Abstract
Endometrial cancer is the most common female genital tract cancer in developed countries. We systematically reviewed the current health-economic evidence on early detection and prevention strategies for endometrial cancer based on a search in relevant databases (Medline/Embase/Cochrane Library/CRD/EconLit). Study characteristics and results including life-years gained (LYG), quality-adjusted life-years (QALY) gained, and incremental cost-effectiveness ratios (ICERs) were summarized in standardized evidence tables. Economic results were transformed into 2019 euros using standard conversion methods (GDP-PPP, CPI). Seven studies were included, evaluating (1) screening for endometrial cancer in women with different risk profiles, (2) risk-reducing interventions for women at increased or high risk for endometrial cancer, and (3) genetic testing for germline mutations followed by risk-reducing interventions for diagnosed mutation carriers. Compared to no screening, screening with transvaginal sonography (TVS), biomarker CA-125, and endometrial biopsy yielded an ICER of 43,600 EUR/LYG (95,800 EUR/QALY) in women with Lynch syndrome at high endometrial cancer risk. For women considering prophylactic surgery, surgery was more effective and less costly than screening. In obese women, prevention using Levonorgestrel as of age 30 for five years had an ICER of 72,000 EUR/LYG; the ICER for using oral contraceptives for five years as of age 50 was 450,000 EUR/LYG. Genetic testing for mutations in women at increased risk for carrying a mutation followed by risk-reducing surgery yielded ICERs below 40,000 EUR/QALY. Based on study results, preventive surgery in mutation carriers and genetic testing in women at increased risk for mutations are cost-effective. Except for high-risk women, screening using TVS and endometrial biopsy is not cost-effective and may lead to overtreatment. Model-based analyses indicate that future biomarker screening in women at increased risk for cancer may be cost-effective, dependent on high test accuracy and moderate test costs. Future research should reveal risk-adapted early detection and prevention strategies for endometrial cancer.
1. Introduction
Endometrial cancer is the sixth most common cancer in women and the most common female genital tract cancer in the developed world with rising incidences since 1990 [1,2]. North America and West Europe are the countries with highest incidences with age-standardized incidences of 20.5 and 22.2 per 100,000 women in the year 2018 [3,4]. Moreover, it is speculated that endometrial cancer incidences are rising due to the ageing population, and influencing life style factors (e.g., obesity, diabetes, lower parity) [5]. The large majority of endometrial cancer cases occur at ages over 50 years, with a peak incidence between 65 and 75 years of age [5,6]. The five-year survival rate depends on the cancer stage and is as high as 95% for local stage endometrial cancer, but as low as 18% for advanced stages [7].
Germline mutations such as Lynch syndrome may increase the lifetime risk for endometrial cancer. Women with Lynch syndrome have a 40–60% chance of developing endometrial cancer and a 3–14% chance of developing ovarian cancer [8,9]. Lynch syndrome accounts for less than one percent of all endometrial cancer cases [10]. Prevention strategies to reduce mortality in women with Lynch syndrome include chemoprevention and cancer risk-reducing surgery such as hysterectomy and bilateral salpingo-oophorectomy [11]. A large retrospective study demonstrated that none of the women with Lynch syndrome who underwent hysterectomy with or without bilateral salpingo-oophorectomy developed endometrial cancer (average follow-up time was 13.3 years after surgery) compared with 33% of the controls (average follow-up time 7.4 years) [12].
Unlike breast and cervical cancer, for which screening programs are available to the general population, endometrial cancer is most commonly diagnosed after endometrial biopsy in symptomatic patients (e.g., vaginal bleeding) [7,13]. In women with postmenopausal bleeding, the prevalence of endometrial cancer is about ten percent [14].
Identifying women at increased risk for endometrial cancer is crucially important for cancer prevention. Risk-tailored early detection and prevention strategies may have the potential to lower endometrial cancer incidence and mortality at reasonable costs. Benefits of screening and prevention need to be carefully balanced against both the clinical harms and the economic burden as health care resources are limited. Thus, it is critical to evaluate the evidence on cost-effectiveness of potential prevention or early detection strategies.
The purpose of this semi-quantitative systematic review was to identify and summarize current evidence on the long-term effectiveness and cost-effectiveness of different early detection and prevention strategies for endometrial cancer in various population subgroups.
2. Results
Out of 125 identified publication hits, seven studies [15,16,17,18,19,20,21] evaluating early detection and prevention strategies for endometrial cancer, met the inclusion criteria. Figure 1 shows the PRISMA flow diagram of the literature search including criteria for the selection of studies.
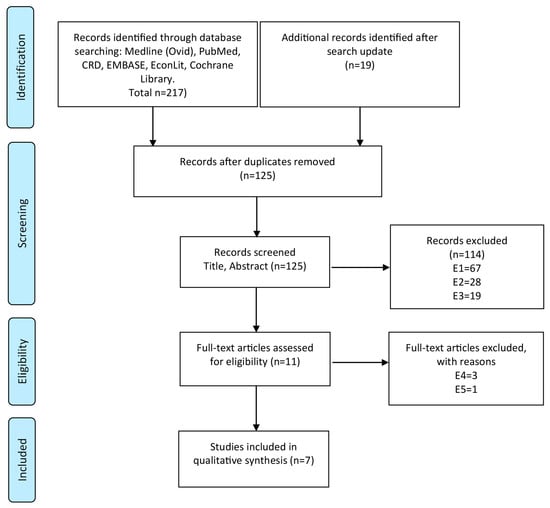
Figure 1.
