Remote Microwave and Field-Effect Sensing Techniques for Monitoring Hydrogel Sensor Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrogel Synthesis
2.1.1. Reagent Materials for Hydrogel Synthesis
2.1.2. Synthesis of Stimulus-Responsive Hydrogel
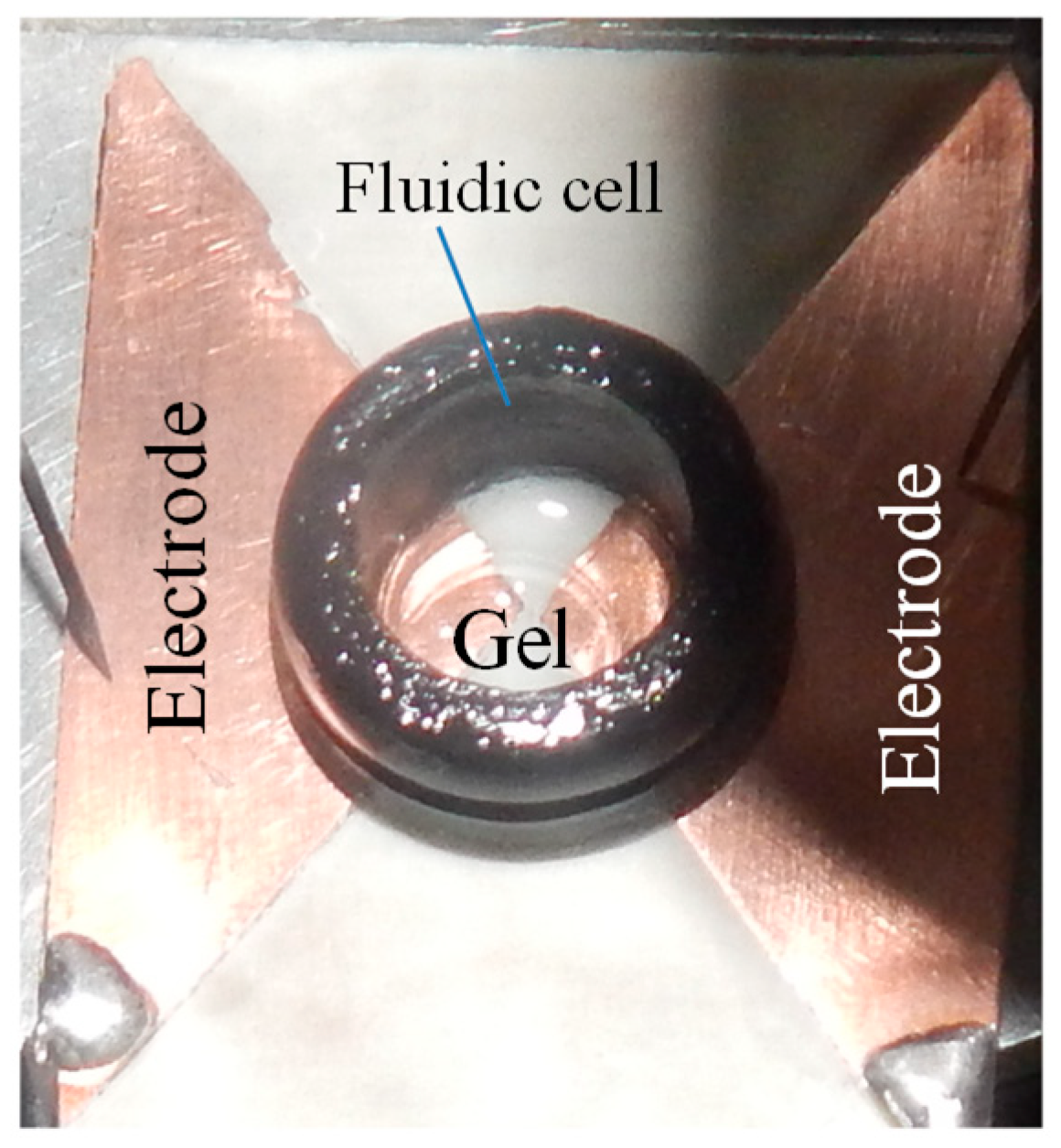
