Targeted Therapies and Immune-Checkpoint Inhibition in Head and Neck Squamous Cell Carcinoma: Where Do We Stand Today and Where to Go?
Abstract
:1. Introduction
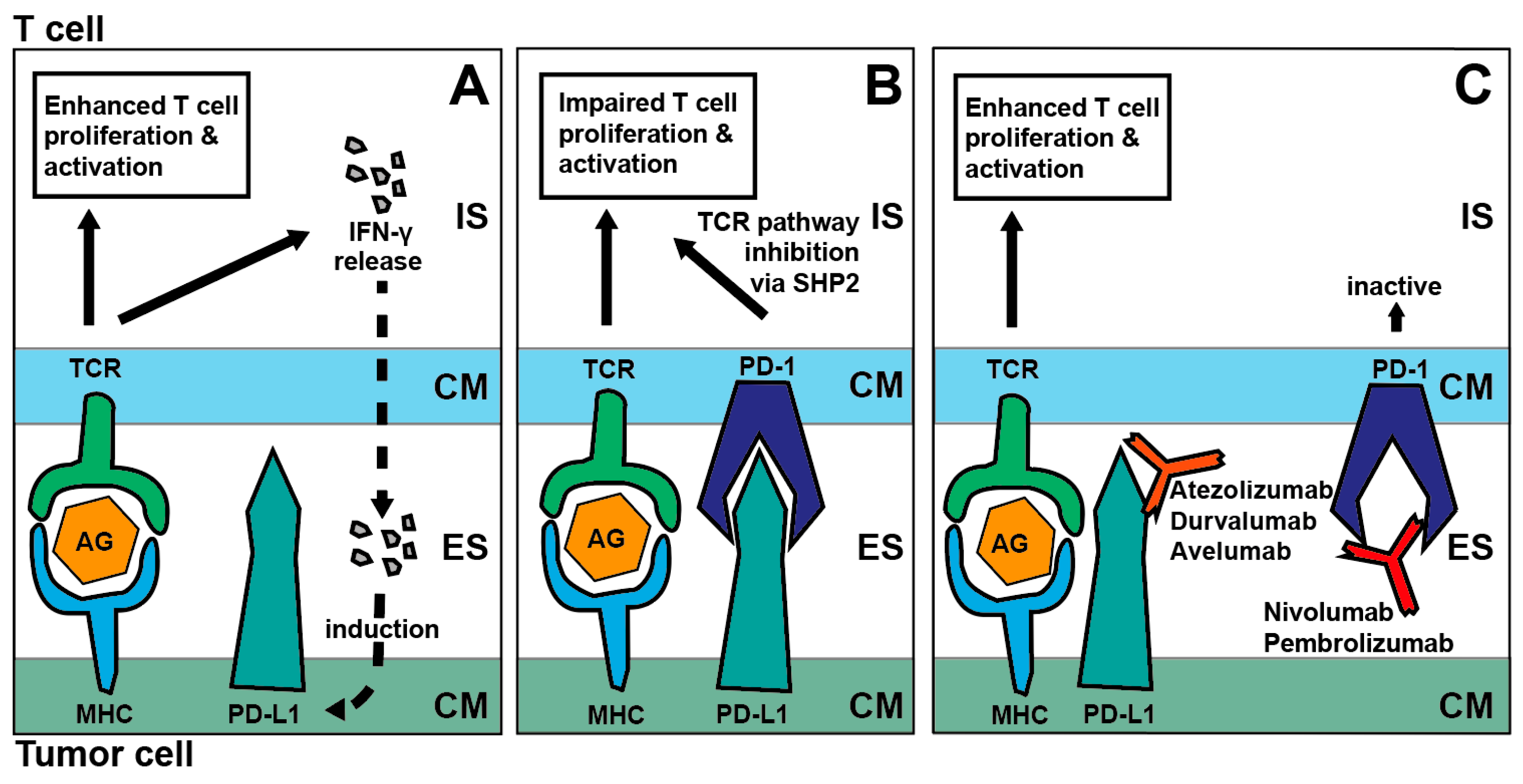
2. Immune-Checkpoint Inhibition in SCCHN: Mode of Action
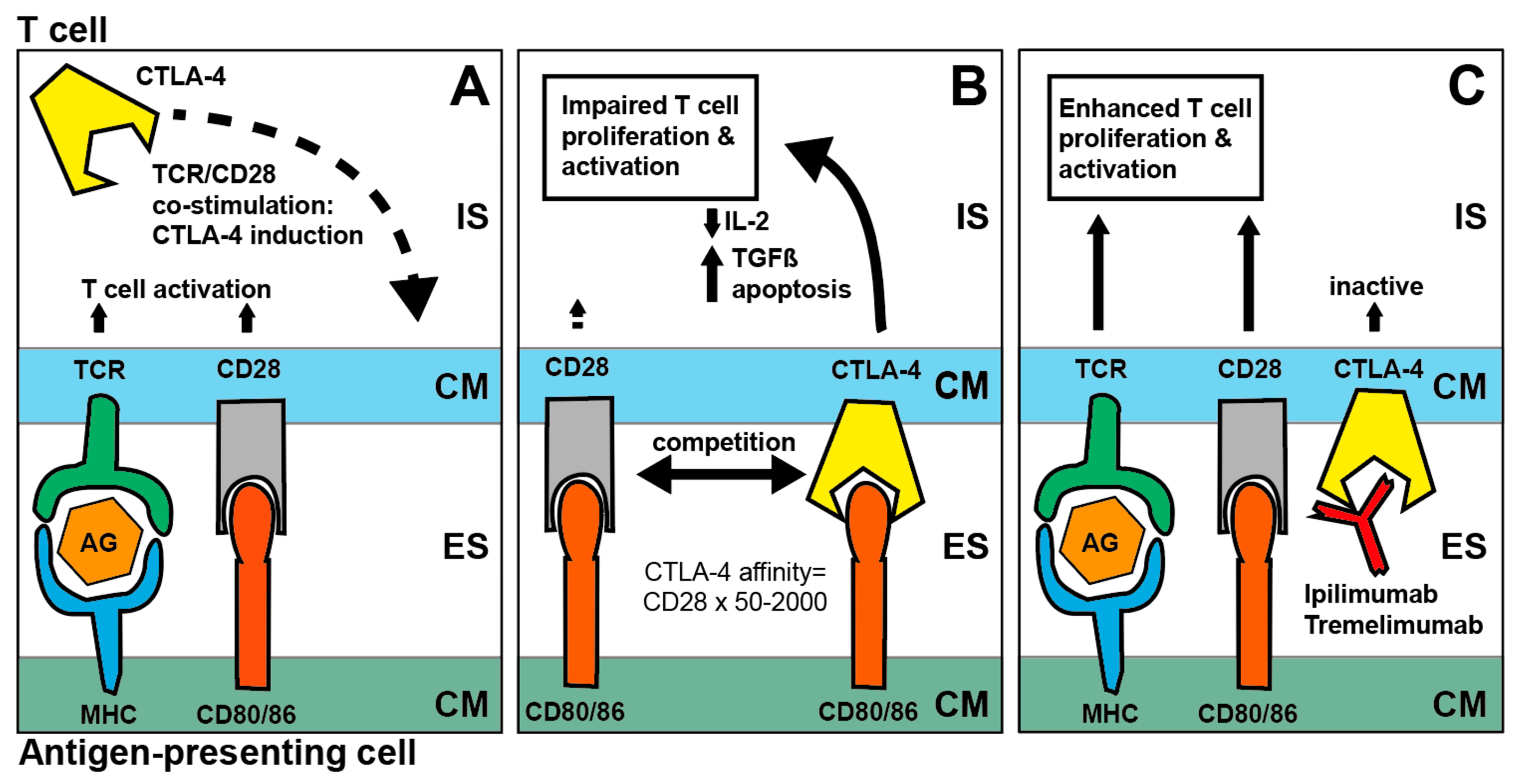
2.1. PD-1/PD-L1 Axis and CTLA4 Blockade
2.2. Immune-Checkpoint Inhibition in the Recurrent/Metastatic Situation
2.2.1. PD-1 Inhibition
2.2.2. Oligometastatic Disease
2.2.3. Combined PD-1 and CTLA-4 Inhibition
2.2.4. PD-L1 Inhibition
2.3. Immune-Checkpoint Inhibition in the Curative Setting
3. Targeted Therapies for SCCHN
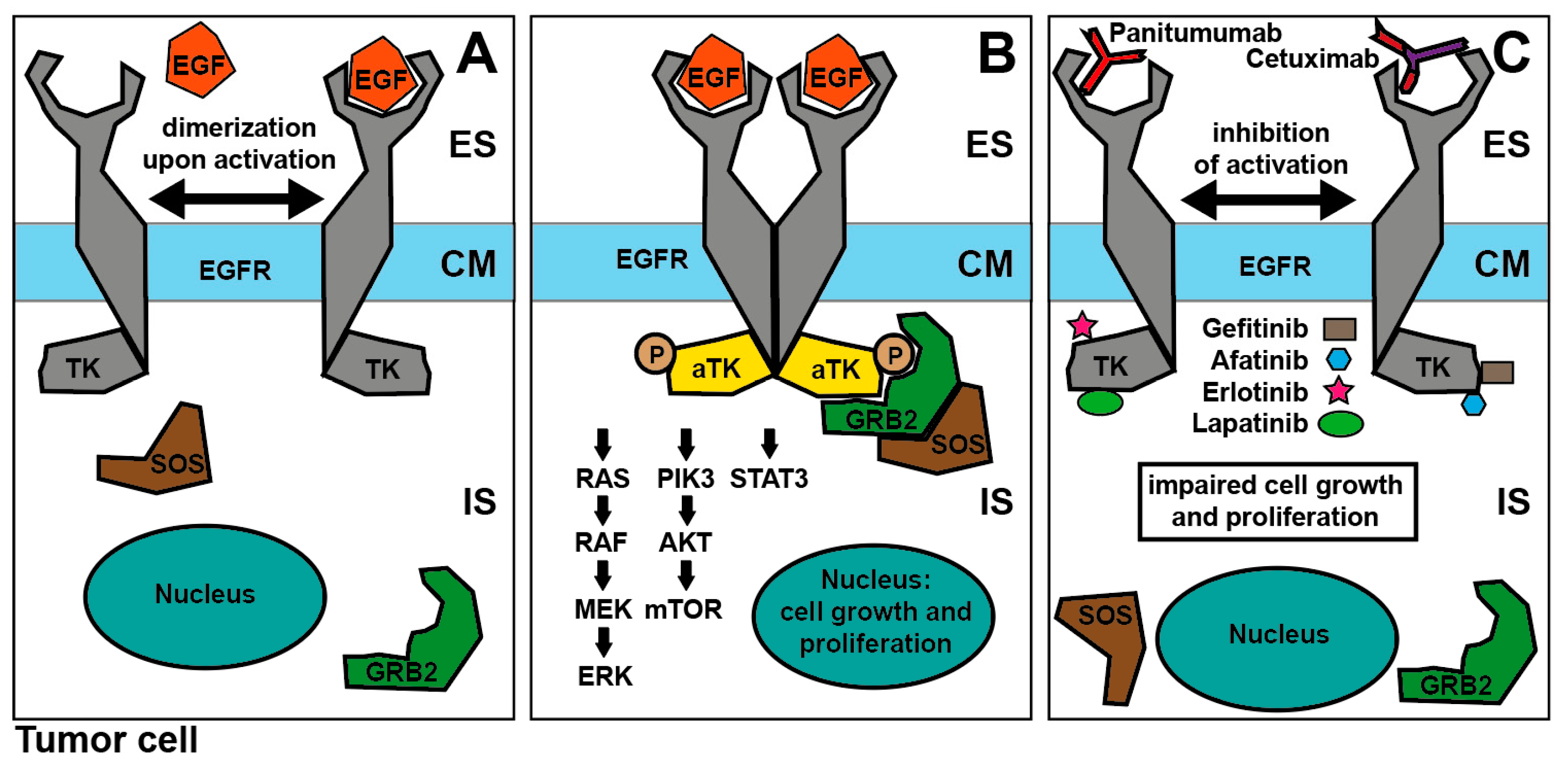
3.1. EGFR-Inhibition: Mode of Action
3.2. EGFR-Inhibition for SCCHN
3.3. Targeted Therapies beyond EGFR-Inhibition
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- Sikora, A.G.; Toniolo, P.; DeLacure, M.D. The changing demographics of head and neck squamous cell carcinoma in the United States. Laryngoscope 2004, 114, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, V.; Lefebvre, J.L.; Licitra, L.; Felip, E.; EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2010, 21 (Suppl. 5), v184–v186. [Google Scholar] [CrossRef] [PubMed]
- Bernier, J.; Domenge, C.; Ozsahin, M.; Matuszewska, K.; Lefebvre, J.L.; Greiner, R.H.; Giralt, J.; Maingon, P.; Rolland, F.; Bolla, M.; et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N. Engl. J. Med. 2004, 350, 1945–1952. [Google Scholar] [CrossRef]
- Cooper, J.S.; Pajak, T.F.; Forastiere, A.A.; Jacobs, J.; Campbell, B.H.; Saxman, S.B.; Kish, J.A.; Kim, H.E.; Cmelak, A.J.; Rotman, M.; et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004, 350, 1937–1944. [Google Scholar] [CrossRef]
- Pignon, J.P.; le Maitre, A.; Maillard, E.; Bourhis, J.; MACH-NC Collaborative Group. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): An update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 2009, 92, 4–14. [Google Scholar] [CrossRef]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Azarnia, N.; Shin, D.M.; Cohen, R.B.; Jones, C.U.; Sur, R.; Raben, D.; Jassem, J.; et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2006, 354, 567–578. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Topalian, S.L.; Drake, C.G.; Pardoll, D.M. Immune checkpoint blockade: A common denominator approach to cancer therapy. Cancer Cell 2015, 27, 450–461. [Google Scholar] [CrossRef]
- Sharabi, A.B.; Lim, M.; DeWeese, T.L.; Drake, C.G. Radiation and checkpoint blockade immunotherapy: Radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015, 16, e498–e509. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Barker, C.A.; Yamada, Y.; Yuan, J.; Kitano, S.; Mu, Z.; Rasalan, T.; Adamow, M.; Ritter, E.; et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 2012, 366, 925–931. [Google Scholar] [CrossRef]
- Golden, E.B.; Demaria, S.; Schiff, P.B.; Chachoua, A.; Formenti, S.C. An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol. Res. 2013, 1, 365–372. [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N. Engl. J. Med. 2016, 375, 1856–1867. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Soulieres, D.; Le Tourneau, C.; Dinis, J.; Licitra, L.; Ahn, M.J.; Soria, A.; Machiels, J.P.; Mach, N.; Mehra, R.; et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): A randomised, open-label, phase 3 study. Lancet 2018. [Google Scholar] [CrossRef]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; De Castro, G., Jr.; Psyrri, A.; Baste Rotllan, N.; Neupane, P.C.; Bratland, Å.; et al. First-line pembrolizumab for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC): Interim results from the phase 3 KEYNOTE-048 study. Ann. Oncol. 2018, 29 (Suppl. 8). [Google Scholar] [CrossRef]
- Baumeister, S.H.; Freeman, G.J.; Dranoff, G.; Sharpe, A.H. Coinhibitory Pathways in Immunotherapy for Cancer. Annu. Rev. Immunol. 2016, 34, 539–573. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Okazaki, T.; Chikuma, S.; Iwai, Y.; Fagarasan, S.; Honjo, T. A rheostat for immune responses: The unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 2013, 14, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Yearley, J.H.; Gibson, C.; Yu, N.; Moon, C.; Murphy, E.; Juco, J.; Lunceford, J.; Cheng, J.; Chow, L.Q.M.; Seiwert, T.Y.; et al. PD-L2 Expression in Human Tumors: Relevance to Anti-PD-1 Therapy in Cancer. Clin. Cancer Res. 2017, 23, 3158–3167. [Google Scholar] [CrossRef]
- Chambers, C.A.; Kuhns, M.S.; Egen, J.G.; Allison, J.P. CTLA-4-mediated inhibition in regulation of T cell responses: Mechanisms and manipulation in tumor immunotherapy. Annu. Rev. Immunol. 2001, 19, 565–594. [Google Scholar] [CrossRef]
