Fluorescence Enhancement Using Bimetal Surface Plasmon-Coupled Emission from 5-Carboxyfluorescein (FAM)
Abstract
:1. Introduction
2. Experimental Apparatus and Techniques
2.1. Experimental Setup for Fluorescence
2.2. Preparation of 5-Carboxyfluorescein (FAM)
3. Results and Discussion
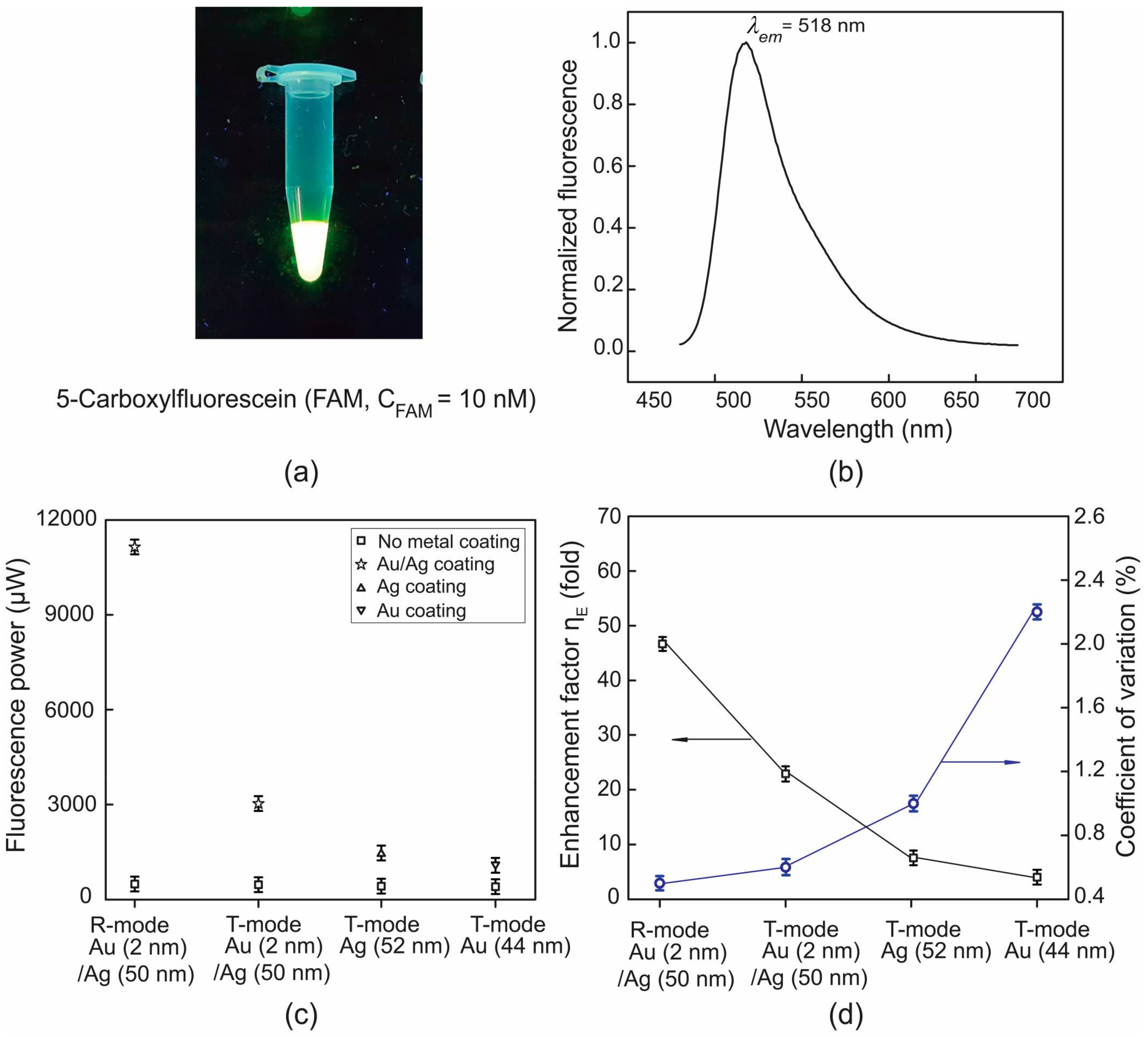
3.1. Fluorescence Enhancement for Plasmonic Chips of Different Metal Layers
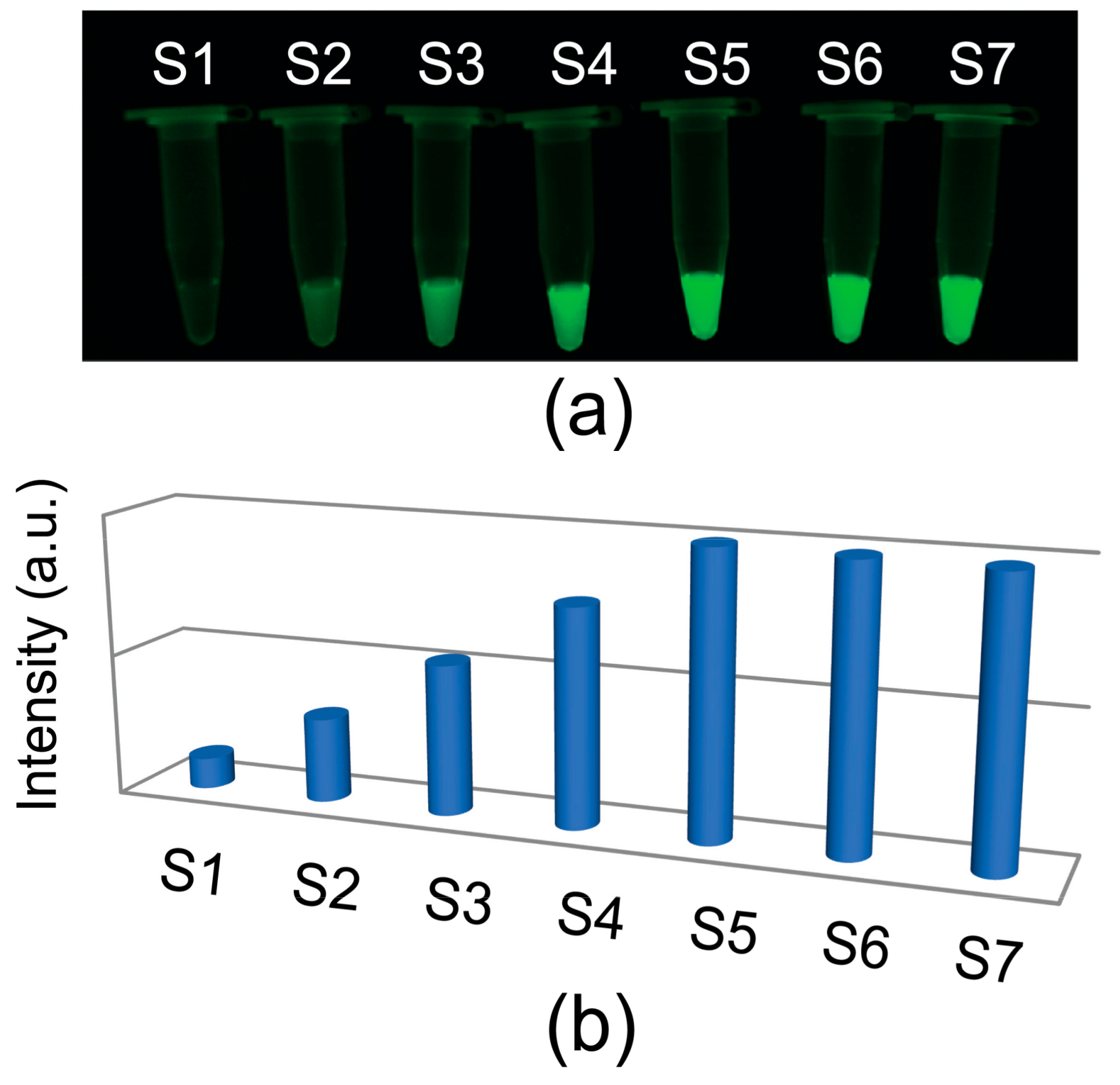
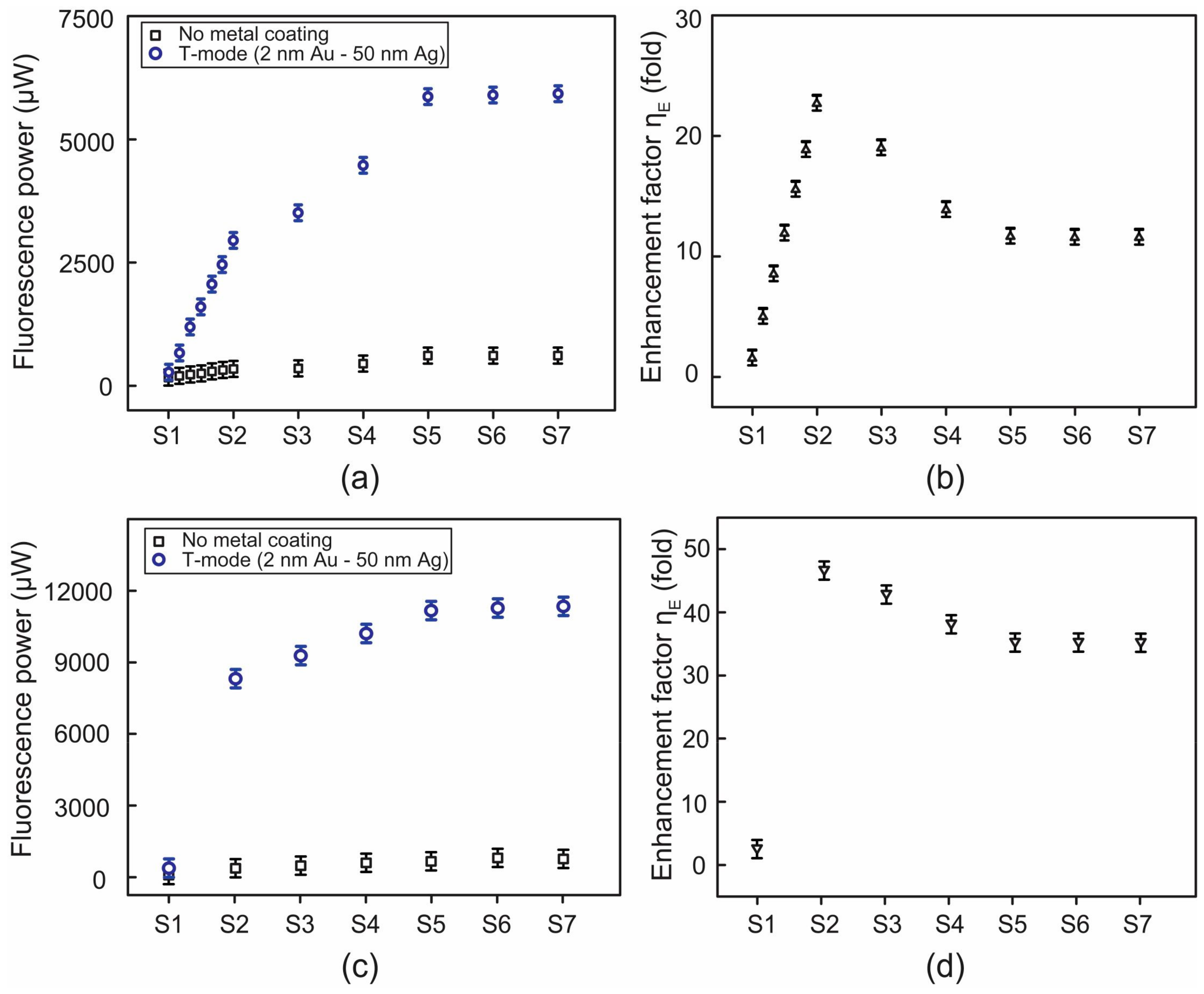
3.2. FAM Concentration-Dependent Enhancement of Fluorescence
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Williams, L.D.; Ghosh, T.; Mastrangelo, C.H. Low noise detection of biomolecular interactions with signal-locking surface plasmon resonance. Anal. Chem. 2010, 82, 6025–6031. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.R.; Alam, M.A. Theory of “selectivity” of label-free nanobiosensors: A geometro-physical perspective. J. Appl. Phys. 2010, 107, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Gao, X.P.; Lieber, C.M. Frequency domain detection of biomolecules using silicon nanowire biosensors. Nano Lett. 2011, 10, 3179–3183. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolo, B.; Collins, J. Biophotonics: Spectroscopy, Imaging, Sensing, and Manipulation (NATO Science for Peace and Security Series B: Physics and Biophysics); Springer: Dordrecht, The Netherlands, 2010; ISBN 978-90-481-9977-8. [Google Scholar]
- Sokolov, K.; Chumanov, G.; Cotton, T.M. Enhancement of molecular fluorescence near the surface of colloidal metal films. Anal. Chem. 1998, 70, 3898–3905. [Google Scholar] [CrossRef] [PubMed]
