Effect of Tumor Burden on Tumor Aggressiveness and Immune Modulation in Prostate Cancer: Association with IL-6 Signaling
Abstract
1. Introduction
2. Materials and Methods
2.1. Tissue Specimens and Patient Characteristics
2.2. Immunohistochemical (IHC) Staining
2.3. Cell Culture and Reagents
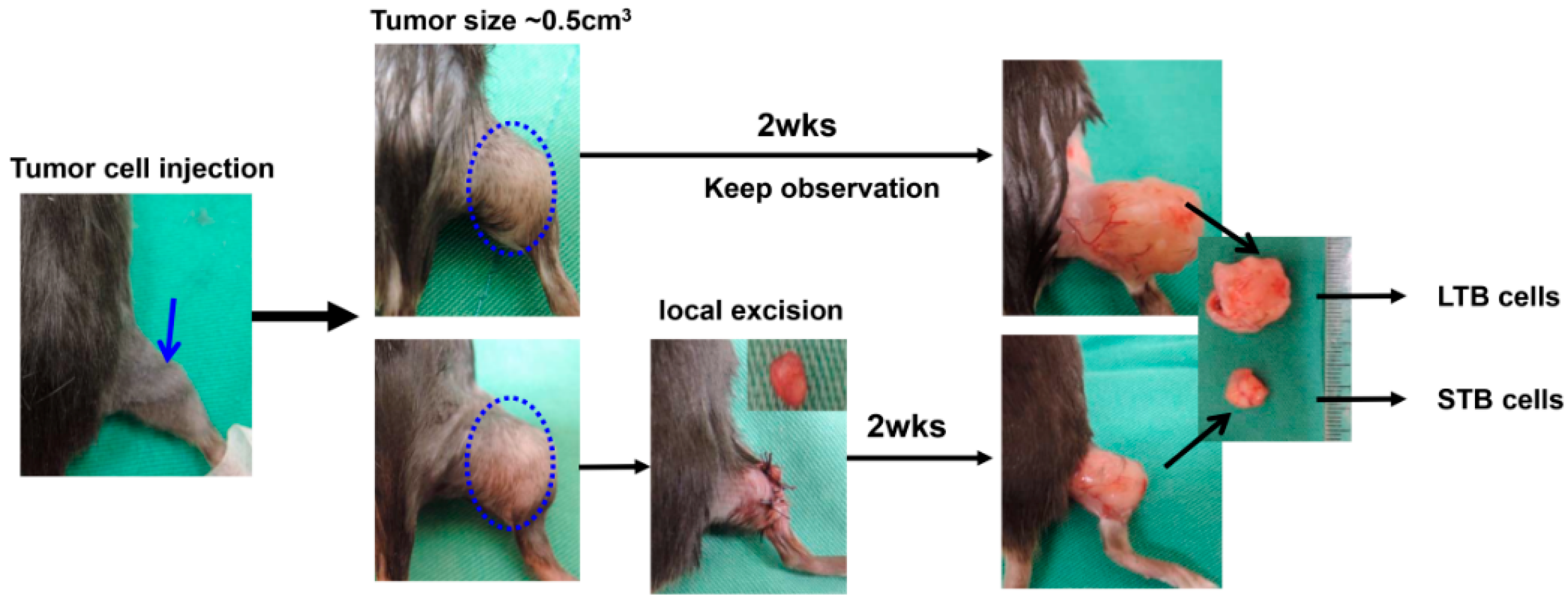
2.4. Mouse Tumor Models (Ectopic and Orthotopic)
2.5. Flow Cytometric Analyses of Myeloid-Derived Suppressor Cells (MDSCs)
2.6. Immunofluorescence (IF) Analyses of Tissue Specimens
2.7. Statistical Analysis
3. Results
3.1. Tumor Burden after Local Treatment Was Correlated with Tumor Aggressiveness
3.2. Role of CD44 in Tumor Aggressiveness
3.3. The Expression of CD44 Is Correlated with CSC and PD-L1 Expression
3.4. Role of IL-6 Signaling in the Expression of CD44 in Prostate Cancer
3.5. Role of IL-6 in Patients with Prostate Cancer
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LAPC | locally advanced prostate cancer |
| SFCs | sphere-forming cells |
| EMT | epithelial-mesenchymal transition |
| TIL | tumor-infiltrating lymphocytes |
| MDSC | myeloid-derived suppressor cells |
| CSCs | Cancer stem cells |
| CRPC | castration-resistant prostate cancer |
| ADT | androgen deprivation therapy |
Appendix A




References
- Attard, G.; Parker, C.; Eeles, R.A.; Schroder, F.; Tomlins, S.A.; Tannock, I.; Drake, C.G.; de Bono, J.S. Prostate cancer. Lancet 2016, 387, 70–82. [Google Scholar] [CrossRef]
- Baade, P.D.; Youlden, D.R.; Cramb, S.M.; Dunn, J.; Gardiner, R.A. Epidemiology of prostate cancer in the Asia-Pacific region. Prostate Int. 2013, 1, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Dall’Era, M.A.; Lo, M.J.; Chen, J.; Cress, R.; Hamilton, A.S. Nine-year prostate cancer survival differences between aggressive versus conservative therapy in men with advanced and metastatic prostate cancer. Cancer 2018, 124, 1921–1928. [Google Scholar] [CrossRef] [PubMed]
- Warde, P.; Mason, M.; Ding, K.; Kirkbride, P.; Brundage, M.; Cowan, R.; Gospodarowicz, M.; Sanders, K.; Kostashuk, E.; Swanson, G.; et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: A randomised, phase 3 trial. Lancet 2011, 378, 2104–2111. [Google Scholar] [CrossRef]
- Mason, M.D.; Parulekar, W.R.; Sydes, M.R.; Brundage, M.; Kirkbride, P.; Gospodarowicz, M.; Cowan, R.; Kostashuk, E.C.; Anderson, J.; Swanson, G.; et al. Final Report of the Intergroup Randomized Study of Combined Androgen-Deprivation Therapy Plus Radiotherapy Versus Androgen-Deprivation Therapy Alone in Locally Advanced Prostate Cancer. J. Clin. Oncol. 2015, 33, 2143–2150. [Google Scholar] [CrossRef] [PubMed]
- James, N.D.; Spears, M.R.; Clarke, N.W.; Dearnaley, D.P.; Mason, M.D.; Parker, C.C.; Ritchie, A.W.; Russell, J.M.; Schiavone, F.; Attard, G.; et al. Failure-Free Survival and Radiotherapy in Patients with Newly Diagnosed Nonmetastatic Prostate Cancer: Data from Patients in the Control Arm of the STAMPEDE Trial. JAMA Oncol. 