Discrimination of Head and Neck Squamous Cell Carcinoma Patients and Healthy Adults by 10-Color Flow Cytometry: Development of a Score Based on Leukocyte Subsets
Abstract
1. Introduction
2. Results
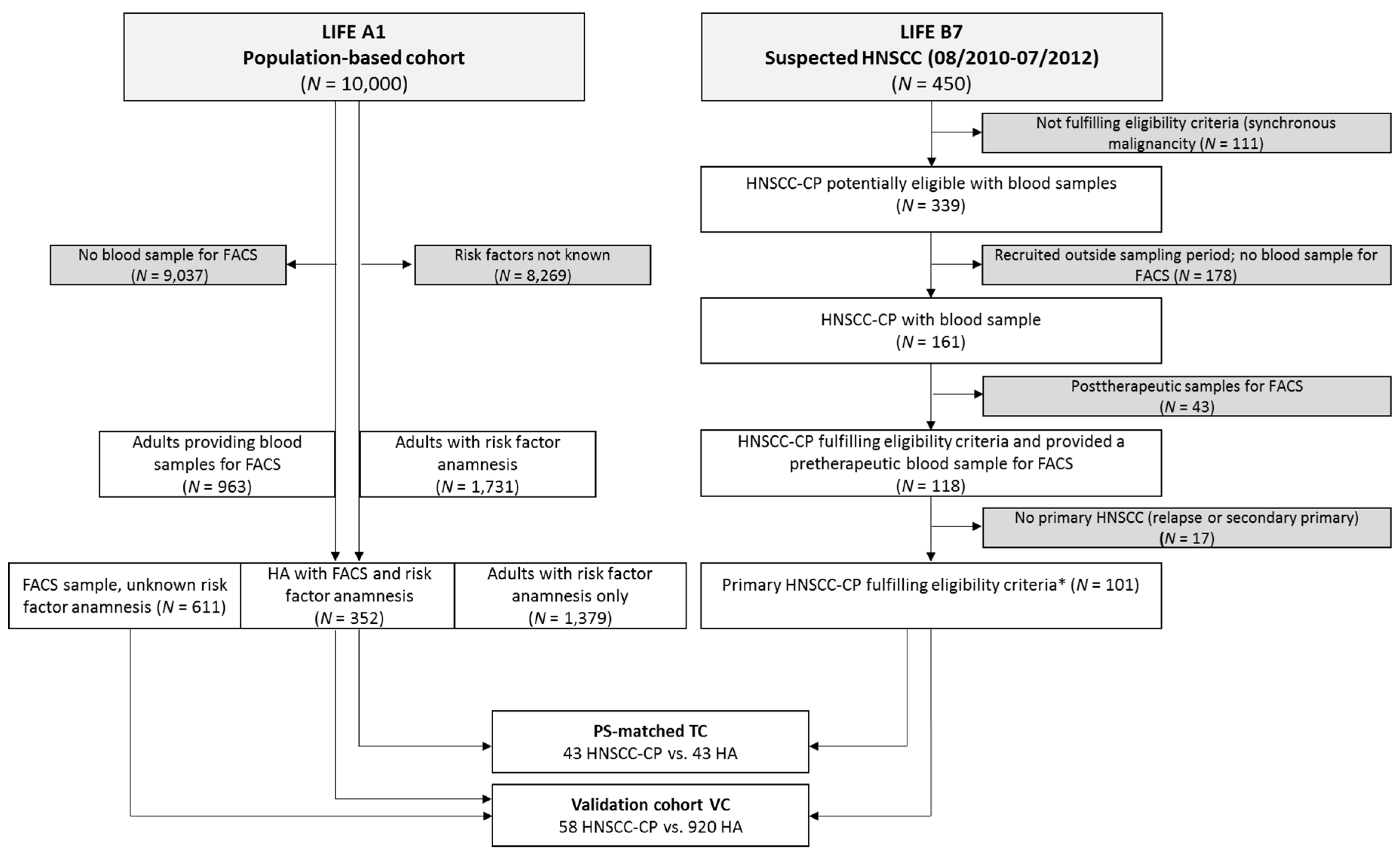
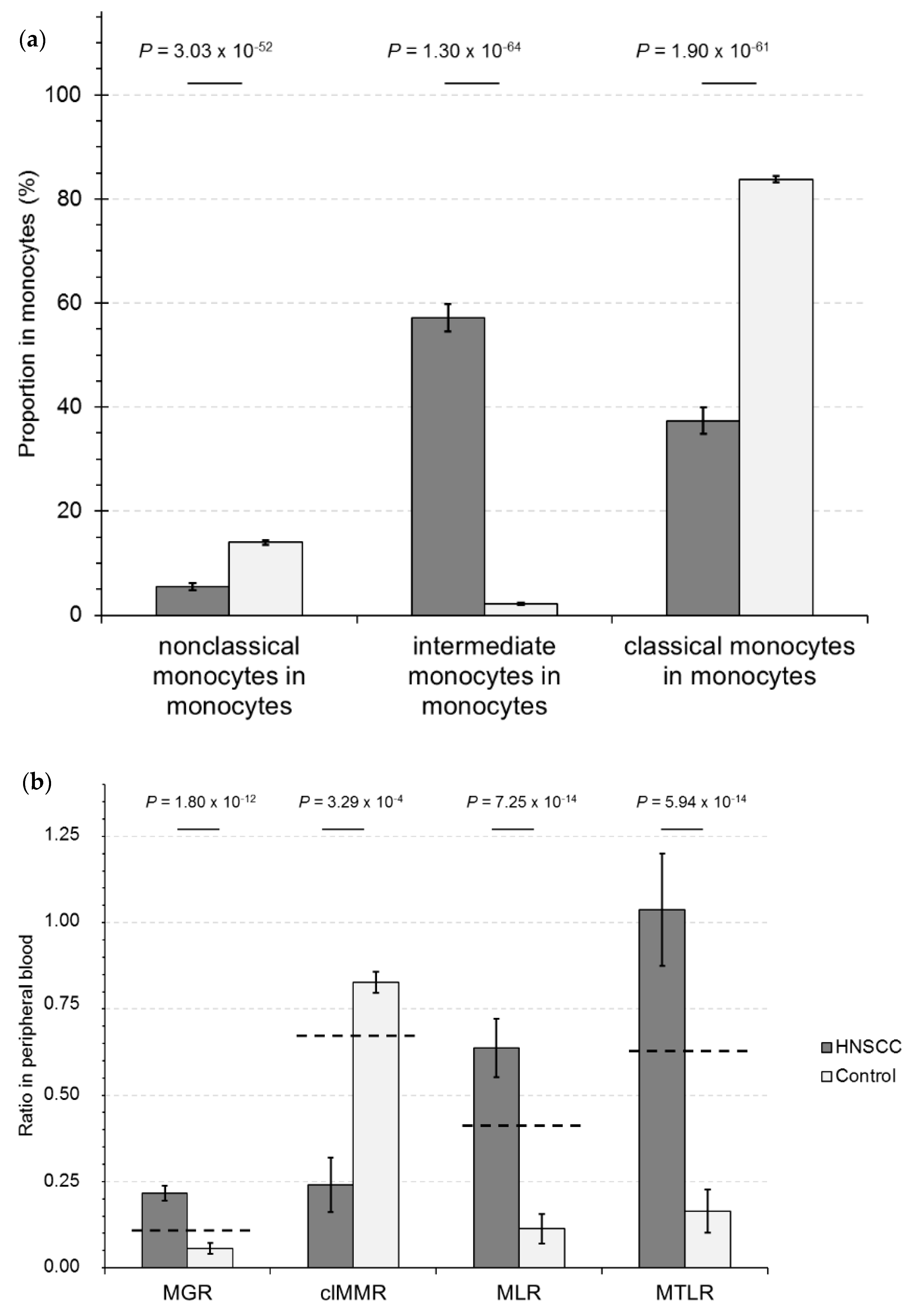
2.1. Calculation of RelCC and PS-Matching
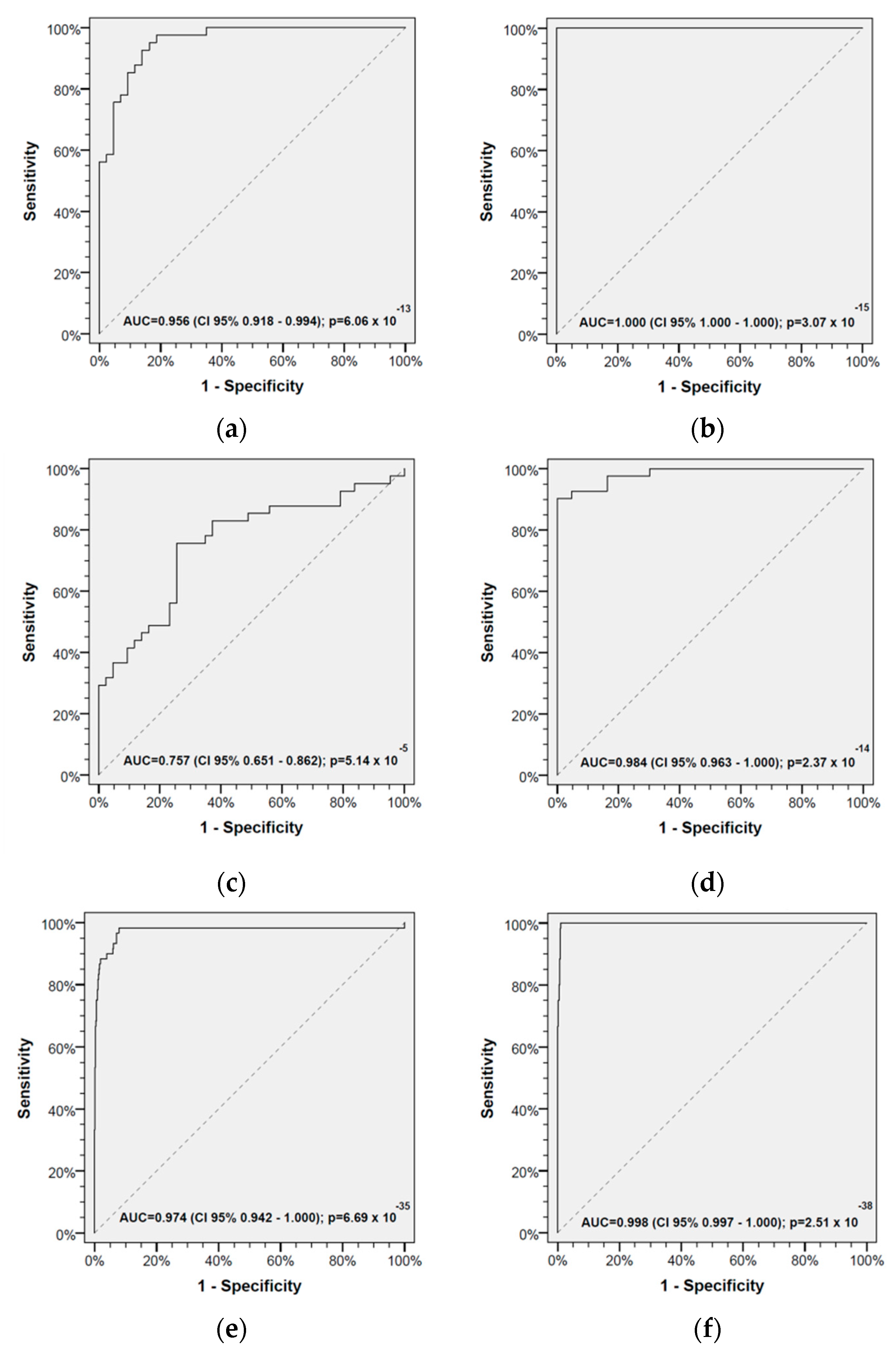
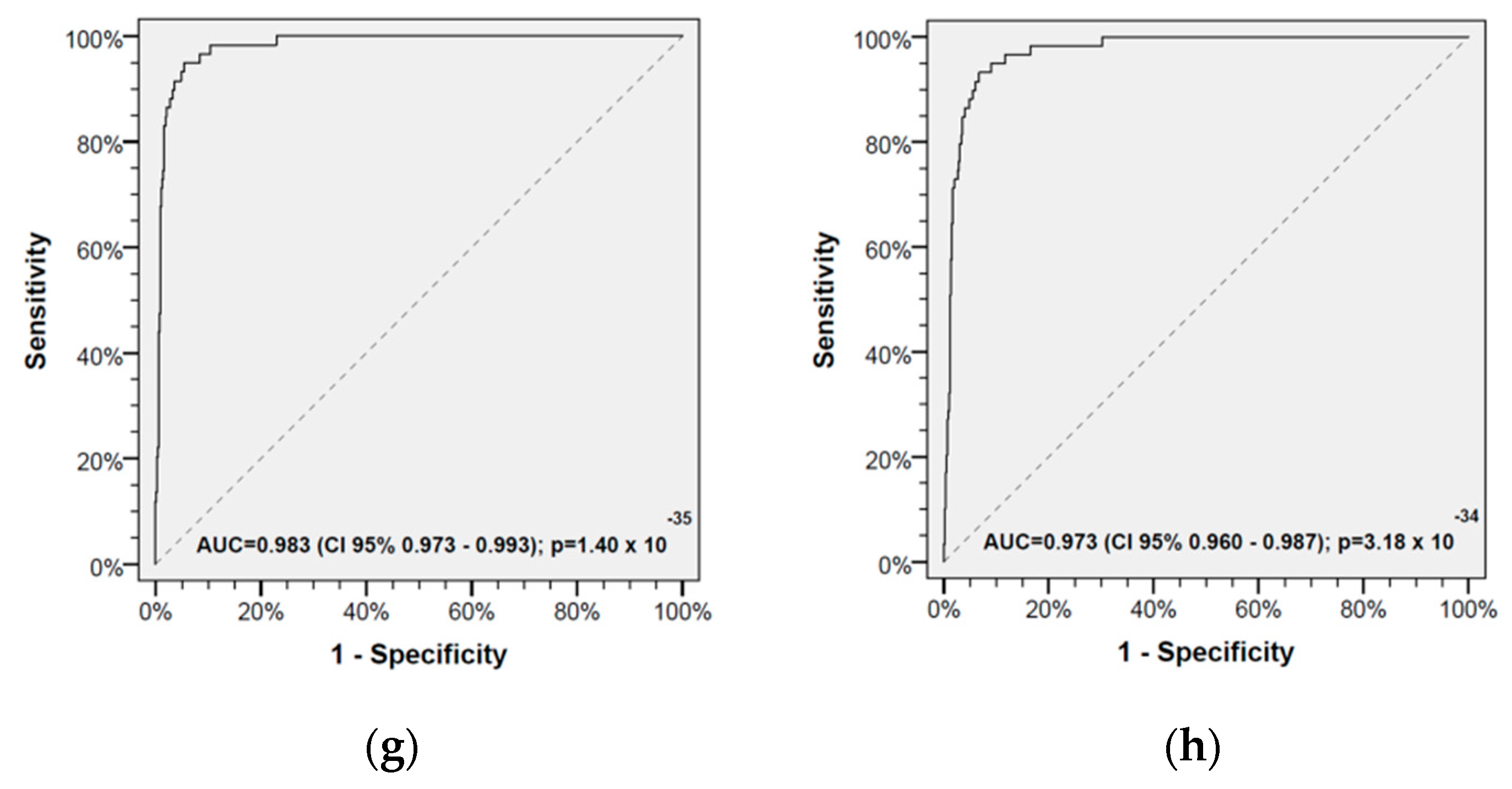
2.2. Receiver Operating Characteristics and Cut-Offs for Discriminating HAs and HNSCC-CPs
2.3. Development of a Leukocyte Score to Identify HNSCC-CPs
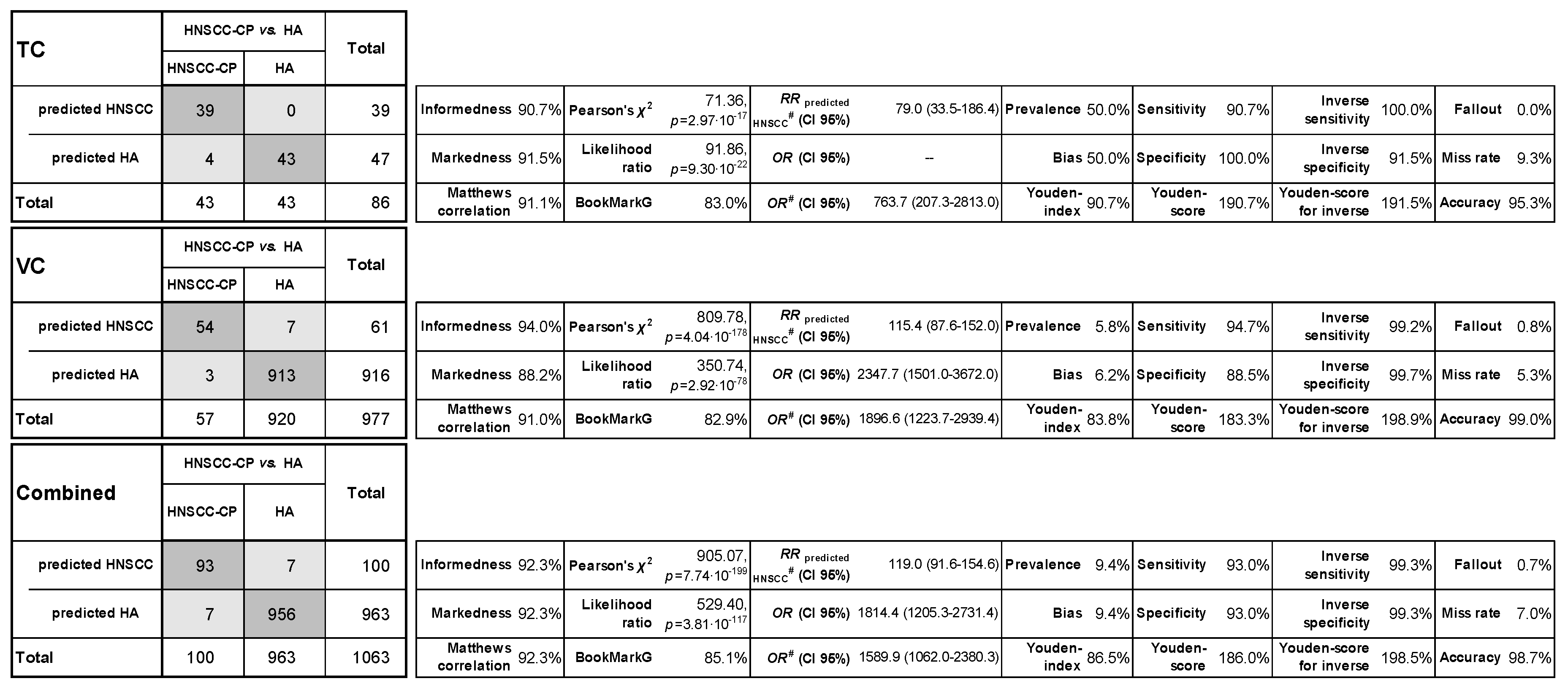
2.4. Classification Characteristics of the Leukocyte Score
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Blood Sample Collection, Preparation, and Staining
4.3. Flow Cytometry (FCM)
4.4. Quality Control
4.5. Manual Gating
4.6. Statistical Considerations and Propensity-Score Matching
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Union for International Cancer Control (UICC). Review of Cancer Medicines on the WHO List of Essential Medicines—Head and Neck; UICC: Geneva, Switzerland, 2014; pp. 1–8. [Google Scholar]
- Leemans, C.R.; Braakhuis, B.J.M.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 1, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, G.; Herchenhahn, C.; Boehm, A.; Mozet, C.; Hofer, M.; Fischer, M.; Kolb, M.; Dietz, A. HLA traits linked to development of head and neck squamous cell carcinoma affect the progression-free survival of patients. Oral Oncol. 2017, 69, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, G.; Lehmann, C.; Herchenhahn, C.; Kolb, M.; Hofer, M.; Wiegand, S.; Dietz, A. Development of a Human Leukocyte Antigen Score to Predict Progression-Free Survival in Head and Neck Squamous Cell Carcinoma Patients. Front. Oncol. 2018, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Schneiders, F.L.; de Bruin, R.C.G.; van den Eertwegh, A.J.M.; Scheper, R.J.; Leemans, C.R.; Brakenhoff, R.H.; Langendijk, J.A.; Verheul, H.M.W.; de Gruijl, T.D.; Molling, J.W.; et al. Circulating invariant natural killer T-cell numbers predict outcome in head and neck squamous cell carcinoma: Updated analysis with 10-year follow-up. J. Clin. Oncol. 2012, 5, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Strauss, L.; Wang, Y.; Szczepanski, M.J.; Lang, S.; Johnson, J.T.; Whiteside, T.L. T regulatory type 1 cells in squamous cell carcinoma of the head and neck: Mechanisms of suppression and expansion in advanced disease. Clin. Cancer Res. 2008, 14, 3706–3715. [Google Scholar] [CrossRef]