- Walunas, T.L.; Lenschow, D.J.; Bakker, C.Y.; Linsley, P.S.; Freeman, G.J.; Green, J.M.; Thompson, C.B.; Bluestone, J.A. CTLA-4 can function as a negative regulator of T cell activation. Immunity 1994, 1, 405–413. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef]
- Ribas, A.; Camacho, L.H.; Lopez-Berestein, G.; Pavlov, D.; Bulanhagui, C.A.; Millham, R.; Comin-Anduix, B.; Reuben, J.M.; Seja, E.; Parker, C.A.; et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J. Clin. Oncol. 2005, 23, 8968–8977. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Mihm, M.C.; Soiffer, R.J.; Haluska, F.G.; Butler, M.; Seiden, M.V.; Davis, T.; Henry-Spires, R.; MacRae, S.; Willman, A.; et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc. Natl. Acad. Sci. USA 2003, 100, 4712–4717. [Google Scholar] [CrossRef]
- Collins, A.V.; Brodie, D.W.; Gilbert, R.J.; Iaboni, A.; Manso-Sancho, R.; Walse, B.; Stuart, D.I.; van der Merwe, P.A.; Davis, S.J. The interaction properties of costimulatory molecules revisited. Immunity 2002, 17, 201–210. [Google Scholar] [CrossRef]
- Tivol, E.A.; Borriello, F.; Schweitzer, A.N.; Lynch, W.P.; Bluestone, J.A.; Sharpe, A.H. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995, 3, 541–547. [Google Scholar] [CrossRef]
- Waterhouse, P.; Penninger, J.M.; Timms, E.; Wakeham, A.; Shahinian, A.; Lee, K.P.; Thompson, C.B.; Griesser, H.; Mak, T.W. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995, 270, 985–988. [Google Scholar] [CrossRef]
- Mole, R.H. Whole body irradiation; radiobiology or medicine? Br. J. Radiol. 1953, 26, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Bruce, A.T.; Ikeda, H.; Old, L.J.; Schreiber, R.D. Cancer immunoediting: From immunosurveillance to tumor escape. Nat. Immunol. 2002, 3, 991–998. [Google Scholar] [CrossRef]
- Bhardwaj, N. Harnessing the immune system to treat cancer. J. Clin. Investig. 2007, 117, 1130–1136. [Google Scholar] [CrossRef]
- Miyauchi, S.; Kim, S.S.; Pang, J.; Gold, K.A.; Gutkind, J.S.; Califano, J.; Mell, L.K.; Cohen, E.E.W.; Sharabi, A.B. Immune modulation of head and neck squamous cell carcinoma and the tumor microenvironment by conventional therapeutics. Clin. Cancer Res. 2019. [Google Scholar] [CrossRef]
- Cristescu, R.; Mogg, R.; Ayers, M.; Albright, A.; Murphy, E.; Yearley, J.; Sher, X.; Liu, X.Q.; Lu, H.; Nebozhyn, M.; et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018, 362. [Google Scholar] [CrossRef]
- Sindoni, A.; Minutoli, F.; Ascenti, G.; Pergolizzi, S. Combination of immune checkpoint inhibitors and radiotherapy: Review of the literature. Crit. Rev. Oncol. Hematol. 2017, 113, 63–70. [Google Scholar] [CrossRef]
- Torphy, R.J.; Schulick, R.D.; Zhu, Y. Newly Emerging Immune Checkpoints: Promises for Future Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2642. [Google Scholar] [CrossRef]
- Ward, F.J.; Dahal, L.N.; Abu-Eid, R. On the Road to Immunotherapy-Prospects for Treating Head and Neck Cancers with Checkpoint Inhibitor Antibodies. Front. Immunol. 2018, 9, 2182. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): An open-label, multicentre, phase 1b trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Chow, L.Q.M.; Haddad, R.; Gupta, S.; Mahipal, A.; Mehra, R.; Tahara, M.; Berger, R.; Eder, J.P.; Burtness, B.; Lee, S.H.; et al. Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort. J. Clin. Oncol. 2016, 34, 3838–3845. [Google Scholar] [CrossRef]
- Mehra, R.; Seiwert, T.Y.; Gupta, S.; Weiss, J.; Gluck, I.; Eder, J.P.; Burtness, B.; Tahara, M.; Keam, B.; Kang, H.; et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: Pooled analyses after long-term follow-up in KEYNOTE-012. Br. J. Cancer 2018, 119, 153–159. [Google Scholar] [CrossRef]
- Mitchell, T.C.; Hamid, O.; Smith, D.C.; Bauer, T.M.; Wasser, J.S.; Olszanski, A.J.; Luke, J.J.; Balmanoukian, A.S.; Schmidt, E.V.; Zhao, Y.; et al. Epacadostat Plus Pembrolizumab in Patients with Advanced Solid Tumors: Phase I Results from a Multicenter, Open-Label Phase I/II Trial (ECHO-202/KEYNOTE-037). J. Clin. Oncol. 2018. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Rischin, D.; Pfister, D.G.; Vermorken, J.B.; Zhao, Y.; Gowda, H.; Ge, J.Y.; Jin, F.; Harrington, K.J. A phase 3, randomized, open-label study of epacadostat plus pembrolizumab, pembrolizumab monotherapy, and the EXTREME regimen as first-line treatment for recurrent/metastatic head and neck squamous cell carcinoma (R/M SCCHN): ECHO-304/KEYNOTE-669. J. Clin. Oncol. 2018, 36, TPS6090. [Google Scholar] [CrossRef]