PRISMA flow diagram for the process of literature search and exclusion: Electronic data bases were searched for cost-effectiveness studies evaluating endometrial cancer screening and/or prevention strategies. Numbers of excluded studies are listed for each reason of exclusion. Exclusion criteria: E1—other diseases than endometrial cancer or already have endometrial cancer, recurrent cancer or metastases; E2—studies evaluating cost-effectiveness of other interventions (e.g., therapy of endometrial cancer); E3—not decision-analytic modelling studies; E4—not full health-economic studies (cost-effectiveness studies); E5—editorials, reviews, abstracts; E6—not in German or English language.
The included studies were published between 2008 and 2017. All studies were performed for the health care system of the United States of America. No European study was found evaluating early detection or prevention strategies for endometrial cancer. The included studies showed heterogeneity regarding target population, study perspective, analytic approach, and compared intervention strategies.
We distinguished between (1) screening for endometrial cancer, (2) preventive interventions and (3) genetic screening for germline mutations. If studies reported more than one type of intervention, we separated the interventions and sorted them into these three categories. We found four studies reporting screening strategies for four different risk groups, five studies reporting risk-reducing interventions targeting women with high risk, and one study on genetic screening. Key characteristics of the included studies are summarized in Table 1. Application of the CHEERS checklist for reporting quality of cost-effectiveness studies resulted in similar scores for the included studies (18–21 out of 24 possible points) (Table 2).

Table 1.
Characteristics of Cost-Effectiveness Studies Evaluating Endometrial Cancer Screening and/or Prevention Strategies Sorted by Intervention Category.

Table 2.
CHEERS checklist for included studies.
In the following paragraphs, the overall health-economic results are summarized for each category and target group presenting incremental cost-effectiveness ratios (ICERs) and incremental cost-utility ratios (ICURs) compared to the next non-dominated strategy.
2.1. Screening for Endometrial Cancer in Women with Different Risk Profiles
In total, four studies [17,18,19,20] evaluated different screening strategies for endometrial cancer in women with different cancer risk profiles (Table 3).

Table 3.
Discounted Costs, Life Years, Quality-Adjusted Life-Years (QALYs), and Incremental Cost-Effectiveness Ratios of Endometrial Cancer Screening Strategies in Women with Different Risk Profiles.
Of those, two studies [19,20] evaluated screening strategies including annual gynecological examination, transvaginal sonography (TVS), the biomarker CA-125, and endometrial biopsy in women with Lynch syndrome with 40–60% lifetime risk for endometrial cancer. Annual screening with TVS, the biomarker CA-125, and endometrial biopsy was considered cost-effective compared with no intervention for 30-year-old women with Lynch syndrome yielding an ICER of 43,600 EUR/LYG [19]. When considering quality of life, the ICUR of this screening strategy increased to 95,800 EUR/QALY compared with no intervention [19]. Annual screening with TVS, CA-125, and endometrial biopsy was less costly and more effective (i.e., dominant) compared with gynecological screening with TVS alone in the other study [20].
Two studies [17,18] evaluated screening strategies for endometrial cancer in obese (body mass index [BMI] > 30 kg/m²) women with at least 3% lifetime risk for endometrial cancer. One of these studies [17] evaluated the impact of specific test characteristics and costs of a potential new biomarker (hypothetical biomarker) on its potential clinical utility and cost-effectiveness in women at different risks for endometrial cancer. In particular, annual screening with a high-performing hypothetical serum test consisting of a multiple biomarker panel including prolactin (sensitivity: 0.98; specificity: 0.98) compared to annual endometrial biopsy or annual TVS was evaluated. Annual screening of obese women (age 45–80 years) with this hypothetical serum test yielded an ICER of 46,700 EUR/LYG compared with no screening [17]. Annual screening with TVS or endometrial biopsy alone was dominated, and therefore not cost-effective [17]. Starting screening at age 25 years generated a higher ICER [17]. In the other study, annual screening of obese women (age 30–80 years) with gynecological examination and endometrial biopsy alone was not cost-effective, having an ICER of over 1.5 million EUR/LYG compared with no screening [18].
Annual screening using the hypothetical serum biomarker panel in women older than 60 years with a breast cancer history at increased risk for endometrial cancer (6% lifetime risk), who had been using tamoxifen for up to five years, achieved an ICER of about 23,000 EUR/LYG compared with no screening [17]. Annual screening with TVS or with endometrial biopsy alone was less effective and more costly and therefore dominated. In women, from the general population (2.5% lifetime risk for endometrial cancer), annual screening between age 50 and 75 years using the hypothetical serum biomarker panel resulted in an ICER of about 68,400 EUR/LYG [17].
2.2. Risk-Reducing Interventions for Women at Increased or High Risk for Endometrial Cancer
Five studies [16,18,19,20,21] evaluated risk-reducing interventions in women at increased or high risk for endometrial cancer (Table 4).

Table 4.
Discounted Costs, Life Years, QALYs, and Incremental Cost-Effectiveness Ratios of Risk-Reducing Interventions for Women at Increased or High Risk for Endometrial Cancer.
Two of these studies [19,20] evaluated risk-reducing interventions including prophylactic surgery (at age 30 or 40 years) for the prevention of future endometrial and additionally ovarian or colon cancer in women with Lynch syndrome at 40–60% lifetime risk for endometrial cancer. Both studies also included screening strategies in their analyses, which were less effective and more costly, and therefore, dominated by prophylactic surgery (Table 4). In a 30-year-old women with Lynch syndrome, prophylactic hysterectomy and prophylactic bilateral salpingo-oophorectomy (PBSO) was considered cost-effective with an ICER of 2800 EUR/LYG compared with no intervention [19]. Prophylactic hysterectomy plus PBSO at age 40 with or without prior screening with endometrial biopsy was more costly and less effective, and was therefore dominated. When quality of life was taken into consideration, prophylactic hysterectomy and PBSO at age 40 dominated prophylactic hysterectomy and PBSO at age 30 with an ICUR of 5700 EUR/QALY compared with no intervention [19]. In the other study, prophylactic hysterectomy with PBSO was more effective and less costly compared with annual gynecologic screening including TVS with or without CA-125 [20].
