A Novel Combination Treatment with Honokiol and Rapamycin Effectively Restricts c-Met-Induced Growth of Renal Cancer Cells, and also Inhibits the Expression of Tumor Cell PD-L1 Involved in Immune Escape
Abstract
1. Introduction
2. Results
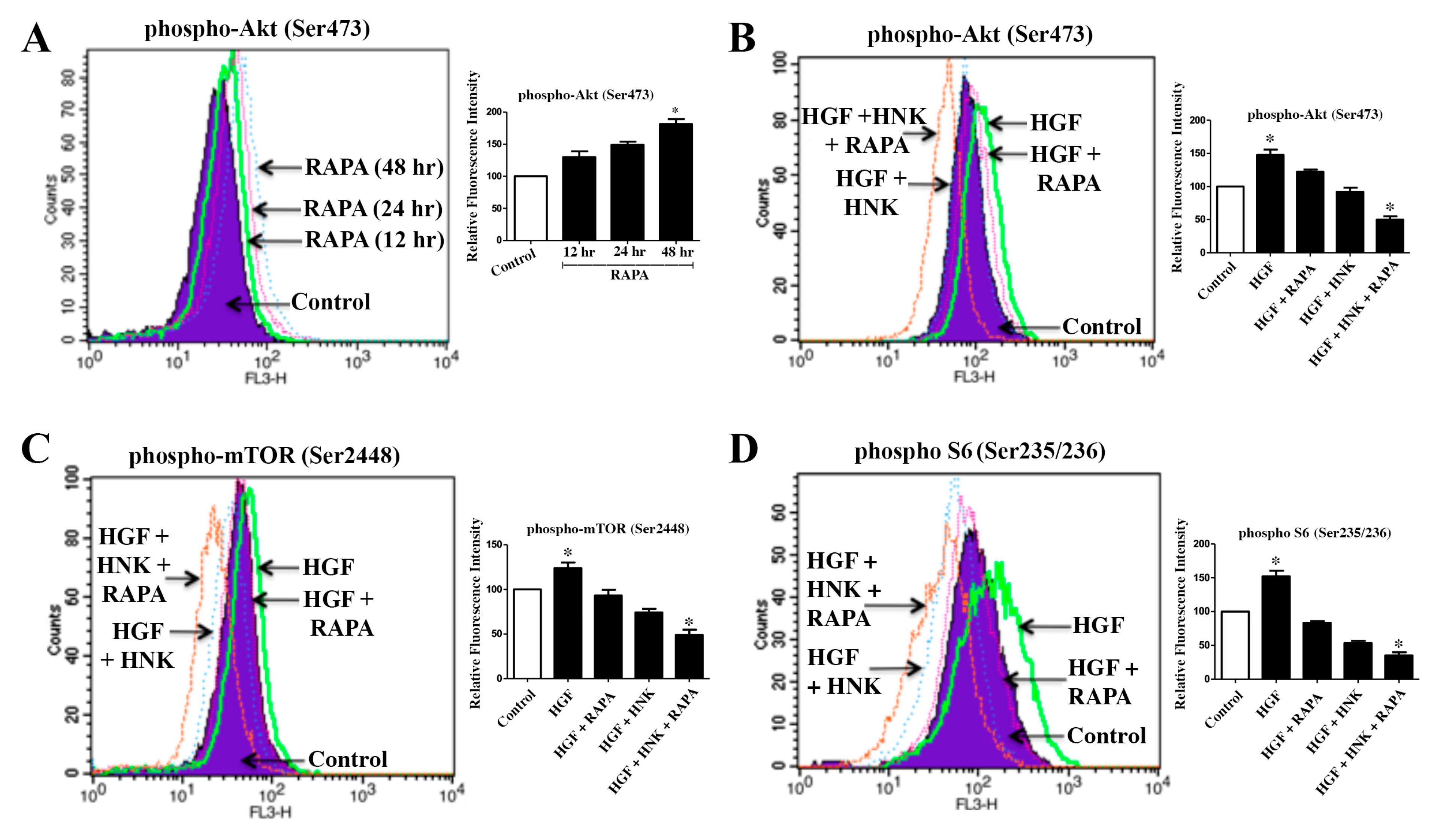
2.1. A Combination Treatment with RAPA and Honokiol Effectively Down-Regulates c-Met-Induced Phosphorylation of Akt, mTOR and S6 in Renal Cancer Cells
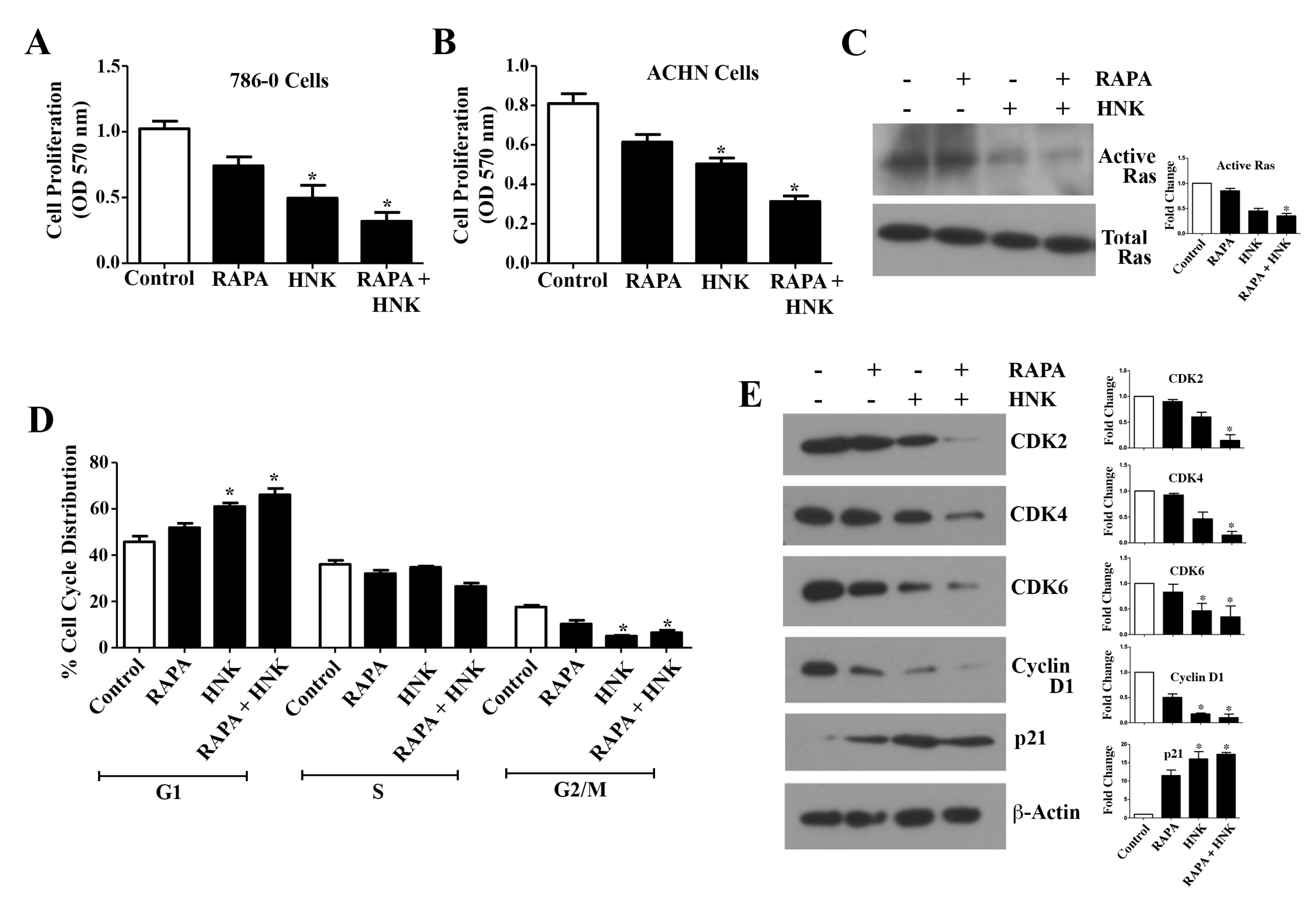
2.2. RAPA and Honokiol Inhibits Renal Cancer Cell Proliferation, Down-Regulates Active Ras, and Induces G1 Phase Cell Cycle Arrest
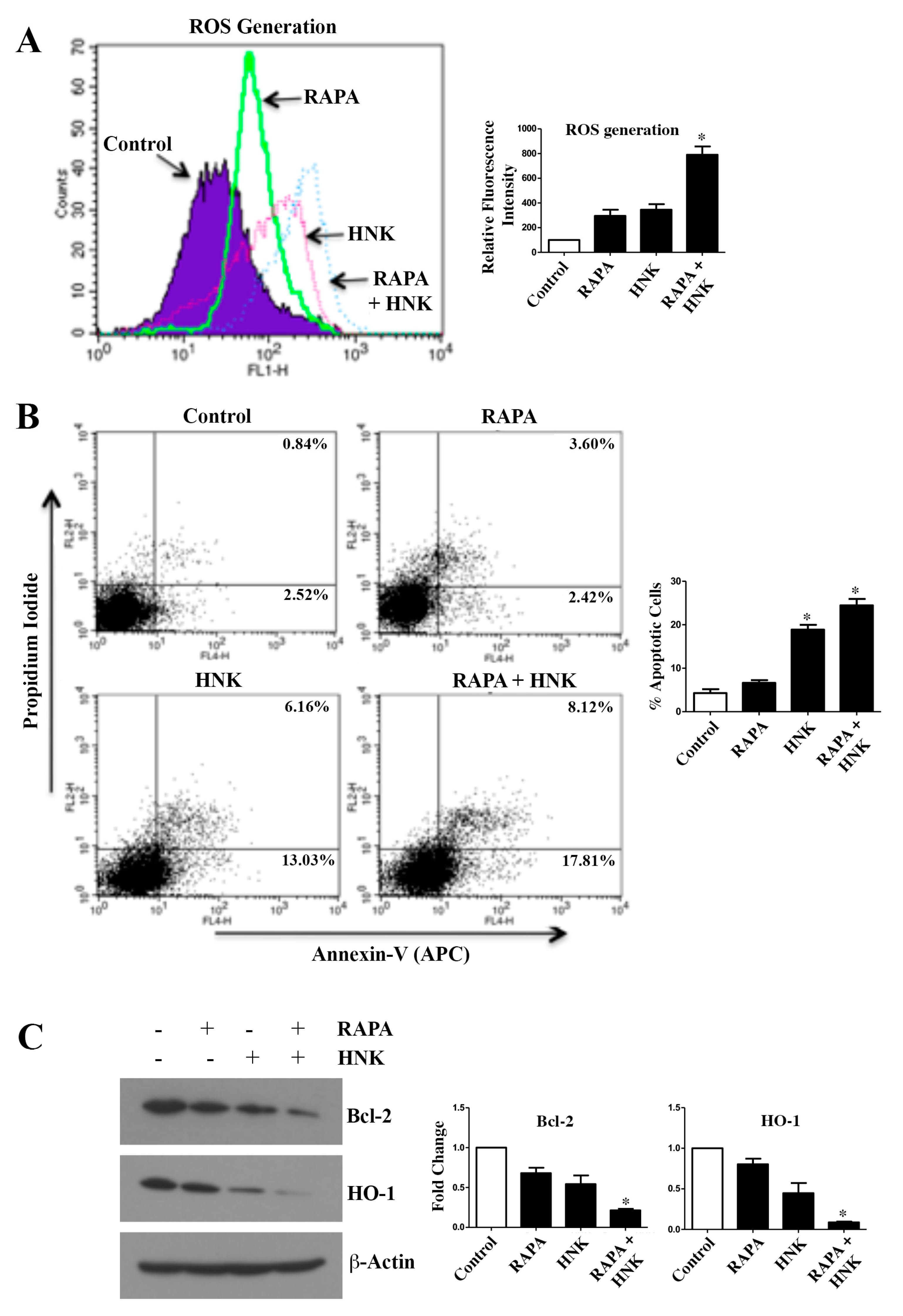
2.3. RAPA and Honokiol Combination Treatment Promotes Reactive Oxygen Species (ROS) Generation, and Induces Apoptosis of Renal Cancer Cells
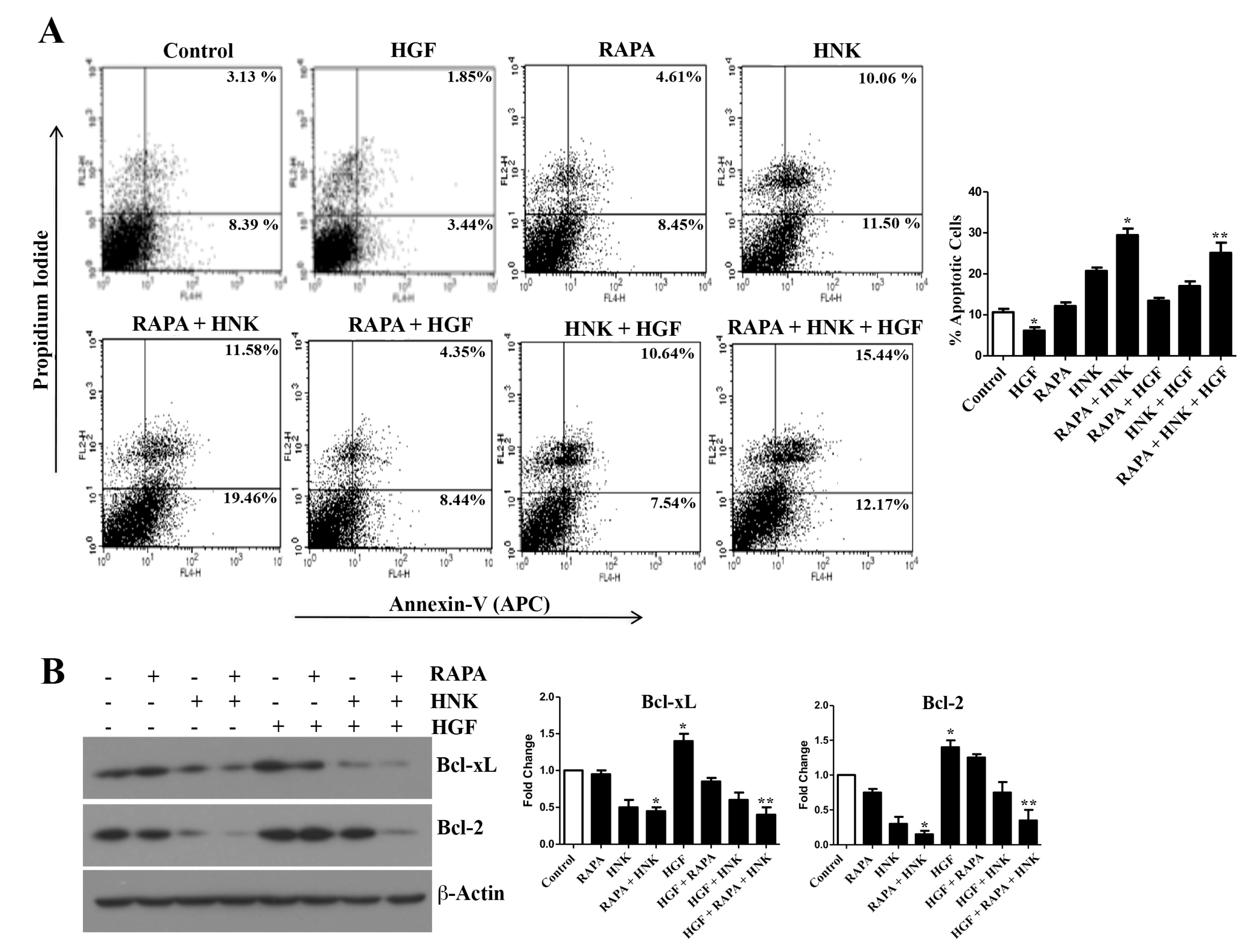
2.4. RAPA and Honokiol Treatment Effectively Restricts c-Met-Induced Survival of Renal Cancer Cells
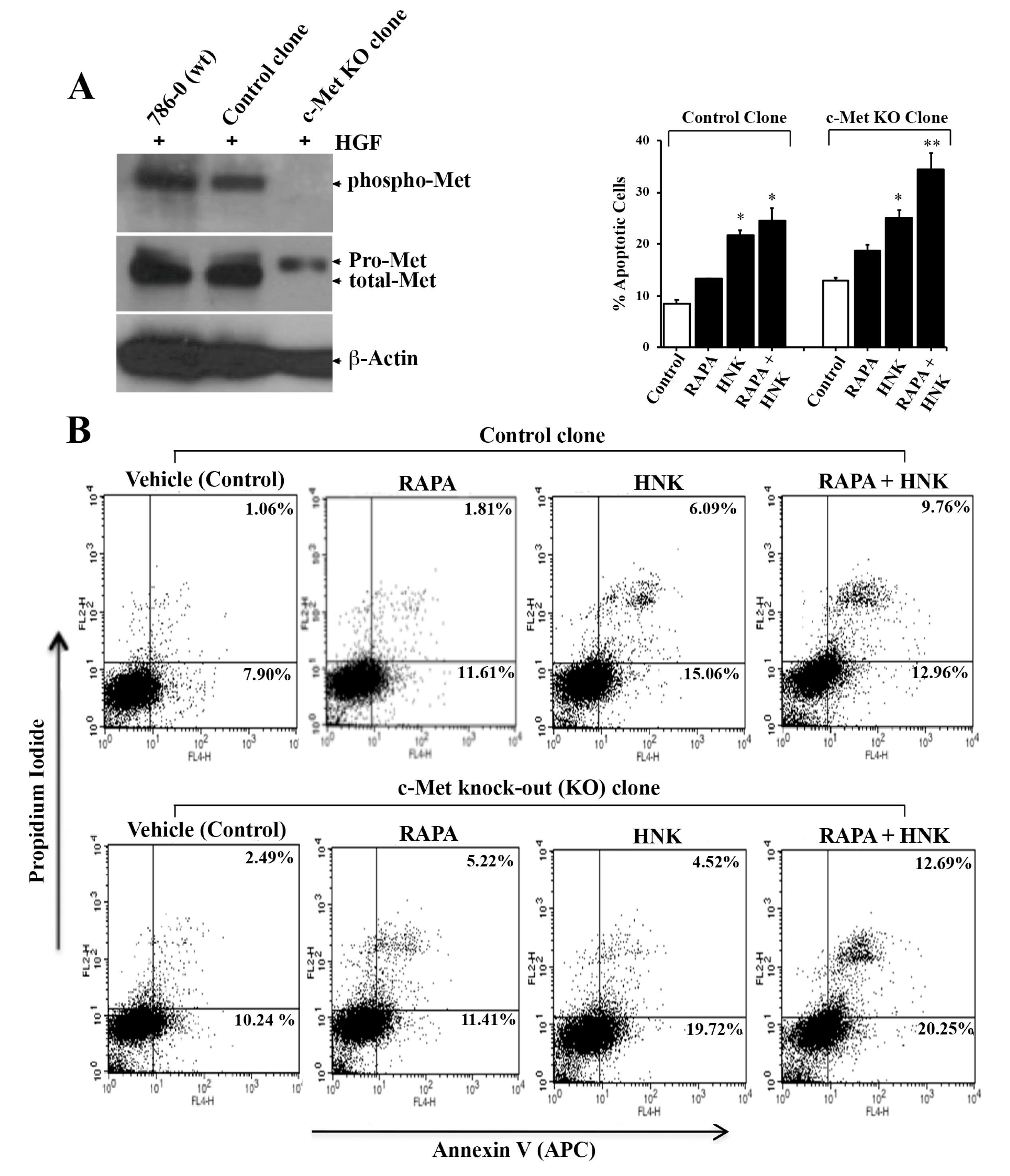
2.5. The Efficiency of RAPA and Honokiol to Induce Tumor Cell Apoptosis is Increased in c-Met Knockout Cells
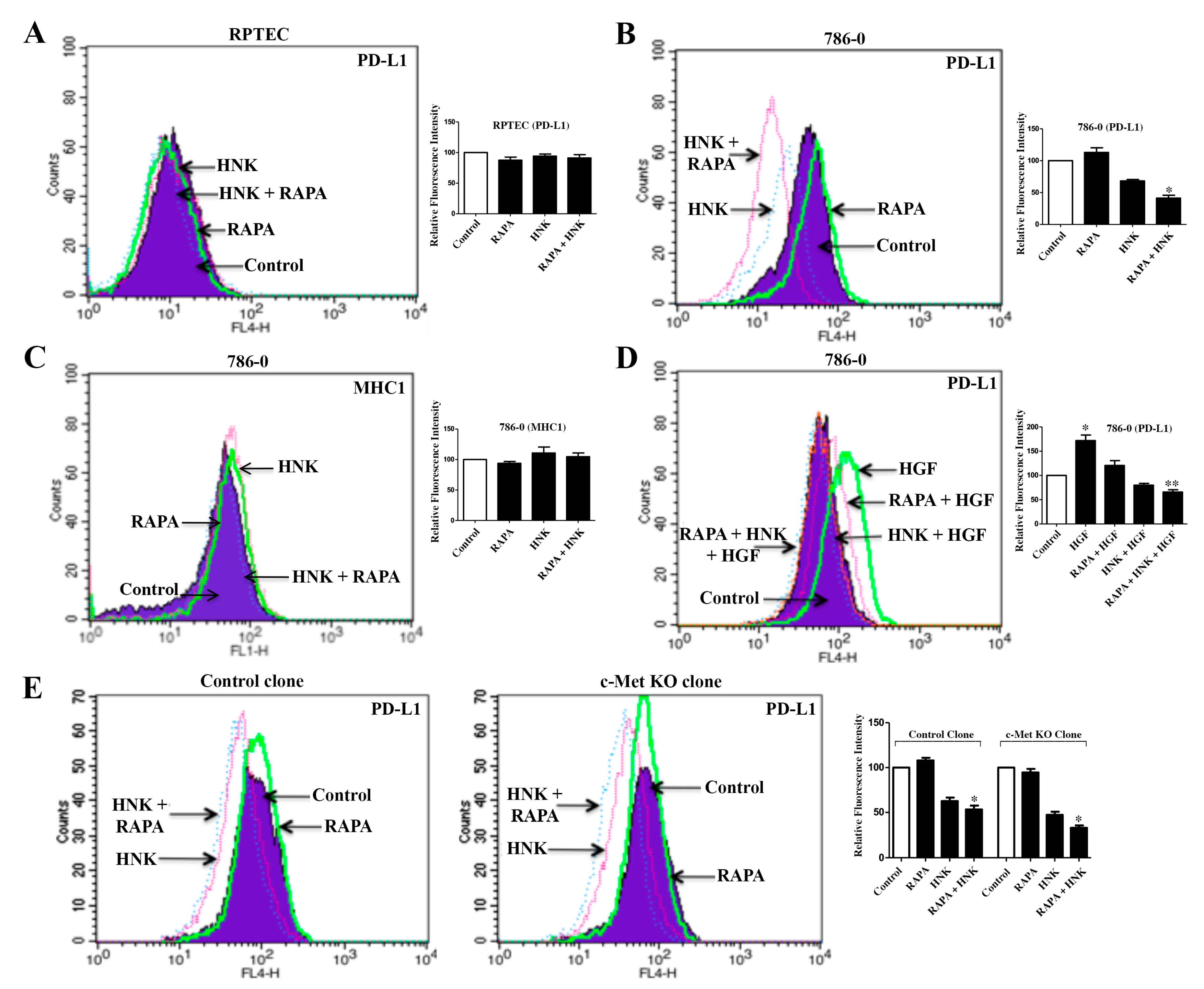
2.6. The c-Met-Induced PD-L1 Expression on Renal Cancer Cells is Down-Regulated by RAPA and Honokiol Combination Treatment
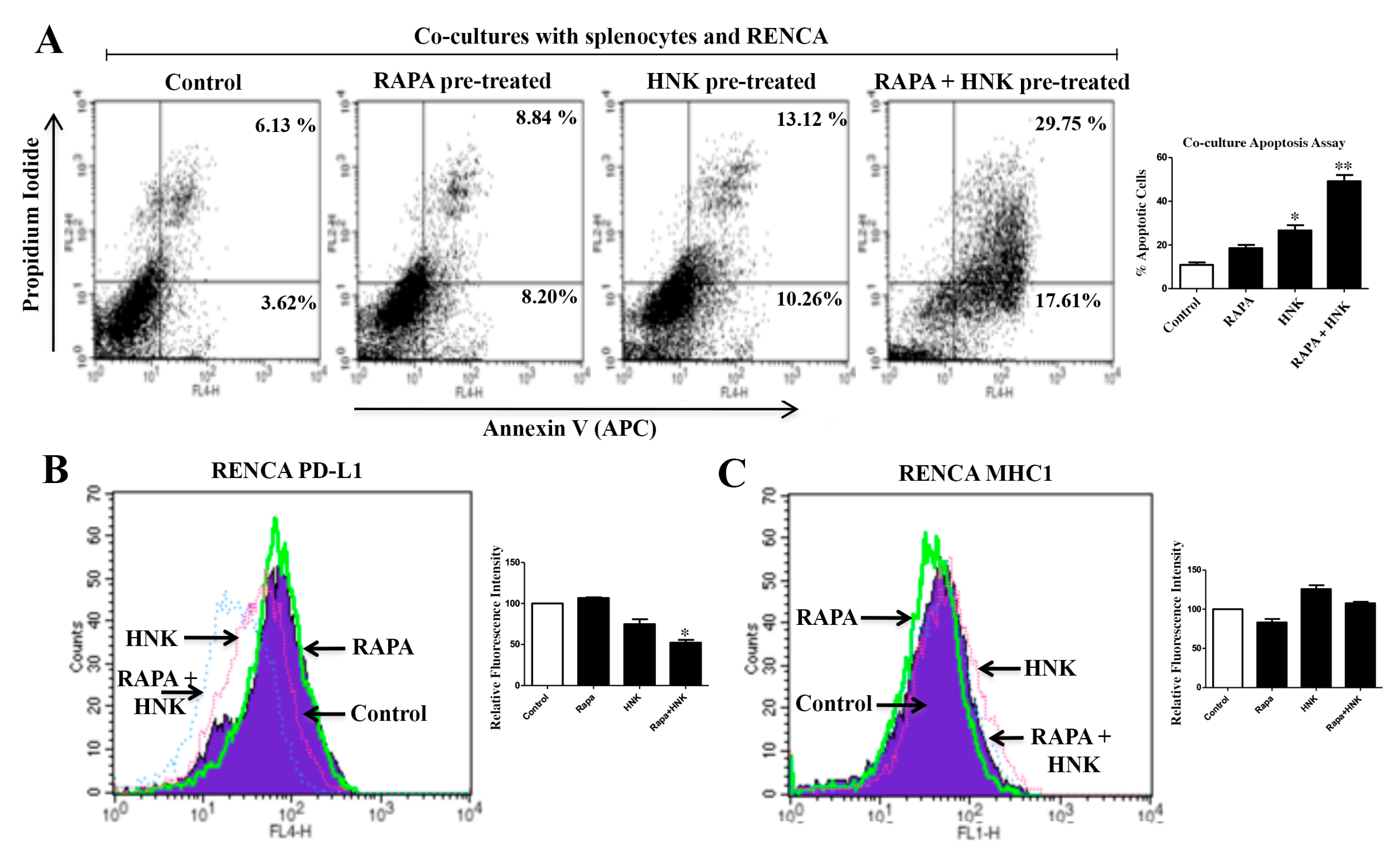
2.7. RAPA and Honokiol Combination Treatment Promotes Immune-Mediated Apoptosis of Renal Cancer Cells, Possibly through the Down-Regulation of PD-L1
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Lines
4.3. Generation of CRISPR/Cas9-Mediated c-Met Knock-out Cells
4.4. Cell Proliferation Assay
4.5. Cell Cycle Analysis
4.6. Apoptosis Assay