- Bauml, J.; Seiwert, T.Y.; Pfister, D.G.; Worden, F.; Liu, S.V.; Gilbert, J.; Saba, N.F.; Weiss, J.; Wirth, L.; Sukari, A.; et al. Pembrolizumab for Platinum- and Cetuximab-Refractory Head and Neck Cancer: Results from a Single-Arm, Phase II Study. J. Clin. Oncol. 2017, 35, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Harrington, K.J.; Tourneau, C.L.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Pembrolizumab (pembro) vs standard of care (SOC) for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC): Phase 3 KEYNOTE-040 trial. Ann. Oncol. 2017, 28 (Suppl. 5). [Google Scholar] [CrossRef]
- Soulieres, D.; Cohen, E.; Tourneau, C.L.; Dinis, J.; Licitra, L.; Ahn, M.-J.; Soria, A.; Machiels, J.-P.; Mach, N.; Mehra, R.; et al. Updated survival results of the KEYNOTE-040 study of pembrolizumab vs standard-of-care chemotherapy for recurrent or metastatic head and neck squamous cell carcinoma. Cancer Res. 2018, 78 (Suppl. 13). [Google Scholar] [CrossRef]
- Ferris, R.L.; Blumenschein, G., Jr.; Fayette, J.; Guigay, J.; Colevas, A.D.; Licitra, L.; Harrington, K.J.; Kasper, S.; Vokes, E.E.; Even, C.; et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018, 81, 45–51. [Google Scholar] [CrossRef] [PubMed]
- McBride, S.M.; Sherman, E.J.; Tsai, C.J.; Baxi, S.S.; Aghalar, J.; Eng, J.; Zhi, W.I.; McFarland, D.C.; Michel, L.S.; Spielsinger, D.; et al. A phase II randomized trial of nivolumab with stereotactic body radiotherapy (SBRT) versus nivolumab alone in metastatic (M1) head and neck squamous cell carcinoma (HNSCC). J. Clin. Oncol. 2018, 36, 6009. [Google Scholar] [CrossRef]
- Haddad, R.; Gillison, M.; Ferris, R.L.; Harrington, K.; Monga, M.; Baudelet, C.; Geese, W.J.; Argiris, A. Double-blind, two-arm, phase 2 study of nivolumab (nivo) in combination with ipilimumab (ipi) versus nivo and ipi-placebo (PBO) as first-line (1L) therapy in patients (pts) with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN)—CheckMate 714. Ann. Oncol. 2016, 27, 1017TiP. [Google Scholar] [CrossRef]
- Argiris, A.; Gillison, M.; Ferris, R.L.; Harrington, K.; Sanchez, T.K.; Baudelet, C.; Geese, W.J.; Shaw, J.; Haddad, R. A randomized, open-label, phase 3 study of nivolumab in combination with ipilimumab vs. extreme regimen (cetuximab + cisplatin/carboplatin + fluorouracil) as first-line therapy in patients with recurrent or metastatic squamous cell carcinoma of the head and neck-CheckMate 651. Ann. Oncol. 2016, 27, 1016TiP. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Segal, N.H.; Ou, S.-H.I.; Balmanoukian, A.S.; Fury, M.G.; Massarelli, E.; Brahmer, J.R.; Weiss, J.; Schoffski, P.; Antonia, S.J.; Massard, C.; et al. Safety and efficacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. J. Clin. Oncol. 2015, 33, 3011. [Google Scholar] [CrossRef]
- Segal, N.H.; Ou, S.-H.I.; Balmanoukian, A.S.; Massarelli, E.; Brahmer, J.R.; Weiss, J.; Schoffski, P.; Antonia, S.J.; Massard, C.; Zandberg, D.P.; et al. Updated safety and efficacy of durvalumab (MEDI4736), an anti-PD-L 1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. Ann. Oncol. 2016, 27, 949O. [Google Scholar] [CrossRef]
- Siu, L.L.; Even, C.; Mesia, R.; Remenar, E.; Daste, A.; Delord, J.P.; Krauss, J.; Saba, N.F.; Nabell, L.; Ready, N.E.; et al. Safety and Efficacy of Durvalumab with or without Tremelimumab in Patients with PD-L1-Low/Negative Recurrent or Metastatic HNSCC: The Phase 2 CONDOR Randomized Clinical Trial. JAMA Oncol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zandberg, D.P.; Algazi, A.P.; Jimeno, A.; Good, J.S.; Fayette, J.; Bouganim, N.; Ready, N.E.; Clement, P.M.; Even, C.; Jang, R.W.; et al. Durvalumab for recurrent or metastatic head and neck squamous cell carcinoma: Results from a single-arm, phase II study in patients with >/=25% tumour cell PD-L1 expression who have progressed on platinum-based chemotherapy. Eur. J. Cancer 2019, 107, 142–152. [Google Scholar] [CrossRef]
- Ferris, R.L.; Even, C.; Haddad, R.; Tahara, M.; Goswami, T.; Franks, A.; Emeribe, U.; Jarkowski, A., III; Melillo, G.; Licitra, L. Phase III, randomized, open-label study of durvalumab (MEDI4736) monotherapy, or durvalumab + tremelimumab, versus standard of care (SoC), in recurrent or metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN): Eagle. J. Immunother. Cancer 2015, 3, P150. [Google Scholar] [CrossRef]
- AstraZeneca. Update on the Phase III EAGLE Trial of Imfinzi and Tremelimumab in Advanced Head and Neck Cancer. 2018. Available online: https://www.astrazeneca.com/media-centre/press-releases/2018/update-on-the-phase-iii-eagle-trial-of-imfinzi-and-tremelimumab-in-advanced-head-and-neck-cancer-07122018.html (accessed on 27 January 2019).
