Targeting Cyclooxygenase-2 in Pheochromocytoma and Paraganglioma: Focus on Genetic Background
Abstract
1. Introduction
2. Results
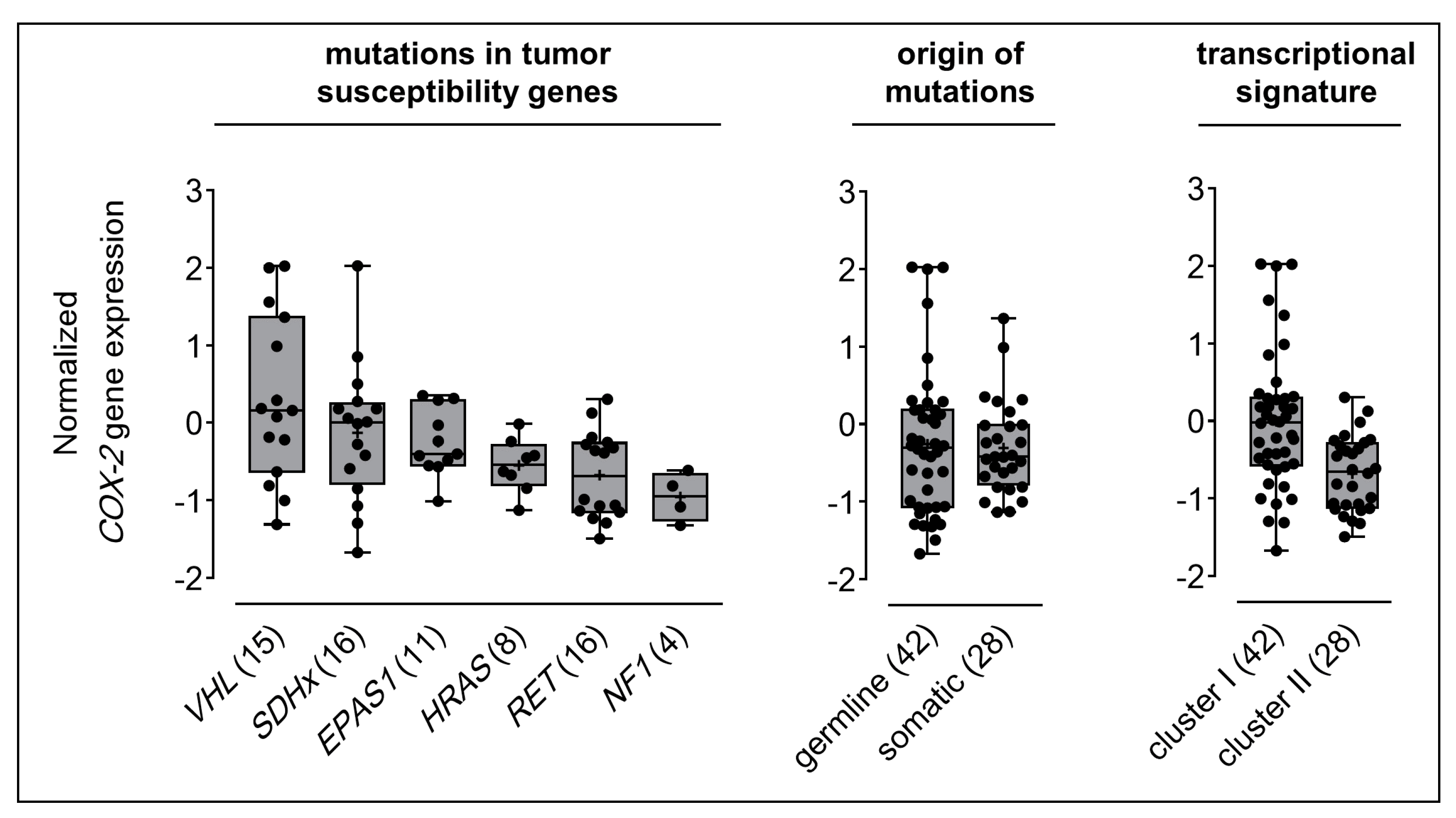
2.1. COX-2 Gene Expression in Clinical PPGL Samples
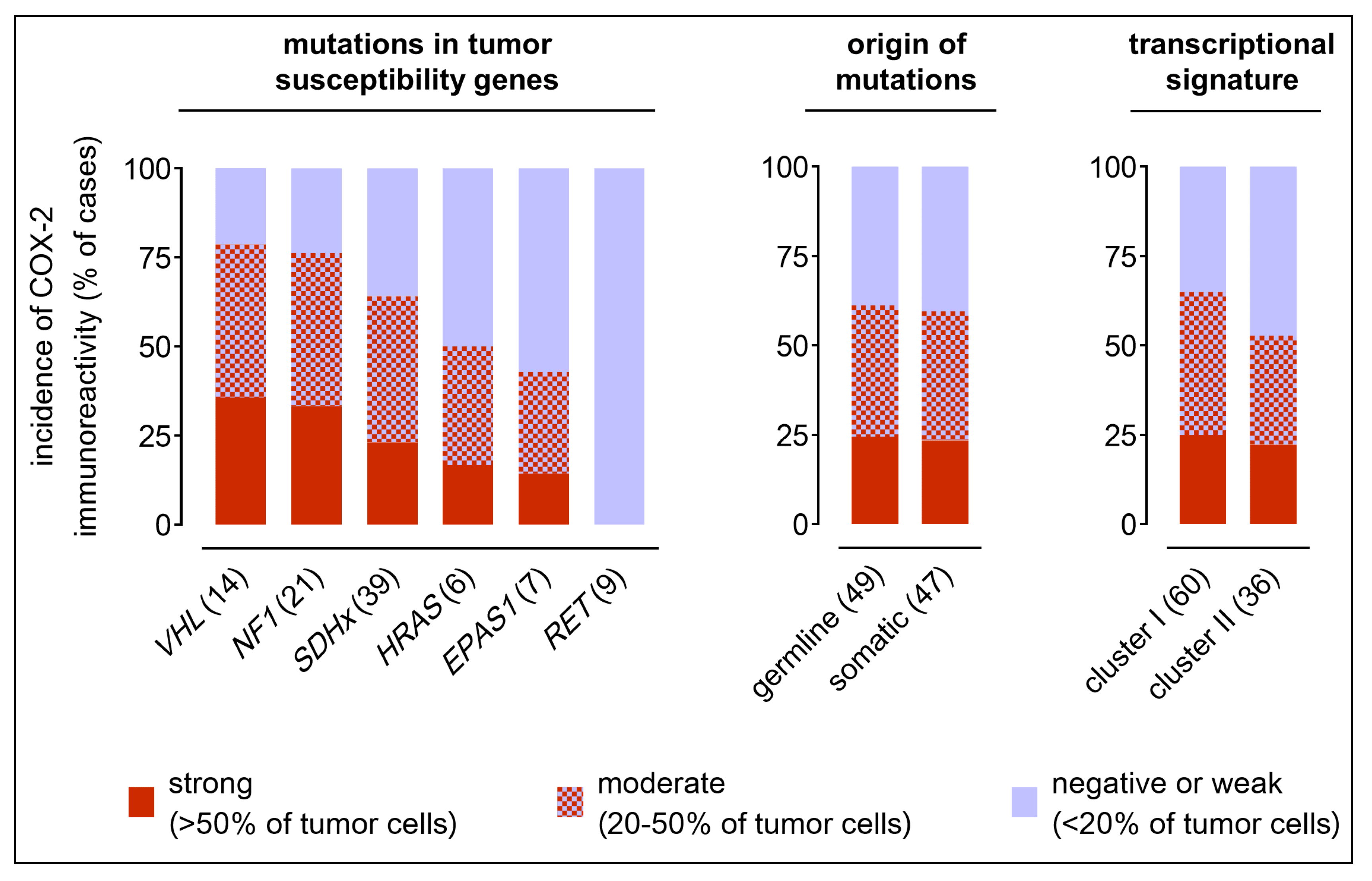
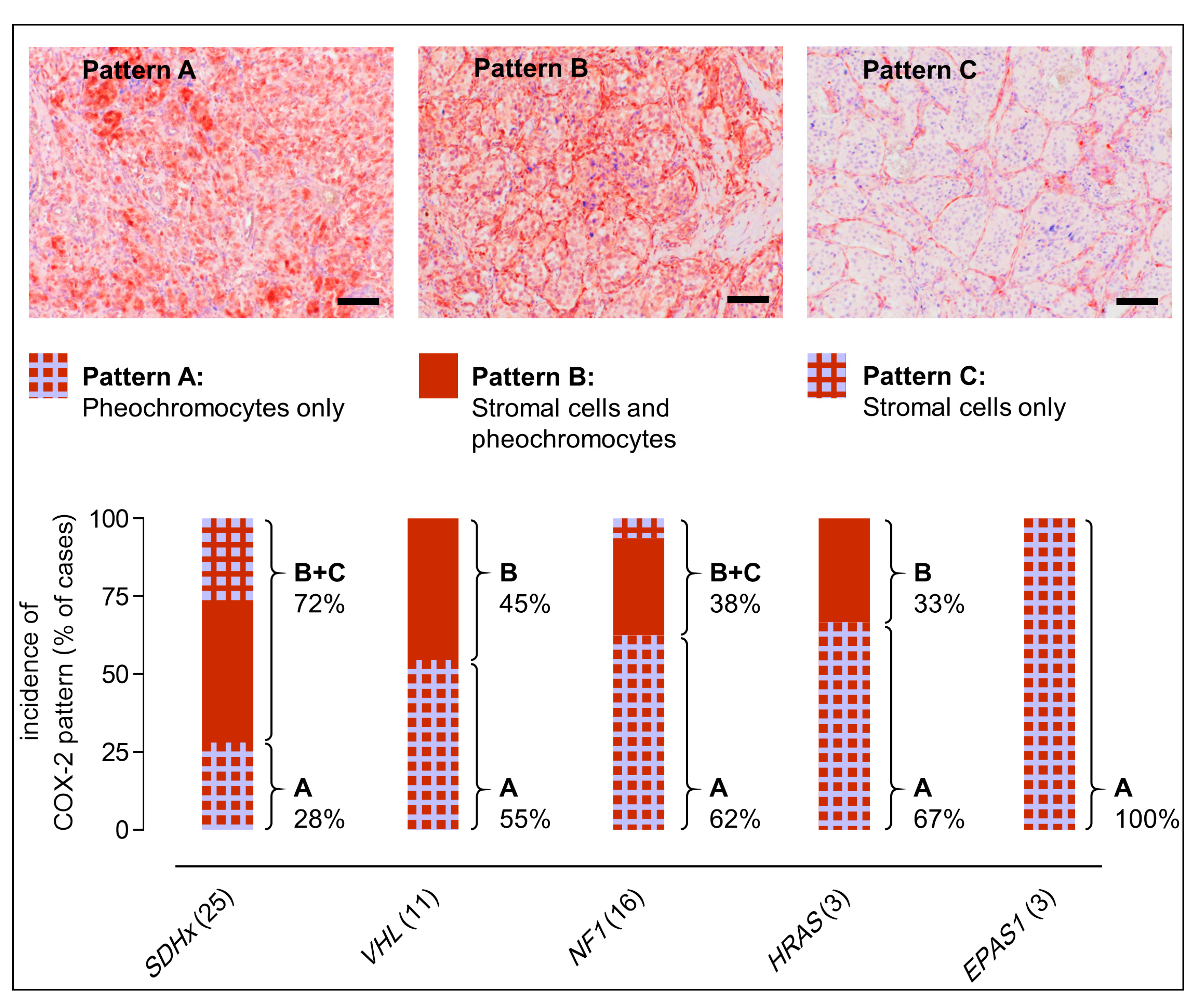
2.2. COX-2 Immunoreactivity in Clinical PPGL Tissue Samples of a Second Cohort
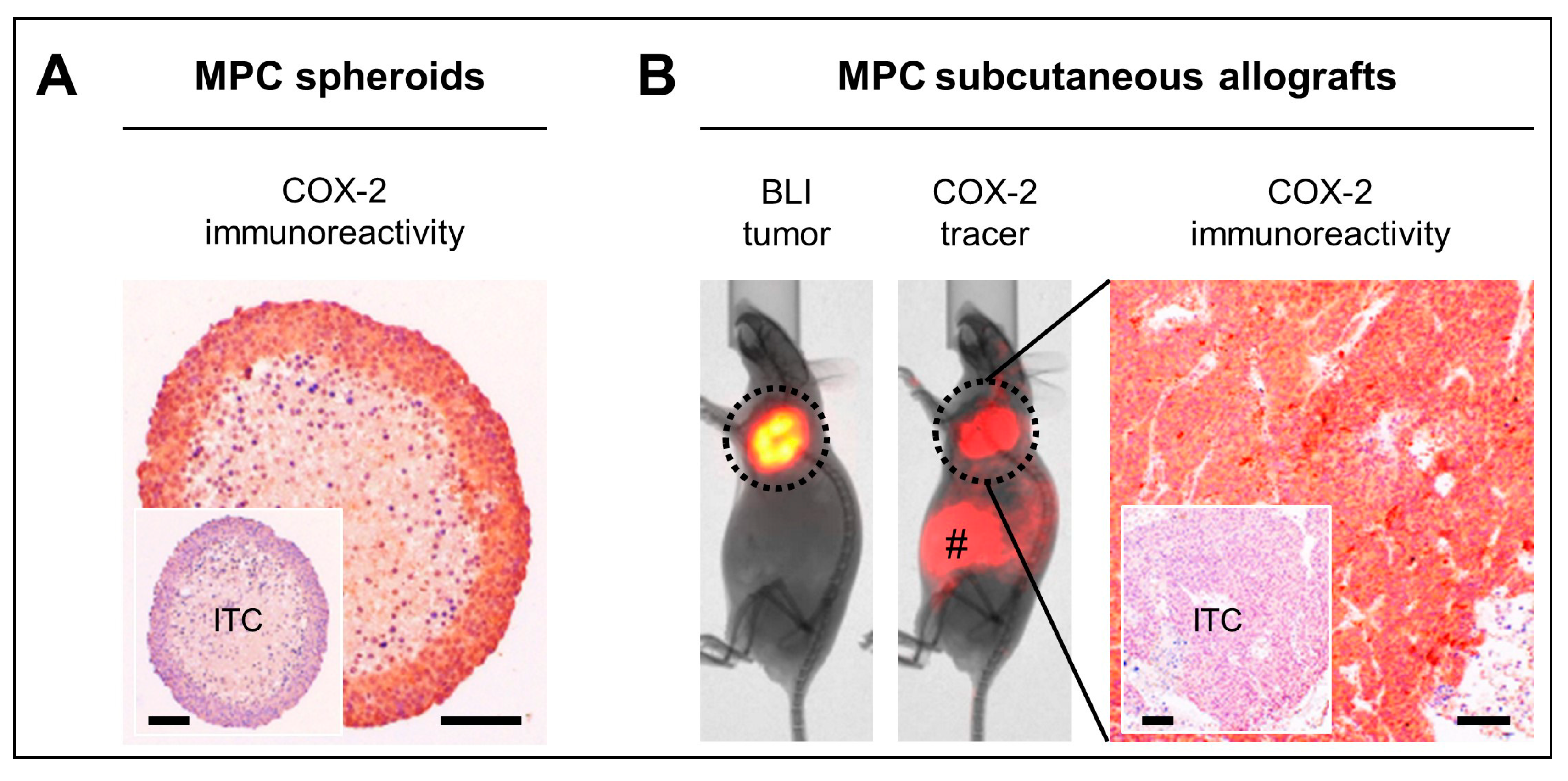
2.3. COX-2 as Molecular Target in Preclinical PPGL Models
3. Discussion
4. Materials and Methods
4.1. Tumor Samples and Genetic Testing
4.2. Gene Expression Profiling and Data Processing
4.3. COX-2 Immunohistochemistry
4.4. Scoring of COX-2 Immunoreactivity
4.5. Spheroid Models
4.6. Tumor Allograft Models
4.7. Optical In Vivo Imaging
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gimenez-Roqueplo, A.P.; Dahia, P.L.; Robledo, M. An update on the genetics of paraganglioma, pheochromocytoma, and associated hereditary syndromes. Horm. Metab. Res. 2012, 44, 328–333. [Google Scholar] [CrossRef]
- Crona, J.; Delgado Verdugo, A.; Maharjan, R.; Stalberg, P.; Granberg, D.; Hellman, P.; Bjorklund, P. Somatic mutations in h-ras in sporadic pheochromocytoma and paraganglioma identified by exome sequencing. J. Clin. Endocrinol. Metab. 2013, 98, E1266–E1271. [Google Scholar] [CrossRef]
- Jochmanova, I.; Pacak, K. Genomic landscape of pheochromocytoma and paraganglioma. Trends Cancer 2018, 4, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, L.; Leshchiner, I.; Walter, V.; Danilova, L.; Robertson, A.G.; Johnson, A.R.; Lichtenberg, T.M.; Murray, B.A.; Ghayee, H.K.; Else, T.; et al. Comprehensive molecular characterization of pheochromocytoma and paraganglioma. Cancer Cell 2017, 31, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, F.R.; Yang, C.; Ng Tang Fui, M.; Vankayalapati, H.; Zhuang, Z.; Huynh, T.; Grossmann, M.; Pacak, K.; Prchal, J.T. A novel EPAS1/hif2a germline mutation in a congenital polycythemia with paraganglioma. J. Mol. Med. (Berl.) 2013, 91, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Comino-Mendez, I.; de Cubas, A.A.; Bernal, C.; Alvarez-Escola, C.; Sanchez-Malo, C.; Ramirez-Tortosa, C.L.; Pedrinaci, S.; Rapizzi, E.; Ercolino, T.; Bernini, G.; et al. Tumoral EPAS1 (hif2a) mutations explain sporadic pheochromocytoma and paraganglioma in the absence of erythrocytosis. Hum. Mol. Genet. 2013, 22, 2169–2176. [Google Scholar] [CrossRef]
- Dahia, P.L. The genetic landscape of pheochromocytomas and paragangliomas: Somatic mutations take center stage. J. Clin. Endocrinol. Metab. 2013, 98, 2679–2681. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Yao, L.; King, E.E.; Buddavarapu, K.; Lenci, R.E.; Chocron, E.S.; Lechleiter, J.D.; Sass, M.; Aronin, N.; Schiavi, F.; et al. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat. Genet. 2010, 42, 229–233. [Google Scholar] [CrossRef]
