6-Thioguanine and Its Analogs Promote Apoptosis of Castration-Resistant Prostate Cancer Cells in a BRCA2-Dependent Manner
Abstract
1. Introduction
2. Results
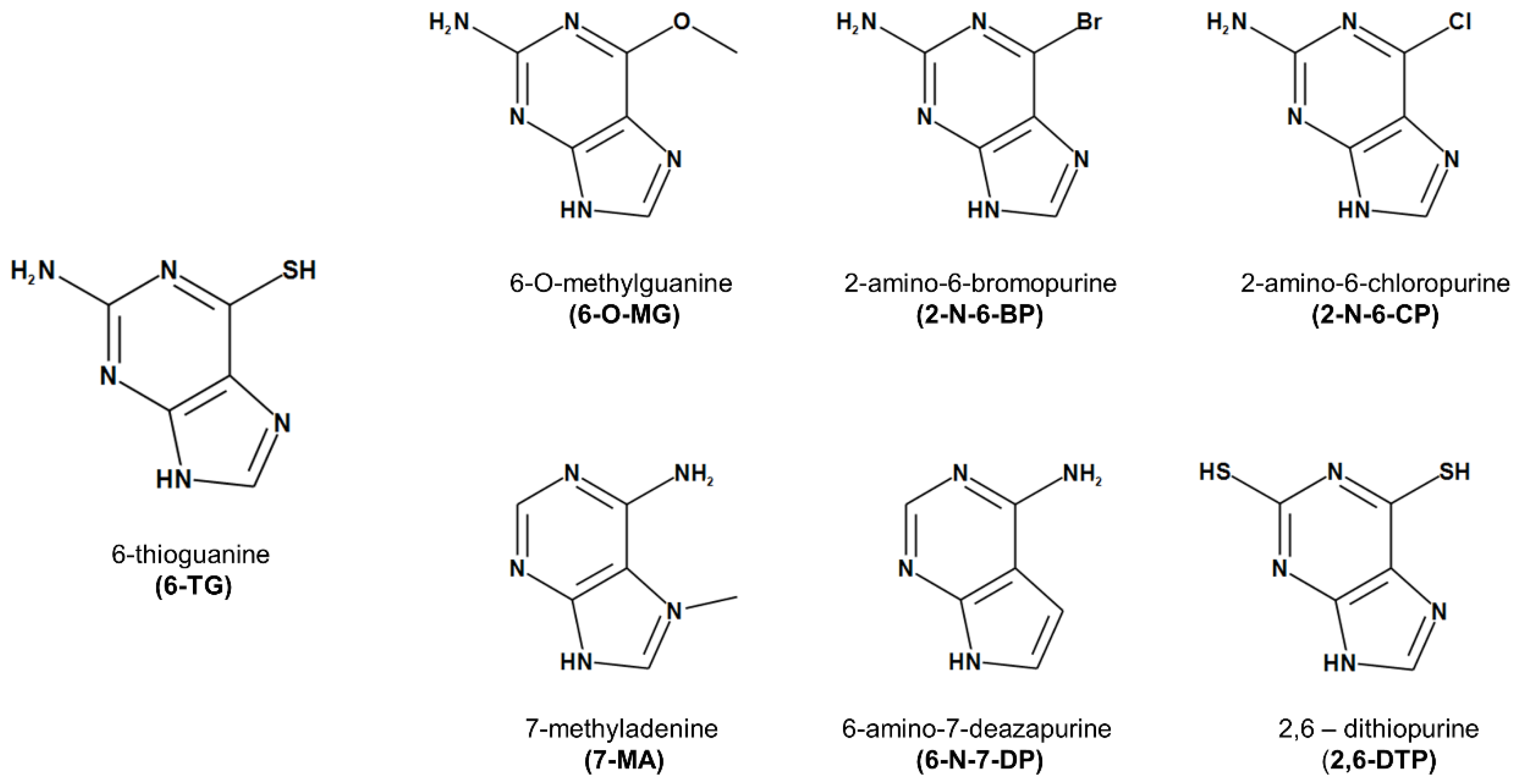
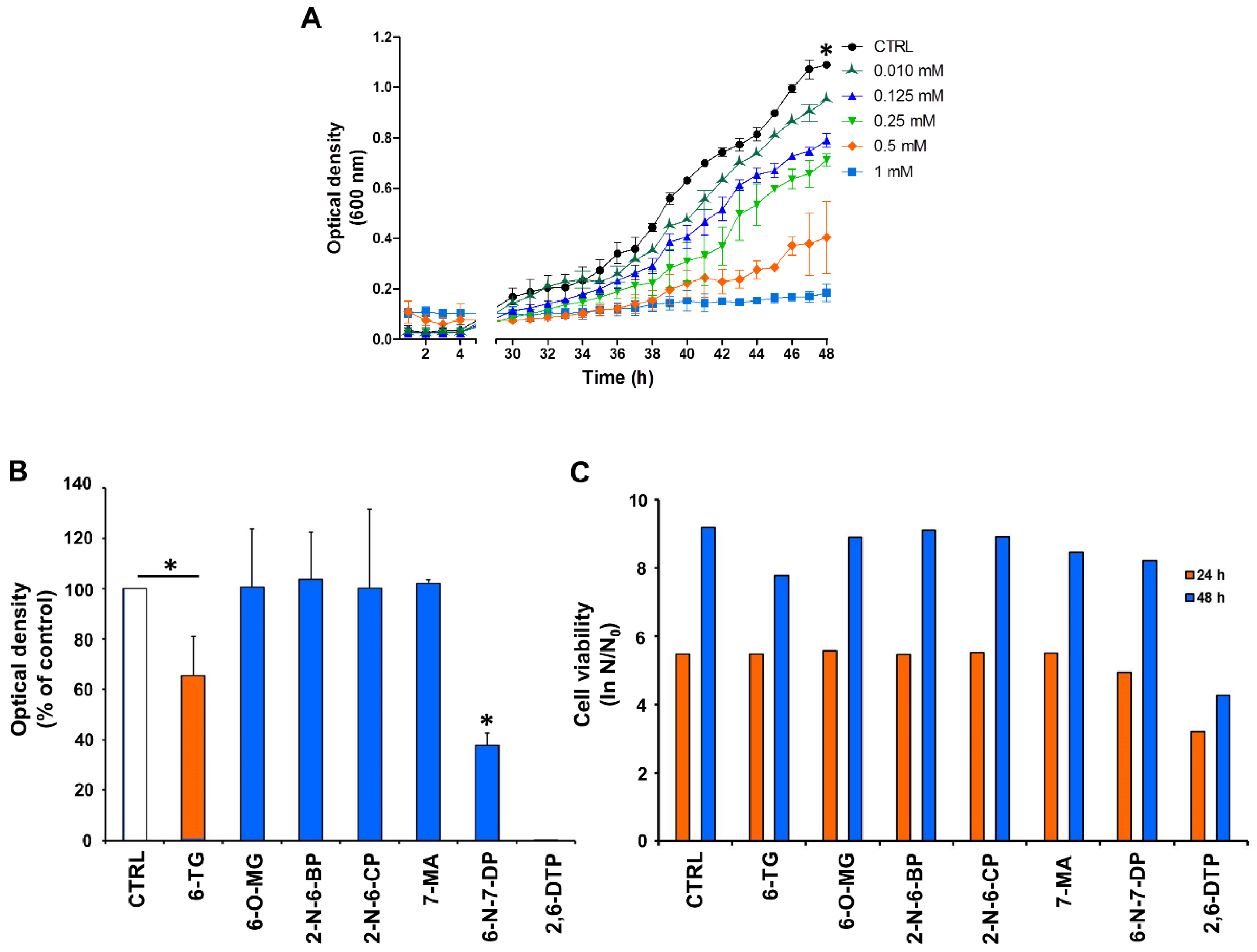
2.1. Effect of 6-TG and Its Analogues on Yeast Cell Growth and Viability
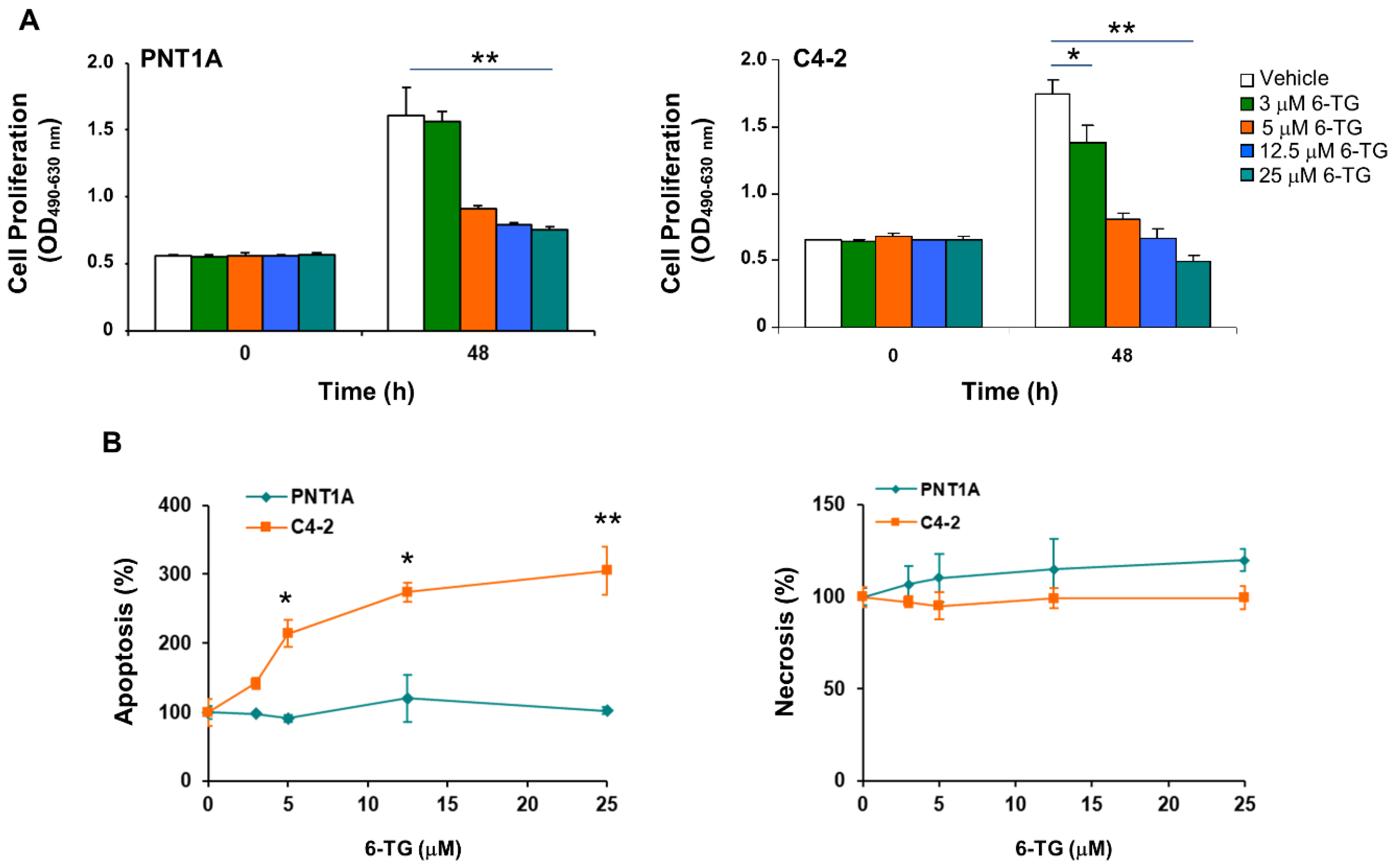
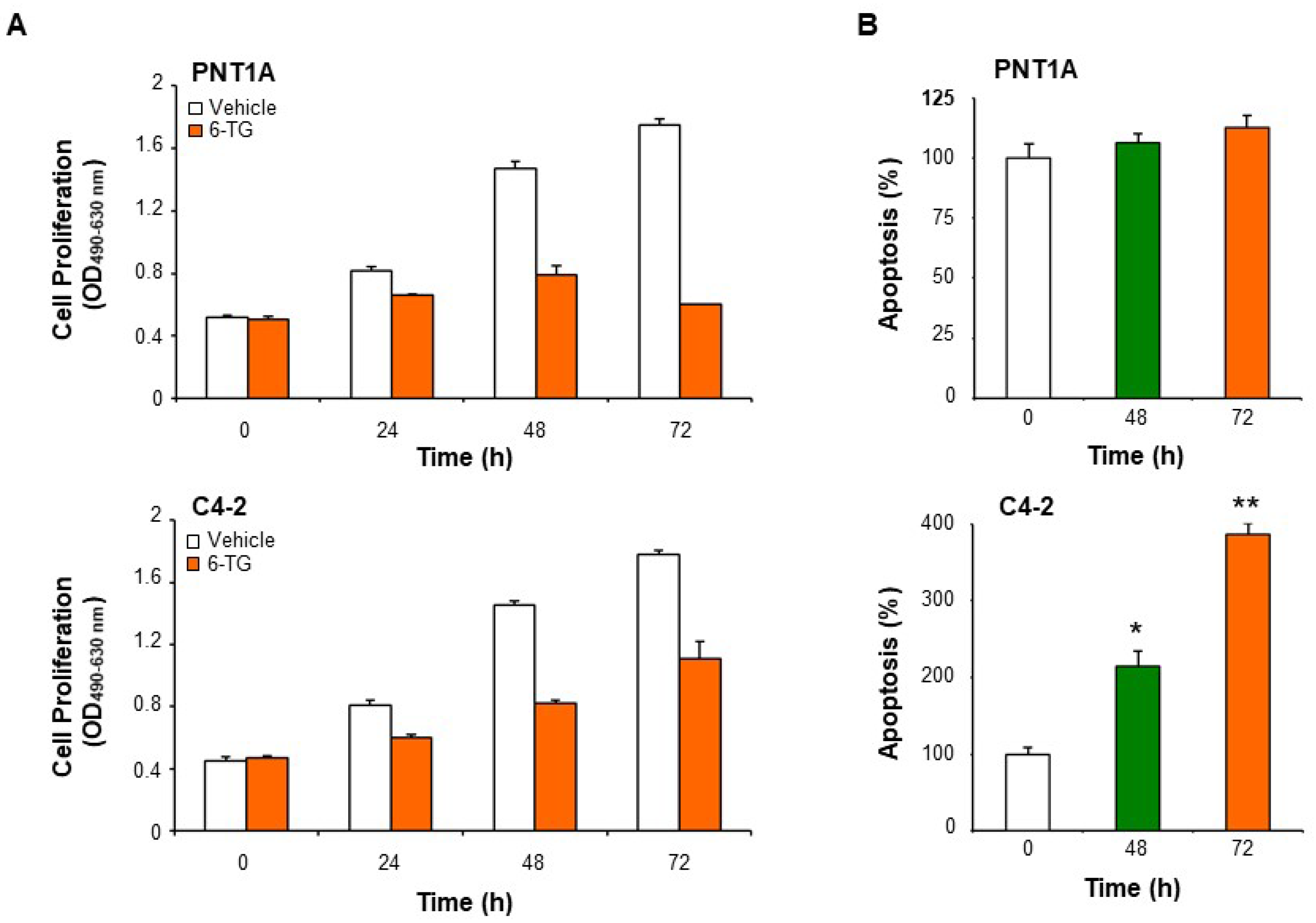
2.2. Anti-Proliferative and Pro-Apoptotic Effect of 6-TG in Castration-Resistant Prostate Cancer Cells
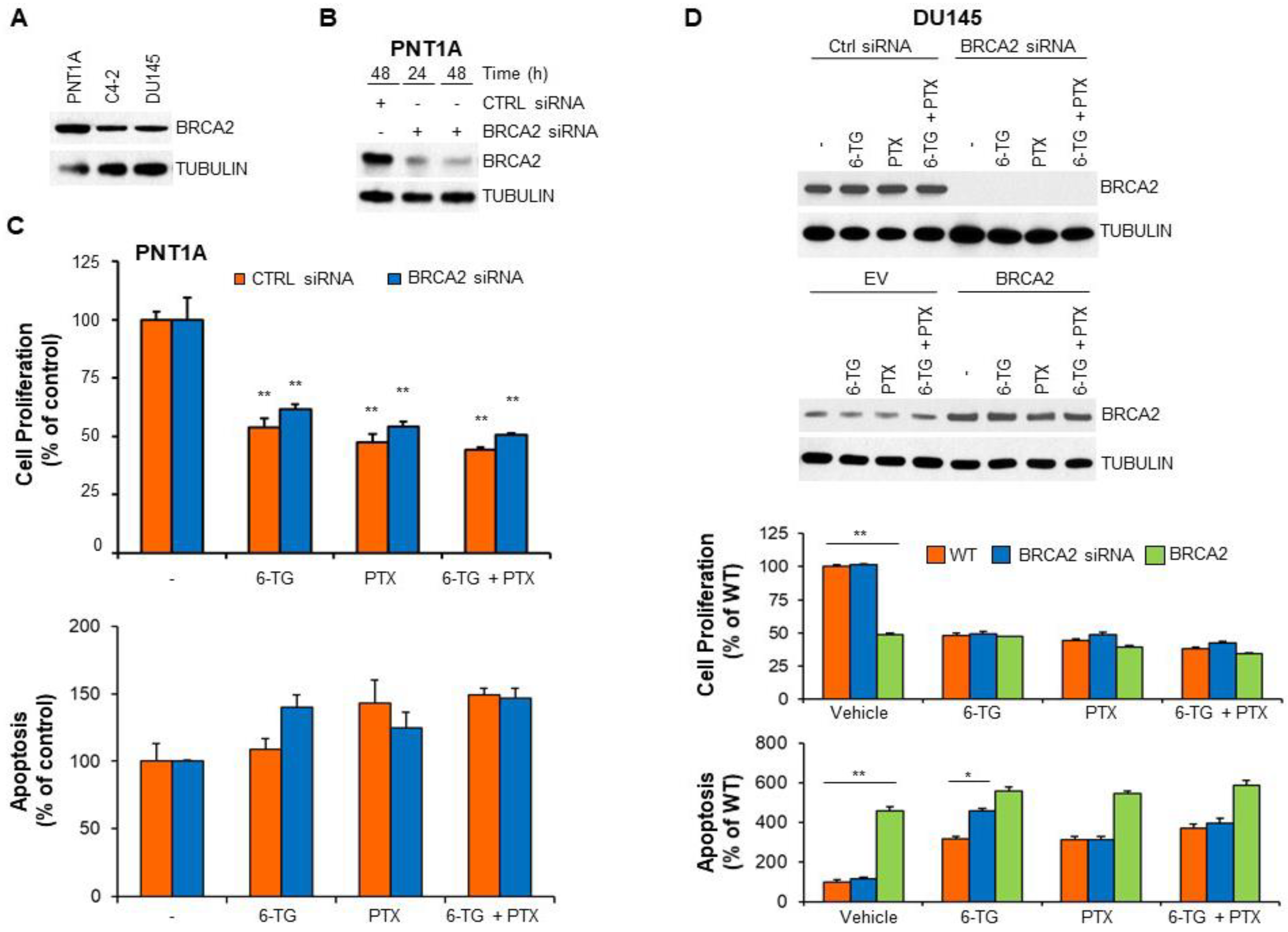
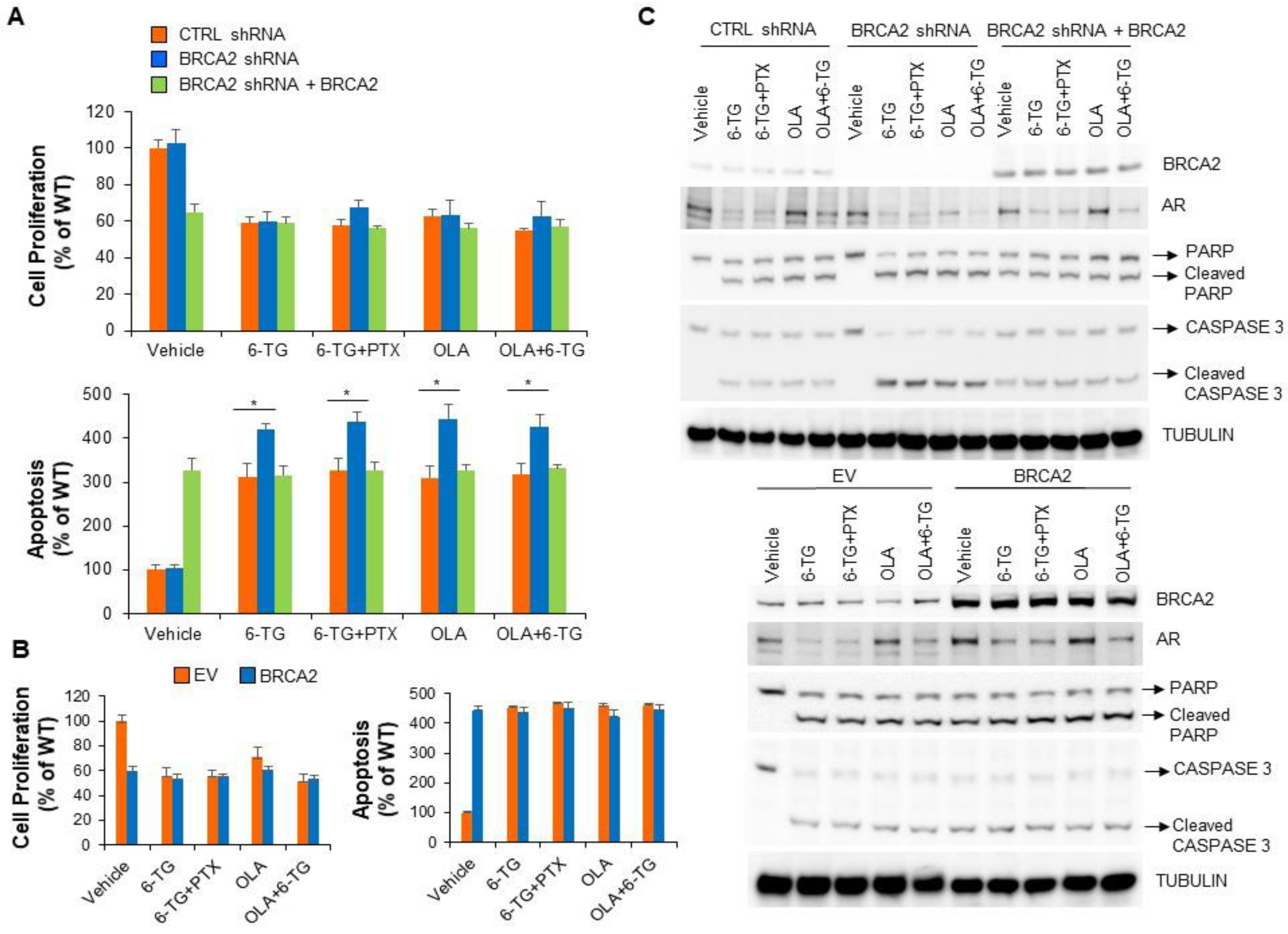
2.3. BRCA2 Levels Affect the Sensitivity of Prostate Cancer Cells to 6-TG
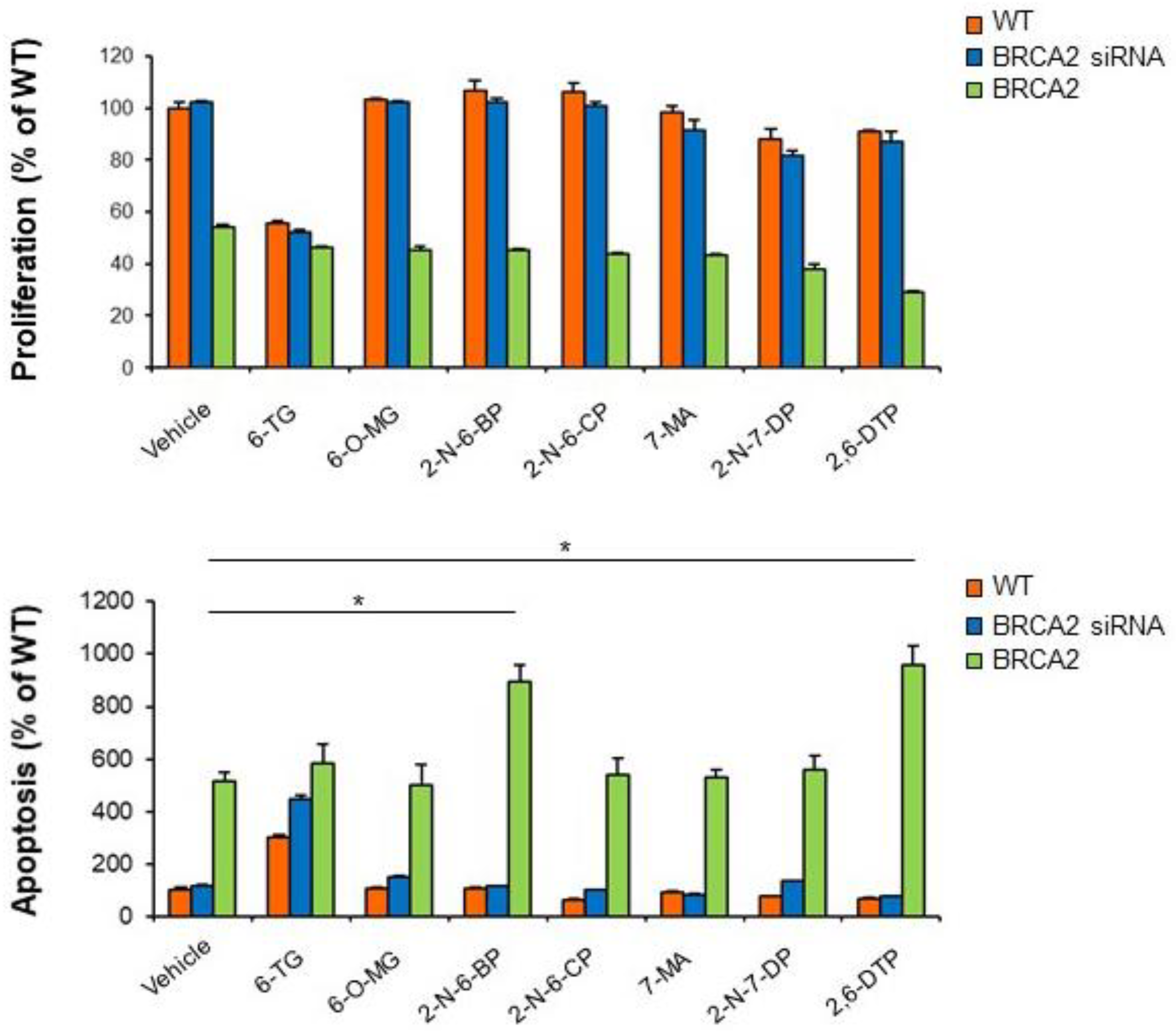
2.4. BRCA2 Expression Promotes Prostate Cancer Cell Sensitivity to 2-N-6-BP and 2,6-DTP
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Cell Lines and Yeast Cell Cultures
4.3. Cell Cytotoxicity Tests
4.3.1. Cell Proliferation and Cell Death Assay
4.3.2. Yeast Growth and Cell Viability Assays
4.4. Immunoblotting
4.5. Transient and Stable Transfection
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jin, J.K.; Dayyani, F.; Gallick, G.E. Steps in prostate cancer progression that lead to bone metastasis. Int. J. Cancer 2011, 128, 2545–2561. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, M.E.; Soung, P.; Perera, S.; Kaplan, I.; Loda, M.; Sellers, W.R. Loss of PTEN expression in paraffin-embedded primary prostate cancer correlates with high Gleason score and advanced stage. Cancer Res. 1999, 59, 4291–4296. [Google Scholar] [PubMed]