- Seiwert, T.Y.; Weiss, J.; Baxi, S.S.; Ahn, M.-J.; Fayette, J.; Gillison, M.L.; Machiels, J.-P.H.; Takahashi, S.; Melillo, G.; Franks, A.; et al. A phase 3, randomized, open-label study of first-line durvalumab (MEDI4736) ± tremelimumab versus standard of care (SoC.; EXTREME regimen) in recurrent/metastatic (R/M) SCCHN: KESTREL. J. Clin. Oncol. 2016, 34. [Google Scholar] [CrossRef]
- Colevas, A.D.; O’Hear, C.; Kabbinavar, F.; Fassò, M.; Powderly, J.; Bahleda, R.; Braiteh, F.; Balmanoukian, A.; Brana, I.; Chau, N.G.; et al. Safety and clinical activity of atezolizumab in head and neck cancer: Results from a phase I trial. Ann. Oncol. 2018, 29, 2247–2253. [Google Scholar] [CrossRef] [PubMed]
- Haddad, R.; Raben, D.; Wong, D.J.; Guo, Y.; Fayette, J.; Cohen, E.E.W.; Kowgier, M.; Sandler, A.; Matheny, C.; Kabbinavar, F. 1117TiPIMvoke010: Randomized phase III study of atezolizumab (atezo) as adjuvant monotherapy after definitive therapy of squamous cell carcinoma of the head and neck (SCCHN). Ann. Oncol. 2018, 29. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ferris, R.L.; Beck, J.T.; Harrington, K.; Haddad, R.I.; Bourhis, J.; Tahara, M.; Geraldes, M.; Nuyten, D.S.A.; Goldberg, Z.; et al. JAVELIN head and neck 100: A phase 3 trial of avelumab in combination with chemoradiotherapy (CRT) vs. CRT for 1st-line treatment of locally advanced squamous cell carcinoma of the head and neck (LA SCCHN). J. Clin. Oncol. 2017, 35. [Google Scholar] [CrossRef]
- Sun, X.S.; Sire, C.; Tao, Y.; Martin, L.; Alfonsi, M.; Prevost, J.B.; Rives, M.; Lafond, C.; Tourani, J.-M.; Biau, J.; et al. A phase II randomized trial of pembrolizumab versus cetuximab, concomitant with radiotherapy (RT) in locally advanced (LA) squamous cell carcinoma of the head and neck (SCCHN): First results of the GORTEC 2015-01 “PembroRad” trial. J. Clin. Oncol. 2018, 36, 6018. [Google Scholar] [CrossRef]
- Powell, S.F.; Gitau, M.M.; Sumey, C.J.; Reynolds, J.T.; Lohr, M.; McGraw, S.; Nowak, R.K.; Terrell, A.M.; Jensen, A.W.; Blanchard, M.J.; et al. Safety of pembrolizumab with chemoradiation (CRT) in locally advanced squamous cell carcinoma of the head and neck (LA-SCCHN). J. Clin. Oncol. 2017, 35, 6011. [Google Scholar] [CrossRef]
- Tao, Y.; Auperin, A.; Sun, X.S.; Sire, C.; Martin, L.; Bera, G.; Coutte, A.; Miroir, J.; Lafond, C.; Colin-Batailhou, N.; et al. Avelumab-cetuximab-radiotherapy (RT) versus standards of care (SoC) in locally advanced squamous cell carcinoma of the head and neck (SCCHN): Safety phase of the randomized trial GORTEC 2017-01 (REACH). J. Clin. Oncol. 2018, 36, 6076. [Google Scholar] [CrossRef]
- Siu, L.L.; Licitra, L.; Tao, Y.G.; Yen, C.-J.; Rischin, D.; Waldron, J.; Burtness, B.; Gregoire, V.; Agarwala, S.; Yorio, J.; et al. Abstract CT163: KEYNOTE-412: Pembrolizumab plus chemoradiation vs chemoradiation alone for locally advanced head and neck squamous cell carcinoma. Cancer Res. 2018, 78, CT163. [Google Scholar] [CrossRef]
- Ceresa, B.P.; Peterson, J.L. Cell and molecular biology of epidermal growth factor receptor. Int. Rev. Cell Mol. Biol. 2014, 313, 145–178. [Google Scholar] [CrossRef]
- Rödel, F.; Balermpas, P. Anti-epidermal growth factor receptor immunotherapy in combination with cisplatin chemoradiation for patients with advanced head and neck carcinoma—Biological and clinical limitations of the triple treatment. Transl. Cancer Res. 2016, 5, 199–202. [Google Scholar] [CrossRef]
- Burtness, B. The role of cetuximab in the treatment of squamous cell cancer of the head and neck AU—Burtness, Barbara. Expert Opin. Biol. Ther. 2005, 5, 1085–1093. [Google Scholar] [CrossRef] [PubMed]
- Milas, L.; Mason, K.; Hunter, N.; Petersen, S.; Yamakawa, M.; Ang, K.; Mendelsohn, J.; Fan, Z. In Vivo Enhancement of Tumor Radioresponse by C225 Antiepidermal Growth Factor Receptor Antibody. Clin. Cancer Res. 2000, 6, 701–708. [Google Scholar] [PubMed]
- Baselga, J.; Pfister, D.; Cooper, M.R.; Cohen, R.; Burtness, B.; Bos, M.; D’Andrea, G.; Seidman, A.; Norton, L.; Gunnett, K.; et al. Phase I Studies of Anti–Epidermal Growth Factor Receptor Chimeric Antibody C225 Alone and in Combination with Cisplatin. J. Clin. Oncol. 2000, 18, 904. [Google Scholar] [CrossRef]
- Robert, F.; Ezekiel, M.P.; Spencer, S.A.; Meredith, R.F.; Bonner, J.A.; Khazaeli, M.B.; Saleh, M.N.; Carey, D.; LoBuglio, A.F.; Wheeler, R.H.; et al. Phase I Study of Anti–Epidermal Growth Factor Receptor Antibody Cetuximab in Combination with Radiation Therapy in Patients With Advanced Head and Neck Cancer. J. Clin. Oncol. 2001, 19, 3234–3243. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Carbonell, X.; Castañeda-Soto, N.-J.; Clemens, M.; Green, M.; Harvey, V.; Morales, S.; Barton, C.; Ghahramani, P. Phase II Study of Efficacy, Safety, and Pharmacokinetics of Trastuzumab Monotherapy Administered on a 3-Weekly Schedule. J. Clin. Oncol. 2005, 23, 2162–2171. [Google Scholar] [CrossRef]
- Herbst, R.S.; Arquette, M.; Shin, D.M.; Dicke, K.; Vokes, E.E.; Azarnia, N.; Hong, W.K.; Kies, M.S. Phase II Multicenter Study of the Epidermal Growth Factor Receptor Antibody Cetuximab and Cisplatin for Recurrent and Refractory Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2005, 23, 5578–5587. [Google Scholar] [CrossRef]
- Pfister, D.G.; Su, Y.B.; Kraus, D.H.; Wolden, S.L.; Lis, E.; Aliff, T.B.; Zahalsky, A.J.; Lake, S.; Needle, M.N.; Shaha, A.R.; et al. Concurrent Cetuximab, Cisplatin, and Concomitant Boost Radiotherapy for Locoregionally Advanced, Squamous Cell Head and Neck Cancer: A Pilot Phase II Study of a New Combined-Modality Paradigm. J. Clin. Oncol. 2006, 24, 1072–1078. [Google Scholar] [CrossRef]
- Tahara, M.; Suzuki, M.; Minato, K.; Yane, K.; Ueda, S.; Hara, H.; Saijo, K.; Yamanaka, T.; Kiyota, N.; Yokota, T.; et al. Phase II trial of combination treatment with paclitaxel, carboplatin and cetuximab (PCE) as first-line treatment in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck (CSPOR-HN02). Ann. Oncol. 2018, 29, 1004–1009. [Google Scholar] [CrossRef] [PubMed]
- The National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Head and Neck Cancers Version 2.2018. Available online: https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf (accessed on 6 February 2019).
- Balermpas, P.; Keller, C.; Hambek, M.; Wagenblast, J.; Seitz, O.; Rödel, C.; Weiss, C. Reirradiation with Cetuximab in Locoregional Recurrent and Inoperable Squamous Cell Carcinoma of the Head and Neck: Feasibility and First Efficacy Results. Int. J. Radiat. Oncol. Biol. Phys. 2012, 83, e377–e383. [Google Scholar] [CrossRef] [PubMed]
- Dornoff, N.; Weiß, C.; Rödel, F.; Wagenblast, J.; Ghanaati, S.; Atefeh, N.; Rödel, C.; Balermpas, P. Re-irradiation with cetuximab or cisplatin-based chemotherapy for recurrent squamous cell carcinoma of the head and neck. Strahlentherapie und Onkologie 2015, 191, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Bonner, J.A.; Harari, P.M.; Giralt, J.; Cohen, R.B.; Jones, C.U.; Sur, R.K.; Raben, D.; Baselga, J.; Spencer, S.A.; Zhu, J.; et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 2010, 11, 21–28. [Google Scholar] [CrossRef]
- Mesia, R.; Henke, M.; Fortin, A.; Minn, H.; Yunes Ancona, A.C.; Cmelak, A.; Markowitz, A.B.; Hotte, S.J.; Singh, S.; Chan, A.T.; et al. Chemoradiotherapy with or without panitumumab in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-1): A randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015, 16, 208–220. [Google Scholar] [CrossRef]
- Giralt, J.; Trigo, J.; Nuyts, S.; Ozsahin, M.; Skladowski, K.; Hatoum, G.; Daisne, J.F.; Yunes Ancona, A.C.; Cmelak, A.; Mesia, R.; et al. Panitumumab plus radiotherapy versus chemoradiotherapy in patients with unresected, locally advanced squamous-cell carcinoma of the head and neck (CONCERT-2): A randomised, controlled, open-label phase 2 trial. Lancet Oncol. 2015, 16, 221–232. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Mortenson, E.D.; Radkevich-Brown, O.; Wang, Y.; Fu, Y.-X. Cetuximab-mediated tumor regression depends on innate and adaptive immune responses. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 91–100. [Google Scholar] [CrossRef]