- Schaefer, C.; Kim, G.G.; Albers, A.; Hoermann, K.; Myers, E.N.; Whiteside, T.L. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br. J. Cancer 2005, 92, 913–920. [Google Scholar] [CrossRef]
- Zeidler, R.; Csanady, M.; Gires, O.; Lang, S.; Schmitt, B.; Wollenberg, B. Tumor cell-derived prostaglandin E2 inhibits monocyte function by interfering with CCR5 and Mac-1. FASEB J. 2000, 14, 661–668. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Lang, S.; Brandau, S. Modulation of neutrophil granulocytes in the tumor microenvironment: Mechanisms and consequences for tumor progression. Semin. Cancer Biol. 2013, 23, 141–148. [Google Scholar] [CrossRef]
- Sumner, W.A.; Stokes, W.A.; Oweida, A.; Berggren, K.L.; McDermott, J.D.; Raben, D.; Abbott, D.; Jones, B.; Gan, G.; Karam, S.D. Survival impact of pre-treatment neutrophils on oropharyngeal and laryngeal cancer patients undergoing definitive radiotherapy. J. Trans. Med. 2017, 15, 168. [Google Scholar] [CrossRef]
- Krieg, C.; Nowicka, M.; Guglietta, S.; Schindler, S.; Hartmann, F.J.; Weber, L.M.; Dummer, R.; Robinson, M.D.; Levesque, M.P.; Becher, B. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat. Med. 2018, 24, 144–153. [Google Scholar] [CrossRef]
- Tham, T.; Olson, C.; Khaymovich, J.; Herman, S.W.; Costantino, P.D. The lymphocyte-to-monocyte ratio as a prognostic indicator in head and neck cancer: A systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2018, 275, 1663–1670. [Google Scholar] [CrossRef] [PubMed]
- Kano, S.; Homma, A.; Hatakeyama, H.; Mizumachi, T.; Sakashita, T.; Kakizaki, T.; Fukuda, S. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for head and neck cancer. Head Neck 2017, 39, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: A systematic review and meta-analysis. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Bocsi, J.; Melzer, S.; Dähnert, I.; Tárnok, A. OMIP-023: 10-color, 13 antibody panel for in-depth phenotyping of human peripheral blood leukocytes. Cytom. Part A 2014, 85, 781–784. [Google Scholar] [CrossRef] [PubMed]
- Melzer, S.; Zachariae, S.; Bocsi, J.; Engel, C.; Löffler, M.; Tárnok, A. Reference intervals for leukocyte subsets in adults: Results from a population-based study using 10-color flow cytometry. Cytom. Part B Clin. Cytom. 2015, 88, 270–281. [Google Scholar] [CrossRef] [PubMed]
- Powers, D. Evaluation: From precision, recall and F-measure to ROC, informedness, markedness and correlation. JMLT 2011, 2, 37–63. [Google Scholar]