4.7. RENCA/Splenocytes Co-Culture
4.8. Measurement of Active/GTP-Bound Ras
4.9. Reactive Oxygen Species (ROS) Detection
4.10. Analysis of Proteins through Flow Cytometry
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Makhov, P.; Joshi, S.; Ghatalia, P.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Resistance to Systemic Therapies in Clear Cell Renal Cell Carcinoma: Mechanisms and Management Strategies. Mol. Cancer Ther. 2018, 17, 1355–1364. [Google Scholar] [CrossRef]
- Siska, P.J.; Beckermann, K.E.; Rathmell, W.K.; Haake, S.M. Strategies to overcome therapeutic resistance in renal cell carcinoma. Urol. Oncol. 2017, 35, 102–110. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Motzer, R.J. Systemic Therapy for Metastatic Renal-Cell Carcinoma. N. Engl. J. Med. 2017, 376, 354–366. [Google Scholar] [CrossRef]
- Wells, J.C.; Graham, J.; Beuselinck, B.; Bjarnason, G.A.; Donskov, F.; Hansen, A.R.; McKay, R.R.; Vaishampayan, U.; De Velasco, G.; Duh, M.S.; et al. Clinical Outcomes of First-line Sunitinib Followed by Immuno-oncology Checkpoint Inhibitors in Patients With Metastatic Renal Cell Carcinoma. Clin. Genitourin. Cancer 2019. [Google Scholar] [CrossRef]
- Gallagher, M.P.; Kelly, P.J.; Jardine, M.; Perkovic, V.; Cass, A.; Craig, J.C.; Eris, J.; Webster, A.C. Long-term cancer risk of immunosuppressive regimens after kidney transplantation. J. Am. Soc. Nephrol. 2010, 21, 852–858. [Google Scholar] [CrossRef]
- Balan, M.; Chakraborty, S.; Pal, S. Signaling Molecules in Posttransplantation Cancer. Clin. Lab. Med. 2019, 39, 171–183. [Google Scholar] [CrossRef]