2016, 2, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Satkunasivam, R.; Kim, A.E.; Desai, M.; Nguyen, M.M.; Quinn, D.I.; Ballas, L.; Lewinger, J.P.; Stern, M.C.; Hamilton, A.S.; Aron, M.; et al. Radical Prostatectomy or External Beam Radiation Therapy vs No Local Therapy for Survival Benefit in Metastatic Prostate Cancer: A SEER-Medicare Analysis. J. Urol. 2015, 194, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, O.; Khairul-Asri, M.G.; Hadi, S.; Gakis, G.; Stenzl, A. The Role of Radical Prostatectomy and Radiotherapy in Treatment of Locally Advanced Prostate Cancer: A Systematic Review and Meta-Analysis. Urol. Int. 2017, 99, 249–256. [Google Scholar] [CrossRef]
- Cifuentes, F.F.; Valenzuela, R.H.; Contreras, H.R.; Castellon, E.A. Surgical cytoreduction of the primary tumor reduces metastatic progression in a mouse model of prostate cancer. Oncol. Rep. 2015, 34, 2837–2844. [Google Scholar] [CrossRef]
- Won, A.C.; Gurney, H.; Marx, G.; De Souza, P.; Patel, M.I. Primary treatment of the prostate improves local palliation in men who ultimately develop castrate-resistant prostate cancer. BJU Int. 2013, 112, E250–E255. [Google Scholar] [CrossRef]
- Grinis, G.; Targonski, P.; Shaw, M.; Rubenstein, M.; Guinan, P.D. Cytoreductive surgery impedes metastasis and enhances the immune response: A preliminary report. J. Surg. Oncol. 1991, 48, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-J.; Ma, C.-G.; Ye, D.-W.; Yao, X.-D.; Zhang, S.-L.; Dai, B.; Zhang, H.-L.; Shen, Y.-J.; Zhu, Y.; Zhu, Y.-P.; et al. Tumor cytoreduction results in better response to androgen ablation—a preliminary report of palliative transurethral resection of the prostate in metastatic hormone sensitive prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2012, 30, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Bayne, C.E.; Williams, S.B.; Cooperberg, M.R.; Gleave, M.E.; Graefen, M.; Montorsi, F.; Novara, G.; Smaldone, M.C.; Sooriakumaran, P.; Wiklund, P.N.; et al. Treatment of the Primary Tumor in Metastatic Prostate Cancer: Current Concepts and Future Perspectives. Eur. Urol. 2016, 69, 775–787. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Vijayalekshmi, R.V.; Sung, B. Targeting inflammatory pathways for prevention and therapy of cancer: Short-term friend, long-term foe. Clin. Cancer Res. 2009, 15, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.T.; Brugge, J.S. IL-6 involvement in epithelial cancers. J. Clin. Investig. 2007, 117, 3660–3663. [Google Scholar] [CrossRef]
- Setrerrahmane, S.; Xu, H. Tumor-related interleukins: Old validated targets for new anti-cancer drug development. Mol. Cancer 2017, 16, 153. [Google Scholar] [CrossRef]
- Tsai, M.-S.; Chen, W.-C.; Lu, C.-H.; Chen, M.-F. The prognosis of head and neck squamous cell carcinoma related to immunosuppressive tumor microenvironment regulated by IL-6 signaling. Oral Oncol. 2019, 91, 47–55. [Google Scholar] [CrossRef]
- Wu, C.-T.; Hsieh, C.-C.; Lin, C.-C.; Chen, W.-C.; Hong, J.-H.; Chen, M.-F. Significance of IL-6 in the transition of hormone-resistant prostate cancer and the induction of myeloid-derived suppressor cells. J. Mol. Med. 2012, 90, 1343–1355. [Google Scholar] [CrossRef]