- Burnichon, N.; Briere, J.J.; Libe, R.; Vescovo, L.; Riviere, J.; Tissier, F.; Jouanno, E.; Jeunemaitre, X.; Benit, P.; Tzagoloff, A.; et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum. Mol. Genet. 2010, 19, 3011–3020. [Google Scholar] [CrossRef]
- Burnichon, N.; Vescovo, L.; Amar, L.; Libe, R.; de Reynies, A.; Venisse, A.; Jouanno, E.; Laurendeau, I.; Parfait, B.; Bertherat, J.; et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum. Mol. Genet. 2011, 20, 3974–3985. [Google Scholar] [CrossRef]
- Comino-Mendez, I.; Gracia-Aznarez, F.J.; Schiavi, F.; Landa, I.; Leandro-Garcia, L.J.; Leton, R.; Honrado, E.; Ramos-Medina, R.; Caronia, D.; Pita, G.; et al. Exome sequencing identifies max mutations as a cause of hereditary pheochromocytoma. Nat. Genet. 2011, 43, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Zhuang, Z.; Fliedner, S.M.; Shankavaram, U.; Sun, M.G.; Bullova, P.; Zhu, R.; Elkahloun, A.G.; Kourlas, P.J.; Merino, M.; et al. Germ-line PHD1 and PHD2 mutations detected in patients with pheochromocytoma/paraganglioma-polycythemia. J. Mol. Med. (Berl.) 2015, 93, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Gupta, G.; Yang, C.; Wang, H.; Huynh, T.T.; Abdullaev, Z.; Pack, S.D.; Percy, M.J.; Lappin, T.R.J.; Zhuang, Z.; et al. A novel splicing site IRP1 somatic mutation in a patient with pheochromocytoma and JAK2(V617F) positive polycythemia vera: A case report. BMC Cancer 2018, 18, 286. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Gieldon, L.; Pang, Y.; Peitzsch, M.; Huynh, T.; Leton, R.; Viana, B.; Ercolino, T.; Mangelis, A.; Rapizzi, E.; et al. Metabolome-guided genomics to identify pathogenic variants in isocitrate dehydrogenase, fumarate hydratase, and succinate dehydrogenase genes in pheochromocytoma and paraganglioma. Genet. Med. 2019, 21, 705–717. [Google Scholar] [CrossRef]
- Cascon, A.; Comino-Mendez, I.; Curras-Freixes, M.; de Cubas, A.A.; Contreras, L.; Richter, S.; Peitzsch, M.; Mancikova, V.; Inglada-Perez, L.; Perez-Barrios, A.; et al. Whole-exome sequencing identifies MDH2 as a new familial paraganglioma gene. J. Natl. Cancer Inst. 2015, 107, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Remacha, L.; Comino-Mendez, I.; Richter, S.; Contreras, L.; Curras-Freixes, M.; Pita, G.; Leton, R.; Galarreta, A.; Torres-Perez, R.; Honrado, E.; et al. Targeted exome sequencing of krebs cycle genes reveals candidate cancer-predisposing mutations in pheochromocytomas and paragangliomas. Clin. Cancer Res. 2017, 23, 6315–6324. [Google Scholar] [CrossRef]