- Visakorpi, T.; Hyytinen, E.; Koivisto, P.; Tanner, M.; Keinanen, R.; Palmberg, C.; Palotie, A.; Tammela, T.; Isola, J.; Kallioniemi, O.P. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat. Genet. 1995, 9, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Quigley, D.A.; Dang, H.X.; Zhao, S.G.; Lloyd, P.; Aggarwal, R.; Alumkal, J.J.; Foye, A.; Kothari, V.; Perry, M.D.; Bailey, A.M.; et al. Genomic Hallmarks and Structural Variation in Metastatic Prostate Cancer. Cell 2018, 174, 758–769. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Di Santi, A.; Cernera, G.; Rossi, V.; Abbondanza, C.; Moncharmont, B.; Sinisi, A.A.; et al. Prostate cancer stem cells: The role of androgen and estrogen receptors. Oncotarget 2016, 7, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Cassinello, J.; Carballido Rodriguez, J.; Anton Aparicio, L. Role of taxanes in advanced prostate cancer. Clin. Transl. Oncol. 2016, 18, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Caffo, O.; Veccia, A.; Kinspergher, S.; Rizzo, M.; Maines, F. Aberrations of DNA Repair Pathways in Prostate Cancer: Future Implications for Clinical Practice? Front. Cell Dev. Biol. 2018, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Bonkhoff, H. Estrogen receptor signaling in prostate cancer: Implications for carcinogenesis and tumor progression. Prostate 2018, 78, 2–10. [Google Scholar] [CrossRef]
- Martin, A.M.; Blackwood, M.A.; Antin-Ozerkis, D.; Shih, H.A.; Calzone, K.; Colligon, T.A.; Seal, S.; Collins, N.; Stratton, M.R.; Weber, B.L.; et al. Germline mutations in BRCA1 and BRCA2 in breast-ovarian families from a breast cancer risk evaluation clinic. J. Clin. Clin. Oncol. 2001, 19, 2247–2253. [Google Scholar] [CrossRef]
- Breast Cancer Linkage, C. Cancer risks in BRCA2 mutation carriers. J. Natl. Cancer Inst. 1999, 91, 1310–1316. [Google Scholar] [CrossRef]
- Thorslund, T.; West, S.C. BRCA2: A universal recombinase regulator. Oncogene 2007, 26, 7720–7730. [Google Scholar] [CrossRef] [PubMed]
