Role of Curcumin and (−)-Epigallocatechin-3-O-Gallate in Bladder Cancer Treatment: A Review
Abstract
1. Introduction
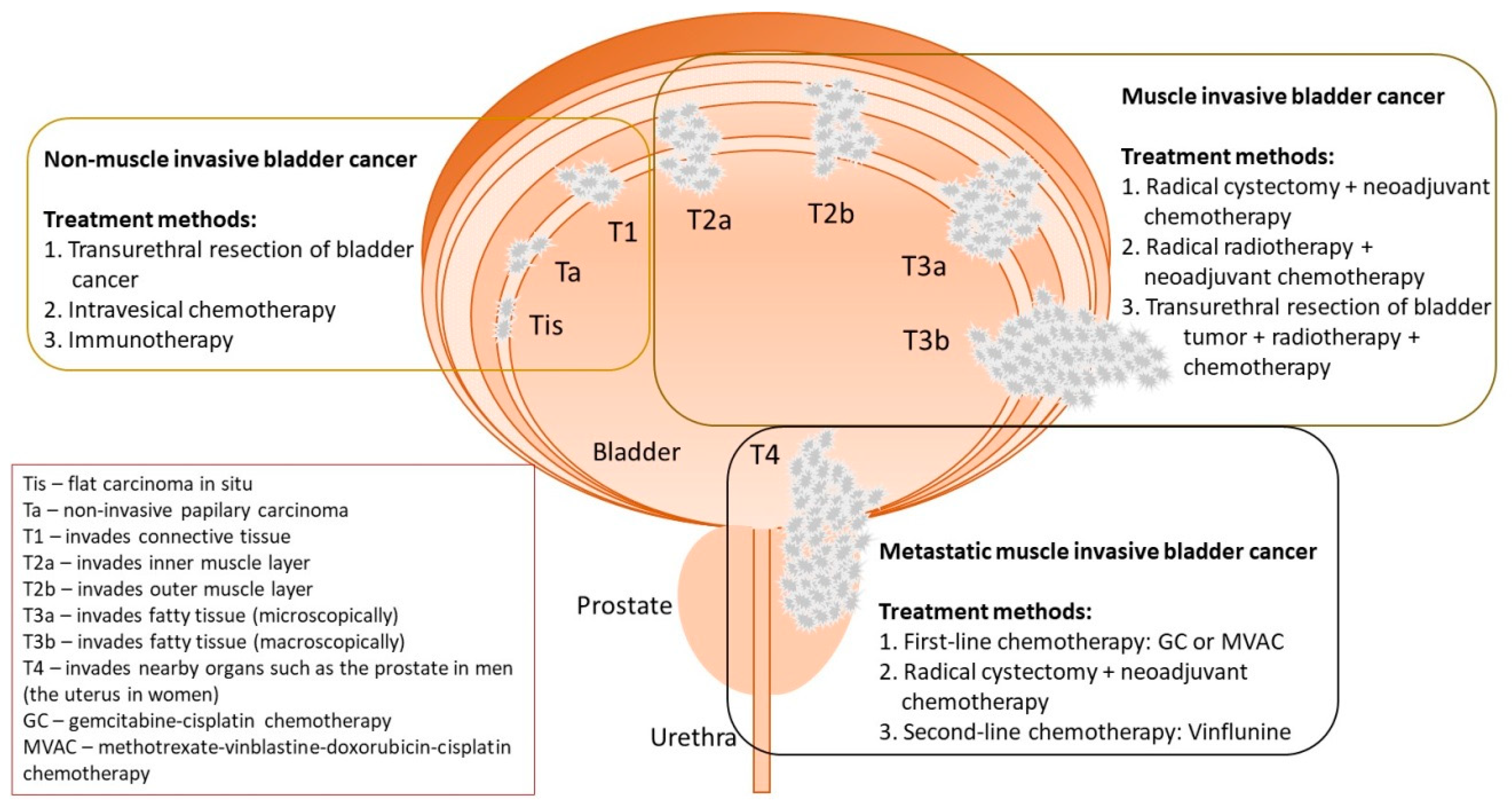
2. The Diagnostics and Treatment Methods of BC
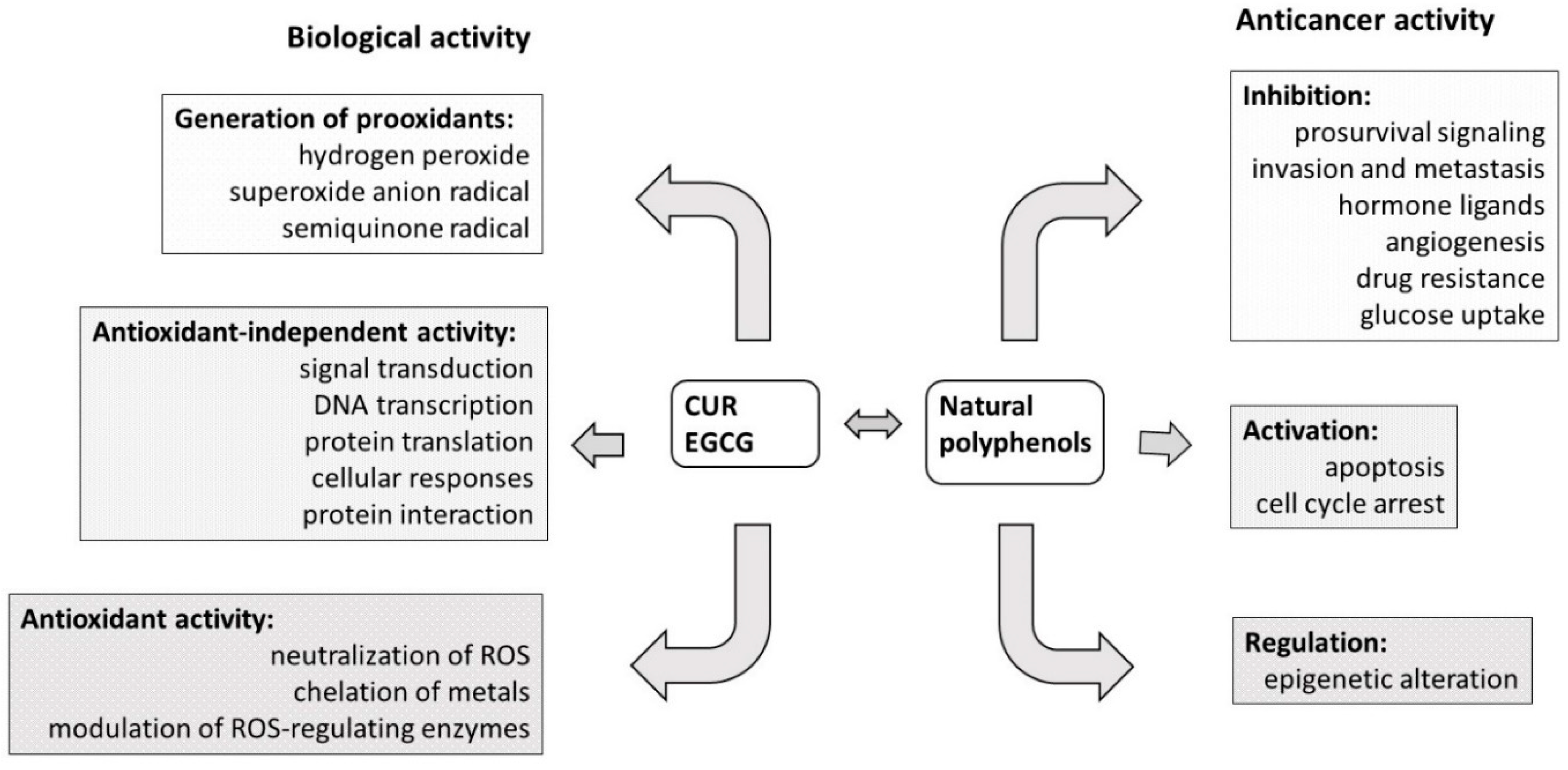
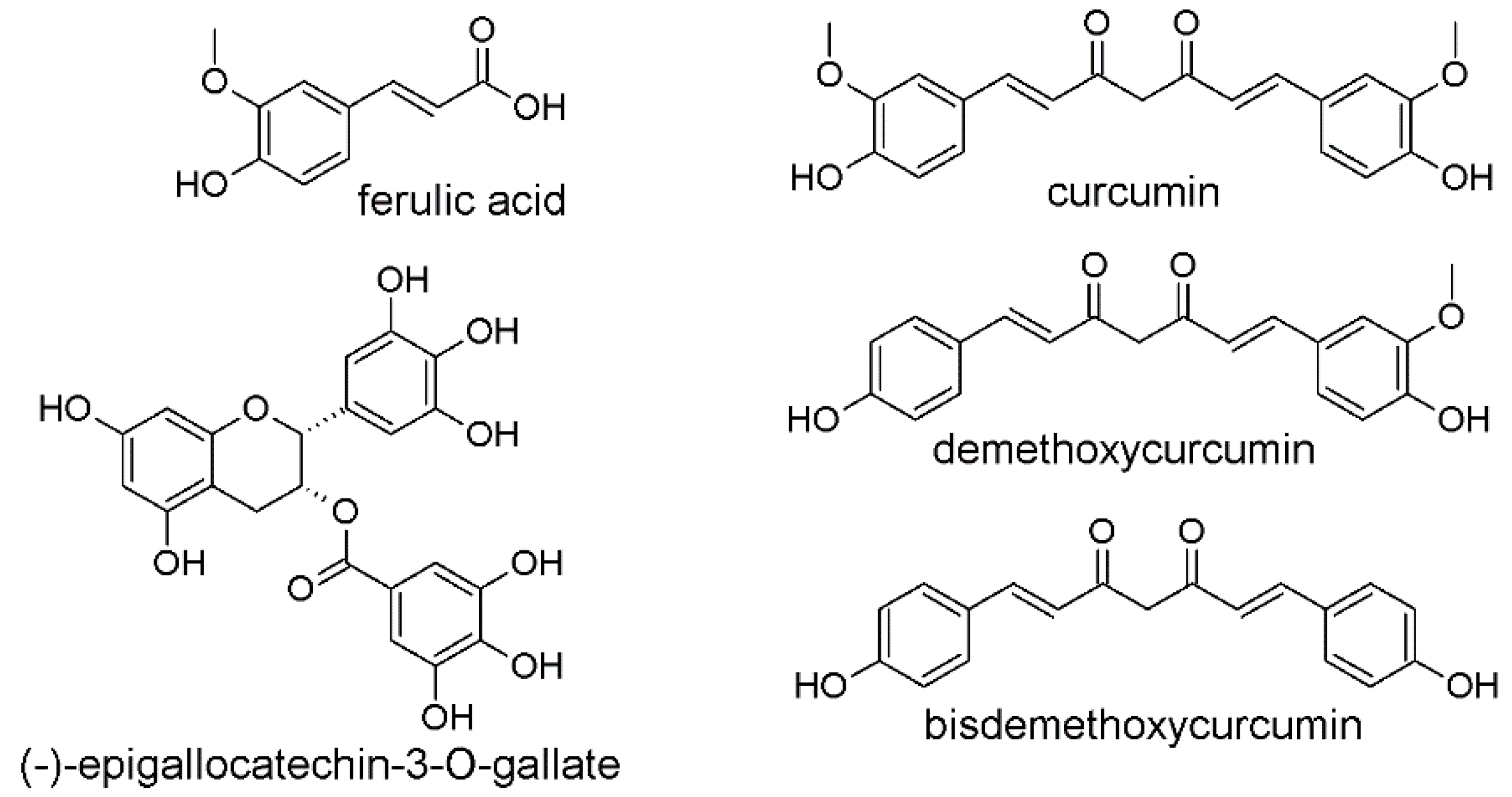
3. The Biological Activities of CUR and EGCG
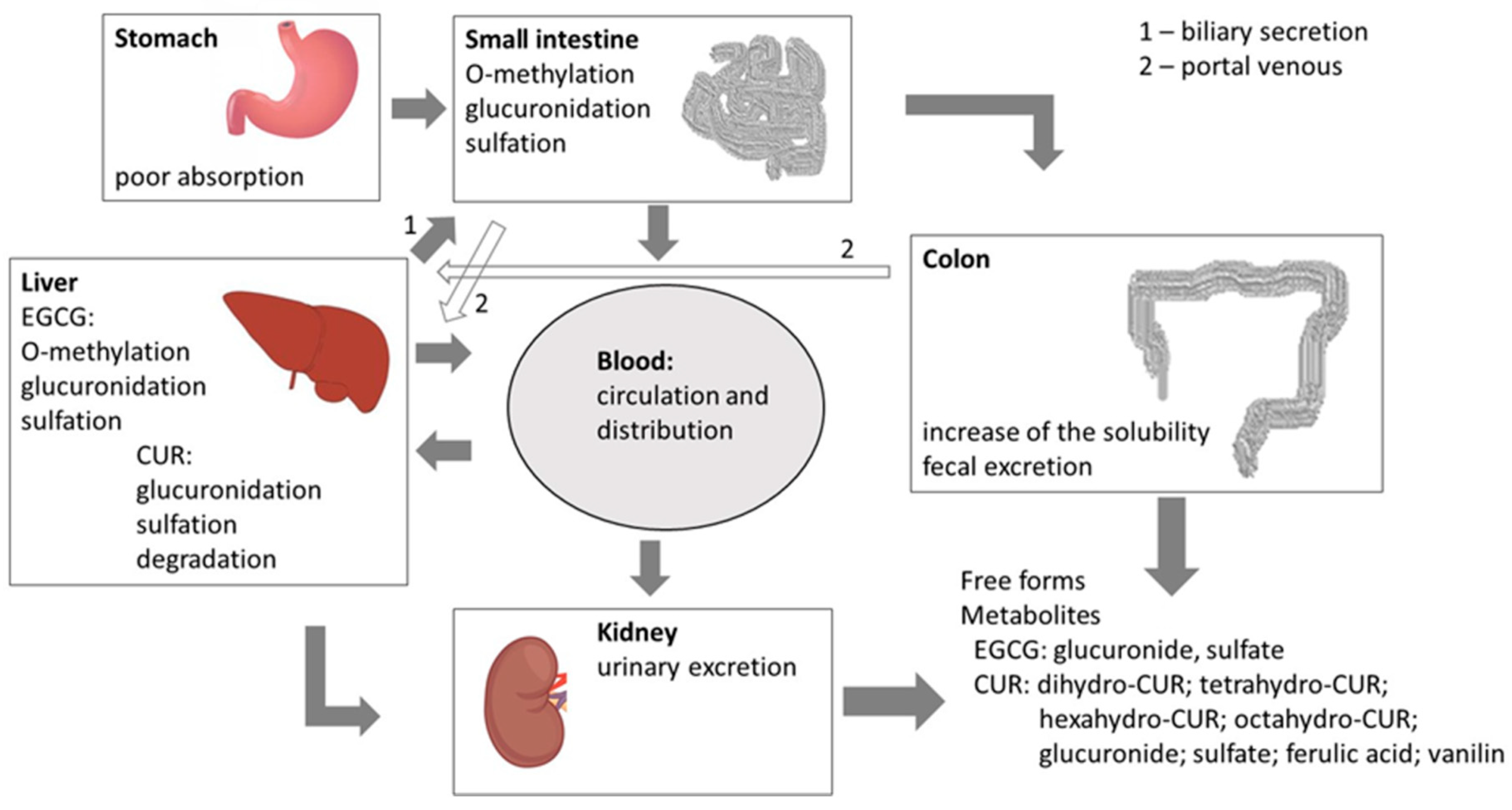
4. The Bioavailability of CUR and EGCG
5. The Role of CUR and EGCG in BC Treatment
5.1. New Perspectives of CUR and EGCG in the Treatment of BC
5.2. The Role of CUR and EGCG in Inhibiting Cancer Stem Cells in BC
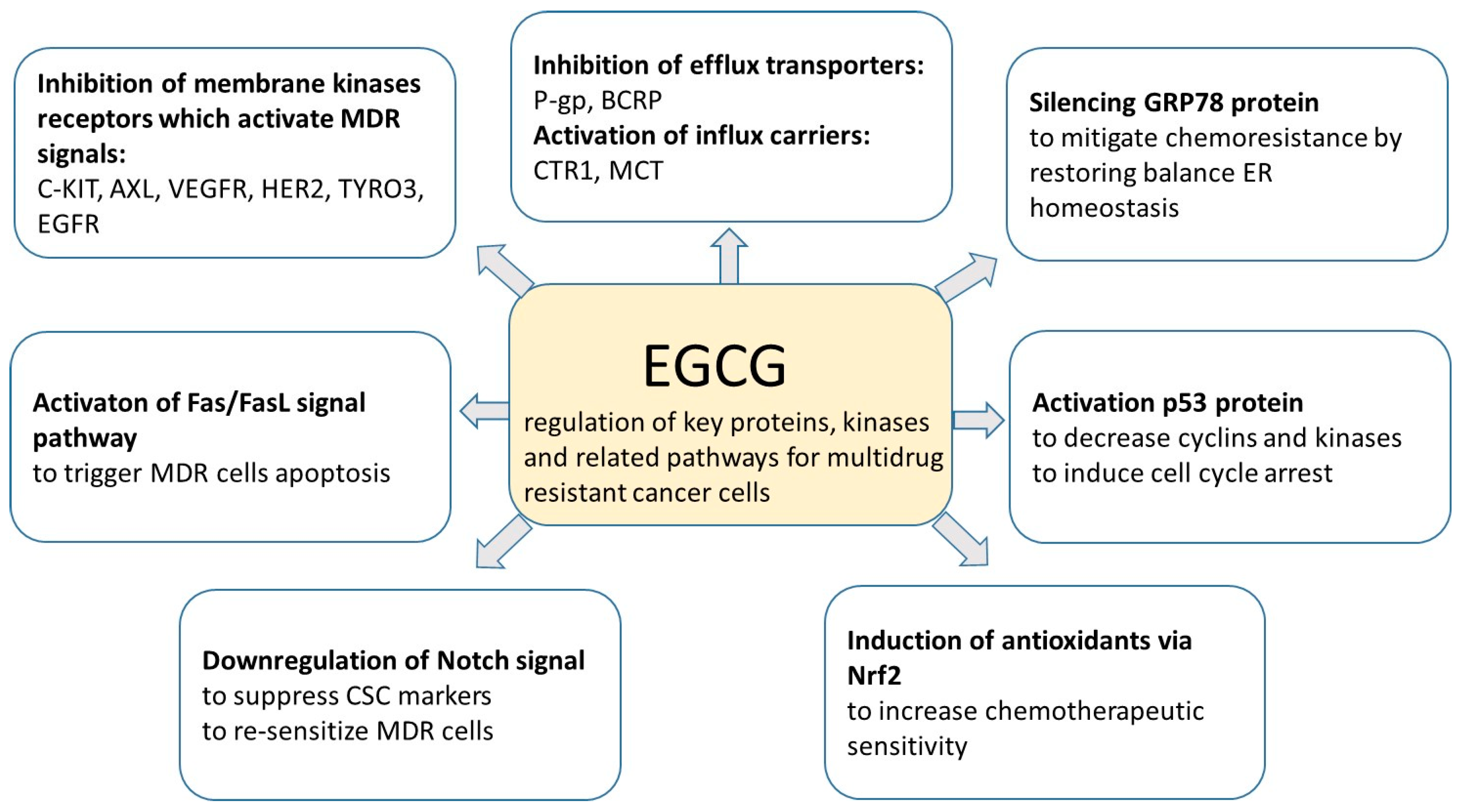
5.3. The Role of CUR and EGCG in Reversing Multidrug Resistance
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Padala, S.A.; Barsouk, A. Epidemiology of Bladder Cancer. Med. Sci. 2020, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Mandelli, G.E.; Missale, F.; Bresciani, D.; Gatta, L.B.; Scapini, P.; Caveggion, E.; Roca, E.; Bugatti, M.; Monti, M.; Cristinelli, L.; et al. Tumor Infiltrating Neutrophils Are Enriched in Basal-Type Urothelial Bladder Cancer. Cells 2020, 9, 291. [Google Scholar] [CrossRef] [PubMed]
- Babjuk, M.; Oosterlinck, W.; Sylvester, R.; Kaasinen, E.; Böhle, A.; Palou-Redorta, J.; Rouprêt, M. EAU Guidelines on Non-Muscle-Invasive Urothelial Carcinoma of the Bladder, the 2011 Update. Eur. Urol. 2011, 59, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Bellmunt, J.; Petrylak, D.P. New Therapeutic Challenges in Advanced Bladder Cancer. Semin. Oncol. 