- Cox, D.R. The Analysis of Binary Data, 1st ed.; Methuen: London, UK, 1970; p. 142. ISBN 0416104002. [Google Scholar]
- Moses, L.E.; Shapiro, D.; Littenberg, B. Combining independent studies of a diagnostic test into a summary ROC curve: Data-analytic approaches and some additional considerations. Stat. Med. 1993, 12, 1293–1316. [Google Scholar] [CrossRef]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef]
- Kuss, O.; Blettner, M.; Börgermann, J. Propensity Score: An Alternative Method of Analyzing Treatment Effects: Part 23 of a Series on Evaluation of Scientific Publications. Dtsch. Ärztebl. Int. 2016, 113, 597–603. [Google Scholar] [CrossRef]
- Millrud, C.R.; Månsson Kvarnhammar, A.; Uddman, R.; Björnsson, S.; Riesbeck, K.; Cardell, L.O. The activation pattern of blood leukocytes in head and neck squamous cell carcinoma is correlated to survival. PLoS ONE 2012, 7, e51120. [Google Scholar] [CrossRef]
- Trellakis, S.; Farjah, H.; Bruderek, K.; Dumitru, C.A.; Hoffmann, T.K.; Lang, S.; Brandau, S. Peripheral blood neutrophil granulocytes from patients with head and neck squamous cell carcinoma functionally differ from their counterparts in healthy donors. Int. J. Immunopathol. Pharmacol. 2011, 24, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Kågedal, Å.; Rydberg Millrud, C.; Häyry, V.; Kumlien Georén, S.; Lidegran, M.; Munck-Wikland, E.; Cardell, L.-O. Oropharyngeal squamous cell carcinoma induces an innate systemic inflammation, affected by the size of the tumour and the lymph node spread. Clin. Otolaryngol. 2018, 43, 1117–1121. [Google Scholar] [CrossRef]
- Chikamatsu, K.; Sakakura, K.; Toyoda, M.; Takahashi, K.; Yamamoto, T.; Masuyama, K. Immunosuppressive activity of CD14+ HLA-DR- cells in squamous cell carcinoma of the head and neck. Cancer Sci. 2012, 103, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Cropet, C.; van Glabbeke, M.; Sebban, C.; Le Cesne, A.; Judson, I.; Tredan, O.; Verweij, J.; Biron, P.; Labidi, I.; et al. Lymphopenia as a prognistic factor for overall survival in advanced carcinomas, sarcomas and lymphomas. Cancer Res. 2009, 69, 5383–5391. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Q.; Huang, X.S. The significance of lymphocyte to monocyte ratio in peripheral blood of patients with benign and malignant laryngeal lesions. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 2017, 31, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hsueh, C.-Y.; Cao, W.; Zhou, L. Pretreatment lymphocyte-to-monocyte ratio as an independent prognostic factor for hypopharyngeal squamous cell carcinoma. Acta Otolaryngol. 2018, 138, 734–740. [Google Scholar] [CrossRef] [PubMed]