- Peng, D.; Fan, Z.; Lu, Y.; DeBlasio, T.; Scher, H.; Mendelsohn, J. Anti-Epidermal Growth Factor Receptor Monoclonal Antibody 225 Up-Regulates p27KIP1 and Induces G1 Arrest in Prostatic Cancer Cell Line DU145. Cancer Res. 1996, 56, 3666–3669. [Google Scholar]
- Soulieres, D.; Senzer, N.N.; Vokes, E.E.; Hidalgo, M.; Agarwala, S.S.; Siu, L.L. Multicenter Phase II Study of Erlotinib, an Oral Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor, in Patients with Recurrent or Metastatic Squamous Cell Cancer of the Head and Neck. J. Clin. Oncol. 2004, 22, 77–85. [Google Scholar] [CrossRef]
- Elser, C.; Siu, L.L.; Winquist, E.; Agulnik, M.; Pond, G.R.; Chin, S.F.; Francis, P.; Cheiken, R.; Elting, J.; McNabola, A.; et al. Phase II Trial of Sorafenib in Patients with Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck or Nasopharyngeal Carcinoma. J. Clin. Oncol. 2007, 25, 3766–3773. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Davis, D.W.; Karrison, T.G.; Seiwert, T.Y.; Wong, S.J.; Nattam, S.; Kozloff, M.F.; Clark, J.I.; Yan, D.-H.; Liu, W.; et al. Erlotinib and bevacizumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck: A phase I/II study. Lancet Oncol. 2009, 10, 247–257. [Google Scholar] [CrossRef]
- Gross, N.D.; Bauman, J.E.; Gooding, W.E.; Denq, W.H.; Thomas, S.M.; Wang, L.; Chiosea, S.; Hood, B.L.; Flint, M.S.; Sun, M.; et al. Erlotinib, erlotinib-sulindac vs. placebo: A randomized, double-blind, placebo-controlled window trial in operable head and neck cancer. Clin. Cancer Res. 2014. [Google Scholar] [CrossRef] [PubMed]
- William, W.N., Jr.; Tsao, A.S.; Feng, L.; Ginsberg, L.E.; Lee, J.J.; Kies, M.S.; Glisson, B.S.; Kim, E.S. Single Arm, Phase II Study of Cisplatin, Docetaxel, and Erlotinib in Patients with Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinomas. Oncologist 2018, 23, e526–e549. [Google Scholar] [CrossRef]
- Yao, M.; Woods, C.; Lavertu, P.; Fu, P.; Gibson, M.; Rezaee, R.; Zender, C.; Wasman, J.; Sharma, N.; Machtay, M.; et al. Phase II study of erlotinib and docetaxel with concurrent intensity-modulated radiotherapy in locally advanced head and neck squamous cell carcinoma. Head Neck 2016, 38, E1770–E1776. [Google Scholar] [CrossRef]
- Gilbert, J.; Rudek, M.A.; Higgins, M.J.; Zhao, M.; Bienvenu, S.; Tsottles, N.; Wahl, R.; Forastiere, A.; Gillison, M. A Phase I Trial of Erlotinib and Concurrent Chemoradiotherapy for Stage III and IV (M0) Squamous Cell Carcinoma of the Head and Neck. Clin. Cancer Res. 2012. [Google Scholar] [CrossRef] [PubMed]
- Herchenhorn, D.; Dias, F.L.; Viegas, C.M.P.; Federico, M.H.; Araújo, C.M.M.; Small, I.; Bezerra, M.; Fontão, K.; Knust, R.E.; Ferreira, C.G.; et al. Phase I/II Study of Erlotinib Combined with Cisplatin and Radiotherapy in Patients with Locally Advanced Squamous Cell Carcinoma of the Head and Neck. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Rusthoven, K.E.; Feigenberg, S.J.; Raben, D.; Kane, M.; Song, J.I.; Nicolaou, N.; Mehra, R.; Burtness, B.; Ridge, J.; Swing, R.; et al. Initial Results of a Phase I Dose-Escalation Trial of Concurrent and Maintenance Erlotinib and Reirradiation for Recurrent and New Primary Head-and-Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Ahn, P.H.; Machtay, M.; Anne, P.R.; Cognetti, D.; Keane, W.M.; Wuthrick, E.; Dicker, A.P.; Axelrod, R.S. Phase I Trial Using Induction Ciplatin, Docetaxel, 5-FU and Erlotinib Followed by Cisplatin, Bevacizumab and Erlotinib With Concurrent Radiotherapy for Advanced Head and Neck Cancer. Am. J. Clin. Oncol. 2018, 41, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, E.; Lin, H.; Ginsberg, L.E.; Tran, H.T.; Lee, J.J.; Canales, J.R.; Williams, M.D.; Blumenschein, G.R., Jr.; Lu, C.; Heymach, J.V.; et al. Phase II trial of everolimus and erlotinib in patients with platinum-resistant recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2015, 26, 1476–1480. [Google Scholar] [CrossRef]
- Martins, R.G.; Parvathaneni, U.; Bauman, J.E.; Sharma, A.K.; Raez, L.E.; Papagikos, M.A.; Yunus, F.; Kurland, B.F.; Eaton, K.D.; Liao, J.J.; et al. Cisplatin and Radiotherapy with or Without Erlotinib in Locally Advanced Squamous Cell Carcinoma of the Head and Neck: A Randomized Phase II Trial. J. Clin. Oncol. 2013, 31, 1415–1421. [Google Scholar] [CrossRef]
- Stewart, J.S.W.; Cohen, E.E.W.; Licitra, L.; Van Herpen, C.M.L.; Khorprasert, C.; Soulieres, D.; Vodvarka, P.; Rischin, D.; Garin, A.M.; Hirsch, F.R.; et al. Phase III Study of Gefitinib Compared with Intravenous Methotrexate for Recurrent Squamous Cell Carcinoma of the Head and Neck. J. Clin. Oncol. 2009, 27, 1864–1871. [Google Scholar] [CrossRef]
- Argiris, A.; Ghebremichael, M.; Gilbert, J.; Lee, J.-W.; Sachidanandam, K.; Kolesar, J.M.; Burtness, B.; Forastiere, A.A. Phase III Randomized, Placebo-Controlled Trial of Docetaxel with or Without Gefitinib in Recurrent or Metastatic Head and Neck Cancer: An Eastern Cooperative Oncology Group Trial. J. Clin. Oncol. 2013, 31, 1405–1414. [Google Scholar] [CrossRef]
- Cohen, E.E.W.; Haraf, D.J.; Kunnavakkam, R.; Stenson, K.M.; Blair, E.A.; Brockstein, B.; Lester, E.P.; Salama, J.K.; Dekker, A.; Williams, R.; et al. Epidermal growth factor receptor inhibitor gefitinib added to chemoradiotherapy in locally advanced head and neck cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 3336–3343. [Google Scholar] [CrossRef]
- Ang, K.K.; Zhang, Q.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Sherman, E.J.; Weber, R.S.; Galvin, J.M.; Bonner, J.A.; Harris, J.; El-Naggar, A.K.; et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin with or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. J. Clin. Oncol. 2014, 32, 2940–2950. [Google Scholar] [CrossRef]
- Gregoire, V.; Hamoir, M.; Chen, C.; Kane, M.; Kawecki, A.; Julka, P.K.; Wang, H.M.; Prasad, S.; D’Cruz, A.K.; Radosevic-Jelic, L.; et al. Gefitinib plus cisplatin and radiotherapy in previously untreated head and neck squamous cell carcinoma: A phase II, randomized, double-blind, placebo-controlled study. Radiother. Oncol. 2011, 100, 62–69. [Google Scholar] [CrossRef]
- Sharp, H.; Morris, J.C.; Van Waes, C.; Gius, D.; Cooley-Zgela, T.; Singh, A.K. High incidence of oral dysesthesias on a trial of gefitinib, Paclitaxel, and concurrent external beam radiation for locally advanced head and neck cancers. Am. J. Clin. Oncol. 2008, 31, 557–560. [Google Scholar] [CrossRef]
- Van Waes, C.; Allen, C.T.; Citrin, D.; Gius, D.; Colevas, A.D.; Harold, N.A.; Rudy, S.; Nottingham, L.; Muir, C.; Chen, Z.; et al. Molecular and clinical responses in a pilot study of gefitinib with paclitaxel and radiation in locally advanced head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 447–454. [Google Scholar] [CrossRef]
- Harrington, K.; Temam, S.; Mehanna, H.; D’Cruz, A.; Jain, M.; D’Onofrio, I.; Manikhas, G.; Horvath, Z.; Sun, Y.; Dietzsch, S.; et al. Postoperative Adjuvant Lapatinib and Concurrent Chemoradiotherapy Followed by Maintenance Lapatinib Monotherapy in High-Risk Patients with Resected Squamous Cell Carcinoma of the Head and Neck: A Phase III, Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Oncol. 2015, 33, 4202–4209. [Google Scholar] [CrossRef]
- Machiels, J.P.; Haddad, R.I.; Fayette, J.; Licitra, L.F.; Tahara, M.; Vermorken, J.B.; Clement, P.M.; Gauler, T.; Cupissol, D.; Grau, J.J.; et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): An open-label, randomised phase 3 trial. Lancet Oncol. 2015, 16, 583–594. [Google Scholar] [CrossRef]