- Sprangers, B.; Nair, V.; Launay-Vacher, V.; Riella, L.V.; Jhaveri, K.D. Risk factors associated with post-kidney transplant malignancies: An article from the Cancer-Kidney International Network. Clin. Kidney J. 2018, 11, 315–329. [Google Scholar] [CrossRef]
- Engels, E.A.; Pfeiffer, R.M.; Fraumeni, J.F., Jr.; Kasiske, B.L.; Israni, A.K.; Snyder, J.J.; Wolfe, R.A.; Goodrich, N.P.; Bayakly, A.R.; Clarke, C.A.; et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA 2011, 306, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- van de Wetering, J.; Roodnat, J.I.; Hemke, A.C.; Hoitsma, A.J.; Weimar, W. Patient survival after the diagnosis of cancer in renal transplant recipients: A nested case-control study. Transplantation 2010, 90, 1542–1546. [Google Scholar] [CrossRef] [PubMed]
- Karami, S.; Yanik, E.L.; Moore, L.E.; Pfeiffer, R.M.; Copeland, G.; Gonsalves, L.; Hernandez, B.Y.; Lynch, C.F.; Pawlish, K.; Engels, E.A. Risk of Renal Cell Carcinoma Among Kidney Transplant Recipients in the United States. Am. J. Transplant. 2016, 16, 3479–3489. [Google Scholar] [CrossRef] [PubMed]
- Dantal, J.; Soulillou, J.P. Immunosuppressive drugs and the risk of cancer after organ transplantation. N. Engl. J. Med. 2005, 352, 1371–1373. [Google Scholar] [CrossRef] [PubMed]
- Frasca, G.M.; Sandrini, S.; Cosmai, L.; Porta, C.; Asch, W.; Santoni, M.; Salviani, C.; D′Errico, A.; Malvi, D.; Balestra, E.; et al. Renal cancer in kidney transplanted patients. J. Nephrol. 2015, 28, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Balan, M.; Chakraborty, S.; Flynn, E.; Zurakowski, D.; Pal, S. Honokiol inhibits c-Met-HO-1 tumor-promoting pathway and its cross-talk with calcineurin inhibitor-mediated renal cancer growth. Sci. Rep. 2017, 7, 5900. [Google Scholar] [CrossRef] [PubMed]