2012, 39, 598–607. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef]
- Chung, S.S.; Vadgama, J.V. Curcumin and Epigallocatechin Gallate Inhibit the Cancer Stem Cell Phenotype via Down-regulation of STAT3–NFκB signaling. Anticancer Res. 2015, 35, 39–46. [Google Scholar]
- Yunos, N.M.; Beale, P.; Yu, J.Q.; Huq, F. Synergism from Sequenced Combinations of Curcumin and Epigallocatechin-3-gallate with Cisplatin in the Killing of Human Ovarian Cancer Cells. Anticancer Res. 2011, 31, 1131–1140. [Google Scholar]
- Gianfredi, V.; Nucci, D.; Vannini, S.; Villarini, M.; Moretti, M. In vitro Biological Effects of Sulforaphane (SFN), Epigallocatechin-3-gallate (EGCG), and Curcumin on Breast Cancer Cells: A Systematic Review of the Literature. Nutr. Cancer 2017, 69, 969–978. [Google Scholar] [CrossRef]
- Mokbel, K.; Wazir, U.; Mokbel, K. Chemoprevention of Prostate Cancer by Natural Agents: Evidence from Molecular and Epidemiological Studies. Anticancer Res. 2019, 39, 5231–5259. [Google Scholar] [CrossRef]
- Su, H.; Jiang, H.; Tao, T.; Kang, X.; Zhang, X.; Kang, D.; Li, S.; Li, C.; Wang, H.; Yang, Z.; et al. Hope and challenge: Precision medicine in bladder cancer. Cancer Med. 2019, 8, 1806–1816. [Google Scholar] [CrossRef]