- Remacha, L.; Pirman, D.; Mahoney, C.E.; Coloma, J.; Calsina, B.; Curras-Freixes, M.; Leton, R.; Torres-Perez, R.; Richter, S.; Pita, G.; et al. Recurrent germline dlst mutations in individuals with multiple pheochromocytomas and paragangliomas. Am. J. Hum. Genet. 2019, 104, 651–664. [Google Scholar] [CrossRef]
- Remacha, L.; Curras-Freixes, M.; Torres-Ruiz, R.; Schiavi, F.; Torres-Perez, R.; Calsina, B.; Leton, R.; Comino-Mendez, I.; Roldan-Romero, J.M.; Montero-Conde, C.; et al. Gain-of-function mutations in dnmt3a in patients with paraganglioma. Genet. Med. 2018, 20, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Buffet, A.; Morin, A.; Castro-Vega, L.J.; Habarou, F.; Lussey-Lepoutre, C.; Letouze, E.; Lefebvre, H.; Guilhem, I.; Haissaguerre, M.; Raingeard, I.; et al. Germline mutations in the mitochondrial 2-oxoglutarate/malate carrier slc25a11 gene confer a predisposition to metastatic paragangliomas. Cancer Res. 2018, 78, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Letouze, E.; Martinelli, C.; Loriot, C.; Burnichon, N.; Abermil, N.; Ottolenghi, C.; Janin, M.; Menara, M.; Nguyen, A.T.; Benit, P.; et al. Sdh mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 2013, 23, 739–752. [Google Scholar] [CrossRef]
- Calsina, B.; Curras-Freixes, M.; Buffet, A.; Pons, T.; Contreras, L.; Leton, R.; Comino-Mendez, I.; Remacha, L.; Calatayud, M.; Obispo, B.; et al. Role of MDH2 pathogenic variant in pheochromocytoma and paraganglioma patients. Genet. Med. 2018, 20, 1652–1662. [Google Scholar] [CrossRef]
- Dahia, P.L.; Ross, K.N.; Wright, M.E.; Hayashida, C.Y.; Santagata, S.; Barontini, M.; Kung, A.L.; Sanso, G.; Powers, J.F.; Tischler, A.S.; et al. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005, 1, 72–80. [Google Scholar] [CrossRef]
- Dahia, P.L. Pheochromocytoma and paraganglioma pathogenesis: Learning from genetic heterogeneity. Nat. Rev. Cancer 2014, 14, 108–119. [Google Scholar] [CrossRef]
- Deorukhkar, A.; Krishnan, S. Targeting inflammatory pathways for tumor radiosensitization. Biochem. Pharmacol. 2010, 80, 1904–1914. [Google Scholar] [CrossRef]
- Jochmanova, I.; Yang, C.; Zhuang, Z.; Pacak, K. Hypoxia-inducible factor signaling in pheochromocytoma: Turning the rudder in the right direction. J. Natl. Cancer Inst. 2013, 105, 1270–1283. [Google Scholar] [CrossRef]
- Xue, X.; Shah, Y.M. Hypoxia-inducible factor-2alpha is essential in activating the COX2/mpges-1/PGE2 signaling axis in colon cancer. Carcinogenesis 2013, 34, 163–169. [Google Scholar] [CrossRef]
- Kaidi, A.; Qualtrough, D.; Williams, A.C.; Paraskeva, C. Direct transcriptional upregulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006, 66, 6683–6691. [Google Scholar] [CrossRef]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef]