- Balan, M.; Mier y Teran, E.; Waaga-Gasser, A.M.; Gasser, M.; Choueiri, T.K.; Freeman, G.; Pal, S. Novel roles of c-Met in the survival of renal cancer cells through the regulation of HO-1 and PD-L1 expression. J. Biol. Chem. 2015, 290, 8110–8120. [Google Scholar] [CrossRef] [PubMed]
- Augustine, J.J.; Bodziak, K.A.; Hricik, D.E. Use of sirolimus in solid organ transplantation. Drugs 2007, 67, 369–391. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Nagy, J.A.; Manseau, E.J.; Phung, T.L.; Dvorak, H.F.; Benjamin, L.E. Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1172–1178. [Google Scholar] [CrossRef][Green Version]
- Rozengurt, E.; Soares, H.P.; Sinnet-Smith, J. Suppression of feedback loops mediated by PI3K/mTOR induces multiple overactivation of compensatory pathways: An unintended consequence leading to drug resistance. Mol. Cancer Ther. 2014, 13, 2477–2488. [Google Scholar] [CrossRef]
- Carracedo, A.; Pandolfi, P.P. The PTEN-PI3K pathway: Of feedbacks and cross-talks. Oncogene 2008, 27, 5527–5541. [Google Scholar] [CrossRef]
- Fried, L.E.; Arbiser, J.L. Honokiol, a multifunctional antiangiogenic and antitumor agent. Antioxid. Redox Signal. 2009, 11, 1139–1148. [Google Scholar] [CrossRef]
- Ma, L.; Chen, J.; Wang, X.; Liang, X.; Luo, Y.; Zhu, W.; Wang, T.; Peng, M.; Li, S.; Jie, S.; et al. Structural modification of honokiol, a biphenyl occurring in Magnolia officinalis: The evaluation of honokiol analogues as inhibitors of angiogenesis and for their cytotoxicity and structure-activity relationship. J. Med. Chem. 2011, 54, 6469–6481. [Google Scholar] [CrossRef]