- Nadal, R.; Bellmunt, J. New Treatments for Bladder Cancer: When Will We Make Progress? Curr. Treat. Options Oncol. 2014, 15, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Premo, C.; Apolo, A.B.; Agarwal, P.K.; Citrin, D.E. Trimodality Therapy in Bladder Cancer. Urol. Clin. North Am. 2015, 42, 169–180. [Google Scholar] [CrossRef]
- Sanli, O.; Dobruch, J.; Knowles, M.A.; Burger, M.; Alemozaffar, M.; Nielsen, M.; Lotan, Y. Bladder Cancer. Nat. Rev. Dis. Primer 2017, 3, 17022. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.K.; Mishra, P.K. Curcumin and its analogues: Potential anticancer agents. Med. Res. Rev. 2010, 30, 818–860. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2016, 7, 205–233. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its’ Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Nagesh, P.K.B.; Jaggi, M.; Chauhan, S.C. Therapeutic Applications of Curcumin Nanoformulations. AAPS J. 2015, 17, 1341–1356. [Google Scholar] [CrossRef]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Lopresti, A.L. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- European Medicines Agency. European Union Herbal Monograph on Curcuma longa L. 2018. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/draft-european-union-herbal-monograph-curcuma-longa-l-c-domestica-valeton-rhizome-revision-1_en.pdf (accessed on 7 April 2020).