One study [21] evaluated risk-reducing interventions in 40-year-old women with BRCA-1 mutations at high risk for uterine cancers additionally considering the risk for developing breast cancer and ovarian cancer. In these women, PBM plus PBSO with hysterectomy yielded more life years and was less costly compared with PBM plus PBSO alone, considering the risk for developing breast and ovarian cancer in addition to endometrial cancer (3.5% lifetime risk) [21]. When quality of life was taken into account, PBSO alone was more effective and was cost-effective with an ICUR of 13,000 EUR/QALY [21].
Two other studies [16,18] evaluated risk-reducing interventions to prevent endometrial cancer in obese women compared to no intervention or usual care.
Application of oral contraceptives for five years in 30-year-old obese women (lifetime risk for endometrial cancer of 3%) to prevent endometrial cancer was not cost-effective with an ICER of 458,800 EUR/LYG compared with no intervention [18]. However, using Levonorgestrel intrauterine devices for five years in 50-year-old obese women (age-dependent lifetime risk for endometrial cancer between 4% and 7%) yielded an ICER of 72,000 EUR/LYG in comparison with usual care [16].
2.3. Genetic Testing for Germline Mutations Followed by Risk-Reducing Interventions for Diagnosed Mutation Carriers
Only one study [15] evaluated genetic testing for mutations in unaffected individuals having a family history of sporadic and/or Lynch syndrome-associated with endometrial and/or colorectal cancer (Table 5). Identified mutation carriers were then screened with TVS and endometrial biopsy (and colonoscopy to early detect colorectal cancer), and were offered prophylactic procedures such as total abdominal hysterectomy and PBSO (and polypectomy to prevent colorectal cancer).

Table 5.
Discounted Costs, Life Years, QALYs, and Incremental Cost-Effectiveness Ratios of Genetic Testing for Germline Mutations Followed by Risk-Reducing Interventions for Diagnosed Mutation Carriers.
The ICURs varied widely depending on the age of the individual and the risk threshold for carrying a mutation, that was considered for genetic testing, with a range from 8200 EUR/QALY for performing genetic testing in populations with a risk of at least 10% to carry a mismatch repair gene mutation in individuals as of age 40, to over 7.4 million EUR/QALY for universal genetic testing in individuals as of age 20. The authors concluded that genetic testing for mismatch repair gene mutations in women age 25–35 years with a pretest risk of carrying a mutation higher than 5% may be cost-effective with an ICUR below 43,300 EUR/QALY gained compared with current practice.
While decision-making should be based on the incremental cost-effectiveness ratios reported above (Table 3, Table 4 and Table 5), determined in a step-wise approach [23], we additionally present cost-effectiveness and cost-utility ratios for each strategy in comparison to no intervention in Figure 2. This provides an overview over strategies across categories. As reference points, the figure shows EU GDP, but decisions should be made according to country-specific willingness-to-pay thresholds.
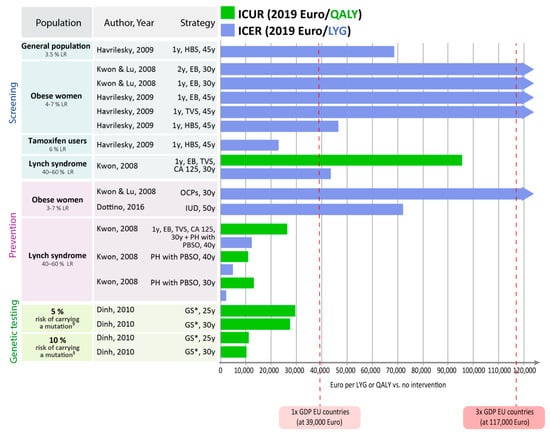
Figure 2.
Cost-effectiveness results for each intervention in comparison to no intervention. EB: endometrial biopsy; GS: genetic screening + EB, TVS (plus colonoscopy to early detect colorectal cancer); HBS: hypothetical biomarker panel screening including prolactin (sensitivity and specificity of 0.98, both); IUD: Levonorgestrel intrauterine device; LR: lifetime risk; OCPs: oral contraceptive pills; PBSO: prophylactic bilateral salpingo-oophorectomy; PH: prophylactic hysterectomy; TVS: transvaginal sonography. § associated with Lynch syndrome. * Including risk-reducing surgery (e.g., hysterectomy, PBSO, polypectomy to prevent colorectal cancer) in mutation carrier.
The risk of developing endometrial cancer in the target population and the impact of interventions on the quality of life are the most important influence factors for cost-effectiveness results. The cost-effectiveness results of the different endometrial cancer early detection and prevention strategies depend mainly on the overall risk for developing endometrial cancer in the target populations, and on the intervention’s impact on the quality of life.
In women at high risk for endometrial cancer (e.g., mutation carrier), risk-reducing prophylactic hysterectomy and PBSO compared with no intervention were highly cost-effective achieving ICERs and ICURs below the threshold of once the per capita GDP [19]. Although annual screening with TVS, CA-125, and endometrial biopsy compared with no intervention achieved also ICERs and ICURs below twice the per capita GDP (e.g., EUR 78,000), prophylactic surgery achieved much lower ICERs and ICURs [19]. Consideration of quality of life suggests that performing prophylactic surgeries at higher age results in a better quality of life in women [19].