- Arora, S.; Singh, S.; Piazza, G.A.; Contreras, C.M.; Panyam, J.; Singh, A.P. Honokiol: A novel natural agent for cancer prevention and therapy. Curr. Mol. Med. 2012, 12, 1244–1252. [Google Scholar] [CrossRef]
- Chakraborty, S.; Balan, M.; Flynn, E.; Zurakowski, D.; Choueiri, T.K.; Pal, S. Activation of c-Met in cancer cells mediates growth-promoting signals against oxidative stress through Nrf2-HO-1. Oncogenesis 2019, 8, 7. [Google Scholar] [CrossRef]
- Huang, K.; Chen, Y.; Zhang, R.; Wu, Y.; Ma, Y.; Fang, X.; Shen, S. Honokiol induces apoptosis and autophagy via the ROS/ERK1/2 signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2018, 9, 157. [Google Scholar] [CrossRef]
- Hsiao, C.H.; Yao, C.J.; Lai, G.M.; Lee, L.M.; Whang-Peng, J.; Shih, P.H. Honokiol induces apoptotic cell death by oxidative burst and mitochondrial hyperpolarization of bladder cancer cells. Exp. Ther. Med. 2019, 17, 4213–4222. [Google Scholar] [CrossRef]
- Pan, J.; Zhang, Q.; Liu, Q.; Komas, S.M.; Kalyanaraman, B.; Lubet, R.A.; Wang, Y.; You, M. Honokiol inhibits lung tumorigenesis through inhibition of mitochondrial function. Cancer Prev. Res. (Phila.) 2014, 7, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Chao, L.K.; Liao, P.C.; Ho, C.L.; Wang, E.I.; Chuang, C.C.; Chiu, H.W.; Hung, L.B.; Hua, K.F. Anti-inflammatory bioactivities of honokiol through inhibition of protein kinase C, mitogen-activated protein kinase, and the NF-kappaB pathway to reduce LPS-induced TNFalpha and NO expression. J. Agric. Food Chem. 2010, 58, 3472–3478. [Google Scholar] [CrossRef] [PubMed]