2.2. Impedance Spectroscopy of Hydrogel
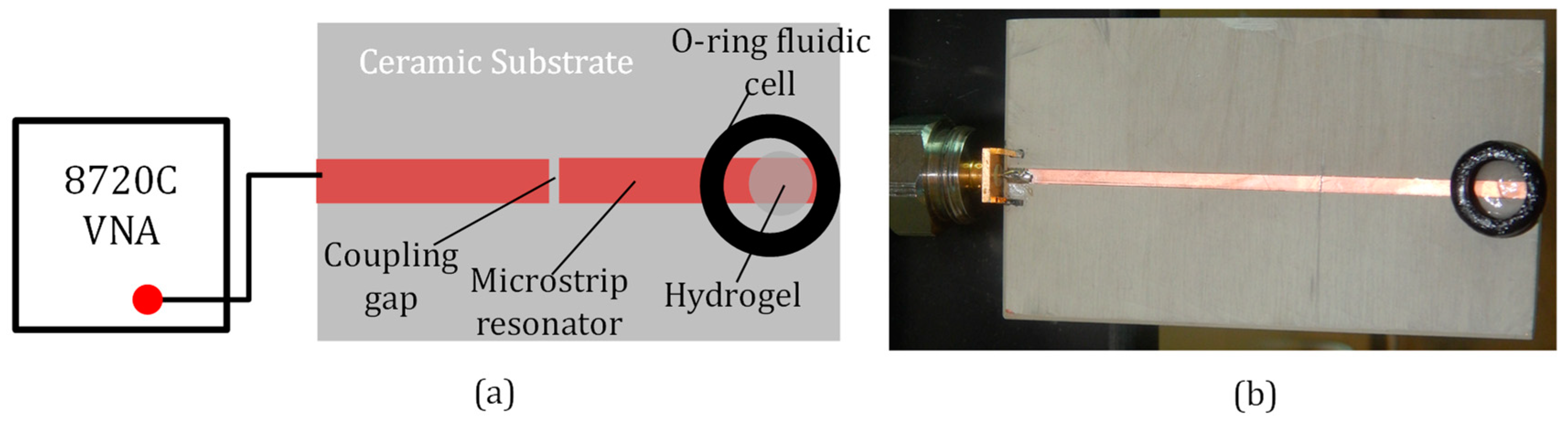
2.3. Contact-Mode Microwave Hydrogel Measurements