- Nix, P.; Lind, M.; Greenman, J.; Stafford, N.; Cawkwell, L. Expression of cox-2 protein in radioresistant laryngeal cancer. Ann. Oncol. 2004, 15, 797–801. [Google Scholar] [CrossRef]
- Lin, F.; Luo, J.; Gao, W.; Wu, J.; Shao, Z.; Wang, Z.; Meng, J.; Ou, Z.; Yang, G. COX-2 promotes breast cancer cell radioresistance via p38/mapk-mediated cellular anti-apoptosis and invasiveness. Tumor Biol. 2013, 34, 2817–2826. [Google Scholar] [CrossRef]
- Suzuki, K.; Gerelchuluun, A.; Hong, Z.; Sun, L.; Zenkoh, J.; Moritake, T.; Tsuboi, K. Celecoxib enhances radiosensitivity of hypoxic glioblastoma cells through endoplasmic reticulum stress. Neuro-Oncology 2013, 15, 1186–1199. [Google Scholar] [CrossRef]
- Terakado, N.; Shintani, S.; Yano, J.; Chunnan, L.; Mihara, M.; Nakashiro, K.; Hamakawa, H. Overexpression of cyclooxygenase-2 is associated with radioresistance in oral squamous cell carcinoma. Oral Oncol. 2004, 40, 383–389. [Google Scholar] [CrossRef]
- Choy, H.; Milas, L. Enhancing radiotherapy with cyclooxygenase-2 enzyme inhibitors: A rational advance? J. Natl. Cancer Inst. 2003, 95, 1440–1452. [Google Scholar] [CrossRef]
- Tessner, T.G.; Muhale, F.; Riehl, T.E.; Anant, S.; Stenson, W.F. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving akt activation and bax translocation. J. Clin. Investig. 2004, 114, 1676–1685. [Google Scholar] [CrossRef]
- Laube, M.; Kniess, T.; Pietzsch, J. Development of antioxidant COX-2 inhibitors as radioprotective agents for radiation therapy-a hypothesis-driven review. Antioxidants (Basel) 2016, 5, 14. [Google Scholar] [CrossRef]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef]
- Bechmann, N.; Hauser, S.; Hofheinz, F.; Kniess, T.; Pietzsch, J. Nitric oxide-releasing selective cyclooxygenase-2 inhibitors as promising radiosensitizers in melanoma cells in vitro. Ann. Radiat. Ther. Oncol. 2017, 1, 1010. [Google Scholar]
- van Essen, M.; Krenning, E.P.; Kam, B.L.; de Herder, W.W.; van Aken, M.O.; Kwekkeboom, D.J. Report on short-term side effects of treatments with 177Lu-octreotate in combination with capecitabine in seven patients with gastroenteropancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 743–748. [Google Scholar] [CrossRef]
- Castinetti, F.; Kroiss, A.; Kumar, R.; Pacak, K.; Taieb, D. 15 years of paraganglioma: Imaging and imaging-based treatment of pheochromocytoma and paraganglioma. Endocr. Relat. Cancer 2015, 22, T135–T145. [Google Scholar] [CrossRef]
- Ullrich, M.; Bergmann, R.; Peitzsch, M.; Zenker, E.F.; Cartellieri, M.; Bachmann, M.; Ehrhart-Bornstein, M.; Block, N.L.; Schally, A.V.; Eisenhofer, G.; et al. Multimodal somatostatin receptor theranostics using [64Cu]Cu-/[177Lu]Lu-DOTA-(Tyr3)octreotate and AN-238 in a mouse pheochromocytoma model. Theranostics 2016, 6, 650–665. [Google Scholar] [CrossRef]