- Banumathy, G.; Cairns, P. Signaling pathways in renal cell carcinoma. Cancer Biol. Ther. 2010, 10, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Teppo, H.R.; Soini, Y.; Karihtala, P. Reactive Oxygen Species-Mediated Mechanisms of Action of Targeted Cancer Therapy. Oxid. Med. Cell. Longev. 2017, 2017, 1485283. [Google Scholar] [CrossRef]
- Marona, P.; Gorka, J.; Kotlinowski, J.; Majka, M.; Jura, J.; Miekus, K. C-Met as a Key Factor Responsible for Sustaining Undifferentiated Phenotype and Therapy Resistance in Renal Carcinomas. Cells 2019, 8, 272. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S.; Horn, L.A.; Haile, S.T. The programmed death-1 immune-suppressive pathway: Barrier to antitumor immunity. J. Immunol. 2014, 193, 3835–3841. [Google Scholar] [CrossRef]
- Saunders, R.N.; Metcalfe, M.S.; Nicholson, M.L. Rapamycin in transplantation: A review of the evidence. Kidney Int. 2001, 59, 3–16. [Google Scholar] [CrossRef]
- Sabarwal, A.; Agarwal, R.; Singh, R.P. Fisetin inhibits cellular proliferation and induces mitochondria-dependent apoptosis in human gastric cancer cells. Mol. Carcinog. 2017, 56, 499–514. [Google Scholar] [CrossRef]
- Lu, C.H.; Chen, S.H.; Chang, Y.S.; Liu, Y.W.; Wu, J.Y.; Lim, Y.P.; Yu, H.I.; Lee, Y.R. Honokiol, a potential therapeutic agent, induces cell cycle arrest and program cell death in vitro and in vivo in human thyroid cancer cells. Pharmacol. Res. 2017, 115, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.B.; Bao, X.J.; Xu, S.B.; Zhang, X.D.; Liu, H.Y. Honokiol induces cell cycle arrest and apoptosis via p53 activation in H4 human neuroglioma cells. Int. J. Clin. Exp. Med. 2015, 8, 7168–7175. [Google Scholar] [PubMed]