- Tayyem, R.F.; Heath, D.D.; Al-Delaimy, W.K.; Rock, C.L. Curcumin content of turmeric and curry powders. Nutr. Cancer 2006, 55, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Kazantzis, K.T.; Koutsonikoli, K.; Mavroidi, B.; Zachariadis, M.; Alexiou, P.; Pelecanou, M.; Politopoulos, K.; Alexandratou, E.; Sagnou, M. Curcumin derivatives as photosensitizers in photodynamic therapy: Photophysical properties and in vitro studies with prostate cancer cells. Photochem. Photobiol. Sci. 2020, 19, 193–206. [Google Scholar] [CrossRef]
- Leite, D.P.V.; Paolillo, F.R.; Parmesano, T.N.; Fontana, C.R.; Bagnato, V.S. Effects of Photodynamic Therapy with Blue Light and Curcumin as Mouth Rinse for Oral Disinfection: A Randomized Controlled Trial. Photomed. Laser Surg. 2014, 32, 627–632. [Google Scholar] [CrossRef]
- Pröhl, M.; Schubert, U.S.; Weigand, W.; Gottschaldt, M. Metal complexes of curcumin and curcumin derivatives for molecular imaging and anticancer therapy. Coord. Chem. Rev. 2016, 307, 32–41. [Google Scholar] [CrossRef]
- Chacko, S.M.; Thambi, P.T.; Kuttan, R.; Nishigaki, I. Beneficial effects of green tea: A literature review. Chin. Med. 2010, 5, 13. [Google Scholar] [CrossRef]
- Granja, A.; Frias, I.; Neves, A.R.; Pinheiro, M.; Reis, S. Therapeutic Potential of Epigallocatechin Gallate Nanodelivery Systems. BioMed Res. Int. 2017, 2017, 5813793. [Google Scholar] [CrossRef]
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Dettlaff, K.; Stawny, M.; Ogrodowczyk, M.; Jelińska, A.; Bednarski, W.; Wątróbska-Świetlikowska, D.; Keck, R.W.; Khan, O.A.; Mostafa, I.H.; Jankun, J. Formulation and characterization of EGCG for the treatment of superficial bladder cancer. Int. J. Mol. Med. 2017, 40, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of Tea Catechins and Its Improvement. Molecules 2018, 23, 2346. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.R.; Saltzstein, D.R.; Kim, K.; Kolesar, J.; Huang, W.; Havighurst, T.C.; Wollmer, B.W.; Stublaski, J.; Downs, T.; Mukhtar, H.; et al. A Phase II Randomized, Double-blind, Presurgical Trial of Polyphenon E in Bladder Cancer Patients to Evaluate Pharmacodynamics and Bladder Tissue Biomarkers. Cancer Prev. Res. 2017, 10, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Ubeyitogullari, A.; Ciftci, O.N. A novel and green nanoparticle formation approach to forming low-crystallinity curcumin nanoparticles to improve curcumin’s bioaccessibility. Sci. Rep. 2019, 9, 19112. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef] [PubMed]
- Sari, T.P.; Mann, B.; Kumar, R.; Singh, R.R.B.; Sharma, R.; Bhardwaj, M.; Athira, S. Preparation and characterization of nanoemulsion encapsulating curcumin. Food Hydrocoll. 2015, 43, 540–546. [Google Scholar] [CrossRef]
- Mani, J.; Fleger, J.; Rutz, J.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Relja, B.; Juengel, E.; et al. Curcumin combined with exposure to visible light blocks bladder cancer cell adhesion and migration by an integrin dependent mechanism. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10564–10574. [Google Scholar] [CrossRef]
- Roos, F.; Binder, K.; Rutz, J.; Maxeiner, S.; Bernd, A.; Kippenberger, S.; Zöller, N.; Chun, F.K.-H.; Juengel, E.; Blaheta, R.A. The Antitumor Effect of Curcumin in Urothelial Cancer Cells Is Enhanced by Light Exposure In Vitro. Evid. Based Complement. Altern. Med. 2019, 2019, 6374940. [Google Scholar] [CrossRef]
- Buss, S.; Dobra, J.; Goerg, K.; Hoffmann, S.; Kippenberger, S.; Kaufmann, R.; Hofmann, M.; Bernd, A. Visible Light Is a Better Co-Inducer of Apoptosis for Curcumin-Treated Human Melanoma Cells than UVA. PLoS ONE 2013, 8, e79748. [Google Scholar] [CrossRef]
- Beyer, K.; Nikfarjam, F.; Butting, M.; Meissner, M.; König, A.; Ramirez Bosca, A.; Kaufmann, R.; Heidemann, D.; Bernd, A.; Kippenberger, S.; et al. Photodynamic Treatment of Oral Squamous Cell Carcinoma Cells with Low Curcumin Concentrations. J. Cancer 2017, 8, 1271–1283. [Google Scholar] [CrossRef]
- Hamidi, A.; Hassani, L.; Mohammadi, F.; Jahangoshayi, P.; Mohammadi, K. The biological effects of vanadyl curcumin and vanadyl diacetylcurcumin complexes: The effect on structure, function and oxidative stability of the peroxidase enzyme, antibacterial activity and cytotoxic effect. J. Enzyme Inhib. Med. Chem. 2016, 31, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Jahangoshaei, P.; Hassani, L.; Mohammadi, F.; Hamidi, A.; Mohammadi, K. Investigating the effect of gallium curcumin and gallium diacetylcurcumin complexes on the structure, function and oxidative stability of the peroxidase enzyme and their anticancer and antibacterial activities. J. Biol. Inorg. Chem. 2015, 20, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Samaddar, S.; Mazur, J.; Boehm, D.; Thompson, D.H. Development And In Vitro Characterization Of Bladder Tumor Cell Targeted Lipid-Coated Polyplex For Dual Delivery Of Plasmids And Small Molecules. Int. J. Nanomed. 2019, 14, 9547–9561. [Google Scholar] [CrossRef]