Genetic testing for mutations in women at increased or high risk for carrying a mutation followed by risk-reducing surgery for mutation carriers compared with no intervention were highly cost-effective, achieving ICERs and ICURs even below the threshold of once the per capita GDP [15].
In women at increased risk for endometrial cancer (e.g., obese women), intrauterine devices with Levonorgestrel for five years at age 50 achieved an ICER below twice the per capita GDP, which can be considered cost-effective [16]. Whereas, oral contraceptive pills for five years in women at age 30 years were not cost-effective [18]. Further, annual screening with TVS or endometrial biopsy in women at increased risk for endometrial cancer (e.g., obese women or tamoxifen users) was not cost-effective, achieving ICERs above three times the per capita GDP [17,18].
An analysis assuming a hypothetical future screening test with high test accuracy (sensitivity: 98%, specificity: 98%) reported this screening test to be highly cost-effective (once the per capita GDP) in women at increased risk for endometrial cancer (e.g., women 61–80 years with a breast cancer history, who have been using tamoxifen for up to five years) and to be cost-effective (twice the per capita GDP) in postmenopausal women from the general population or in obese women as of age 45 years [17].
3. Discussion
We conducted a systematic literature search with the objective to give an overview of the current evidence on the effectiveness and cost-effectiveness of screening and prevention strategies for endometrial cancer. According to our study, the cost-effectiveness is influenced strongly by the risk level in the chosen target population. It also depends to a large extent on the intervention’s impact on quality of life and finally on the willingness-to-pay threshold of the specific country or health care system.
Study results suggest that preventive surgery, for example, hysterectomy plus PBSO, may be considered highly cost-effective in women at high risk (e.g., mutation carriers) for developing endometrial cancer. In women at increased risk, younger age at intervention (e.g., 30 versus 40 years) results in very low ICERs, but in higher ICURs when including quality of life. This points to the importance of including quality of life in the evaluation in addition to mortality reductions and remaining life expectancy. As prophylactic surgery may have severe side effects, which strongly impact the quality of life of the individual woman (e.g., preterm menopause and infertility), the age at which the prophylactic surgery is offered should be chosen wisely. For women at high risk for endometrial cancer not willing to undergo prophylactic surgery, annual screening with TVS, CA-125, and endometrial biopsy may also be a cost-effective alternative. One study on genetic testing for mutations in women with familial risk found a 5% threshold for pre-test risk of carrying a mutation above which genetic testing is highly cost-effective if followed by risk-reducing surgery for mutation carriers.
In obese women at increased risk for endometrial cancer, Levonorgestrel to prevent endometrial cancer may be considered cost-effective when treating women as of age 50 for five years. In contrast, treating obese women as of age 30 with oral contraceptives for five years cannot be considered cost-effective, neither can be screening approaches with annual TVS or endometrial biopsy.
In women at average risk for endometrial cancer, no reliable early detection, and no prevention strategy are currently available. In our review, one study reported that annual screening with a high-performing hypothetical test (98% sensitivity and 98% specificity) may be effective and cost-effective to early detect endometrial cancer in women from the general population as well as in women at increased cancer risk. These results emphasize that a future screening test for endometrial cancer not only needs to detect malignancies at a very high rate, but also needs to be very accurate in order to avoid unnecessary treatment associated with false-positive results.
Current evidence advises against endometrial cancer screening in asymptomatic women in the general population. Presently applied screening tests such as TVS or endometrial biopsy have not yet demonstrated to reduce endometrial cancer mortality. Controversies still exist on what endometrial thickness threshold to apply when deciding on biopsy. Using the same threshold for asymptomatic women as is used for women with abnormal vaginal bleeding (4 mm cut-off) [24] will lead to poor sensitivity and a high number of false-positive rates. In our review, one decision-analytic study [17] applied a cut-off value of 5 mm in annual TVS screening, but results were not cost-effective for the asymptomatic general population. New cut-off values with higher sensitivity of TVS have been evaluated by Jacobs et al. [25] and Smith-Bindman [26]. However, there is no evidence suggesting a reduction in cancer-related mortality, yet. As such, TVS cannot be implemented for endometrial cancer screening for now, and further research as well as a consensus for the recommended cut-off are needed.
Today’s guidelines recommend either prophylactic surgery or annual screening with TVS and endometrial biopsy as of age 35 years for women with Lynch syndrome. In modeling studies, both screening and preventive surgery in women as of age 30 years were shown to be cost-effective, but only when quality of life was not considered. Consideration of quality of life is of high importance though as each early detection or prevention strategy might have possible implications for the physical and psychological health of women. Invasive interventions such as an endometrial biopsy or a prophylactic surgery (e.g., hysterectomy and PBSO) often have moderate to severe side effects. Preventive surgeries may lead to infertility and artificial menopause in women at childbearing age. Preventive surgeries may affect body image negatively, cause disturbances in sexual relationships, and generate psychological distress [27]. For women with increased or high risk, the decision about preventive interventions is difficult and highly personal. It is important that clinicians are qualified to inform and consult carefully and communicate clearly and sensitively with women in the decision process.
To our best knowledge, this is the first study systematically summarizing current evidence on the cost-effectiveness of early detection or preventive strategies for endometrial cancer for women with different risk profiles.
Our study has particular strengths. One specific strength of our review is that we performed additional calculations with the published data such as the incremental cost-effectiveness ratios based on the reported effects and cost of the individual studies. All ICERs/ICURs are consistently calculated in the step-wise manner considering dominance and extended dominance as is necessary to be relevant for decision-making [23,28]. To facilitate quantitative comparison across studies, all cost data have been converted to 2019 euros, which is a particular strength of this semi-quantitative review. In addition, we provide a visual overview of results for all strategies when compared to a common baseline. While reporting results, we highlighted the role of risk-adaptation for screening and prevention strategies for endometrial cancer, and we performed a critical assessment of the model aspects.