- Guaragnella, N.; Marra, E.; Galli, A.; Moro, L.; Giannattasio, S. Silencing of BRCA2 decreases anoikis and its heterologous expression sensitizes yeast cells to acetic acid-induced programmed cell death. Apoptosis 2014, 19, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; San Juan, B.P.; Lim, E.; Weinberg, R.A. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016, 35, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Tryggvadottir, L.; Vidarsdottir, L.; Thorgeirsson, T.; Jonasson, J.G.; Olafsdottir, E.J.; Olafsdottir, G.H.; Rafnar, T.; Thorlacius, S.; Jonsson, E.; Eyfjord, J.E.; et al. Prostate cancer progression and survival in BRCA2 mutation carriers. J. Natl. Cancer Inst. 2007, 99, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.M.; Evans, D.G.; Hope, Q.; Norman, A.R.; Barbachano, Y.; Bullock, S.; Kote-Jarai, Z.; Meitz, J.; Falconer, A.; Osin, P.; et al. Prostate cancer in BRCA2 germline mutation carriers is associated with poorer prognosis. Br. J. Cancer 2010, 103, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, E.K.; Page, E.C.; Castro, E.; Lilja, H.; Vickers, A.; Sjoberg, D.; Assel, M.; Foster, C.S.; Mitchell, G.; Drew, K.; et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: Results from the initial screening round of the IMPACT study. Eur. Urol. 2014, 66, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.J.; Gaudet, M.M.; Pal, P.; Kirchhoff, T.; Balistreri, L.; Vora, K.; Bhatia, J.; Stadler, Z.; Fine, S.W.; Reuter, V.; et al. Germline BRCA mutations denote a clinicopathologic subset of prostate cancer. Clin. Cancer Res. 2010, 16, 2115–2121. [Google Scholar] [CrossRef]
- Castro, E.; Goh, C.; Leongamornlert, D.; Saunders, E.; Tymrakiewicz, M.; Dadaev, T.; Govindasami, K.; Guy, M.; Ellis, S.; Frost, D.; et al. Effect of BRCA Mutations on Metastatic Relapse and Cause-specific Survival After Radical Treatment for Localised Prostate Cancer. Eur. Urol. 2015, 68, 186–193. [Google Scholar] [CrossRef]