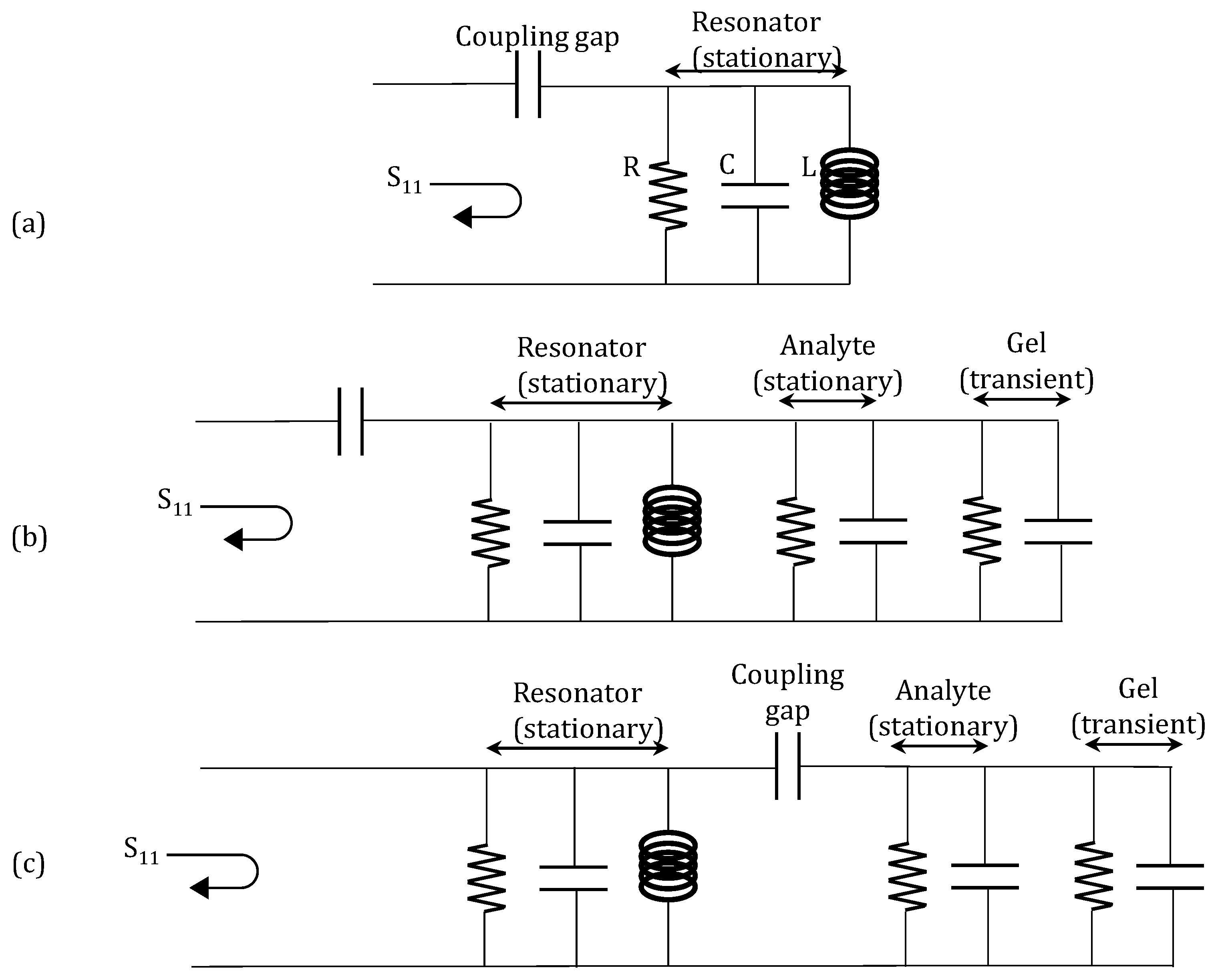
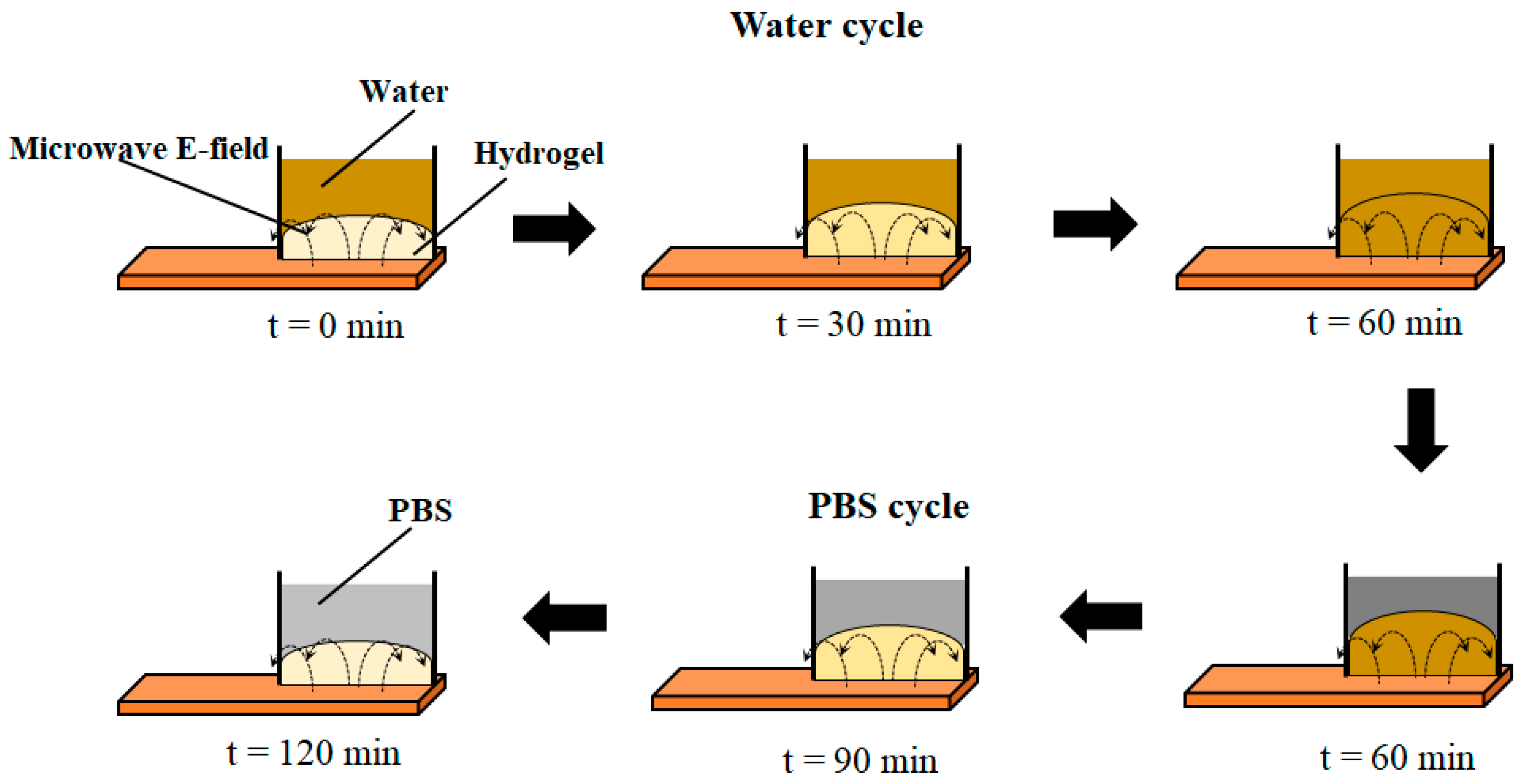
2.4. Contactless (Remote) Microwave Monitoring