- Burtness, B.; Haddad, R.I.; Dinis, J.; Trigo Perez, J.M.; Yokota, T.; Viana, L.D.S.; Romanov, I.; Vermorken, J.B.; Bourhis, J.; Tahara, M.; et al. LUX-head and neck 2: Randomized, double-blind, placebo-controlled, phase III trial of afatinib as adjuvant therapy after chemoradiation (CRT) in primary unresected, high/intermediate-risk, squamous cell cancer of the head and neck (HNSCC) patients (pts). J. Clin. Oncol. 2017, 35, 6001. [Google Scholar] [CrossRef]
- Gillison, M.L.; Trotti, A.M.; Harris, J.; Eisbruch, A.; Harari, P.M.; Adelstein, D.J.; Sturgis, E.M.; Burtness, B.; Ridge, J.A.; Ringash, J.; et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): A randomised, multicentre, non-inferiority trial. Lancet 2019, 393, 40–50. [Google Scholar] [CrossRef]
- Mehanna, H.; Robinson, M.; Hartley, A.; Kong, A.; Foran, B.; Fulton-Lieuw, T.; Dalby, M.; Mistry, P.; Sen, M.; O’Toole, L.; et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): An open-label randomised controlled phase 3 trial. Lancet 2019, 393, 51–60. [Google Scholar] [CrossRef]
- Geoffrois, L.; Martin, L.; De Raucourt, D.; Sun, X.S.; Tao, Y.; Maingon, P.; Buffet, J.; Pointreau, Y.; Sire, C.; Tuchais, C.; et al. Induction Chemotherapy Followed by Cetuximab Radiotherapy Is Not Superior to Concurrent Chemoradiotherapy for Head and Neck Carcinomas: Results of the GORTEC 2007-02 Phase III Randomized Trial. J. Clin. Oncol. 2018, 36, 3077–3083. [Google Scholar] [CrossRef] [PubMed]
- Saleh, K.; Daste, A.; Martin, N.; Pons-Tostivint, E.; Auperin, A.; Herrera-Gómez, R.G.; Baste, N.; Bidault, F.; Guigay, J.; Le Tourneau, C.; et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with squamous cell carcinoma of the head and neck. J. Clin. Oncol. 2018, 36, 6015. [Google Scholar] [CrossRef]
- Ciardiello, F.; Tortora, G. Epidermal growth factor receptor (EGFR) as a target in cancer therapy: Understanding the role of receptor expression and other molecular determinants that could influence the response to anti-EGFR drugs. Eur. J. Cancer 2003, 39, 1348–1354. [Google Scholar] [CrossRef]
- Soulieres, D.; Faivre, S.; Mesia, R.; Remenar, E.; Li, S.H.; Karpenko, A.; Dechaphunkul, A.; Ochsenreither, S.; Kiss, L.A.; Lin, J.C.; et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2017, 18, 323–335. [Google Scholar] [CrossRef]
- Bauman, J.E.; Arias-Pulido, H.; Lee, S.J.; Fekrazad, M.H.; Ozawa, H.; Fertig, E.; Howard, J.; Bishop, J.; Wang, H.; Olson, G.T.; et al. A phase II study of temsirolimus and erlotinib in patients with recurrent and/or metastatic, platinum-refractory head and neck squamous cell carcinoma. Oral Oncol. 2013, 49, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Geiger, J.L.; Bauman, J.E.; Gibson, M.K.; Gooding, W.E.; Varadarajan, P.; Kotsakis, A.; Martin, D.; Gutkind, J.S.; Hedberg, M.L.; Grandis, J.R.; et al. Phase II trial of everolimus in patients with previously treated recurrent or metastatic head and neck squamous cell carcinoma. Head Neck 2016, 38, 1759–1764. [Google Scholar] [CrossRef]
- Jimeno, A.; Shirai, K.; Choi, M.; Laskin, J.; Kochenderfer, M.; Spira, A.; Cline-Burkhardt, V.; Winquist, E.; Hausman, D.; Walker, L.; et al. A randomized, phase II trial of cetuximab with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer. Ann. Oncol. 2015, 26, 556–561. [Google Scholar] [CrossRef]
- Li, S.H.; Lin, W.C.; Huang, T.L.; Chen, C.H.; Chiu, T.J.; Fang, F.M.; Huang, W.T.; Hsu, C.M.; Luo, S.D.; Lai, C.C.; et al. Significance of mammalian target of rapamycin in patients with locally advanced stage IV head and neck squamous cell carcinoma receiving induction chemotherapy with docetaxel, cisplatin, and fluorouracil. Head Neck 2016, 38 (Suppl. 1), E844–E852. [Google Scholar] [CrossRef]
- Dunn, L.A.; Fury, M.G.; Xiao, H.; Baxi, S.S.; Sherman, E.J.; Korte, S.; Pfister, C.; Haque, S.; Katabi, N.; Ho, A.L.; et al. A phase II study of temsirolimus added to low-dose weekly carboplatin and paclitaxel for patients with recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC). Ann. Oncol. 2017, 28, 2533–2538. [Google Scholar] [CrossRef] [PubMed]
- John, K.; Rosner, I.; Keilholz, U.; Gauler, T.; Bantel, H.; Grunwald, V. Baseline caspase activity predicts progression free survival of temsirolimus-treated head neck cancer patients. Eur. J. Cancer 2015, 51, 1596–1602. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, V.; Keilholz, U.; Boehm, A.; Guntinas-Lichius, O.; Hennemann, B.; Schmoll, H.J.; Ivanyi, P.; Abbas, M.; Lehmann, U.; Koch, A.; et al. TEMHEAD: A single-arm multicentre phase II study of temsirolimus in platin- and cetuximab refractory recurrent and/or metastatic squamous cell carcinoma of the head and neck (SCCHN) of the German SCCHN Group (AIO). Ann. Oncol. 2015, 26, 561–567. [Google Scholar] [CrossRef]
- Saba, N.F.; Hurwitz, S.J.; Magliocca, K.; Kim, S.; Owonikoko, T.K.; Harvey, D.; Ramalingam, S.S.; Chen, Z.; Rogerio, J.; Mendel, J.; et al. Phase 1 and pharmacokinetic study of everolimus in combination with cetuximab and carboplatin for recurrent/metastatic squamous cell carcinoma of the head and neck. Cancer 2014, 120, 3940–3951. [Google Scholar] [CrossRef]
- Fury, M.G.; Sherman, E.; Ho, A.; Katabi, N.; Sima, C.; Kelly, K.W.; Nwankwo, O.; Haque, S.; Pfister, D.G. A phase I study of temsirolimus plus carboplatin plus paclitaxel for patients with recurrent or metastatic (R/M) head and neck squamous cell cancer (HNSCC). Cancer Chemother. Pharmacol. 2012, 70, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Fury, M.G.; Lee, N.Y.; Sherman, E.; Ho, A.L.; Rao, S.; Heguy, A.; Shen, R.; Korte, S.; Lisa, D.; Ganly, I.; et al. A phase 1 study of everolimus + weekly cisplatin + intensity modulated radiation therapy in head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 87, 479–486. [Google Scholar] [CrossRef]
- Day, T.A.; Shirai, K.; O’Brien, P.E.; Matheus, M.G.; Godwin, K.; Sood, A.J.; Kompelli, A.; Vick, J.A.; Martin, D.; Vitale-Cross, L.; et al. Inhibition of mTOR Signaling and Clinical Activity of Rapamycin in Head and Neck Cancer in a Window of Opportunity Trial. Clin. Cancer Res. 2019, 25, 1156–1164. [Google Scholar] [CrossRef]
- Villaflor, V.M.; Melotek, J.M.; Karrison, T.G.; Brisson, R.J.; Blair, E.A.; Portugal, L.; De Souza, J.A.; Ginat, D.T.; Stenson, K.M.; Langerman, A.; et al. Response-adapted volume de-escalation (RAVD) in locally advanced head and neck cancer. Ann. Oncol. 2016, 27, 908–913. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Adkins, D.; Worden, F.; Wade, J.L.; Hu, S.; Price, K.; Zavala, J.; Lussier, Y.; Vokes, E.E.; Cohen, E.E.W. Activity of Temsirolimus Added to Cetuximab in Patients with Cetuximab-Resistant, Recurrent/Metastatic Head-and-Neck Cancer: Results of the Randomized Phase 2 Maestro-HN Study: Molecular Biology and Therapeutics. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 510. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Hilpert, F.; Weber, B.; Reuss, A.; Poveda, A.; Kristensen, G.; Sorio, R.; Vergote, I.; Witteveen, P.; Bamias, A.; et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: The AURELIA open-label randomized phase III trial. J. Clin. Oncol. 2014, 32, 1302–1308. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; Michael, H.E.; et al. Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017, 390, 1654–1663. [Google Scholar] [CrossRef]
- Holsinger, F.C.; Piha-Paul, S.A.; Janku, F.; Hong, D.S.; Atkins, J.T.; Tsimberidou, A.M.; Kurzrock, R. Biomarker-Directed Therapy of Squamous Carcinomas of the Head and Neck: Targeting PI3K/PTEN/mTOR Pathway. J. Clin. Oncol. 2013, 31, e137–e140. [Google Scholar] [CrossRef]