- Keck, M.K.; Zuo, Z.; Khattri, A.; Stricker, T.P.; Brown, C.D.; Imanguli, M.; Rieke, D.; Endhardt, K.; Fang, P.; Brägelmann, J.; et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin. Cancer Res. 2015, 21, 870–881. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 2015, 517, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, G.; Rosolowski, M.; Krohn, K.; Kreuz, M.; Boehm, A.; Reiche, A.; Scharrer, U.; Halama, D.; Bertolini, J.; Bauer, U.; et al. The role of HPV RNA transcription, immune response-related gene expression and disruptive TP53 mutations in diagnostic and prognostic profiling of head and neck cancer. Int. J. Cancer 2015, 137, 2846–2857. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, M.; Engel, C.; Ahnert, P.; Alfermann, D.; Arelin, K.; Baber, R.; Beutner, F.; Binder, H.; Brahler, E.; Burkhardt, R.; et al. The LIFE-Adult-Study: Objectives and design of a population-based cohort study with 10,000 deeply phenotyped adults in Germany. BMC Public Health 2015, 15, 691. [Google Scholar] [CrossRef] [PubMed]
| Leukocyte Subsets | Marker | Healthy Adults (HAs) Mean (95% CI) | HNSCC Patients (HNSCC-CPs) (95% CI) | p-Value * | ||
|---|---|---|---|---|---|---|
| Combined a | n | 935 | 101 | |||
| Granulocytes | SSChigh | 3.93 | (3.84–4.02) | 4.81 | (4.41–5.22) | 0.0001 |
| Neutrophils | SSChighCD16+ | 3.64 | (3.55–3.73) | 4.67 | (4.27–5.07) | <0.0001 |
| Eosinophils | SSChighCD16− | 0.14 | (0.13–0.15) | 0.14 | (0.12–0.16) | 0.8979 |
| Monocytes | SSCmedCD14high | 0.26 | (0.25–0.27) | 0.36 | (0.33–0.40) | <0.0001 |
| nonclassical m. | SSCmedCD14dimCD16high | 0.03 | (0.03–0.04) | 0.04 | (0.03–0.04) | 0.3414 |
| classical m. | SSCmedCD14highCD16dim | 0.22 | (0.21–0.23) | 0.32 | (0.29–0.35) | <0.0001 |
| Lymphocytes | SSClow | 1.77 | (1.73–1.81) | 1.01 | (0.91–1.10) | <0.0001 |
| B-cells | CD3−CD16/CD56− CD19+ | 0.19 | (0.18–0.20) | 0.17 | (0.15–0.20) | 0.2814 |
| T-cells | CD3+CD16/CD56− | 1.23 | (1.20–1.26) | 0.91 | (0.82–0.99) | <0.0001 |
| Tc cells | CD3+CD8+ | 0.24 | (0.23–0.26) | 0.19 | (0.16–0.22) | 0.0011 |
| DPT | CD3+CD4+CD8+ | 0.02 | (0.02–0.02) | 0.01 | (0.01–0.02) | <0.0001 |
| Th cells | CD3+CD4+ | 0.89 | (0.86–0.92) | 0.75 | (0.67–0.82) | 0.0005 |
| regulatory T-cells e | CD3+CD4+CD25+CD127+ | 0.07 | (0.07–0.07) | 0.07 | (0.06–0.08) | 0.5573 |
| NKT cells | CD3+CD8+ CD16/CD56+ | 0.10 | (0.10–0.11) | 0.10 | (0.08–0.12) | 0.8046 |