- Malicka, J.; Gryczynski, I.; Gryczynski, Z.; Lakowicz, J.R. Effects of fluorophore-to-silver distance on the emission of cyanine-dye-labeled oligonucleotides. Anal. Biochem. 2003, 315, 57–66. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Shen, Y.; D’Auria, S.; Malickam, J.; Fang, J.; Gryczynski, Z.; Gryczynski, I. Radiative Decay Engineering. Anal. Biochem. 2002, 301, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.D.; Lakowicz, J.R. Metal-Enhanced Fluorescence. J. Fluoresc. 2002, 12, 121–129. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative decay engineering: Biophysical and biomedical applications. Anal. Biochem. 2001, 298, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.D.; Parfenov, A.; Lakowicz, J.R. Photodeposition of silver can result in metal-enhanced fluorescence. Appl. Spectrosc. 2003, 57, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Punj, D.; Ghenuche, P.; Moparthi, S.B.; de Torres, J.; Grigoriev, V.; Rigneault, H.; Wenger, J. Plasmonic antennas and zero-mode waveguides to enhance single molecule fluorescence detection and fluorescence correlation spectroscopy toward physiological concentrations. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 268–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Jackman, J.A.; Yang, H.H.; Chen, P.; Cho, N.J.; Kim, D.H. Strategies for enhancing the sensitivity of plasmonic nanosensors. Nano Today 2015, 10, 213–239. [Google Scholar] [CrossRef]
- Lim, T.-S.; Fu, C.-C.; Lee, K.-C.; Lee, H.-Y.; Chen, K.; Cheng, W.-F.; Pai, W.W.; Chang, H.-C.; Fann, W. Fluorescence enhancement and lifetime modification of single nanodiamonds near a nanocrystalline silver surface. Phys. Chem. Chem. Phys. 2009, 11, 1508–1514. [Google Scholar] [CrossRef] [PubMed]
- Takmakov, P.; Vlassiouk, I.; Smirnov, S. Application of anodized aluminum in fluorescence detection of biological species. Anal. Bioanal. Chem. 2006, 385, 954–958. [Google Scholar] [CrossRef] [PubMed]