- Yao, M.; Galanopoulos, N.; Lavertu, P.; Fu, P.; Gibson, M.; Argiris, A.; Rezaee, R.; Zender, C.; Wasman, J.; Machtay, M.; et al. Phase II study of bevacizumab in combination with docetaxel and radiation in locally advanced squamous cell carcinoma of the head and neck. Head Neck 2015, 37, 1665–1671. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.S.; Kirkpatrick, J.P.; Craciunescu, O.; Broadwater, G.; Peterson, B.L.; Carroll, M.D.; Clough, R.; MacFall, J.R.; Hoang, J.; Scher, R.L.; et al. Prospective trial of synchronous bevacizumab, erlotinib, and concurrent chemoradiation in locally advanced head and neck cancer. Clin. Cancer Res. 2012, 18, 1404–1414. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Haraf, D.J.; Cohen, E.E.; Stenson, K.; Witt, M.E.; Dekker, A.; Kocherginsky, M.; Weichselbaum, R.R.; Chen, H.X.; Vokes, E.E. Phase I study of bevacizumab added to fluorouracil- and hydroxyurea-based concomitant chemoradiotherapy for poor-prognosis head and neck cancer. J. Clin. Oncol. 2008, 26, 1732–1741. [Google Scholar] [CrossRef]
- Fury, M.G.; Xiao, H.; Sherman, E.J.; Baxi, S.; Smith-Marrone, S.; Schupak, K.; Gewanter, R.; Gelblum, D.; Haque, S.; Schoder, H.; et al. Phase II trial of bevacizumab + cetuximab + cisplatin with concurrent intensity-modulated radiation therapy for patients with stage III/IVB head and neck squamous cell carcinoma. Head Neck 2016, 38 (Suppl. 1), E566–E570. [Google Scholar] [CrossRef] [PubMed]
- Fury, M.G.; Lee, N.Y.; Sherman, E.; Lisa, D.; Kelly, K.; Lipson, B.; Carlson, D.; Stambuk, H.; Haque, S.; Shen, R.; et al. A phase 2 study of bevacizumab with cisplatin plus intensity-modulated radiation therapy for stage III/IVB head and neck squamous cell cancer. Cancer 2012, 118, 5008–5014. [Google Scholar] [CrossRef]
- Nyflot, M.J.; Kruser, T.J.; Traynor, A.M.; Khuntia, D.; Yang, D.T.; Hartig, G.K.; McCulloch, T.M.; Wiederholt, P.A.; Gentry, L.R.; Hoang, T.; et al. Phase 1 trial of bevacizumab with concurrent chemoradiation therapy for squamous cell carcinoma of the head and neck with exploratory functional imaging of tumor hypoxia, proliferation, and perfusion. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Argiris, A.; Li, S.; Savvides, P.; Ohr, J.; Gilbert, J.; Levine, M.A.; Haigentz, M.; Saba, N.F.; Chakravarti, A.; Ikpeazu, C.; et al. Phase III randomized trial of chemotherapy with or without bevacizumab (B) in patients (pts) with recurrent or metastatic squamous cell carcinoma of the head and neck (R/M SCCHN): Survival analysis of E1305, an ECOG-ACRIN Cancer Research Group trial. J. Clin. Oncol. 2017, 35, 6000. [Google Scholar] [CrossRef]
- Hoang, T.; Huang, S.; Armstrong, E.; Eickhoff, J.C.; Harari, P.M. Enhancement of radiation response with bevacizumab. J. Exp. Clin. Cancer Res. 2012, 31, 37. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Zhang, Q.; Pfister, D.G.; Kim, J.; Garden, A.S.; Mechalakos, J.; Hu, K.; Le, Q.T.; Colevas, A.D.; Glisson, B.S.; et al. Addition of bevacizumab to standard chemoradiation for locoregionally advanced nasopharyngeal carcinoma (RTOG 0615): A phase 2 multi-institutional trial. Lancet Oncol. 2012, 13, 172–180. [Google Scholar] [CrossRef]
- Machiels, J.P.; Henry, S.; Zanetta, S.; Kaminsky, M.C.; Michoux, N.; Rommel, D.; Schmitz, S.; Bompas, E.; Dillies, A.F.; Faivre, S.; et al. Phase II study of sunitinib in recurrent or metastatic squamous cell carcinoma of the head and neck: GORTEC 2006-01. J. Clin. Oncol. 2010, 28, 21–28. [Google Scholar] [CrossRef]
- Fountzilas, G.; Fragkoulidi, A.; Kalogera-Fountzila, A.; Nikolaidou, M.; Bobos, M.; Calderaro, J.; Andreiuolo, F.; Marselos, M. A phase II study of sunitinib in patients with recurrent and/or metastatic non-nasopharyngeal head and neck cancer. Cancer Chemother. Pharmacol. 2010, 65, 649–660. [Google Scholar] [CrossRef]
- Williamson, S.K.; Moon, J.; Huang, C.H.; Guaglianone, P.P.; LeBlanc, M.; Wolf, G.T.; Urba, S.G. Phase II evaluation of sorafenib in advanced and metastatic squamous cell carcinoma of the head and neck: Southwest Oncology Group Study S0420. J. Clin. Oncol. 2010, 28, 3330–3335. [Google Scholar] [CrossRef]
- Adkins, D.; Mehan, P.; Ley, J.; Siegel, M.J.; Siegel, B.A.; Dehdashti, F.; Jiang, X.; Salama, N.N.; Trinkaus, K.; Oppelt, P. Pazopanib plus cetuximab in recurrent or metastatic head and neck squamous cell carcinoma: an open-label, phase 1b and expansion study. Lancet Oncol. 2018, 19, 1082–1093. [Google Scholar] [CrossRef]
- Argiris, A.; Kotsakis, A.P.; Hoang, T.; Worden, F.P.; Savvides, P.; Gibson, M.K.; Gyanchandani, R.; Blumenschein, G.R., Jr.; Chen, H.X.; Grandis, J.R.; et al. Cetuximab and bevacizumab: Preclinical data and phase II trial in recurrent or metastatic squamous cell carcinoma of the head and neck. Ann. Oncol. 2013, 24, 220–225. [Google Scholar] [CrossRef]
- Argiris, A.; Bauman, J.E.; Ohr, J.; Gooding, W.E.; Heron, D.E.; Duvvuri, U.; Kubicek, G.J.; Posluszny, D.M.; Vassilakopoulou, M.; Kim, S.; et al. Phase II randomized trial of radiation therapy, cetuximab, and pemetrexed with or without bevacizumab in patients with locally advanced head and neck cancer. Ann. Oncol. 2016, 27, 1594–1600. [Google Scholar] [CrossRef]
- Argiris, A.; Karamouzis, M.V.; Gooding, W.E.; Branstetter, B.F.; Zhong, S.; Raez, L.E.; Savvides, P.; Romkes, M. Phase II trial of pemetrexed and bevacizumab in patients with recurrent or metastatic head and neck cancer. J. Clin. Oncol. 2011, 29, 1140–1145. [Google Scholar] [CrossRef]
- Hainsworth, J.D.; Spigel, D.R.; Greco, F.A.; Shipley, D.L.; Peyton, J.; Rubin, M.; Stipanov, M.; Meluch, A. Combined modality treatment with chemotherapy, radiation therapy, bevacizumab, and erlotinib in patients with locally advanced squamous carcinoma of the head and neck: A phase II trial of the Sarah Cannon oncology research consortium. Cancer J. 2011, 17, 267–272. [Google Scholar] [CrossRef]
- Bonomo, P.; Desideri, I.; Loi, M.; Mangoni, M.; Sottili, M.; Marrazzo, L.; Talamonti, C.; Greto, D.; Pallotta, S.; Livi, L. Anti PD-L1 DUrvalumab combined with Cetuximab and RadiOtherapy in locally advanced squamous cell carcinoma of the head and neck: A phase I/II study (DUCRO). Clin. Transl. Radiat. Oncol. 2018, 9, 42–47. [Google Scholar] [CrossRef]
- Ferris, R.L.; Lenz, H.J.; Trotta, A.M.; Garcia-Foncillas, J.; Schulten, J.; Audhuy, F.; Merlano, M.; Milano, G. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: Harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat. Rev. 2018, 63, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Bridges, K.A.; Hirai, H.; Buser, C.A.; Brooks, C.; Liu, H.; Buchholz, T.A.; Molkentine, J.M.; Mason, K.A.; Meyn, R.E. MK-1775, a novel Wee1 kinase inhibitor, radiosensitizes p53-defective human tumor cells. Clin. Cancer Res. 2011, 17, 5638–5648. [Google Scholar] [CrossRef]
- Kim, J.H.; Jenrow, K.A.; Brown, S.L. Novel biological strategies to enhance the radiation therapeutic ratio. Radiat. Oncol. J. 2018, 36, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Morales-Orue, I.; Chicas-Sett, R.; Lara, P.C. Nanoparticles as a promising method to enhance the abscopal effect in the era of new targeted therapies. Rep. Pract. Oncol. Radiother. 2019, 24, 86–91. [Google Scholar] [CrossRef]
| PI/Author | Phase | Trial/NCT Number | Trial Design# | Substance | No. of Patients‡ | Status |
|---|---|---|---|---|---|---|
| Colevas et al. [56] | Ia | PCD4989g NCT01375842 | single-arm, multicentric | Atezolizumab*,† | 32 | complete |
| Seiwert et al. [36] | Ib | Keynote-012 NCT01848834 | single-arm, multicentric | Pembrolizumab† | 60 | complete |
| Segal et al. [49] | I/II | Study 1108 NCT01693562 | single-arm, multicentric | Durvalumab† | 62 | complete |
| Zandberg et al. [52] | II | HAWK NCT02207530 | single-arm, multicentric | Durvalumab† | 111 | complete |
| Siu et al. [51] | II | CONDOR NCT02319044 | randomized, multicentric | Durvalumab + Tremelimumab† vs. Durvalumab vs. Tremelimumab | 267 | complete |
| Bauml et al. [41] | II | Keynote-055 NCT02255097 | single-arm, multicentric | Pembrolizumab† | 171 | complete |
| Grünwald et al. | II | ELDORANDO NCT03193931 | randomized, multicentric | Pembrolizumab* vs. Methotrexate | e.e. 100 | recruiting |
| Grünwald et al. | II | OPTIM NCT03620123 | randomized, multicentric | Nivolumab + Ipilimumab† vs.¥ Docetaxel | e.e. 280 | recruiting |