- Falke, J.; Parkkinen, J.; Vaahtera, L.; Hulsbergen-van de Kaa, C.A.; Oosterwijk, E.; Witjes, J.A. Curcumin as Treatment for Bladder Cancer: A Preclinical Study of Cyclodextrin-Curcumin Complex and BCG as Intravesical Treatment in an Orthotopic Bladder Cancer Rat Model. BioMed Res. Int. 2018, 2018, 9634902. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Zhu, J.; Wang, Q.; Du, X.; Liu, F.; Jiang, J.; Song, J.; Xing, J.; Sun, D.; Hou, Q.; et al. Melatonin potentiates the antitumor effect of curcumin by inhibiting IKKβ/NF-κB/COX-2 signaling pathway. Int. J. Oncol. 2017, 51, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Zhang, R.; Dong, L.; Chen, H.; Bo, J.; Xue, W.; Huang, Y. Curcumin inhibits cell proliferation and motility via suppression of TROP2 in bladder cancer cells. Int. J. Oncol. 2018, 53, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, Y.; Jia, Z.; Gao, Y.; Zhao, C.; Yao, Y. Curcumin inhibits bladder cancer progression via regulation of β-catenin expression. Tumor Biol. 2017, 39, 1010428317702548. [Google Scholar] [CrossRef]
- Cho, C.; Yang, C.; Wu, C.; Ho, J.; Yu, C.; Wu, S.; Yu, D. The modulation study of multiple drug resistance in bladder cancer by curcumin and resveratrol. Oncol. Lett. 2019, 18, 6869–6876. [Google Scholar] [CrossRef]
- Xu, R.; Li, H.; Wu, S.; Qu, J.; Yuan, H.; Zhou, Y.; Lu, Q. MicroRNA-1246 regulates the radio-sensitizing effect of curcumin in bladder cancer cells via activating P53. Int. Urol. Nephrol. 2019, 51, 1771–1779. [Google Scholar] [CrossRef]
- Wang, K.; Tan, S.-L.; Lu, Q.; Xu, R.; Cao, J.; Wu, S.-Q.; Wang, Y.-H.; Zhao, X.-K.; Zhong, Z.-H. Curcumin Suppresses microRNA-7641-Mediated Regulation of p16 Expression in Bladder Cancer. Am. J. Chin. Med. 2018, 46, 1357–1368. [Google Scholar] [CrossRef]
- Liang, Z.; Lu, L.; Mao, J.; Li, X.; Qian, H.; Xu, W. Curcumin reversed chronic tobacco smoke exposure induced urocystic EMT and acquisition of cancer stem cells properties via Wnt/β-catenin. Cell Death Dis. 2017, 8, e3066. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Kong, X.; Li, Y.; Qian, W.; Ma, J.; Wang, D.; Yu, D.; Zhong, C. Curcumin inhibits bladder cancer stem cells by suppressing Sonic Hedgehog pathway. Biochem. Biophys. Res. Commun. 2017, 493, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, X.; Shi, T.; Li, H. Antitumor effects of curcumin in human bladder cancer in vitro. Oncol. Lett. 2017, 14, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhao, Y.; Liang, T.; Ye, X.; Li, Z.; Yan, D.; Fu, Q.; Li, Y. Curcumin inhibits urothelial tumor development by suppressing IGF2 and IGF2-mediated PI3K/AKT/mTOR signaling pathway. J. Drug Target. 2017, 25, 626–636. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Zhao, L.; Geng, H.; Ma, J.; Zhang, Z.; Yu, D.; Zhong, C. Curcumin reverses benzidine-induced epithelial-mesenchymal transition via suppression of ERK5/AP-1 in SV-40 immortalized human urothelial cells. Int. J. Oncol. 2017, 50, 1321–1329. [Google Scholar] [CrossRef]
- Park, B.H.; Lim, J.E.; Jeon, H.G.; Seo, S.I.; Lee, H.M.; Choi, H.Y.; Jeon, S.S.; Jeong, B.C. Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2. Oncotarget 2016, 7, 63870–63886. [Google Scholar] [CrossRef]
- Sun, X.; Deng, Q.-F.; Liang, Z.-F.; Zhang, Z.-Q.; Zhao, L.; Geng, H.; Xie, D.-D.; Wang, Y.; Yu, D.-X.; Zhong, C.-Y. Curcumin reverses benzidine-induced cell proliferation by suppressing ERK1/2 pathway in human bladder cancer T24 cells. Exp. Toxicol. Pathol. 2016, 68, 215–222. [Google Scholar] [CrossRef]
- Liang, Z.; Xie, W.; Wu, R.; Geng, H.; Zhao, L.; Xie, C.; Li, X.; Zhu, M.; Zhu, W.; Zhu, J.; et al. Inhibition of tobacco smoke-induced bladder MAPK activation and epithelial-mesenchymal transition in mice by curcumin. Int. J. Clin. Exp. Pathol. 2015, 8, 4503–4513. [Google Scholar]
- Pan, Z.-J.; Deng, N.; Zou, Z.-H.; Chen, G.-X. The effect of curcumin on bladder tumor in rat model. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 884–889. [Google Scholar]
- Afsharmoghadam, N.; Haghighatian, Z.; Mazdak, H.; Mirkheshti, N.; Mehrabi Koushki, R.; Alavi, S.A. Concentration- Dependent Effects of Curcumin on 5-Fluorouracil Efficacy in Bladder Cancer Cells. Asian Pac. J. Cancer Prev. 2017, 18, 3225–3230. [Google Scholar] [CrossRef]
- Shao, Y.; Zhu, W.; Da, J.; Xu, M.; Wang, Y.; Zhou, J.; Wang, Z. Bisdemethoxycurcumin in combination with α-PD-L1 antibody boosts immune response against bladder cancer. OncoTargets Ther. 2017, 10, 2675–2683. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Ho, J.-N.; Kook, H.R.; Lee, S.; Oh, J.J.; Hong, S.K.; Lee, S.E.; Byun, S.-S. Theracurmin® efficiently inhibits the growth of human prostate and bladder cancer cells via induction of apoptotic cell death and cell cycle arrest. Oncol. Rep. 2016, 35, 1463–1472. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Chen, Y.-J.; Chang, W.-A.; Li, W.-M.; Ke, H.-L.; Wu, W.-J.; Kuo, P.-L. Effects of Epigallocatechin Gallate (EGCG) on Urinary Bladder Urothelial Carcinoma―Next-Generation Sequencing and Bioinformatics Approaches. Medicina 2019, 55, 768. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Song, J.; Li, E.; Geng, H.; Li, Y.; Yu, D.; Zhong, C. (-)-Epigallocatechin-3-gallate inhibits bladder cancer stem cells via suppression of sonic hedgehog pathway. Oncol. Rep. 2019, 42, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.-W.; Lung, W.-Y.; Xie, C.; Luo, X.-L.; Huang, W.-R. EGCG inhibited bladder cancer T24 and 5637 cell proliferation and migration via PI3K/AKT pathway. Oncotarget 2018, 9, 12261–12272. [Google Scholar] [CrossRef]
- Luo, K.-W.; Chen, W.; Lung, W.-Y.; Wei, X.-Y.; Cheng, B.-H.; Cai, Z.-M.; Huang, W.-R. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-κB and MMP-9. J. Nutr. Biochem. 2017, 41, 56–64. [Google Scholar] [CrossRef]