- Bharti, R.; Dey, G.; Mandal, M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: A snapshot of IL-6 mediated involvement. Cancer Lett. 2016, 375, 51–61. [Google Scholar] [CrossRef]
- Yu, S.C.; Bian, X.W. Enrichment of cancer stem cells based on heterogeneity of invasiveness. Stem. Cell Rev. 2009, 5, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Frank, N.Y.; Schatton, T.; Frank, M.H. The therapeutic promise of the cancer stem cell concept. J. Clin. Investig. 2010, 120, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhou, S.; Wang, L.; Wang, J.; Zou, Q.; Zhao, W.; Fu, Q.; Fang, X. Current Stem Cell Biomarkers and Their Functional Mechanisms in Prostate Cancer. Int. J. Mol. Sci. 2016, 17, 1163. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Rycaj, K.; Liu, X.; Tang, D.G. New insights into prostate cancer stem cells. Cell Cycle 2013, 12, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived-suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-Mesenchymal Transitions in Development and Disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.Y. Expression of CD44 in prostate cancer cells. Cancer Lett. 1994, 76, 63–69. [Google Scholar] [CrossRef]
- Zeisberg, M.; Neilson, E.G. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Investig. 2009, 119, 1429–1437. [Google Scholar] [CrossRef]
- Maccalli, C.; Volontè, A.; Cimminiello, C.; Parmiani, G. Immunology of cancer stem cells in solid tumours. A review. Eur. J. Cancer 2014, 50, 649–655. [Google Scholar] [CrossRef]
- Silver, D.J.; Sinyuk, M.; Vogelbaum, M.A.; Ahluwalia, M.S.; Lathia, J.D. The intersection of cancer, cancer stem cells, and the immune system: Therapeutic opportunities. Neuro Oncol. 2016, 18, 153–159. [Google Scholar] [CrossRef]
- Ricci-Vitiani, L.; Lombardi, D.G.; Pilozzi, E.; Biffoni, M.; Todaro, M.; Peschle, C.; De Maria, R. Identification and expansion of human colon-cancer-initiating cells. Nature 2007, 445, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Thiery, J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2002, 2, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Li, R.-M.; Kong, F.-M.; Li, H.; Yu, J.-P.; Ren, X.-B. How do tumor stem cells actively escape from host immunosurveillance? Biochem. Biophys. Res. Commun. 2012, 420, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Marotta, L.L.; Almendro, V.; Marusyk, A.; Shipitsin, M.; Schemme, J.; Walker, S.R.; Bloushtain-Qimron, N.; Kim, J.J.; Choudhury, S.A.; Maruyama, R.; et al. The JAK2/STAT3 signaling pathway is required for growth of CD44(+)CD24(-) stem cell-like breast cancer cells in human tumors. J. Clin. Investig. 2011, 121, 2723–2735. [Google Scholar] [CrossRef] [PubMed]
- Brady, J.J.; Li, M.; Suthram, S.; Jiang, H.; Wong, W.H.; Blau, H.M. Early role for IL-6 signalling during generation of induced pluripotent stem cells revealed by heterokaryon RNA-Seq. Nature 2013, 15, 1244–1252. [Google Scholar] [CrossRef] [PubMed]