| Fietkau et al. | II | IMPORTANCE NCT03386357 | randomized, multicentric | Pembrolizumab† | e.e. 130 | recruiting |
| McBride et al. [45] | II | NCT02684253 | randomized, multicentric | Nivolumab + SBRT vs. Nivolumab | 66 | complete |
| Haddad et al. [46] | II | Checkmate714 NCT02823574 | double-blind, randomized, multicentric | Nivolumab + Ipilimumab vs, Nivolumab + Placebo | e.e. 315 | recruiting |
| Cohen et al. [14] | III | Keynote-040 NCT02252042 | randomized, multicentric | Pembrolizumab† vs. Methotrexate, Docetaxel or Cetuximab | 495 | complete |
| Burtness et al. [15] | III | Keynote-048 NCT02358031 | randomized, multicentric | Pembrolizumab* vs. Pembrolizumab + Cisplatin + 5FU vs. EXTREME | 825 | recruitment completed |
| Ferris et al. [13] | III | CheckMate141 NCT02105636 | randomized, multicentric | Nivolumab† vs. Methotrexate, Docetaxel or Cetuximab | 361 | complete |
| Argiris et al. [47] | III | CheckMate651 NCT02741570 | randomized, multicentric | Nivolumab + Ipilimumab vs. EXTREME | e.e. 490 | recruiting |
| Seiwert et al. [55] | III | KESTREL NCT02551159 | randomized, multicentric | Durvalumab + Tremelimumab* vs. Durvalumab vs. EXTREME | 823 | recruitment completed |
| Ferris et al. [53] | III | EAGLE NCT02369874 | randomized, multicentric | Durvalumab + Tremelimumab vs. Durvalumab vs. Cetuximab, Taxane, Methotrexate, or Fluoropyrimidine | 720 | recruitment completed |
| PI/Author | Trial/NCT Number | Substance and Treatment# | Primary Endpoint/Design | Estimated Enrollment‡/Primary Completion¥ |
|---|---|---|---|---|
| Lee et al. [58] | Javelin Head and Neck 100 NCT02952586 | 70Gy RT + Cisplatin + Avelumab vs. Placebo | PFS/DB | N = 640 04/2021 |
| GORTEC [61] | REACH NCT02999087 | FIT: 70Gy RT + Cisplatin vs. Cetuximab + Avelumab UNFIT: 70Gy RT + Cetuximab vs. Cetuximab + Avelumab | PFS/OL | N = 640 10/2019 |
| GORTEC | NIVOPOSTOP NCT03576417 | 66Gy PO RT Randomization: Cisplatin vs. Cisplatin + Nivolumab | DFS/OL | N = 484 12/2012 |
| Busch et al. | IMSTAR-HN NCT03700905 | Surgery +PO RT/CRT vs. Nivolumab + Surgery + PO RT/CRT + Nivolumab/Nivolumab + Ipilimumab | DFS/OL | N = 276 05/2024 |
| Siu et al. [62] | Keynote-412 NCT03040999 | 70Gy RT + Cisplatin + Pembrolizumab vs. Placebo | EFS/DB | N = 780 04/2021 |
| MSD | Keynote-689 NCT03765918 | Pembrolizumab + Surgery + PO RT/CRT vs. Surgery + PO RT/CRT | mPR, EFS /OL | N = 600 01/2023 |
| EORTC | ADHERE NCT03673735 | 66Gy PO RT + Cisplatin Randomization: Nivolumab vs. Placebo | DFS/DB | N = 650 07/2026 |
| Mell et al. | NRG-HN004 NCT03258554 | 70Gy RT + Cetuximab vs. Durvalumab | DLT,PFS,OS/OL | N = 523 12/2025 |
| Haddad et al. [57] | IMvoke010 NCT03452137 | Atezolizumab vs. Placebo | EFS/DB | N = 400 08/2023 |
| Author/ Trial/ NCT Number | Phase | No. of Patients | Setting | Regimen | Endpoint | Status |
|---|---|---|---|---|---|---|
| GERCOR NCT01333085 | I/II | 49 | Curative-LA | Induction with Everolimus, Carboplatin, Paclitaxel followed by RT or surgery | I: MTD II: ORR | Completed No results |
| Saba et al. [116] NCT01283334 | I/II | 20 | Palliative, 1st line | Carboplatin, Cetuximab, Everolimus | I: MTD II: PFS | MTD: 2.5 mg every other day, median PFS: 8.15 month |
| NCT01009346 | I/II | 9 | Palliative, 1st line | Everolimus, Cetuximab and Cisplatin | I: MTD II: PFS | Terminated due to toxicity |
| Villaflor et al. [120] NCT01133678 | I/II | 94 | Curative-LA | Induction Cisplatin, Paclitaxel, Cetuximab, Everolimus, followed by reduced-field RT | Tumor response rate | No benefit of everolimus |
| Massarelli et al. [91] NCT00942734 | II | 49 | Palliative, 2nd line | Everolimus, Erlotinib | Tumor response rate | No benefit |
| NCT01016769 | I/II | 48 | Palliative, 1st line | Temsirolimus, Paclitaxel, Carboplatin | I: dose finding II: ORR | Completed Results pending |
| TEMHEAD, Grünwald et al. [115] NCT01172769 | II | 40 | Palliative, 1st/2nd line | Temsirolimus | PFS | Median PFS: 56 d Median OS: 152 d |
| Seiwert et al. [121] MAESTRO HN NCT01256385 | II | 86 | Palliative 2nd line | Temsirolimus +/− Cetuximab (arms A vs. B) | PFS | Median PFS: 105 d in both arms 4-m-PFS: 41.3 vs 36.4% |
| Geiger et al. [110] NCT01051791 | II | 13 | Palliative 1st line | Everolimus 10 mg/d | CBR | Terminated 28%; PFS: 1.5 mo, OS: 4.5 month |
| NCT01195922 | II | 37, only 16 treated | Curative-LA | Rapamycin (sirolimus) → surgery | %change in levels of pS6, pAKt473, Ki-67 | Completed, no oncological results |
| NCT01015664 | I/II | 11 | Palliative, 1st line | Cisplatin, Cetuximab, Temsirolimus | I: MTD II: PFS | Terminated |
| Author/Trial/NCT Number | Phase | No. of Patients | Setting | Regimen | Endpoint | Status |
|---|---|---|---|---|---|---|
| Argiris et al. [138] NCT00409565 | II | 46 | Palliative, 1 st/2 nd line | Bevacizumab (15 mg/kg, q21) and Cetuximab | ORR | ORR: 16% Median PFS: 2.8 mo |
| NCT00203905 | II | 23/30 | Curative-LA | CRT+FHX +/− Bevacizumab, randomized | PFS | Completed, no results |
| Fury et al. [129] NCT00423930 | II | 44 | Curative-LA | IMRT + Cisplatin + Bevacizumab | 2y-PFS | 2-y-PFS: 75.9; 2-y-OS: 88% |
| Yao et al. [125] NCT00281840 | I/II | 30 | Curative-LA | RT + weekly docetaxel 20 mg/qm+ Bevacizumab (5mg/kg biweekly) | Time to progression | 3-y-PFS: 61.7%; 25/30 not completed |
| Argiris et al. [139] NCT00703976 | II | 80 | Curative-LA | RT + Cetuximab + Pemetrexed +/− Bevacizumab (15 mg/kg, q21) randomized | 2y-PFS | 2y-PFS: 79% vs. 75%; 2y-OS: 91% vs. 87% |
| Argiris et al. [140] NCT00222729 | II | 42 | Palliative, 1 st/2 nd line | Pemetrexed + Bevacizumab (15 mg/kg, q21) | Time to progression | Time to progression 5 mo; ORR: 30% |
| Cohen et al. [83] NCT00055913 | I/II | 58 | Palliative, 1 st/2 nd line | Erlotinib + Bevacizumab (q21) | I: MTD; II: ORR | 15 mg/kg, q21 ORR: 15.2% |
| Yoo et al. [126] NCT00140556 | I | 28 | Curative-LA | Induction Bevacizumab + Erlotinib followed by CRT with Cisplatin, Bevacizumab + Erlotinib | Tumor resolution | 25/26 patients |
| Hainsworth et al. [141] NCT00392704 | II | 60 | Curative-LA | Induction Carboplatin, Paclitaxel, Bevacizumab (15 mg/kg, d 1+22) followed by CRT with Paclitaxel, Erlotinib, Bevacizumab (15 mg/kg, d 50+71) | 2y-PFS | 2y-PFS: 83% |
| ATHENA NCT03818061 | II | Estimated 110 | Palliative, 1st line | Bevacizumab (15 mg/kg, q21) + Atezolizumab | ORR | Not yet recruiting |
| NCT00392665 | II | 36/82 | Palliative, 1 st/2 nd line | Bevacizumab + Erlotinib vs. Sulindac + Erlotinib randomized | PFS | 9.38 vs 7.01 mo terminated due to slow accrual |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
von der Grün, J.; Rödel, F.; Brandts, C.; Fokas, E.; Guckenberger, M.; Rödel, C.; Balermpas, P. Targeted Therapies and Immune-Checkpoint Inhibition in Head and Neck Squamous Cell Carcinoma: Where Do We Stand Today and Where to Go? Cancers 2019, 11, 472. https://doi.org/10.3390/cancers11040472
von der Grün J, Rödel F, Brandts C, Fokas E, Guckenberger M, Rödel C, Balermpas P. Targeted Therapies and Immune-Checkpoint Inhibition in Head and Neck Squamous Cell Carcinoma: Where Do We Stand Today and Where to Go? Cancers. 2019; 11(4):472. https://doi.org/10.3390/cancers11040472
Chicago/Turabian Stylevon der Grün, Jens, Franz Rödel, Christian Brandts, Emmanouil Fokas, Matthias Guckenberger, Claus Rödel, and Panagiotis Balermpas. 2019. "Targeted Therapies and Immune-Checkpoint Inhibition in Head and Neck Squamous Cell Carcinoma: Where Do We Stand Today and Where to Go?" Cancers 11, no. 4: 472. https://doi.org/10.3390/cancers11040472
APA Stylevon der Grün, J., Rödel, F., Brandts, C., Fokas, E., Guckenberger, M., Rödel, C., & Balermpas, P. (2019). Targeted Therapies and Immune-Checkpoint Inhibition in Head and Neck Squamous Cell Carcinoma: Where Do We Stand Today and Where to Go? Cancers, 11(4), 472. https://doi.org/10.3390/cancers11040472