Decision-analytic models, as a simplification of reality, have their limitations. Analyses outcomes will depend greatly on the model assumptions, methodology, and structure of the model [29].
First, the studies varied significantly in the study perspective, time horizon, and in the evaluated interventions, which makes comparison between studies difficult. Compared interventions in different studies for the same population subgroup differ greatly in the type and frequency of the interventions. Three studies reported to adopt a societal perspective but included only direct medical costs. Incomplete cost assessments (e.g., excluding indirect costs) may lead either to underestimated or overestimated ICERs and ICURs. Thus, these study findings may be biased or rather considered as findings adopting a payer’s perspective.
Second, an important limitation of most of the studies was a lack of reporting model validations. International guidelines for modeling recommend that the “validation of a model should include an evaluation of face validity of the structure, evidence, problem formulation, and results of the model.” Models that are not validated may bear a potential risk of bias [30].
One study [20] used a decision tree model to evaluate the cost-effectiveness of preventive surgery compared to annual gynecological examination in high-risk women. Although some questions may be answered with this approach, results of this analysis should be interpreted cautiously. A decision tree approach does not easily allow considering time to an event and a change in probabilities over time. Decision trees are mainly useful for modeling diseases and intervention effects with short time horizons [31]. Some effects of screening interventions, especially lead-time and overdiagnosis, as well as overtreatment, cannot fully be represented in decision tree models.
Third, not all studies considered quality of life, and none of the included studies considered the anxiety that women might experience when they receive a positive test result. While in some studies the impact of a preventive intervention such as surgery on quality of life was considered, the impact of receiving a positive test result itself was not incorporated in any of the analyses. Studies not including quality of life measures in women receiving a positive test result and/or undergoing surgery might not be able to thoroughly evaluate the trade-off between clinical benefits and psychological harms of screening.
Fourth, in modeling studies evaluating screening strategies, false-positive test results leading to unnecessary surgeries (e.g., hysterectomy, PBSO) could potentially have a positive effect if they prevent endometrial cancer in women who would have developed it later in life (and in the model) without the surgery. Studies neglecting the effect on quality of life caused by positive test results and necessary or unnecessary surgery may overestimate benefits and underestimate cost-effectiveness ratios. Both may lead to falsely favoring strategies with lower specificity and therefore more false-positive results and unnecessary surgeries. A similar effect may occur in modeling studies when not all downstream costs of positive test results are carefully included.
Finally, it is crucial that all relevant strategies (variations on screening intervals, start and stop age) are included as comparators in the analysis and that cost-effectiveness ratios are reported for each non-dominated strategy and always in comparison to the next best non-dominated strategy [32]. This principle was not always followed in the evaluated studies.
Besides the limitations of individual studies, our review has limitations of its own. Our search for studies was focused on cost-effectiveness studies based on a decision-analytic model, evaluating screening and prevention for endometrial cancer in asymptomatic women. We may have missed evidence from effectiveness studies not reporting costs. In addition, our results may be not representative for women presenting with symptoms such as abnormal bleeding (e.g., vaginal bleeding in postmenopausal women). While we searched in a range of relevant electronic databases, we may have missed further studies in the gray literature. Publications in languages other than English or German were also not considered.
Future research should consider all of the issues discussed above and may also focus on risk-adapted strategies to early detect and prevent endometrial cancer considering genomics, epigenetics, and lifestyle information, including individual characteristics (e.g., age, time since menopause, reproductive factors). To date, only a few endometrial cancer prediction models have been developed especially for asymptomatic women [33]. Likewise, there are currently no established tests available to predict the individual risk to develop endometrial cancer—especially poor prognostic cancer—which may help to tailor different screening and prevention strategies according to women’s risk. This kind of biomarker test would be of critical value for the implementation of risk-stratified interventions. Further, the evaluation of public health programs to facilitate lifestyle changes for specific women at increased risk would be of interest. In general, future studies should include all relevant invasive (e.g., prophylactic surgery) and non-invasive comparator strategies for the prevention or early-detection of endometrial cancer in order to make a comprehensive comparison.
This review can guide further research into risk-adapted, personalized early detection and prevention strategies for endometrial cancer and provide guidance for informed decisions in health care resource allocation.
4. Materials and Methods
This semi-quantitative systematic review consists of several steps. In the first step, we systematically searched for studies evaluating both the clinical long-term effectiveness and cost-effectiveness of early detection and prevention strategies for endometrial cancer in the electronic databases Medline (Ovid and PubMed), Embase (Ovid), the Cochrane Library, CRD databases (NHS EED, DARE, HTA Database), and EconLit (last update: January 2020). The search codes were developed separately for each database using medical subject headings (MeSH) and search term combinations for endometrial cancer, detection or prevention, effectiveness, costs, and modeling (Table S1). Additionally, we screened the reference lists of identified reviews for relevant literature. All references were imported into a literature database (Endnote version X7, Thomson Corp., Stamford, CT, USA). Two authors (AG, GS) screened reference titles and abstracts for relevant articles. Subsequently, references were selected based on a-priori defined inclusion and exclusion, after reading the full-text documents. If there was any discrepancy among reviewers, a third reviewer (ACF) took the decision. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist [34] was applied to all included studies (Table 2).
We included decision-analytic modeling studies assessing both the long-term effectiveness and the cost-effectiveness of different early detection and prevention strategies for endometrial cancer, reporting outcome measures such as quality-adjusted life-years gained (QALY), life-years gained (LYG), incremental cost-utility ratios (ICUR; in cost/QALY), or incremental cost-effectiveness ratios (ICER; in cost/LYG). We included studies with a time horizon sufficiently long to reflect that cancer and preventive interventions affect life expectancy and overall costs [35,36]. We excluded studies in languages other than English or German, unsystematic reviews, editorials, letters, abstracts, and studies which were not full health-economic evaluations or evaluating follow-up or treatment strategies, as well as costing studies not using a decision-analytic model.