- Akbari, M.R.; Wallis, C.J.; Toi, A.; Trachtenberg, J.; Sun, P.; Narod, S.A.; Nam, R.K. The impact of a BRCA2 mutation on mortality from screen-detected prostate cancer. Br. J. Cancer 2014, 111, 1238–1240. [Google Scholar] [CrossRef]
- Pritchard, C.C.; Mateo, J.; Walsh, M.F.; De Sarkar, N.; Abida, W.; Beltran, H.; Garofalo, A.; Gulati, R.; Carreira, S.; Eeles, R.; et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N. Engl. J. Med. 2016, 375, 443–453. [Google Scholar] [CrossRef]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, K.K.; Swisher, E.M.; Taniguchi, T. Secondary mutations of BRCA1/2 and drug resistance. Cancer Sci. 2011, 102, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A. Drug resistance caused by reversion mutation. Cancer Res. 2008, 68, 10021–10023. [Google Scholar] [CrossRef] [PubMed]
- Elgemeie, G.H. Thioguanine, mercaptopurine: Their analogs and nucleosides as antimetabolites. Curr. Pharm. Des. 2003, 9, 2627–2642. [Google Scholar] [CrossRef] [PubMed]
- Munshi, P.N.; Lubin, M.; Bertino, J.R. 6-thioguanine: A drug with unrealized potential for cancer therapy. Oncologist 2014, 19, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Choi, Y.S.; Song, J.H.; Choi, E.A.; Park, S.; Lee, E.J.; Rhee, J.K.; Kim, S.C.; Chang, S. A drug-repositioning screen for primary pancreatic ductal adenocarcinoma cells identifies 6-thioguanine as an effective therapeutic agent for TPMT-low cancer cells. Mol. Oncol. 2018, 12, 1526–1539. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.J.; Cheng, S.C.; Tang, H.C.; Sun, C.Y.; Chou, C.Y. 6-Thioguanine is a noncompetitive and slow binding inhibitor of human deubiquitinating protease USP2. Sci. Rep. 2018, 8, 3102. [Google Scholar] [CrossRef] [PubMed]