of Hyrogel Response
2.5. Potentiometric Method for Monitoring Hydrogel Response
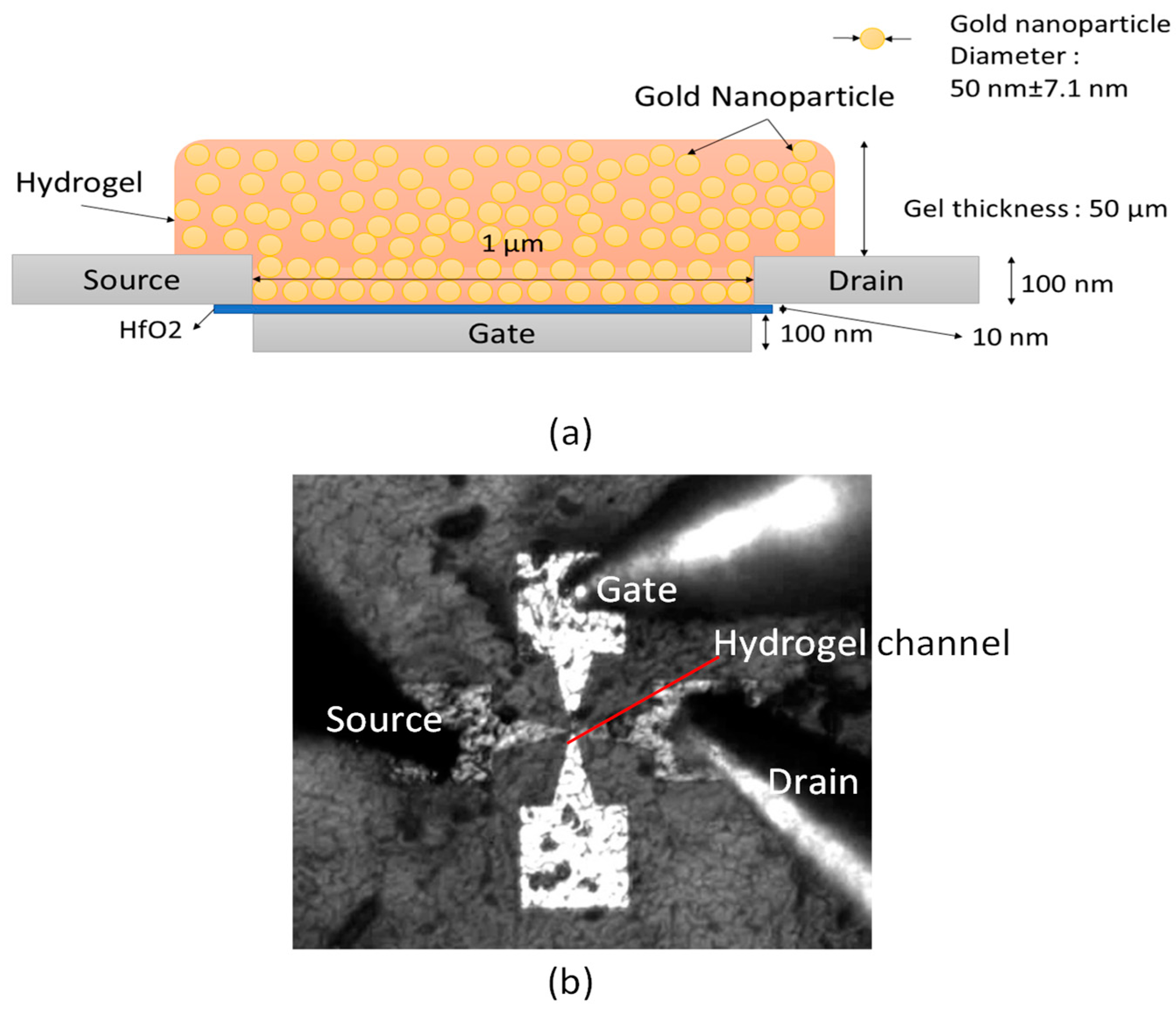