| NK cells | CD3−CD8− CD16/CD56+ | 0.31 | (0.30–0.32) | 0.29 | (0.25–0.32) | 0.2452 |
| PS-matched TC b | n | 43 | 43 | |||
| Granulocytes | SSChigh | 3.84 | (3.41–4.28) | 4.69 | (4.05–5.32) | 0.0370 |
| Neutrophils | SSChighCD16+ | 3.66 | (3.24–4.08) | 4.56 | (3.94–5.19) | 0.0234 |
| Eosinophils | SSChighCD16− | 0.17 | (0.12–0.21) | 0.12 | (0.09–0.16) | 0.1334 |
| Monocytes | SSCmedCD14high | 0.31 | (0.28–0.34) | 0.37 | (0.31–0.42) | 0.0838 |
| nonclassical m. | SSCmedCD14dimCD16high | 0.03 | (0.03–0.04) | 0.04 | (0.03–0.05) | 0.3462 |
| classical m. | SSCmedCD14highCD16dim | 0.27 | (0.24–0.30) | 0.32 | (0.27–0.37) | 0.1235 |
| Lymphocytes | SSClow | 1.84 | (1.66–2.02) | 1.02 | (0.89–1.15) | <0.0001 |
| B-cells | CD3−CD16/CD56− CD19+ | 0.21 | (0.17–0.24) | 0.17 | (0.14–0.20) | 0.0979 |
| T-cells | CD3+CD16/CD56− | 1.20 | (1.08–1.33) | 0.91 | (0.79–1.03) | 0.0015 |
| Tc cells | CD3+CD8+ | 0.20 | (0.17–0.24) | 0.20 | (0.15–0.25) | 0.9290 |
| DPT | CD3+CD4+CD8+ | 0.01 | (0.01–0.02) | 0.02 | (0.01–0.02) | 0.4755 |
| Th cells | CD3+CD4+ | 0.92 | (0.81–1.02) | 0.73 | (0.63–0.83) | 0.0147 |
| regulatory T-cells | CD3+CD4+CD25+CD127+ | 0.08 | (0.07–0.09) | 0.07 | (0.06–0.08) | 0.2114 |
| NKT cells | CD3+CD8+ CD16/CD56+ | 0.09 | (0.07–0.10) | 0.11 | (0.07–0.14) | 0.2775 |
| NK cells | CD3−CD8− CD16/CD56+ | 0.40 | (0.32–0.47) | 0.29 | (0.23–0.34) | 0.0222 |
| VC c | n | 892 | 58 | |||
| Granulocytes | SSChigh | 3.93 | (3.84–4.03) | 4.91 | (4.39–5.43) | 0.0007 |
| Neutrophils | SSChighCD16+ | 3.64 | (3.55–3.73) | 4.75 | (4.23–5.27) | 0.0001 |
| Eosinophils | SSChighCD16− | 0.14 | (0.13–0.15) | 0.15 | (0.13–0.18) | 0.2848 |
| Monocytes | SSCmedCD14high | 0.26 | (0.25–0.26) | 0.36 | (0.32–0.40) | <0.0001 |
| nonclassical m. | SSCmedCD14dimCD16high | 0.03 | (0.03–0.04) | 0.04 | (0.03–0.04) | 0.5644 |
| classical m. | SSCmedCD14highCD16dim | 0.22 | (0.21–0.22) | 0.32 | (0.28–0.36) | <0.0001 |
| Lymphocytes | SSClow | 1.76 | (1.72–1.81) | 1.00 | (0.87–1.13) | <0.0001 |
| B-cells | CD3−CD16/CD56− CD19+ | 0.19 | (0.17–0.20) | 0.18 | (0.15–0.21) | 0.4031 |
| T-cells | CD3+CD16/CD56− | 1.23 | (1.20–1.27) | 0.91 | (0.79–1.03) | <0.0001 |
| Tc cells | CD3+CD8+ | 0.25 | (0.23–0.26) | 0.18 | (0.14–0.21) | 0.0011 |
| DPT | CD3+CD4+CD8+ | 0.02 | (0.02–0.02) | 0.01 | (0.01–0.01) | <0.0001 |
| Th cells | CD3+CD4+ | 0.89 | (0.86–0.91) | 0.76 | (0.65–0.86) | 0.0221 |
| regulatory T-cells e | CD3+CD4+CD25+CD127+ | 0.07 | (0.07–0.07) | 0.07 | (0.06–0.08) | 0.7916 |
| NKT cells | CD3+CD8+ CD16/CD56+ | 0.10 | (0.10–0.11) | 0.09 | (0.07–0.11) | 0.3587 |