- Guaragnella, N.; Palermo, V.; Galli, A.; Moro, L.; Mazzoni, C.; Giannattasio, S. The expanding role of yeast in cancer research and diagnosis: Insights into the function of the oncosuppressors p53 and BRCA1/2. FEMS Yeast Res. 2014, 14, 2–16. [Google Scholar] [CrossRef]
- Ferreira, R.; Limeta, A.; Nielsen, J. Tackling Cancer with Yeast-Based Technologies. Trends Biotechnol. 2018. [Google Scholar] [CrossRef]
- Spugnesi, L.; Balia, C.; Collavoli, A.; Falaschi, E.; Quercioli, V.; Caligo, M.A.; Galli, A. Effect of the expression of BRCA2 on spontaneous homologous recombination and DNA damage-induced nuclear foci in Saccharomyces cerevisiae. Mutagenesis 2013, 28, 187–195. [Google Scholar] [CrossRef]
- Veldscholte, J.; Berrevoets, C.A.; Ris-Stalpers, C.; Kuiper, G.G.; Jenster, G.; Trapman, J.; Brinkmann, A.O.; Mulder, E. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J. Steroid Biochem. Mol. Biol. 1992, 41, 665–669. [Google Scholar] [CrossRef]
- Alimirah, F.; Chen, J.; Basrawala, Z.; Xin, H.; Choubey, D. DU-145 and PC-3 human prostate cancer cell lines express androgen receptor: Implications for the androgen receptor functions and regulation. FEBS Lett. 2006, 580, 2294–2300. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Portnoy, D.C.; Wang, H.; Jiang, X.; Chen, S.; Balk, S.P. Androgen receptor expression in prostate cancer cells is suppressed by activation of epidermal growth factor receptor and ErbB2. Cancer Res. 2009, 69, 5202–5209. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.M.; LaSpina, M.; Long, J.; Ho, S.M. Expression of estrogen receptor (ER)-alpha and ER-beta in normal and malignant prostatic epithelial cells: Regulation by methylation and involvement in growth regulation. Cancer Res. 2000, 60, 3175–3182. [Google Scholar] [PubMed]