- Hahm, E.R.; Arlotti, J.A.; Marynowski, S.W.; Singh, S.V. Honokiol, a constituent of oriental medicinal herb magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin. Cancer Res. 2008, 14, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.H.; Gillett, M.D.; Cheville, J.C.; Lohse, C.M.; Dong, H.; Webster, W.S.; Krejci, K.G.; Lobo, J.R.; Sengupta, S.; Chen, L.; et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc. Natl. Acad. Sci. USA 2004, 101, 17174–17179. [Google Scholar] [CrossRef]
- Garcia-Lora, A.; Algarra, I.; Garrido, F. MHC class I antigens, immune surveillance, and tumor immune escape. J. Cell. Physiol. 2003, 195, 346–355. [Google Scholar] [CrossRef]
- Conciatori, F.; Ciuffreda, L.; Bazzichetto, C.; Falcone, I.; Pilotto, S.; Bria, E.; Cognetti, F.; Milella, M. mTOR Cross-Talk in Cancer and Potential for Combination Therapy. Cancers 2018, 10, 23. [Google Scholar] [CrossRef]
- Basu, A.; Liu, T.; Banerjee, P.; Flynn, E.; Zurakowski, D.; Datta, D.; Viklicky, O.; Gasser, M.; Waaga-Gasser, A.M.; Yang, J.; et al. Effectiveness of a combination therapy using calcineurin inhibitor and mTOR inhibitor in preventing allograft rejection and post-transplantation renal cancer progression. Cancer Lett. 2012, 321, 179–186. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Bao, J.; Jia, X.; Liang, Y.; Wang, X.; Chen, M.; Su, H.; Li, P.; Wan, J.B.; et al. Synergistic chemopreventive effects of curcumin and berberine on human breast cancer cells through induction of apoptosis and autophagic cell death. Sci. Rep. 2016, 6, 26064. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabarwal, A.; Chakraborty, S.; Mahanta, S.; Banerjee, S.; Balan, M.; Pal, S. A Novel Combination Treatment with Honokiol and Rapamycin Effectively Restricts c-Met-Induced Growth of Renal Cancer Cells, and also Inhibits the Expression of Tumor Cell PD-L1 Involved in Immune Escape. Cancers 2020, 12, 1782. https://doi.org/10.3390/cancers12071782
Sabarwal A, Chakraborty S, Mahanta S, Banerjee S, Balan M, Pal S. A Novel Combination Treatment with Honokiol and Rapamycin Effectively Restricts c-Met-Induced Growth of Renal Cancer Cells, and also Inhibits the Expression of Tumor Cell PD-L1 Involved in Immune Escape. Cancers. 2020; 12(7):1782. https://doi.org/10.3390/cancers12071782
Chicago/Turabian StyleSabarwal, Akash, Samik Chakraborty, Simran Mahanta, Selina Banerjee, Murugabaskar Balan, and Soumitro Pal. 2020. "A Novel Combination Treatment with Honokiol and Rapamycin Effectively Restricts c-Met-Induced Growth of Renal Cancer Cells, and also Inhibits the Expression of Tumor Cell PD-L1 Involved in Immune Escape" Cancers 12, no. 7: 1782. https://doi.org/10.3390/cancers12071782
APA StyleSabarwal, A., Chakraborty, S., Mahanta, S., Banerjee, S., Balan, M., & Pal, S. (2020). A Novel Combination Treatment with Honokiol and Rapamycin Effectively Restricts c-Met-Induced Growth of Renal Cancer Cells, and also Inhibits the Expression of Tumor Cell PD-L1 Involved in Immune Escape. Cancers, 12(7), 1782. https://doi.org/10.3390/cancers12071782