- Feng, C.; Ho, Y.; Sun, C.; Xia, G.; Ding, Q.; Gu, B. Epigallocatechin gallate inhibits the growth and promotes the apoptosis of bladder cancer cells. Exp. Ther. Med. 2017, 14, 3513–3518. [Google Scholar] [CrossRef]
- Wang, S.; Meng, Q.; Xie, Q.; Zhang, M. Effect and mechanism of resveratrol on drug resistance in human bladder cancer cells. Mol. Med. Rep. 2017, 15, 1179–1187. [Google Scholar] [CrossRef]
- Wang, S.; Lei, T.; Zhang, M. The Reversal Effect and Its Mechanisms of Tetramethylpyrazine on Multidrug Resistance in Human Bladder Cancer. PLoS ONE 2016, 11, e0157759. [Google Scholar] [CrossRef]
- Jankun, J.; Keck, R.W.; Selman, S.H. Epigallocatechin-3-gallate prevents tumor cell implantation/growth in an experimental rat bladder tumor model. Int. J. Oncol. 2014, 44, 147–152. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, H.; Evelson, P.; Sigaud, S.; González-Flecha, B. Mitogen-activated protein kinases modulate H2O2-induced apoptosis in primary rat alveolar epithelial cells. J. Cell. Biochem. 2004, 92, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Golonko, A.; Lewandowska, H.; Świsłocka, R.; Jasińska, U.T.; Priebe, W.; Lewandowski, W. Curcumin as tyrosine kinase inhibitor in cancer treatment. Eur. J. Med. Chem. 2019, 181, 111512. [Google Scholar] [CrossRef]
- Sever, R.; Brugge, J.S. Signal Transduction in Cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a006098. [Google Scholar] [CrossRef]
- Phi, L.T.H.; Sari, I.N.; Yang, Y.-G.; Lee, S.-H.; Jun, N.; Kim, K.S.; Lee, Y.K.; Kwon, H.Y. Cancer Stem Cells (CSCs) in Drug Resistance and their Therapeutic Implications in Cancer Treatment. Stem. Cells Int. 2018, 2018, 5416923. [Google Scholar] [CrossRef]
- Islam, S.S.; Mokhtari, R.B.; Noman, A.S.; Uddin, M.; Rahman, M.Z.; Azadi, M.A.; Zlotta, A.; van der Kwast, T.; Yeger, H.; Farhat, W.A. Sonic hedgehog (Shh) signaling promotes tumorigenicity and stemness via activation of epithelial-to-mesenchymal transition (EMT) in bladder cancer. Mol. Carcinog. 2016, 55, 537–551. [Google Scholar] [CrossRef]
- Yang, M.; Li, H.; Li, Y.; Ruan, Y.; Quan, C. Identification of genes and pathways associated with MDR in MCF-7/MDR breast cancer cells by RNA-seq analysis. Mol. Med. Rep. 2018, 17, 6211–6226. [Google Scholar] [CrossRef]
- Li, H.; Krstin, S.; Wink, M. Modulation of multidrug resistant in cancer cells by EGCG, tannic acid and curcumin. Phytomedicine 2018, 50, 213–222. [Google Scholar] [CrossRef]
- Wen, C.; Fu, L.; Huang, J.; Dai, Y.; Wang, B.; Xu, G.; Wu, L.; Zhou, H. Curcumin reverses doxorubicin resistance via inhibition the efflux function of ABCB4 in doxorubicin-resistant breast cancer cells. Mol. Med. Rep. 2019, 19, 5162–5168. [Google Scholar] [CrossRef]
- Lv, X.-A.; Wang, B.; Xu, X.-H.; Pan, L.; Wang, B.; Dong, X.-X.; Zheng, C.-H.; Du, Q.-W. Curcumin re-sensitizes multidrug resistant (MDR) breast cancer to cisplatin through inducing autophagy by decreasing CCAT1 expression. RSC Adv. 2017, 7, 33572–33579. [Google Scholar] [CrossRef]
- Hassanalilou, T.; Ghavamzadeh, S.; Khalili, L. Curcumin and Gastric Cancer: A Review on Mechanisms of Action. J. Gastrointest. Cancer 2019, 50, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Wu, X.-N.; Chen, J.; Wang, W.-X.; Lu, Z.-F. Resveratrol reverses multidrug resistance in human breast cancer doxorubicin-resistant cells. Exp. Ther. Med. 2014, 7, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, W.; Sun, L.; Xiang, L.; Lai, X.; Li, Q.; Sun, S. The effects and mechanisms of epigallocatechin-3-gallate on reversing multidrug resistance in cancer. Trends Food Sci. Technol. 2019, 93, 221–233. [Google Scholar] [CrossRef]
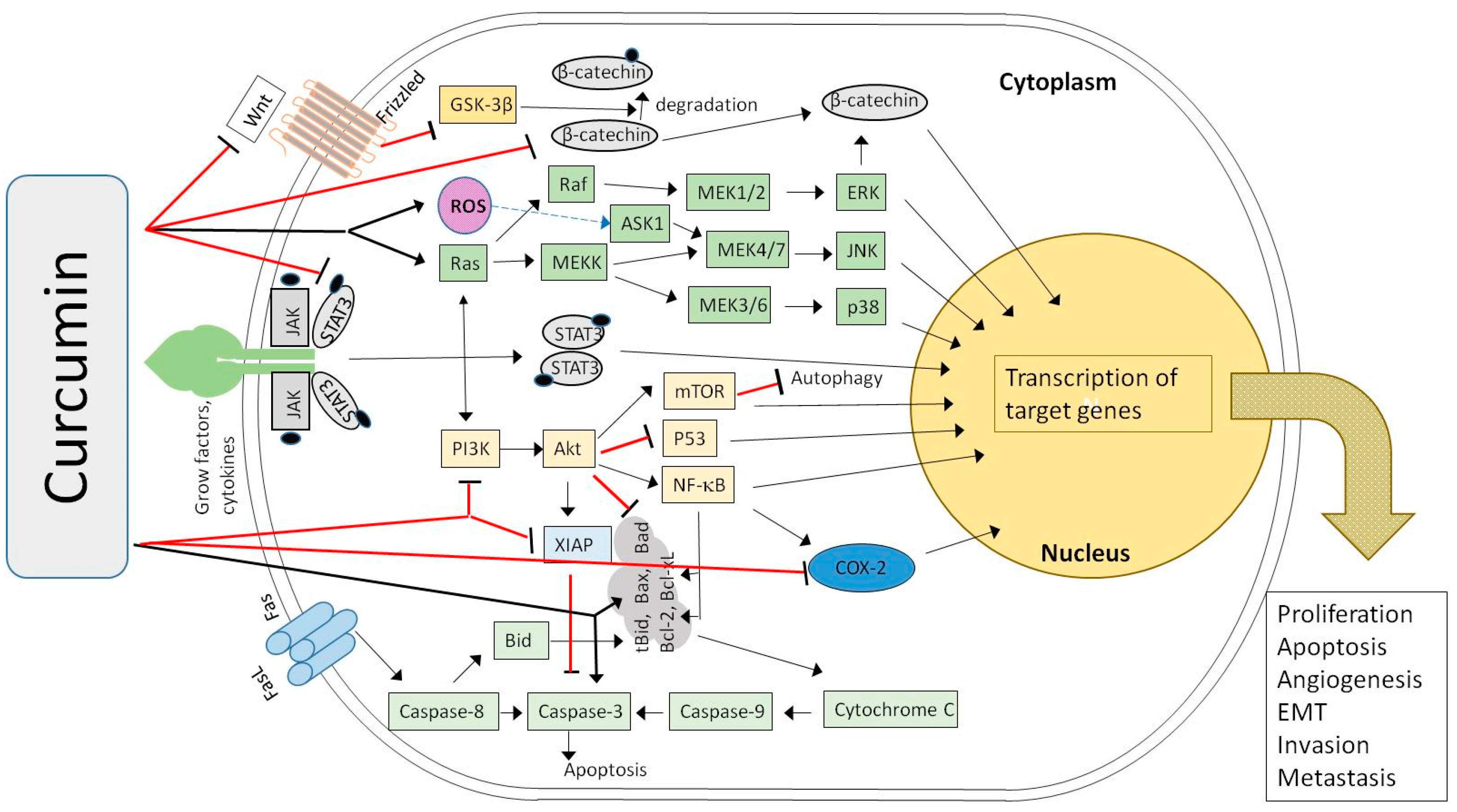
| Main Compound [Ref.] | Study Goal | In Vitro (Cell Line/s) In Vivo (Model) | Main Conclusions |
|---|---|---|---|
| CUR + 400–550 nm light (1.65 J/cm2) [38] | Effect of light on increase in bioavailability and effectiveness of CUR in BC treatment. | In vitro: RT112, UMUC3, TCCSUP | Photoexcitation of CUR altered migration and adhesion of BC cells by integrin-dependent mechanism (α3, α5 and β1). |
| CUR (0.1–0.4 μg/mL) + 400–550 nm light (1.65 J/cm2) [39] | Effect of light exposure on CUR bioavailability and anticancer efficacy. | In vitro: RT112, UMUC3, TCCSUP | Inhibition of BC cells by low-dosed CUR exposed to light (0.27 μmol/L) in various cell phases (G0/G1 for cell line RT112, G2/M for TCCSUP, and G2/M- and S-phase for UMUC3) via various molecular action mechanisms. |
| Vanadyl CUR, vanadyldiacetyl CUR [42] | Effect of vanadyl CUR and vanadyldiacetyl CUR on structure, function and antitumor activity of peroxidase enzyme (HRP). | In vitro: C5637 | Upregulation of horseradish peroxidase (HRP) enzyme stability and activation of peroxidation reaction. Evidence of cytotoxic effect on BC cells. |
| Gallium CUR, gallium diacetylcurcumin [43] | Effect of gallium CUR and gallium diacetylcurcumin on structure, function and antitumor activity of peroxidase enzyme (HRP). | In vitro: C5637 | Upregulation of horseradish peroxidase (HRP) enzyme stability and activation of peroxidation reaction. Evidence of cytotoxic effect on BC cells. |
| Lipid-coated polyplex with CUR and anionic plasmid [44] | Combination therapy involving hydrophilic genes and hydrophobic drugs for treatment of BC. | In vitro: MB49 | Development of a lipid carrier system for dual delivery of plasmid DNA and small hydrophobic molecules into MB49 BC cells. |