- Hu, Q.; Zhang, B.; Chen, R.; Fu, C.; A, J.; Fu, X.; Li, J.; Fu, L.; Zhang, Z.; Dong, J.T. ZFHX3 is indispensable for ERbeta to inhibit cell proliferation via MYC downregulation in prostate cancer cells. Oncogenesis 2019, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.N.; Koo, K.H.; Sung, J.Y.; Yun, U.J.; Kim, H. Anoikis resistance: An essential prerequisite for tumor metastasis. Int. J. Cell. Biol. 2012, 2012, 306879. [Google Scholar] [CrossRef] [PubMed]
- Arbini, A.A.; Greco, M.; Yao, J.L.; Bourne, P.; Marra, E.; Hsieh, J.T.; di Sant’agnese, P.A.; Moro, L. Skp2 overexpression is associated with loss of BRCA2 protein in human prostate cancer. Am. J. Pathol. 2011, 178, 2367–2376. [Google Scholar] [CrossRef] [PubMed]
- Issaeva, N.; Thomas, H.D.; Djureinovic, T.; Jaspers, J.E.; Stoimenov, I.; Kyle, S.; Pedley, N.; Gottipati, P.; Zur, R.; Sleeth, K.; et al. 6-thioguanine selectively kills BRCA2-defective tumors and overcomes PARP inhibitor resistance. Cancer Res. 2010, 70, 6268–6276. [Google Scholar] [CrossRef]
- Chaabane, W.; Appell, M.L. Interconnections between apoptotic and autophagic pathways during thiopurine-induced toxicity in cancer cells: The role of reactive oxygen species. Oncotarget 2016, 7, 75616–75634. [Google Scholar] [CrossRef]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008, 68, 1777–1785. [Google Scholar] [CrossRef]
- Bertino, J.R.; Waud, W.R.; Parker, W.B.; Lubin, M. Targeting tumors that lack methylthioadenosine phosphorylase (MTAP) activity: Current strategies. Cancer Biol. Ther. 2011, 11, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Bistulfi, G.; Affronti, H.C.; Foster, B.A.; Karasik, E.; Gillard, B.; Morrison, C.; Mohler, J.; Phillips, J.G.; Smiraglia, D.J. The essential role of methylthioadenosine phosphorylase in prostate cancer. Oncotarget 2016, 7, 14380–14393. [Google Scholar] [CrossRef] [PubMed]