2.6. Monitoring Hydrogel Response with MOHFET Current-Voltage Characteristics
3. Results
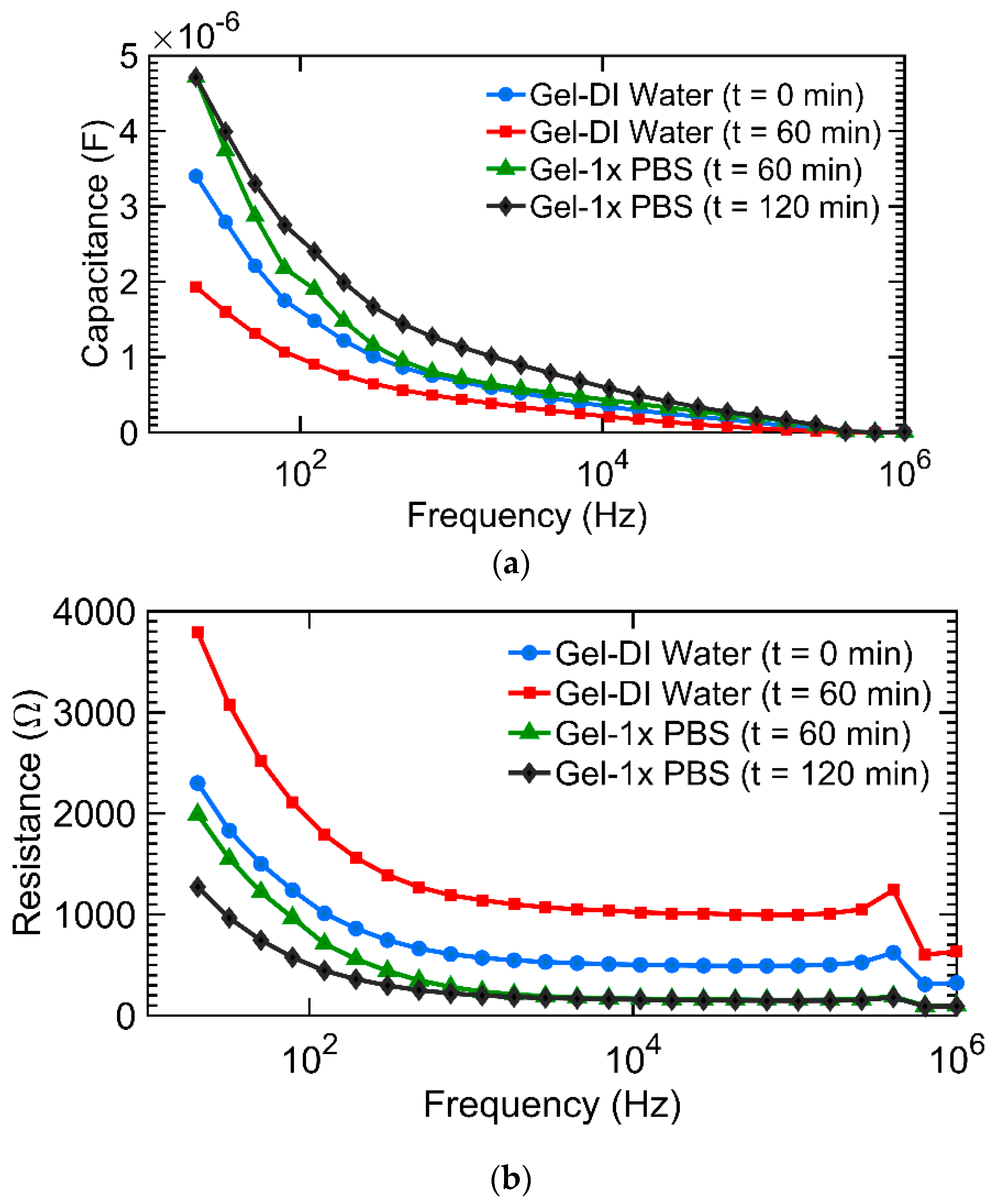