In the second step, we systematically extracted and summarized the following information in standardized evidence tables: model analytic framework and characteristics (target population, study type, perspective, time horizon, discount rate, model type and simulation type, sensitivity analysis, model validation), clinical effectiveness, and cost-effectiveness results of compared strategies. In cases where ICER or ICUR were not reported in the included study, we calculated ICER and ICUR based on the reported study data if possible.
The third step served the comparison across countries, currencies, and study years. We converted all costs into 2019 euros using the gross domestic product (GDP) purchasing power parities (PPP) to adjust for the country-specific purchasing power and the consumer price index (CPI) of Europe to adjust for inflation [37,38,39].
In the fourth step, in order to quantitatively compare cost-effectiveness measures across studies, we calculated ICER or ICUR compared to the next non-dominated strategy, if necessary and if data were available. Strategies are considered dominated if they provide less health benefit at higher costs when compared with any other strategy [40,41]. Similarly, dominated strategies should not be considered by decision-makers and no ICER is calculated. Furthermore, extended dominance is applied to eliminate strategies, for which costs and benefits are dominated by a mix of two other alternatives.
In the fifth step, we performed the judgment of cost-effectiveness. We did not use a global willingness-to-pay (WTP) threshold for incremental cost-effectiveness ratios, as these thresholds vary across different countries [42]. In this review, the reported incremental cost-effectiveness of different studies is based on the reported truly (i.e., stepwise) incremental cost-effectiveness and cost-utility ratios, that is, always comparing the evaluated strategy to the next effective non-dominated strategy.
Finally, we calculated the ICER or ICUR compared to no intervention or standard of care as a simple descriptive point of reference for all included studies, if data were available. This step served to visualize the cost-effectiveness measures across different risk groups, interventions, studies, and countries. Only for this visualization, we used the World Health Organization (WHO) recommended willingness-to-pay threshold of one to three times the GDP per capita for a specific country [43,44] to show the potential lower and upper willingness-to-pay limits for countries of the European Union in comparison with the ICER or ICUR. Thus, when visualizing our results compared to no intervention or standard care, we show the lower and upper limit of this range using the 2019 average GDP per capita in 28 countries of the European Union [37], which is approximately EUR 39,000 to EUR 117,000. This visualization serves as a mere descriptive point of orientation for European countries, as decision-making should be based on country-specific willingness-to-pay thresholds and on truly stepwise incremental cost-effectiveness ratios [23].
All abbreviations used are summarized in Table S2.
5. Conclusions
Based on study results, genetic testing in women at increased risk for mutations followed by preventive surgery in mutation carriers as well as preventive surgery in known mutation carriers can be considered effective and cost-effective. Except for women with diagnosed Lynch syndrome, screening strategies using transvaginal sonography or endometrial biopsy are likely not cost-effective. Screening with transvaginal sonography or endometrial biopsy in women at average risk may lead to overtreatment and cannot be considered cost-effective. Promising model-based results suggest that future biomarker screening in asymptomatic women at increased risk for cancer may be cost-effective if the test accuracy is sufficiently high and test costs are moderate. Future research is needed on risk-adapted early detection and prevention strategies for endometrial cancer, integrating harms of intervention and considering overdiagnosis. Future trials may investigate new biomarkers for preventive strategies based on genomics, epigenetics, and lifestyle information.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/7/1874/s1. Table S1. Search strategies. Databases searched and search codes; Table S2. List of abbreviations.
Author Contributions
G.S.: Project coordinator. Development of study design and research questions. Qualitative analyses and interpretation of results. Results visualization and documentation. Manuscript writing and discussion. A.G.: systematic literature search and data extraction. Qualitative analyses and interpretation of results. Manuscript writing and discussion. A.C.-F.: qualitative analyses and interpretation of results. Manuscript review and discussion. L.R.H.: discussion and interpretation of results. Manuscript writing and discussion. N.P.: discussion and interpretation of results. Manuscript review and discussion. M.W.: FORECEE Principle Investigator and project lead. Discussion and interpretation of results. Manuscript review and discussion. U.S.: guarantor of this study. Responsible key researcher of the project. Qualitative analyses and interpretation of results. Manuscript review and discussion. All authors have read and agreed to the published version of the manuscript.
Funding
The project FORECEE(4C) has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement number 634570 and The Eve Appeal. The funding agreement ensured the authors’ independence in designing the study, interpreting the data, writing, and publishing the report. All authors, external and internal, had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest
The authors declare no conflict of interest. There were no other relationships or activities than those disclosed. Sroczynski, Gogollari, Conrads-Frank, Hallsson, Pashayan, Widschwendter and Siebert report a grant from European Union’s Horizon 2020 research and innovation programme (grant agreement number 634570) during the conduct of this study. MD Gogollari was partly funded by the Erasmus-Western Balkans mobility programme (ERAWEB), a project funded by the European Commission. No other conflict of interest was reported.