| Cyclodextrin–CUR complex (CDC) [45] | Effect of CDC complex on human and rat urothelial cancer cells and in AY-F344 orthotopic BC rat model. | In vitro: RT4, T24, 253J, RT112 AY-27 (rat cell line), In vivo: AY-F344 orthotopic BC rat model | Antiproliferative effect (dose-dependent) on rat AY-27 and various cell lines in vitro. Intravesical instillation of CDC as promising antitumor response. |
| CUR + melatonin [46] | Effectiveness of CUR and melatonin combination therapy by inhibiting proliferation of BC cells. | In vitro: T24, UMUC3, 5637 In vivo: Male BALB/c mice | Antiproliferative and antimigration effects of combined CUR and melatonin on BC cells. Melatonin synergized the ability of CUR to inhibit BC growth, both in vivo and in vitro. The effect of CUR and melatonin on BC cells was related to simultaneous action on cyto c/caspase and IKKβ/NF-κB/COX-2 signaling. |
| CUR [47] | Effect of CUR on human trophoblast cell surface antigen 2 (Trop2) to reduce oncogenic activity of BC cells. | In vitro: RT4, RT24 | Trop2 as CUR target in RT4 and T24 cell lines. Inhibition of migration, growth and invasion of cancer cells by CUR can be related to the decreased expression of Trop2 and its downstream target cyclin E1, and the increased level of p27. Apoptosis of BC cells. |
| CUR [48] | Antitumor action mechanisms of CUR in BC. | In vitro: T24, 5637 | Time- and dose-dependent inhibition of T24 and 5637 cell line proliferation by CUR. Inhibition of epithelial–mesenchymal transition (EMT) and β-catenin signaling pathways. |
| Resveratrol, CUR [49] | Analysis of effects of CUR as potential treatment for reversing drug resistance in BC chemotherapy. | In vitro: T24, T24-GCB (gemcitabine-resistant) | CUR may reverse the multidrug resistance (MDR) of T24-GCB cells. CUR activates apoptosis by regulation of ABCC2 (increased the expression) and DCK, TK1, TK2 (decreased the expression) as well as increasing PARP cleavage. |
| CUR and irradiation [50] | Possibility of increasing radiation sensitivity in BC. | In vitro: T24, HT-1376, SV-HUC-1 | Anticancer effect of irradiated CUR due to involvement of miR-1246 in inhibiting p53 gene translation in BC cells. |
| CUR [51] | Effect of CUR on regulation of microRNA-7641 in BC. | In vitro: J82, TCCSUP, T24, SV-HUC-1 | miR-7641 found to be cancer-stimulating factor. Therapeutic effect of CUR on SV-HUC-1 cells by modulating miR-7641 (downregulation) leading to increased p16 expression being target of miR-7641. CUR revealed proapoptotic effect, which influenced inhibition of proliferation and migration of BC cells. |
| CUR [52] | Preventive action of CUR on cancer stem cells activated by tobacco smoke and role of Wnt/β-catenin pathway in urocystic epithelial–mesenchymal transition. | In vitro: T24, SV-HUC-1 In vivo: Male BALB/c mice | Effective reversal by CUR of the activation of Wnt/β-catenin pathway in vitro and in vivo. |
| CUR [53] | Role of CUR in inhibiting growth of BC stem cells and regulation of the Sonic hedgehog (Shh) pathway. | In vitro: UM-UC-3, EJ | CUR activity against BC stem cells in vitro was observed, especially reducing the cell spheres formation, decreasing expression of cancer stem cells markers, suppressing cell proliferation, and inducing cell apoptosis. Deactivation of the Shh pathway. |
| CUR [54] | Therapeutic effect of CUR in BC treatment. | In vitro: T24, 5637 | Time- and dose-dependent cell growth inhibition by CUR. Proapoptotic and antimigration effects of CUR by suppression of matrix metalloproteinase signaling pathways in vitro. |
| CUR [55] | Antitumor action mechanisms in BC treatment. | In vitro: T24, UMUC2 In vivo: Female Wistar rats | Anticancer activity of CUR by inhibition of IGF2, suppression of PI3K/AKT/mTOR signaling pathway, and inactivation of N-methyl-N-nitrosourea-induced urothelial tumor tissue. |
| CUR [56] | Preventive effect of CUR on bezidine-induced EMT. | In vitro: SV-40 (SV-HUC-1) | CUR as promising BC drug due to inhibition of ERK5/AP-1 pathway. |
| CUR + cisplatin [57] | Combination treatment of CUR and cisplatin in BC cells. | In vitro: T24, 253J-Bv | BC cells apoptosis caused by combination therapy due to reactive oxygen species (ROS) and extracellular regulated kinase (ERK), along with activation of p-MEK and p-ERK1/2. Apoptosis of 253J-Bv cells caused by increased expression of p53 and p21 proteins. Apoptosis of T24 cells caused by decreased p-signal transducer and activator of transcription 3(STAT3) expression. |
| CUR [58] | Effect of CUR on proliferation of benzidine-induced BC cells. | In vitro: T24 | Antiproliferative effect of CUR on benzidine-induced T24 cells through prevention of ERK1/2 activation. Reduced activation of AP-1 proteins (c-Fos and c-Jun) due to downregulation of ERK 1/2. |
| CUR [59] | Effect of TS on activation of MAPK pathway and EMT changes in BC and preventive effect of CUR. | In vivo: Male BALB/c mice | CUR-induced inhibition of activation of ERK1/2, JNK and p38 MAPK pathways, and AP-1 proteins. Prevention of epithelial-mesenchymal transition (EMT) in the BC. |
| CUR (160 μmol/L) [60] | Use of CUR in BC treatment. | In vitro: SPF-grade Wistar rats + N-methyl-N-nitrosourea | Proapoptotic effect of CUR by stopping G1/S phases of cell cycle and increasing Bax protein expression. |
| CUR (5 and 15 μM) + 5-fluorouracil (5-FU) [61] | Effect of CUR on 5-FU toxicity in BC cells | In vitro: EJ138 | 5-FU cytotoxicity was dependent on CUR concentration. |