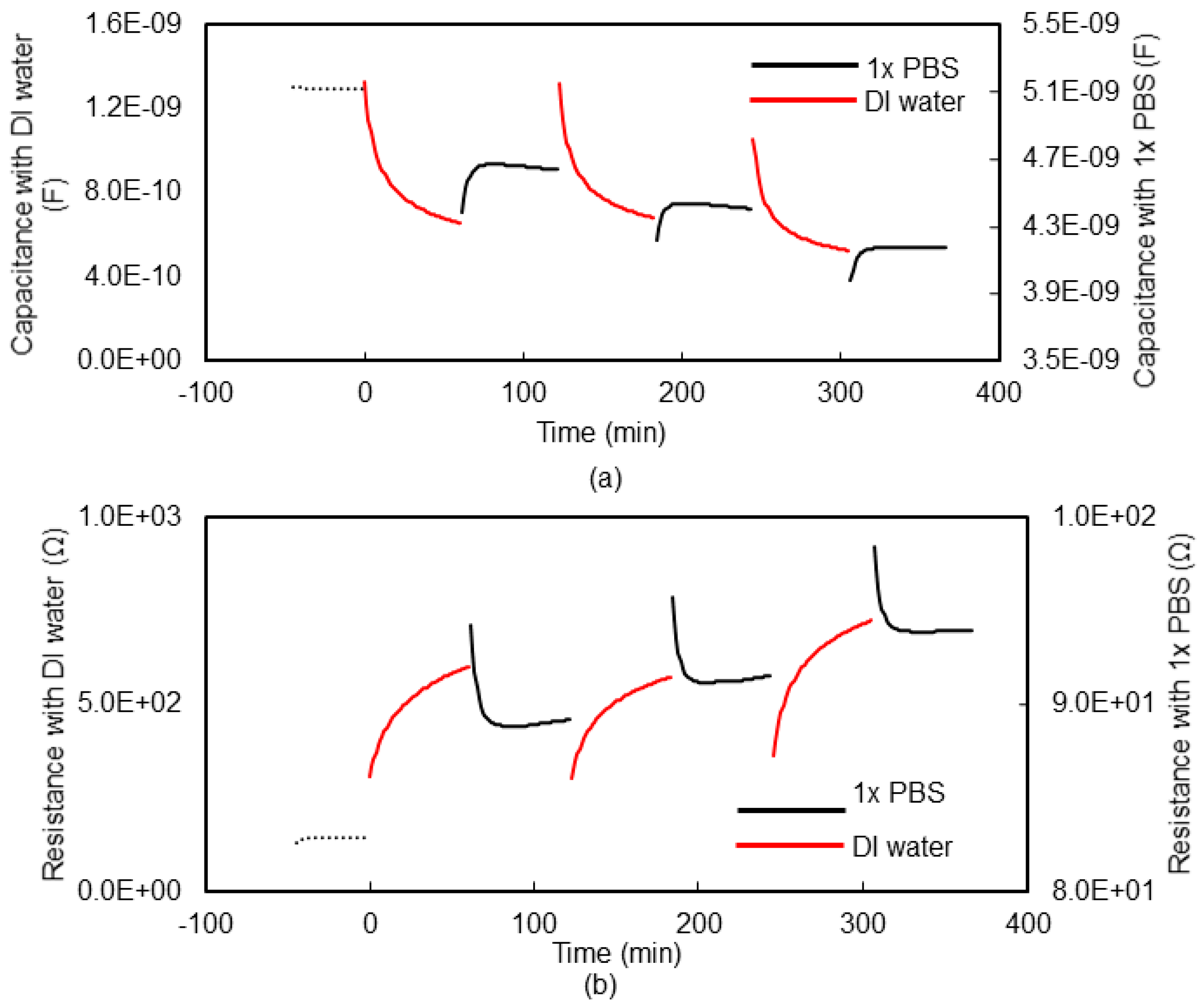
3.1. Impedance Spectroscopy for Monitoring Hydrogel Response
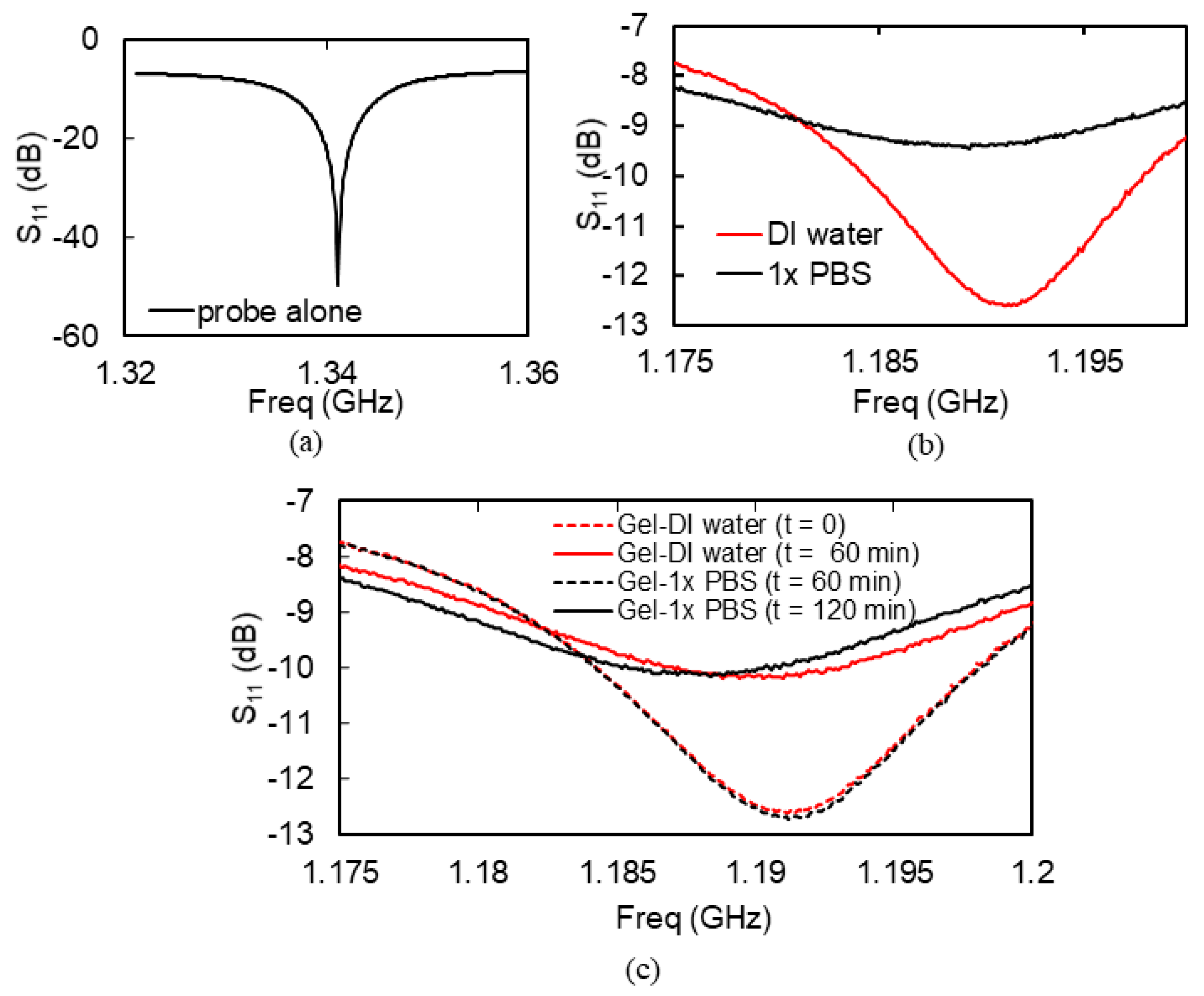