- Salmenkivi, K.; Haglund, C.; Ristimaki, A.; Arola, J.; Heikkila, P. Increased expression of cyclooxygenase-2 in malignant pheochromocytomas. J. Clin. Endocrinol. Metab. 2001, 86, 5615–5619. [Google Scholar] [CrossRef]
- Cadden, I.S.; Atkinson, A.B.; Johnston, B.T.; Pogue, K.; Connolly, R.; McCance, D.; Ardill, J.E.; Russell, C.F.; McGinty, A. Cyclooxygenase-2 expression correlates with phaeochromocytoma malignancy: Evidence for a bcl-2-dependent mechanism. Histopathology 2007, 51, 743–751. [Google Scholar] [CrossRef]
- Zhu, Y.; He, H.C.; Yuan, F.; Zhang, J.; Rui, W.B.; Zhao, J.P.; Shen, Z.J.; Ning, G. Heparanase-1 and cyclooxygenase-2: Prognostic indicators of malignancy in pheochromocytomas. Endocrine 2010, 38, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Zhu, Y.; Wang, X.; Wu, Y.; Zhou, W.; Jin, X.; Zhang, R.; Sun, F.; Kasoma, Z.; Shen, Z. Predictive factors for malignant pheochromocytoma: Analysis of 136 patients. J. Urol. 2011, 185, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Saffar, H.; Sanii, S.; Heshmat, R.; Haghpanah, V.; Larijani, B.; Rajabiani, A.; Azimi, S.; Tavangar, S.M. Expression of galectin-3, nm-23, and cyclooxygenase-2 could potentially discriminate between benign and malignant pheochromocytoma. Am. J. Clin. Pathol. 2011, 135, 454–460. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, R.; Chen, D.; Mao, J.; Shi, R.; Wu, Z.; Kang, J.; Tian, W.; Zhang, C. COX2 is involved in hypoxia-induced TNF-alpha expression in osteoblast. Sci. Rep. 2015, 5, 10020. [Google Scholar] [CrossRef] [PubMed]
- Campillo, N.; Torres, M.; Vilaseca, A.; Nonaka, P.N.; Gozal, D.; Roca-Ferrer, J.; Picado, C.; Montserrat, J.M.; Farre, R.; Navajas, D.; et al. Role of cyclooxygenase-2 on intermittent hypoxia-induced lung tumor malignancy in a mouse model of sleep apnea. Sci. Rep. 2017, 7, 44693. [Google Scholar] [CrossRef]
- Powers, J.F.; Evinger, M.J.; Tsokas, P.; Bedri, S.; Alroy, J.; Shahsavari, M.; Tischler, A.S. Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell Tissue Res. 2000, 302, 309–320. [Google Scholar] [CrossRef]
- Ullrich, M.; Liers, J.; Peitzsch, M.; Feldmann, A.; Bergmann, R.; Sommer, U.; Richter, S.; Bornstein, S.R.; Bachmann, M.; Eisenhofer, G.; et al. Strain-specific metastatic phenotypes in pheochromocytoma allograft mice. Endocr. Relat. Cancer 2018, 25, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimenez, E.; Gomez-Lopez, G.; Leandro-Garcia, L.J.; Munoz, I.; Schiavi, F.; Montero-Conde, C.; de Cubas, A.A.; Ramires, R.; Landa, I.; Leskela, S.; et al. Research resource: Transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol. Endocrinol. 2010, 24, 2382–2391. [Google Scholar] [CrossRef]