| NK cells | CD3−CD8− CD16/CD56+ | 0.30 | (0.29–0.31) | 0.29 | (0.24–0.33) | 0.4768 |
| Characteristics and Leukocyte Subsets | Unit | Healthy Adults (HAs) Mean (95% CI) | HNSCC Patients (HNSCC-CPs) Mean (95% CI) | p-Value * | ||
|---|---|---|---|---|---|---|
| Combined a | n | 963 | 101 | |||
| Male sex | % | 42.3 | 84.2 | <0.0001 | ||
| Age | years | 54.09 | (52.70–55.48) | 60.54 | (58.54–62.53) | <0.0001 |
| Tobacco smoking | pack years d | 5.94 | (4.55–7.32) | 32.16 | (27.25–37.07) | <0.0001 |
| Alcohol (g/d) 0 | % | 14.2 | 16.0 | <0.0001 | ||
| >0–30 | % | 72.4 | 27.0 | |||
| >30–60 | % | 10.2 | 27.0 | |||
| >60 | % | 3.1 | 30.0 | |||
| Cell subsets RelCC | ||||||
| Granulocytes | % | 64.85 | (64.16–65.53) | 69.24 | (67.17–71.31) | 0.0001 |
| Neutrophils | % | 60.04 | (59.33–60.75) | 63.42 | (61.26–65.57) | 0.0044 |
| Lymphocytes e | % | 30.35 | (29.67–31.04) | 24.11 | (22.02–26.19) | <0.0001 |
| T-lymphocytes e | % | 21.04 | (20.49–21.60) | 14.91 | (13.32–16.50) | <0.0001 |
| Tc cells e | % | 4.15 | (3.96–4.34) | 2.31 | (1.92–2.70) | <0.0001 |
| Th cells e | % | 15.23 | (14.81–15.65) | 11.84 | (10.54–13.14) | <0.0001 |
| Monocytes f | % | 4.43 | (4.32–4.53) | 16.43 | (15.46–17.40) | <0.0001 |
| nonclassical m. f | % | 0.59 | (0.56–0.61) | 0.88 | (0.76–1.01) | <0.0001 |
| intermediate m. f | % | 0.09 | (0.08–0.10) | 9.47 | (8.60–10.33) | <0.0001 |
| classical m. f | % | 3.74 | (3.65–3.84) | 5.89 | (5.43–6.35) | <0.0001 |
| PS-matched TC b | n | 43 | 43 | |||
| Male sex | % | 100 | 100 | >0.9999 | ||
| Age | years | 60.25 | (57.15–63.36) | 61.78 | (58.86–64.70) | 0.4830 |
| Tobacco smoking | pack yearsd | 23.36 | (15.42–31.30) | 26.05 | (19.60–32.49) | 0.6075 |
| Alcohol (g/d) 0 | % | 16.3 | 18.6 | 0.9722 | ||
| >0–30 | % | 37.2 | 32.6 | |||
| >30–60 | % | 34.9 | 37.2 | |||
| >60 | % | 11.6 | 11.6 | |||
| Cell subsets RelCC | ||||||
| Granulocytes | % | 62.82 | (59.45–66.19) | 68.46 | (65.16–71.76) | 0.0215 |
| Neutrophils | % | 59.86 | (56.35–63.37) | 63.03 | (59.76–66.31) | 0.1983 |
| Lymphocytes | % | 31.89 | (28.73–35.06) | 24.89 | (21.48–28.31) | 0.0042 |
| T-lymphocytes | % | 20.67 | (18.62–22.72) | 15.27 | (12.79–17.74) | 0.0015 |
| Tc cells | % | 3.50 | (2.97–4.04) | 2.53 | (1.79–3.26) | 0.0381 |
| Th cells | % | 15.68 | (14.05–17.30) | 11.96 | (9.98–13.93) | 0.0056 |
| Monocytes f | % | 5.35 | (4.73–5.97) | 16.57 | (14.88–18.27) | <0.0001 |
| nonclassical m. f | % | 0.57 | (0.46–0.68) | 0.79 | (0.62–0.96) | 0.0344 |
| intermediate m. f | % | 0.04 | (0.00–0.08) | 9.32 | (7.83–10.81) | <0.0001 |