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed]
- De Haydu, C.; Black, J.D.; Schwab, C.L.; English, D.P.; Santin, A.D. An update on the current pharmacotherapy for endometrial cancer. Expert Opin. Pharmacother. 2015, 17, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Bray, F.; Silva, I.D.S.; Moller, H.; Weiderpass, E. Endometrial Cancer Incidence Trends in Europe: Underlying Determinants and Prospects for Prevention. Cancer Epidemiology Biomarkers Prev. 2005, 14, 1132–1142. [Google Scholar] [CrossRef]
- Newell, S.; Overton, C. Postmenopausal bleeding should be referred urgently. Pr. 2012, 256, 13. [Google Scholar]
- Cooper, N.A.M.; Barton, P.M.; Breijer, M.; Caffrey, O.; Opmeer, B.C.; Timmermans, A.; Mol, B.W.J.; Khan, K.S.; Clark, T.J. Cost-effectiveness of diagnostic strategies for the management of abnormal uterine bleeding (heavy menstrual bleeding and post-menopausal bleeding): A decision analysis. Heal. Technol. Assess. 2014, 18, e1. [Google Scholar] [CrossRef]
- Koornstra, J.J.; Mourits, M.J.; Sijmons, R.; Leliveld, A.M.; Hollema, H.; Kleibeuker, J.H. Management of extracolonic tumours in patients with Lynch syndrome. Lancet Oncol. 2009, 10, 400–408. [Google Scholar] [CrossRef]
- Tafe, L.J.; Riggs, E.R.; Tsongalis, G.J. Lynch Syndrome Presenting as Endometrial Cancer. Clin. Chem. 2014, 60, 111–121. [Google Scholar] [CrossRef]
- De La Chapelle, A. The Incidence of Lynch Syndrome. Fam. Cancer 2005, 4, 233–237. [Google Scholar] [CrossRef]
- Stoffel, E.M.; Walsh, C. Chemoprevention of Endometrial Cancer in Lynch Syndrome: A Step Forward. Cancer Prev. Res. 2013, 6, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Schmeler, K.M.; Lynch, H.T.; Chen, L.-M.; Munsell, M.F.; Soliman, P.T.; Clark, M.B.; Daniels, M.; White, K.G.; Boyd-Rogers, S.G.; Conrad, P.G.; et al. Prophylactic Surgery to Reduce the Risk of Gynecologic Cancers in the Lynch Syndrome. New Engl. J. Med. 2006, 354, 261–269. [Google Scholar] [CrossRef] [PubMed]
- NCI. Endometrial Cancer Screening Physician Data Query (PDQ). Available online: www.cancer.gov/cancertopics/pdq/screening/endometrial/HealthProfessional/ (accessed on 4 November 2016).
- Bachmann, L.M.; ter Riet, G.; Clark, T.J.; Gupta, J.K.; Khan, K.S. Probability analysis for diagnosis of endometrial hyperplasia and cancer in postmenopausal bleeding: An approach for a rational diagnostic workup. Acta Obstet Gynecol Scand 2003, 82, 564–569. [Google Scholar] [CrossRef]
- Dinh, T.A.; Rosner, B.I.; Atwood, J.C.; Boland, C.R.; Syngal, S.; Vasen, H.; Gruber, S.B.; Burt, R.W. Health benefits and cost-effectiveness of primary genetic screening for Lynch syndrome in the general population. Cancer Prev. Res. 2010, 4, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Dottino, J.A.; Hasselblad, V.; Secord, A.A.; Myers, E.R.; Chino, J.; Havrilesky, L.J. Levonorgestrel Intrauterine Device as an Endometrial Cancer Prevention Strategy in Obese Women. Obstet. Gynecol. 2016, 128, 747–753. [Google Scholar] [CrossRef]
- Havrilesky, L.J.; Maxwell, G.L.; Myers, E.R. Cost-effectiveness analysis of annual screening strategies for endometrial cancer. Am. J. Obstet. Gynecol. 2009, 200, 640.e1–640.e8. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Lu, K.H. Cost-Effectiveness Analysis of Endometrial Cancer Prevention Strategies for Obese Women. Obstet. Gynecol. 2008, 112, 56–63. [Google Scholar] [CrossRef]
- Kwon, J.; Sun, C.C.; Peterson, S.K.; White, K.G.; Daniels, M.; Boyd-Rogers, S.G.; Lu, K.H. Cost-effectiveness analysis of prevention strategies for gynecologic cancers in Lynch syndrome. Cancer 2008, 113, 326–335. [Google Scholar] [CrossRef]
- Yang, K.Y.; Caughey, A.B.; Little, S.E.; Cheung, M.K.; Chen, L.-M. A cost-effectiveness analysis of prophylactic surgery versus gynecologic surveillance for women from hereditary non-polyposis colorectal cancer (HNPCC) Families. Fam. Cancer 2011, 10, 535–543. [Google Scholar] [CrossRef]
- Havrilesky, L.J.; Moss, H.A.; Chino, J.; Myers, E.R.; Kauff, N. Mortality reduction and cost-effectiveness of performing hysterectomy at the time of risk-reducing salpingo-oophorectomy for prophylaxis against serous/serous-like uterine cancers in BRCA1 mutation carriers. Gynecol. Oncol. 2017, 145, 549–554. [Google Scholar] [CrossRef]
- Kastrinos, F.; Steyerberg, E.W.; Mercado, R.; Balmaña, J.; Holter, S.; Gallinger, S.; Siegmund, K.D.; Church, J.M.; Jenkins, M.A.; Lindor, N.M.; et al. The PREMM1,2,6 Model Predicts Risk of MLH1, MSH2, and MSH6 Germline Mutations Based on Cancer History. Gastroenterol. 2011, 140, 73–81.e5. [Google Scholar] [CrossRef] [PubMed]