- Qin, N.; de Cubas, A.A.; Garcia-Martin, R.; Richter, S.; Peitzsch, M.; Menschikowski, M.; Lenders, J.W.; Timmers, H.J.; Mannelli, M.; Opocher, G.; et al. Opposing effects of HIF1α and HIF2α on chromaffin cell phenotypic features and tumor cell proliferation: Insights from myc-associated factor x. Int. J. Cancer 2014, 135, 2054–2064. [Google Scholar] [CrossRef]
- Pamporaki, C.; Hamplova, B.; Peitzsch, M.; Prejbisz, A.; Beuschlein, F.; Timmers, H.; Fassnacht, M.; Klink, B.; Lodish, M.; Stratakis, C.A.; et al. Characteristics of pediatric vs adult pheochromocytomas and paragangliomas. J. Clin. Endocrinol. Metab. 2017, 102, 1122–1132. [Google Scholar] [CrossRef]
- Deadwyler, G.D.; Dang, I.; Nelson, J.; Srikanth, M.; De Vries, G.H. Prostaglandin E2 metabolism is activated in schwann cell lines derived from human NF1 malignant peripheral nerve sheath tumors. Neuron Glia Biol. 2004, 1, 149–155. [Google Scholar] [CrossRef]
- Kniess, T.; Laube, M.; Bergmann, R.; Sehn, F.; Graf, F.; Steinbach, J.; Wuest, F.; Pietzsch, J. Radiosynthesis of a 18F-labeled 2,3-diarylsubstituted indole via McMurry coupling for functional characterization of cyclooxygenase-2 (COX-2) in vitro and in vivo. Bioorg. Med. Chem. 2012, 20, 3410–3421. [Google Scholar] [CrossRef]
- Laube, M.; Gassner, C.; Sharma, S.K.; Gunther, R.; Pigorsch, A.; Konig, J.; Kockerling, M.; Wuest, F.; Pietzsch, J.; Kniess, T. Diaryl-substituted (dihydro)pyrrolo[3,2,1-hi]indoles, a class of potent COX-2 inhibitors with tricyclic core structure. J. Org. Chem. 2015, 80, 5611–5624. [Google Scholar] [CrossRef]
- Laube, M.; Kniess, T.; Pietzsch, J. Radiolabeled COX-2 inhibitors for non-invasive visualization of COX-2 expression and activity—A critical update. Molecules 2013, 18, 6311–6355. [Google Scholar] [CrossRef]
- Sheng, H.; Williams, C.S.; Shao, J.; Liang, P.; DuBois, R.N.; Beauchamp, R.D. Induction of cyclooxygenase-2 by activated ha-ras oncogene in rat-1 fibroblasts and the role of mitogen-activated protein kinase pathway. J. Biol. Chem. 1998, 273, 22120–22127. [Google Scholar] [CrossRef]
- Sheng, H.; Shao, J.; Dixon, D.A.; Williams, C.S.; Prescott, S.M.; DuBois, R.N.; Beauchamp, R.D. Transforming growth factor-beta1 enhances ha-Ras-induced expression of cyclooxygenase-2 in intestinal epithelial cells via stabilization of mrna. J. Biol. Chem. 2000, 275, 6628–6635. [Google Scholar] [CrossRef]
- Lam, A.K.; Montone, K.T.; Nolan, K.A.; Livolsi, V.A. Ret oncogene activation in papillary thyroid carcinoma: Prevalence and implication on the histological parameters. Hum. Pathol. 1998, 29, 565–568. [Google Scholar] [CrossRef]
- Farhat, N.A.; Powers, J.F.; Shepard-Barry, A.; Dahia, P.; Pacak, K.; Tischler, A.S. A previously unrecognized monocytic component of pheochromocytoma and paraganglioma. Endocr. Pathol. 2019, 1–6. [Google Scholar] [CrossRef]