- Fridlich, R.; Annamalai, D.; Roy, R.; Bernheim, G.; Powell, S.N. BRCA1 and BRCA2 protect against oxidative DNA damage converted into double-strand breaks during DNA replication. DNA Repair 2015, 30, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Zhang, H.; Savarese, T.M. Gene deletion chemoselectivity: Codeletion of the genes for p16(INK4), methylthioadenosine phosphorylase, and the alpha- and beta-interferons in human pancreatic cell carcinoma lines and its implications for chemotherapy. Cancer Res. 1996, 56, 1083–1090. [Google Scholar] [PubMed]
- Banda, K.; Swisher, E.M.; Wu, D.; Pritchard, C.C.; Gadi, V.K. Somatic Reversion of Germline BRCA2 Mutation Confers Resistance to Poly(ADP-ribose) Polymerase Inhibitor Therapy. JCO Precis. Oncol. 2018. [Google Scholar] [CrossRef]
- Cheng, H.H.; Salipante, S.J.; Nelson, P.S.; Montgomery, B.; Pritchard, C.C. Polyclonal BRCA2 Reversion Mutations Detected in Circulating Tumor DNA After Platinum Chemotherapy in a Patient With Metastatic Prostate Cancer. JCO Precis. Oncol. 2018. [Google Scholar] [CrossRef]
- Carneiro, B.A.; Collier, K.A.; Nagy, R.J.; Pamarthy, S.; Sagar, V.; Fairclough, S.; Odegaard, J.; Lanman, R.B.; Costa, R.; Taxter, T.; et al. Acquired Resistance to Poly (ADP-ribose) Polymerase Inhibitor Olaparib in BRCA2-Associated Prostate Cancer Resulting From Biallelic BRCA2 Reversion Mutations Restores Both Germline and Somatic Loss-of-Function Mutations. JCO Precis. Oncol. 2018. [Google Scholar] [CrossRef]
- Reyes-Turcu, F.E.; Ventii, K.H.; Wilkinson, K.D. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu. Rev. Biochem. 2009, 78, 363–397. [Google Scholar] [CrossRef]