| classical m. f | % | 4.74 | (4.18–5.30) | 6.01 | (5.36–6.66) | 0.0048 |
| VC c | n | 920 | 58 | |||
| Male sex | % | 34.3 | 72.4 | <0.0001 | ||
| Age | years | 53.23 | (51.73–54.73) | 59.61 | (56.90–62.32) | 0.0001 |
| Tobacco smoking | pack years d | 3.51 | (2.69–4.34) | 36.70 | (29.79–43.61) | <0.0001 |
| Alcohol (g/d) 0 | % | 13.9 | 14.0 | <0.0001 | ||
| >0–30 | % | 77.3 | 22.8 | |||
| >30–60 | % | 6.8 | 19.3 | |||
| >60 | % | 1.9 | 43.9 | |||
| Cell subsets RelCC | ||||||
| Granulocytes | % | 64.94 | (64.24–65.64) | 69.81 | (67.14–72.48) | 0.0010 |
| Neutrophils | % | 60.04 | (59.32–60.77) | 63.70 | (60.81–66.59) | 0.0199 |
| Lymphocytes e | % | 30.28 | (29.58–30.98) | 23.51 | (20.90–26.13) | <0.0001 |
| T-lymphocytes e | % | 21.06 | (20.49–21.63) | 14.64 | (12.56–16.72) | <0.0001 |
| Tc cells e | % | 4.18 | (3.99–4.37) | 2.14 | (1.73–2.56) | <0.0001 |
| Th cells e | % | 15.21 | (14.77–15.64) | 11.75 | (10.02–13.48) | 0.0004 |
| Monocytes | % | 4.38 | (4.27–4.49) | 16.33 | (15.17–17.48) | <0.0001 |
| nonclassical m. | % | 0.59 | (0.56–0.61) | 0.95 | (0.77–1.13) | 0.0003 |
| intermediate m. | % | 0.10 | (0.09–0.11) | 9.58 | (8.55–10.6) | <0.0001 |
| classical m. | % | 3.70 | (3.60–3.80) | 5.80 | (5.16–6.44) | <0.0001 |
| Characteristic | Category | Score ≥ 3 | Score < 3 | p Value * | ||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
| Sex | Female | 14 | (15.1) | 1 | (14.3) | 0.9562 |
| Male | 79 | (84.9) | 6 | (85.7) | ||
| Age (years) | <50 | 12 | (12.9) | 2 | (28.6) | 0.3932 |
| 50–59 | 37 | (39.8) | 3 | (42.9) | ||
| 60–69 | 23 | (24.7) | -- | -- | ||
| >70 | 21 | (22.6) | 2 | (28.6) | ||
| Tobacco smoking (pack years) | never smoker | 14 | (15.1) | 2 | (28.6) | 0.5024 |
| <30 | 29 | (31.2) | 1 | (14.3) | ||
| >30 | 50 | (53.8) | 4 | (57.1) | ||
| Alcohol (g/d) | 0 | 14 | (15.1) | 1 | (14.3) | 0.8724 |
| 1–30 | 24 | (25.8) | 3 | (42.9) | ||
| 31–60 | 26 | (28.0) | 1 | (14.3) | ||
| >60 | 28 | (30.1) | 2 | (28.6) | ||
| Unknown | 1 | (1.1) | -- | -- | ||
| HPV | p16+ HPV16+ | 16 | (17.2) | -- | -- | 0.2312 |
| p16− HPV− | 77 | (82.8) | 7 | (100.0) | ||
| Localization | Oropharynx | 31 | (33.3) | 3 | (42.9) | 0.8241 |
| Hypopharynx | 10 | (10.8) | 1 | (14.3) | ||
| Larynx | 23 | (24.7) | 2 | (28.6) | ||
| Other | 29 | (31.2) | 1 | (14.3) | ||
| UICC stage 7th ed. | 0 | 4 | (4.3) | -- | -- | 0.3545 |
| I | 10 | (10.8) | 2 | (28.6) | ||
| II | 14 | (15.1) | 1 | (14.3) | ||
| III | 7 | (7.5) | 2 | (28.6) | ||
| IVA | 49 | (52.7) | 2 | (28.6) | ||