- Bessueille, F.; Dugas, V.; Vikulov, V.; Cloarec, J.P.; Souteyrand, E.; Martin, J.R. Assessment of porous silicon substrate for well-characterised sensitive DNA chip implement. Biosens. Bioelectron. 2005, 21, 908–916. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, T.; Yasuda, M. Fluorescence enhancement and reflection of the excitation light observed with a multilayered substrate. Appl. Opt. 2010, 49, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Malicka, J.; Gryczynski, I.; Maliwal, B.P.; Fang, J.; Lakowicz, J.R. Fluorescence spectral properties of cyanine dye labeled DNA near metallic silver particles. Biopolymers 2003, 72, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Malicka, J.; Gryczynski, I.; Lakowicz, J.R. Enhanced emission of highly labeled DNA oligomers near silver metallic surfaces. Anal. Chem. 2003, 75, 4408–4414. [Google Scholar] [CrossRef] [PubMed]
- Malicka, J.; Gryczynski, I.; Lakowicz, J.R. Fluorescence spectral properties of labeled thiolated oligonucleotides bound to silver particles. Biopolymers 2004, 74, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matveeva, E.G.; Gryczynski, I.; Barnett, A.; Leonenko, Z.; Lakowicz, J.R.; Gryczynski, Z. Metal particle-enhanced fluorescent immunoassays on metal mirrors. Anal. Biochem. 2007, 363, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Akselrod, G.M.; Walker, B.J.; Tisdale, W.A.; Bawendi, M.G.; Bulovic, V. Twenty-fold enhancement of molecular fluorescence by coupling to a J-aggregate critically coupled resonator. ACS Nano 2012, 6, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Klantsataya, E.; François, A.; Ebendorff-Heidepriem, H.; Sciacca, B.; Zuber, A.; Monro, T.M. Effect of surface roughness on metal enhanced fluorescence in planar substrates and optical fibers. Opt. Mater. Express 2016, 6, 2128–2138. [Google Scholar] [CrossRef]
- Sevick-Muraca, E.M.; Houston, J.P.; Gurfinkel, M. Fluorescence-enhanced, near infrared diagnostic imaging with contrast agents. Curr. Opin. Chem. Biol. 2002, 6, 642–650. [Google Scholar] [CrossRef]