- Schoenfeld, A.R.; Apgar, S.; Dolios, G.; Wang, R.; Aaronson, S.A. BRCA2 is ubiquitinated in vivo and interacts with USP11, a deubiquitinating enzyme that exhibits prosurvival function in the cellular response to DNA damage. Mol. Cell. Biol. 2004, 24, 7444–7455. [Google Scholar] [CrossRef]
- Foland, T.B.; Dentler, W.L.; Suprenant, K.A.; Gupta, M.L., Jr.; Himes, R.H. Paclitaxel-induced microtubule stabilization causes mitotic block and apoptotic-like cell death in a paclitaxel-sensitive strain of Saccharomyces cerevisiae. Yeast 2005, 22, 971–978. [Google Scholar] [CrossRef]
- Arbini, A.A.; Guerra, F.; Greco, M.; Marra, E.; Gandee, L.; Xiao, G.; Lotan, Y.; Gasparre, G.; Hsieh, J.T.; Moro, L. Mitochondrial DNA depletion sensitizes cancer cells to PARP inhibitors by translational and post-translational repression of BRCA2. Oncogenesis 2013, 2, e82. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laera, L.; Guaragnella, N.; Giannattasio, S.; Moro, L. 6-Thioguanine and Its Analogs Promote Apoptosis of Castration-Resistant Prostate Cancer Cells in a BRCA2-Dependent Manner. Cancers 2019, 11, 945. https://doi.org/10.3390/cancers11070945
Laera L, Guaragnella N, Giannattasio S, Moro L. 6-Thioguanine and Its Analogs Promote Apoptosis of Castration-Resistant Prostate Cancer Cells in a BRCA2-Dependent Manner. Cancers. 2019; 11(7):945. https://doi.org/10.3390/cancers11070945
Chicago/Turabian StyleLaera, Luna, Nicoletta Guaragnella, Sergio Giannattasio, and Loredana Moro. 2019. "6-Thioguanine and Its Analogs Promote Apoptosis of Castration-Resistant Prostate Cancer Cells in a BRCA2-Dependent Manner" Cancers 11, no. 7: 945. https://doi.org/10.3390/cancers11070945
APA StyleLaera, L., Guaragnella, N., Giannattasio, S., & Moro, L. (2019). 6-Thioguanine and Its Analogs Promote Apoptosis of Castration-Resistant Prostate Cancer Cells in a BRCA2-Dependent Manner. Cancers, 11(7), 945. https://doi.org/10.3390/cancers11070945