- Marzec, M.; Zhang, Q.; Goradia, A.; Raghunath, P.N.; Liu, X.; Paessler, M.; Wang, H.Y.; Wysocka, M.; Cheng, M.; Ruggeri, B.A.; et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc. Natl. Acad. Sci. USA 2008, 105, 20852–20857. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, H.; Ohno, Y.; Toyoshima, Y.; Ohtake, J.; Homma, S.; Kawamura, H.; Takahashi, N.; Taketomi, A. Interleukin-6/STAT3 signaling as a promising target to improve the efficacy of cancer immunotherapy. Cancer Sci. 2017, 108, 1947–1952. [Google Scholar] [CrossRef]
- Löppenberg, B.; Dalela, D.; Karabon, P.; Sood, A.; Sammon, J.D.; Meyer, C.P.; Sun, M.; Noldus, J.; Peabody, J.O.; Trinh, Q.-D.; et al. The Impact of Local Treatment on Overall Survival in Patients with Metastatic Prostate Cancer on Diagnosis: A National Cancer Data Base Analysis. Eur. Urol. 2017, 72, 14–19. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Sampieri, K.; Fodde, R. Cancer stem cells and metastasis. Semin. Cancer Biol. 2012, 22, 187–193. [Google Scholar] [CrossRef]
- Ajani, J.A.; Song, S.; Hochster, H.S.; Steinberg, I.B. Cancer stem cells: The promise and the potential. Semin. Oncol. 2015, 42 (Suppl. 1), S3–S17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Huang, X.; Zheng, X.; Wang, X.; Li, S.; Zhang, L.; Yang, Z.; Xia, Z. Enrichment of Prostate Cancer Stem-Like Cells from Human Prostate Cancer Cell Lines by Culture in Serum-Free Medium and Chemoradiotherapy. Int. J. Boil. Sci. 2013, 9, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Ghiso, J.A. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 2007, 7, 834–846. [Google Scholar] [CrossRef] [PubMed]






| Prostate Cancer IHC Staining | No. of Patients | p Value | |
|---|---|---|---|
| IL-6 (−) | IL-6 (+) | ||
| Number | 100 | 67 | |
| Age | 0.73 | ||
| Median | 72.3 | 73.1 | |
| Range | 48~83 | 47~85 | |
| T stage | <0.001 * | ||
| T1–T2 | 83 | 23 | |
| T3–T4 | 17 | 44 | |
| Gleason score | <0.001 * | ||
| <7 | 54 | 14 | |
| ≥7 | 46 | 53 | |
| LN and/or distant | <0.001 * | ||
| Negative | 94 | 49 | |
| Positive | 6 | 18 | |
| Pre-Tx PSA | <0.001 * | ||
| <20 | 66 | 19 | |
| ≥20 | 34 | 48 | |
| NLR | |||
| <3 | 73 | 15 | <0.001 * |
| ≥3 | 12 | 41 | |
| Unknown | 15 | 11 | |
| Survival status | |||
| Alive | 90 | 55 | 0.11 |
| Dead | 10 | 12 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-T.; Huang, Y.-C.; Chen, W.-C.; Chen, M.-F. Effect of Tumor Burden on Tumor Aggressiveness and Immune Modulation in Prostate Cancer: Association with IL-6 Signaling. Cancers 2019, 11, 992. https://doi.org/10.3390/cancers11070992
Wu C-T, Huang Y-C, Chen W-C, Chen M-F. Effect of Tumor Burden on Tumor Aggressiveness and Immune Modulation in Prostate Cancer: Association with IL-6 Signaling. Cancers. 2019; 11(7):992. https://doi.org/10.3390/cancers11070992
Chicago/Turabian StyleWu, Chun-Te, Yun-Ching Huang, Wen-Cheng Chen, and Miao-Fen Chen. 2019. "Effect of Tumor Burden on Tumor Aggressiveness and Immune Modulation in Prostate Cancer: Association with IL-6 Signaling" Cancers 11, no. 7: 992. https://doi.org/10.3390/cancers11070992
APA StyleWu, C.-T., Huang, Y.-C., Chen, W.-C., & Chen, M.-F. (2019). Effect of Tumor Burden on Tumor Aggressiveness and Immune Modulation in Prostate Cancer: Association with IL-6 Signaling. Cancers, 11(7), 992. https://doi.org/10.3390/cancers11070992