- Orrit, M.; Bernard, J. Single pentacene molecules detected by fluorescence excitation in a p-terphenyl crystal. Phys. Rev. Lett. 1990, 65, 2716–2719. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S. Fluorescence spectroscopy of single biomolecules. Science 1999, 283, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.D.; Cao, H.; Gryczynski, I.; Gryczynski, Z.; Fang, J.; Lakowicz, J.R. Metal-enhanced fluorescence (MEF) due to silver colloids on a planar surface: Potential applications of indocyanine green to in vivo Imaging. J. Phys. Chem. A 2003, 107, 3443–3449. [Google Scholar] [CrossRef]
- Xie, F.; Baker, M.S.; Goldys, E.M. Enhanced fluorescence detection on homogeneous gold colloid self-assembled monolayer substrates. Chem. Mater. 2008, 20, 1788–1797. [Google Scholar] [CrossRef]
- Goldys, E.M.; Barnett, A.; Xie, F.; Drozdowicz-Tomsia, K.; Gryczynski, I.; Matveeva, E.G.; Gryczynski, Z.; Shtoyko, T. Plasmon-enhanced fluorescence near metallic nanostructures: Biochemical applications. Appl. Phys. A 2007, 89, 265–271. [Google Scholar] [CrossRef]
- Bauch, M.; Toma, K.; Toma, M.; Zhang, Q.; Dostalek, J. Plasmon-Enhanced Fluorescence Biosensors: A Review. Plasmonics 2014, 9, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Baker, M.S.; Goldys, E.M. Homogeneous silver-coated nanoparticle substrates for enhanced fluorescence detection. J. Phys. Chem. B 2006, 110, 23085–23091. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, J.; Lakowicz, J.R. Plasmon-enhanced fluorescence from single fluorophores end-linked to gold nanorods. J. Am. Chem. Soc. 2010, 132, 5540–5541. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.J.; Chen, B.; Pathan, S.; Bok, S.; Mathai, C.J.; Gangopadhyay, K.; Grant, S.A.; Gangopadhyay, S. Influence of silver grain size, roughness, and profile on the extraordinary fluorescence enhancement capabilities of grating coupled surface plasmon resonance. RSC Adv. 2015, 5, 78534–78544. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-enhanced optical sensors: A review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-F.; Li, C.-Y.; Aroca, R.F. Plasmon-enhanced fluorescence spectroscopy. Chem. Soc. Rev. 2017, 46, 3962–3979. [Google Scholar] [CrossRef] [PubMed]
- Fort, E.; Grésillon, S. Surface enhanced fluorescence. J. Phys. D Appl. Phys. 2008, 41, 013001. [Google Scholar] [CrossRef]
- Tran, N.H.T.; Trinh, K.T.L.; Lee, J.-H.; Yoon, W.J.; Ju, H. Reproducible enhancement of fluorescence by bimetal mediated surface plasmon coupled emission for highly sensitive quantitative diagnosis of double-stranded DNA. Small 2018, 14, 1801385. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.T.; Phan, B.T.; Yoon, W.J.; Khym, S.; Ju, H. Dielectric metal-based multilayers for surface plasmon resonance with enhanced quality factor of the plasmonic waves. J. Electron. Mater. 2017, 46, 3654–3659. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, N.H.T.; Trinh, K.T.L.; Lee, J.-H.; Yoon, W.J.; Ju, H. Fluorescence Enhancement Using Bimetal Surface Plasmon-Coupled Emission from 5-Carboxyfluorescein (FAM). Micromachines 2018, 9, 460. https://doi.org/10.3390/mi9090460
Tran NHT, Trinh KTL, Lee J-H, Yoon WJ, Ju H. Fluorescence Enhancement Using Bimetal Surface Plasmon-Coupled Emission from 5-Carboxyfluorescein (FAM). Micromachines. 2018; 9(9):460. https://doi.org/10.3390/mi9090460
Chicago/Turabian StyleTran, Nhu Hoa Thi, Kieu The Loan Trinh, Jun-Ho Lee, Won Jung Yoon, and Heongkyu Ju. 2018. "Fluorescence Enhancement Using Bimetal Surface Plasmon-Coupled Emission from 5-Carboxyfluorescein (FAM)" Micromachines 9, no. 9: 460. https://doi.org/10.3390/mi9090460




