy of Autologous TIL and PD1-TIL Cells for Patients with Glioblastoma Multiforme | Autologous TIL+ PD1-TIL | NCT03347097 |
| 4/II | Pediatric Trial of Indoximod With Chemotherapy and Radiation for Relapsed Brain Tumors or Newly Diagnosed DIPG | Indoximod + partial radiation or full-dose radiation | NCT04049669 |
| 4/I | Combination of Immunization and Radiotherapy for Malignant Gliomas (InSituVac1) | GM-CSF + Poly I:C or CAR-T or TCR-T + radiation | NCT03392545 |
| 4/I | Avelumab With Laser Interstitial Therapy for Recurrent Glioblastoma | Avelumab + MRI-guided LITT therapy | NCT03341806 |
| 4/I | Genetically Engineered HSV-1 Phase 1 Study for the Treatment of Recurrent Malignant Glioma | M032 (NSC 733972) (a second-generation oncolytic herpes simplex virus (oHSV)) | NCT02062827 |
| 4/II | Non-Viral TCR Gene Therapy | fludarabine + cyclophosphamide + aldesleukin + sleeping beauty transposed PBL | NCT04102436 |
| 4/I | Safety and Immunogenicity of Personalized Genomic Vaccine and Tumor Treating Fields (TTFields) to Treat Glioblastoma | Poly-ICLC + tumor treating fields + peptides vaccine | NCT03223103 |
| 4/I | A Study to Evaluate the Safety, Tolerability and Immunogenicity of EGFR(V)-EDV-Dox in Subjects with Recurrent Glioblastoma Multiforme (GBM) | EGFR(V)-EDV-Dox (a bacterially derived minicell which packages a toxic payload, doxorubicin, into a 400 nm particle which targets specific cancer cells using bispecific antibodies (BsAb)) | NCT02766699 |
| 4/II | Administration of Autologous T-Cells Genetically Engineered to Express T-Cell Receptors Reactive Against Mutated Neoantigens in People with Metastatic Cancer | Cyclophosphamide + fludarabine + aldesleukin + individual patient TCR-Transduced PBL + pembrolizumab | NCT03412877 |
| 4/I/II | Dose-Escalation Study to Evaluate the Safety and Tolerability of GX-I7 in Patients with Glioblastoma | GX-I7 (a protein drug recombining human IL-7 and hybrid Fc (hyFc)) | NCT03619239 |
| 4/I/II | Study to Evaluate Safety, Tolerability, and Optimal Dose of Candidate GBM Vaccine VBI-1901 in Recurrent GBM Subjects | VBI-1901 (a polyvalent therapeutic vaccine against cytomegalovirus antigen gB and pp65) + GM-CSF | NCT03382977 |
| 4/I | Trial of C134 in Patients with Recurrent GBM | C134 (a cancer killing virus (HSV-1)) | NCT03657576 |
| 4/I | GMCI, Nivolumab, and Radiation Therapy in Treating Patients with Newly Diagnosed High-Grade Gliomas | AdV-tk + valacyclovir + radiation + temozolomide + nivolumab | NCT03576612 |
| 4/I | Study of the IDO Pathway Inhibitor, Indoximod, and Temozolomide for Pediatric Patients with Progressive Primary Malignant Brain Tumors | Indoximod + temozolomide + conformal radiation + cyclophosphamide + cyclophosphamide | NCT02502708 |
| 4/I | A Study of the Treatment of Recurrent Malignant Glioma With rQNestin34.5v.2 | rQNestin (an oncolytic viral vector made from the herpes simplex virus type 1 (HSV1)) + cyclophosphamide | NCT03152318 |
| 4/I | Phase 1b Study PVSRIPO on Recurrent Malignant Glioma in Children | polio/rhinovirus recombinant (PVSRIPO) | NCT03043391 |
| 4/I | HSV G207 in Children with Recurrent or Refractory Cerebellar Brain Tumors | G207 (an oncolytic herpes simplex virus-1 (HSV)) | NCT03911388 |
| 4/I | HSV G207 Alone or With a Single Radiation Dose in Children with Progressive or Recurrent Supratentorial Brain Tumors | G207 (an oncolytic herpes simplex virus-1 (HSV)) + radiation | NCT02457845 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyazaki, T.; Ishikawa, E.; Sugii, N.; Matsuda, M. Therapeutic Strategies for Overcoming Immunotherapy Resistance Mediated by Immunosuppressive Factors of the Glioblastoma Microenvironment. Cancers 2020, 12, 1960. https://doi.org/10.3390/cancers12071960
Miyazaki T, Ishikawa E, Sugii N, Matsuda M. Therapeutic Strategies for Overcoming Immunotherapy Resistance Mediated by Immunosuppressive Factors of the Glioblastoma Microenvironment. Cancers. 2020; 12(7):1960. https://doi.org/10.3390/cancers12071960
Chicago/Turabian StyleMiyazaki, Tsubasa, Eiichi Ishikawa, Narushi Sugii, and Masahide Matsuda. 2020. "Therapeutic Strategies for Overcoming Immunotherapy Resistance Mediated by Immunosuppressive Factors of the Glioblastoma Microenvironment" Cancers 12, no. 7: 1960. https://doi.org/10.3390/cancers12071960
APA StyleMiyazaki, T., Ishikawa, E., Sugii, N., & Matsuda, M. (2020). Therapeutic Strategies for Overcoming Immunotherapy Resistance Mediated by Immunosuppressive Factors of the Glioblastoma Microenvironment. Cancers, 12(7), 1960. https://doi.org/10.3390/cancers12071960