| IVB | 8 | (8.6) | -- | -- | ||
| IVC | 1 | (1.1) | -- | -- | ||
| Early vs. advanced | Advanced | 65 | (69.9) | 4 | (57.1) | 0.5612 |
| Early | 24 | (25.8) | 3 | (42.9) | ||
| SINIII | 4 | (4.3) | -- | -- | ||
| T category | Tis | 4 | (4.3) | -- | -- | 0.8318 |
| 1 | 18 | (19.4) | 3 | (42.9) | ||
| 2 | 27 | (29.0) | 2 | (28.6) | ||
| 3 | 14 | (15.1) | 1 | (14.3) | ||
| 4a | 24 | (25.8) | 1 | (14.3) | ||
| 4b | 3 | (3.2) | -- | -- | ||
| X | 3 | (3.2) | -- | -- | ||
| T; locally early vs. advanced | T0–T2 | 52 | (55.9) | 5 | (71.4) | 0.4240 |
| T3–T4 | 41 | (44.1) | 2 | (28.6) | ||
| N category | 0 | 37 | (39.8) | 4 | (57.1) | 0.2369 |
| 1 | 6 | (6.5) | 2 | (28.6) | ||
| 2a | 1 | (1.1) | -- | -- | ||
| 2b | 23 | (24.7) | 1 | (14.3) | ||
| 2c | 22 | (23.7) | -- | -- | ||
| 3 | 4 | (4.3) | -- | -- | ||
| Neck nodes | N0 | 37 | (39.8) | 4 | (57.1) | 0.3679 |
| N+ | 56 | (60.2) | 3 | (42.9) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wichmann, G.; Gaede, C.; Melzer, S.; Bocsi, J.; Henger, S.; Engel, C.; Wirkner, K.; Wenning, J.R.; Wald, T.; Freitag, J.; et al. Discrimination of Head and Neck Squamous Cell Carcinoma Patients and Healthy Adults by 10-Color Flow Cytometry: Development of a Score Based on Leukocyte Subsets. Cancers 2019, 11, 814. https://doi.org/10.3390/cancers11060814
Wichmann G, Gaede C, Melzer S, Bocsi J, Henger S, Engel C, Wirkner K, Wenning JR, Wald T, Freitag J, et al. Discrimination of Head and Neck Squamous Cell Carcinoma Patients and Healthy Adults by 10-Color Flow Cytometry: Development of a Score Based on Leukocyte Subsets. Cancers. 2019; 11(6):814. https://doi.org/10.3390/cancers11060814
Chicago/Turabian StyleWichmann, Gunnar, Clara Gaede, Susanne Melzer, Jozsef Bocsi, Sylvia Henger, Christoph Engel, Kerstin Wirkner, John Ross Wenning, Theresa Wald, Josefine Freitag, and et al. 2019. "Discrimination of Head and Neck Squamous Cell Carcinoma Patients and Healthy Adults by 10-Color Flow Cytometry: Development of a Score Based on Leukocyte Subsets" Cancers 11, no. 6: 814. https://doi.org/10.3390/cancers11060814
APA StyleWichmann, G., Gaede, C., Melzer, S., Bocsi, J., Henger, S., Engel, C., Wirkner, K., Wenning, J. R., Wald, T., Freitag, J., Willner, M., Kolb, M., Wiegand, S., Löffler, M., Dietz, A., & Tárnok, A. (2019). Discrimination of Head and Neck Squamous Cell Carcinoma Patients and Healthy Adults by 10-Color Flow Cytometry: Development of a Score Based on Leukocyte Subsets. Cancers, 11(6), 814. https://doi.org/10.3390/cancers11060814