- Ferrandina, G.; Lauriola, L.; Zannoni, G.F.; Distefano, M.G.; Legge, F.; Salutari, V.; Gessi, M.; Maggiano, N.; Scambia, G.; Ranelletti, F.O. Expression of cyclooxygenase-2 (COX-2) in tumour and stroma compartments in cervical cancer: Clinical implications. Br. J. Cancer 2002, 87, 1145–1152. [Google Scholar] [CrossRef][Green Version]
- Curras-Freixes, M.; Pineiro-Yanez, E.; Montero-Conde, C.; Apellaniz-Ruiz, M.; Calsina, B.; Mancikova, V.; Remacha, L.; Richter, S.; Ercolino, T.; Rogowski-Lehmann, N.; et al. Pheoseq: A targeted next-generation sequencing assay for pheochromocytoma and paraganglioma diagnostics. J. Mol. Diagn. 2017, 19, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Khurana, P.; Sugadev, R.; Jain, J.; Singh, S.B. Hypoxiadb: A database of hypoxia-regulated proteins. Database (Oxf.) 2013, 2013, bat074. [Google Scholar] [CrossRef][Green Version]
- Salmenkivi, K.; Heikkila, P.; Haglund, C.; Arola, J. Malignancy in pheochromocytomas. APMIS 2004, 112, 551–559. [Google Scholar] [CrossRef]
- Unger, P.; Hoffman, K.; Pertsemlidis, D.; Thung, S.; Wolfe, D.; Kaneko, M. S100 protein-positive sustentacular cells in malignant and locally aggressive adrenal pheochromocytomas. Arch. Pathol. Lab. Med. 1991, 115, 484–487. [Google Scholar]
- Seifert, V.; Liers, J.; Kniess, T.; Richter, S.; Bechmann, N.; Feldmann, A.; Bachmann, M.; Eisenhofer, G.; Pietzsch, J.; Ullrich, M. Fluorescent mouse pheochromocytoma spheroids expressing hypoxia-inducible factor 2 alpha: Morphologic and radiopharmacologic characterization. J. Cell. Biotechnol. 2019, in press. [Google Scholar]
- Bechmann, N.; Poser, I.; Seifert, V.; Greunke, C.; Ullrich, M.; Qin, N.; Walch, A.; Peitzsch, M.; Robledo, M.; Pacak, K.; et al. Impact of extrinsic and intrinsic hypoxia on catecholamine biosynthesis in absence or presence of HIF2α in pheochromocytoma cells. Cancers 2019, 11, 594. [Google Scholar] [CrossRef]

| Mutant Gene | VHL | SDHx 1 | EPAS1 | NF1 | RET | HRAS | Total |
|---|---|---|---|---|---|---|---|
| Total cases (n) | 15 | 16 | 11 | 4 | 16 | 8 | 70 |
| Hereditary (n) | 10 | 15 | 0 | 3 | 14 | 0 | 42 |
| Sex (n) | |||||||
| Female | 6 | 9 | 10 | 4 | 8 | 3 | 40 |
| Male | 9 | 7 | 1 | 0 | 8 | 2 | 27 |
| Unknown | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| Tumor location (n) | |||||||
| A | 13 | 1 | 5 | 4 | 16 | 8 | 47 |
| A + TA | 2 | 2 | 2 | 0 | 0 | 0 | 6 |
| TA | 0 | 8 | 4 | 0 | 0 | 0 | 14 |
| HN | 0 | 5 | 0 | 0 | 0 | 0 | 5 |
| Tumor diameter (n) | |||||||
| <4 cm | 1 | 6 | 3 | 0 | 4 | 0 | 14 |
| ≥ 4 and ≤ 8 cm | 4 | 3 | 5 | 0 | 1 | 4 | 17 |
| >8 cm | 0 | 1 | 2 | 0 | 1 | 1 | 5 |
| Unknown | 10 | 6 | 1 | 4 | 10 | 3 | 34 |