| BDMC with α-PD-L1 antibody [62] | Combination treatment of BDMC and α-PD-L1 antibody on survival of BC cells. | In vivo: Female C57BL/6 mice with MB49 mouse BC cells | BDMC with α-PD-L1 antibody appeared as promising therapy against BC. BDMC enhanced CD8+ T cell response, elevated the level of IFN-γ in the blood, and suppressed myeloid-derived suppressor cells due to combination treatment. |
| Theracurmin® (nanocurcumin), CUR [63] | Anticancer effect of Theracurmin® and CUR compounds on BC cells. | In vitro: T24R2, 253J, HTB9 | Comparable efficacy of Theracurmin® and CUR. Dosage and time-dependent antitumor effect of Theracurmin® due to activation of apoptosis and arresting the cell cycle in sub-G1, and dysregulation of S and/or G2/M phases. |
| Main Compound [Ref.] | Main Goal | In Vitro (cell line/s) In Vivo (model) | Main Conclusions |
|---|---|---|---|
| EGCG [32] | Effect of ionizing radiation on EGCG sterilization in treatment of BC. | In vitro: AY-27 | EGCG sterilization by irradiation (25 kGy) and filtration (0.2 µM) with minimal loss of EGCG. Suitability of both methods for development of sterile drug for intravesical infusion. |
| Polyphenon E (main compound: EGCG) [34] | Polyphenon E in prevention of BC with pharmacodynamic and biomarker assessment of bladder tissue. | In vivo: 33 participants in 4–28 days prior to transurethral resection of bladder tumor or cystectomy | EGCG accumulation relative to dosage, and plasma and urine concentrations in benign bladder epithelium. EGCG influenced dose-dependent decrease of biomarkers (cell nuclear antigen-PCNA and clustering). |
| EGCG [64] | New EGCG therapeutic targets in BC. | In vitro: BFTC-905 [human urinary bladder transitional cell carcinoma (TCC) cell line] | Identification of 108 genes of different expression activity and 22 genes showing miRNA interactions in EGCG-treated TCC cell line. Detection of microRNA–mRNA interactions involved in EGCG treatment of TCC [miR-185-3p- ARRB1 (arrestin beta 1), miR-3116- MGAT5B (alpha-1,6-mannosylglycoprotein 6-beta-N-acetylglucosaminyltransferase B), miR-31-5p-TNS1 (tensin 1), miR-642a-5p-TNS1, miR-1226-3p-DLG2 (discs large homolog 2), miR-484-DLG2, and miR-22-3p- PPM1K (protein phosphatase 1K)]. Better understanding of the new targets. |
| EGCG [65] | Effect of molecular action mechanism of EGCG on cancer stem cells (CSCs). | In vitro: EJ, UM-UC-3 | Inhibition of bladder CSCs by targeting Shh signaling pathway (decreased expression of Smo, Shh, Gli1 i Gli2), downregulation of bladder CSCs markers (CD44, CD133, Oct4, ALDH1A and Nanog). Evidence of EGCG proapoptotic activity. |
| EGCG [66] | Effect of EGCG on proliferation and migration of BC cells and role of PI3K/AKT pathway. | In vitro: T24, 5637 In vivo: BALB/c nude mice | EGCG-induced apoptosis (activation of caspase-3 and PARP) and inhibition of migration of T24 and 5637 cells (25–100 μM). 51.2% decrease in tumor growth in EGCG-treated subjects. Reduced expression of phosphorylated PI3K, AKT in tumor, and activated PARP. |
| EGCG [67] | Effect of EGCG on cell proliferation and migration in BC. | In vitro: SW780 In vivo: Female BALB/c mice | EGCG found to reduce cell proliferation and promote apoptosis by activation of caspases-8, -9 and -3, Bax, Bcl-2 and PARP. 68.4% decrease in tumor size in mice by reduction of expression of nuclear factor-kappa B (NF-κB) and matrix metalloproteinase (MMP) -9 at mRNA and protein levels. |
| EGCG [68] | Effect of EGCG on induction of apoptosis by tissue factor pathway inhibitor 2 (TFPI-2). | In vitro: T24 | EGCG-induced restoring of TFPI-2 expression in T24 cells. Proapoptotic effect of EGCG. |
| Ginsenoside Rh2, EGCG, resveratrol [69] | Effects of herbal compounds as potential treatment for reversing MDR in BC chemotherapy. | In vitro: adriamycin-resistant pumc-91 cells (pumc-91/ADM) | EGCG found not to increase anticancer cytotoxic effect on pumc-91/ADM. |
| Ginsenoside Rh2, EGCG, tetramethylpyrazine [70] | |||
| Polyphenon E (main compound: EGCG), mitomycin C (MMC) [71] | Therapeutic efficacy of EGCG and MMC in preventing cell implantation in BC. | In vivo: Female Fisher 344 rats | Concentration- and time-dependent prevention of intravesical tumor growth by EGCG and MMC. Greater therapeutic effect shown by EGCG (downregulation of urokinase and matrix metalloproteinase-9). |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piwowarczyk, L.; Stawny, M.; Mlynarczyk, D.T.; Muszalska-Kolos, I.; Goslinski, T.; Jelińska, A. Role of Curcumin and (−)-Epigallocatechin-3-O-Gallate in Bladder Cancer Treatment: A Review. Cancers 2020, 12, 1801. https://doi.org/10.3390/cancers12071801
Piwowarczyk L, Stawny M, Mlynarczyk DT, Muszalska-Kolos I, Goslinski T, Jelińska A. Role of Curcumin and (−)-Epigallocatechin-3-O-Gallate in Bladder Cancer Treatment: A Review. Cancers. 2020; 12(7):1801. https://doi.org/10.3390/cancers12071801
Chicago/Turabian StylePiwowarczyk, Ludwika, Maciej Stawny, Dariusz T. Mlynarczyk, Izabela Muszalska-Kolos, Tomasz Goslinski, and Anna Jelińska. 2020. "Role of Curcumin and (−)-Epigallocatechin-3-O-Gallate in Bladder Cancer Treatment: A Review" Cancers 12, no. 7: 1801. https://doi.org/10.3390/cancers12071801
APA StylePiwowarczyk, L., Stawny, M., Mlynarczyk, D. T., Muszalska-Kolos, I., Goslinski, T., & Jelińska, A. (2020). Role of Curcumin and (−)-Epigallocatechin-3-O-Gallate in Bladder Cancer Treatment: A Review. Cancers, 12(7), 1801. https://doi.org/10.3390/cancers12071801