- Hoch, J.S.; Dewa, C.S. A clinician’s guide to correct cost-effectiveness analysis: Think incremental not average. Can. J. Psychiatry 2008, 53, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Smith-Bindman, R.; Kerlikowske, K.; Feldstein, V.A.; Subak, L.; Scheidler, J.; Segal, M.; Brand, R.; Grady, D. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA 1998, 280, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, I.J.; Gentry-Maharaj, A.; Burnell, M.; Manchanda, R.; Singh, N.; Sharma, A.; Ryan, A.; Seif, M.W.; Amso, N.; Turner, G.; et al. Sensitivity of transvaginal ultrasound screening for endometrial cancer in postmenopausal women: A case-control study within the UKCTOCS cohort. Lancet Oncol. 2011, 12, 38–48. [Google Scholar] [CrossRef]
- Smith-Bindman, R.; Weiss, E.; Feldstein, V. How thick is too thick? When endometrial thickness should prompt biopsy in postmenopausal women without vaginal bleeding. Ultrasound Obstet. Gynecol. 2004, 24, 558–565. [Google Scholar] [CrossRef]
- Van Oostrom, I.; Meijers-Heijboer, H.E.; Lodder, L.; Duivenvoorden, H.J.; Van Gool, A.R.; Seynaeve, C.; Van Der Meer, C.A.; Klijn, J.G.; Van Geel, B.N.; Burger, C.W.; et al. Long-Term Psychological Impact of Carrying a BRCA1/2 Mutation and Prophylactic Surgery: A 5-Year Follow-Up Study. J. Clin. Oncol. 2003, 21, 3867–3874. [Google Scholar] [CrossRef]
- Siebert, U.; Alagoz, O.; Bayoumi, A.M.; Jahn, B.; Owens, U.K.; Cohen, D.J.; Kuntz, K.M. State-Transition Modeling. Med Decis. Mak. 2012, 32, 690–700. [Google Scholar] [CrossRef]
- Siebert, U.; Mdh, M.U.S. When should decision-analytic modeling be used in the economic evaluation of health care? Eur. J. Heal. Econ. 2003, 4, 143–150. [Google Scholar] [CrossRef]
- Caro, J.J.; Briggs, A.H.; Siebert, U.; Kuntz, K.M. Modeling Good Research Practices—Overview. Med Decis. Mak. 2012, 32, 667–677. [Google Scholar] [CrossRef]
- Roberts, M.; Russell, L.B.; Paltiel, A.D.; Chambers, M.; McEwan, P.; Krahn, M. Conceptualizing a Model. Med Decis. Mak. 2012, 32, 678–689. [Google Scholar] [CrossRef]
- O’Mahony, J.F.; Naber, S.K.; Normand, C.; Sharp, L.; O’Leary, J.J.; De Kok, I.M. Beware of Kinked Frontiers: A Systematic Review of the Choice of Comparator Strategies in Cost-Effectiveness Analyses of Human Papillomavirus Testing in Cervical Screening. Value Heal. 2015, 18, 1138–1151. [Google Scholar] [CrossRef] [PubMed]
- Alblas, M.; Velt, K.B.; Pashayan, N.; Widschwendter, M.; Steyerberg, E.W.; Vergouwe, Y. Prediction models for endometrial cancer for the general population or symptomatic women: A systematic review. Crit. Rev. Oncol. 2018, 126, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Husereau, D.; Drummond, M.; Petrou, S.; Carswell, C.; Moher, D.; Greenberg, D.; Augustovski, F.; Briggs, A.H.; Mauskopf, J.; Loder, E.; et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ 2013, 346, f1049. [Google Scholar] [CrossRef] [PubMed]
- Caro, J.J.; Briggs, A.H.; Siebert, U.; Kuntz, K.M. Modeling Good Research Practices—Overview: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Value Heal. 2012, 15, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Siebert, U.; Alagoz, O.; Bayoumi, A.M.; Jahn, B.; Owens, U.K.; Cohen, D.J.; Kuntz, K.M. State-Transition Modeling: A Report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Value Heal. 2012, 15, 812–820. [Google Scholar] [CrossRef]
- OECD Purchasing power parities (PPP). Conversion rates. 2019. Available online: https://data.oecd.org/conversion/purchasing-power-parities-ppp.htm (accessed on 17 January 2020).
- OECD. Inflation (CPI) (indicator). Available online: https://data.oecd.org/price/inflation-cpi.htm (accessed on 17 January 2020).
- Siebert, U. Using decision-analytic modelling to transfer international evidence from health technology assessment to the context of the German health care system. GMS Heal. Technol Assess 2005, 1. [Google Scholar]
- Hunink, M.M.; Weinstein, M.C.; Wittenberg, E.; Drummond, M.F.; Pliskin, J.S.; Wong, J.B.; Glasziou, P.P.; Fineberg, H.V. Decision Making in Health and Medicine; Cambridge University Press (CUP): Cambridge, UK, 2014. [Google Scholar]
- Neumann, P.J.; Cohen, J.T.; Weinstein, M.C. Updating Cost-Effectiveness — The Curious Resilience of the $50,000-per-QALY Threshold. New Engl. J. Med. 2014, 371, 796–797. [Google Scholar] [CrossRef]
- Schwarzer, R.; Rochau, U.; Saverno, K.; Jahn, B.; Bornschein, B.; Muehlberger, N.; Flatscher-Thoeni, M.; Schnell-Inderst, P.; Sroczynski, G.; Lackner, M.; et al. Systematic overview of cost–effectiveness thresholds in ten countries across four continents. J. Comp. Eff. Res. 2015, 4, 485–504. [Google Scholar] [CrossRef]
- WHO. Macroeconomics and health: Investing in health for economic development. Report of the Commission on Macroeconomics and Health. Available online: http://apps.who.int/iris/bitstream/10665/42435/1/924154550X.pdf.2001 (accessed on 17 January 2020).
- WHO. Choosing interventions that are cost-effective [Internet]. Available online: http://www.who.int/choice/en/2014 (accessed on 17 January 2020).
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).