| Mean (cm) | 4.4 ± 1.0 | 4.6±1.2 | 5.8 ± 1.2 | n.a. | 4.6 ± 1.2 | 5.9 ± 0.9 | 5.1 ± 0.5 |
| Age at diagnosis (years) | |||||||
| Range | 9−47 | 10−95 | 18−78 | 38−58 | 18−62 | 45−79 | 9−97 |
| Unknown | 1 | 0 | 0 | 1 | 0 | 2 | 4 |
| Mean | 24 ± 3.1 † | 27 ± 7.9 | 42 ± 6.4 | 48 ± 5.8 | 38 ± 6.5 | 64 ± 4.6 ‡ | 36 ± 2.3 |
| Metastatic (n) | 0 | 4 | 2 | 0 | 0 | 0 | 6 |
| Mutant Gene | VHL | SDHx 1 | EPAS1 | NF1 | RET | HRAS | Total |
|---|---|---|---|---|---|---|---|
| Total cases (n) | 14 | 39 | 7 | 21 | 9 | 6 | 96 |
| Hereditary (n) | 7 | 38 | 0 | 0 | 4 | 0 | 50 |
| Sex (n) | |||||||
| Female | 6 | 18 | 6 | 9 | 4 | 4 | 47 |
| Male | 8 | 21 | 1 | 12 | 5 | 2 | 49 |
| Tumor location (n) | |||||||
| A | 10 | 4 | 4 | 20 | 8 | 4 | 50 |
| TA | 3 | 15 | 3 | 1 | 1 | 2 | 25 |
| HN | 1 | 20 | 0 | 0 | 0 | 0 | 21 |
| Tumor diameter (n) | |||||||
| <4 cm | 3 | 22 | 2 | 6 | 2 | 2 | 35 |
| ≥4 and ≤8 cm | 7 | 13 | 5 | 11 | 3 | 3 | 44 |
| >8 cm | 0 | 2 | 0 | 0 | 3 | 0 | 5 |
| Unknown | 4 | 2 | 0 | 4 | 1 | 0 | 12 |
| Mean (cm) | 4.6 ± 0.6 | 3.9 ± 0.4 * | 4.5 ± 0.6 | 4.1 ± 0.3 | 7.2 ± 1.5 * | 3.8 ± 0.5 | 4.4 ± 0.3 |
| Age at diagnosis (years) | |||||||
| Range | 9−49 | 14−71 | 17−75 | 20−74 | 33−72 | 28−81 | 9−81 |
| Mean | 25 ± 3.9 ‡ | 38 ± 2.7 † | 42 ± 8.7 | 52 ± 2.8 † | 49 ± 4.2 | 58 ± 7.2 * | 42 ± 1.7 |
| Metastatic (n) | 1 | 8 | 0 | 1 | 1 | 1 | 12 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullrich, M.; Richter, S.; Seifert, V.; Hauser, S.; Calsina, B.; Martínez-Montes, Á.M.; ter Laak, M.; Ziegler, C.G.; Timmers, H.; Eisenhofer, G.; et al. Targeting Cyclooxygenase-2 in Pheochromocytoma and Paraganglioma: Focus on Genetic Background. Cancers 2019, 11, 743. https://doi.org/10.3390/cancers11060743
Ullrich M, Richter S, Seifert V, Hauser S, Calsina B, Martínez-Montes ÁM, ter Laak M, Ziegler CG, Timmers H, Eisenhofer G, et al. Targeting Cyclooxygenase-2 in Pheochromocytoma and Paraganglioma: Focus on Genetic Background. Cancers. 2019; 11(6):743. https://doi.org/10.3390/cancers11060743
Chicago/Turabian StyleUllrich, Martin, Susan Richter, Verena Seifert, Sandra Hauser, Bruna Calsina, Ángel M. Martínez-Montes, Marjolein ter Laak, Christian G. Ziegler, Henri Timmers, Graeme Eisenhofer, and et al. 2019. "Targeting Cyclooxygenase-2 in Pheochromocytoma and Paraganglioma: Focus on Genetic Background" Cancers 11, no. 6: 743. https://doi.org/10.3390/cancers11060743
APA StyleUllrich, M., Richter, S., Seifert, V., Hauser, S., Calsina, B., Martínez-Montes, Á. M., ter Laak, M., Ziegler, C. G., Timmers, H., Eisenhofer, G., Robledo, M., & Pietzsch, J. (2019). Targeting Cyclooxygenase-2 in Pheochromocytoma and Paraganglioma: Focus on Genetic Background. Cancers, 11(6), 743. https://doi.org/10.3390/cancers11060743