3.2. Contact-Mode Microwave Measurements of Hydrogels
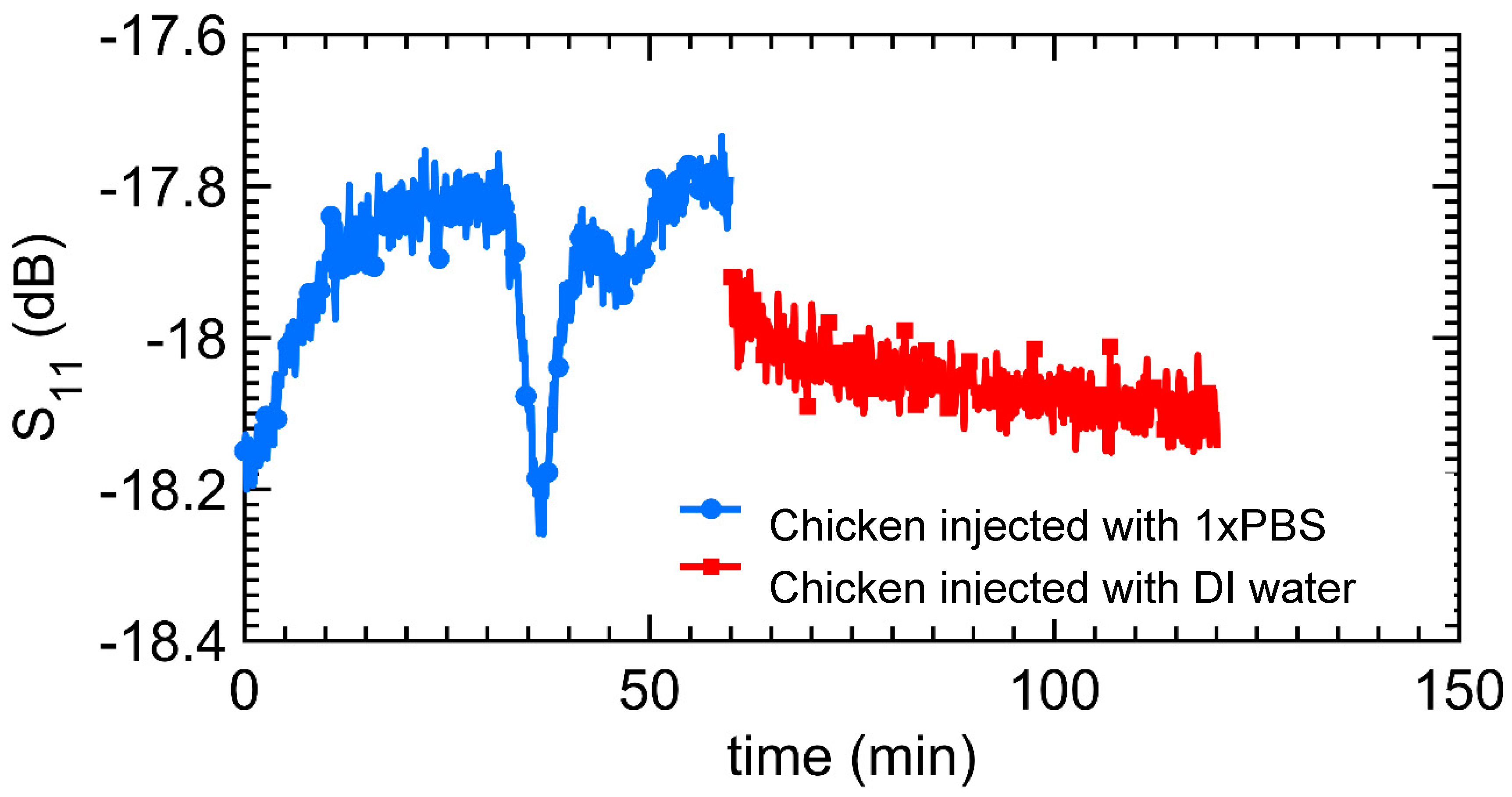
3.3. Remote Microwave Monitoring of Hydrogel Response
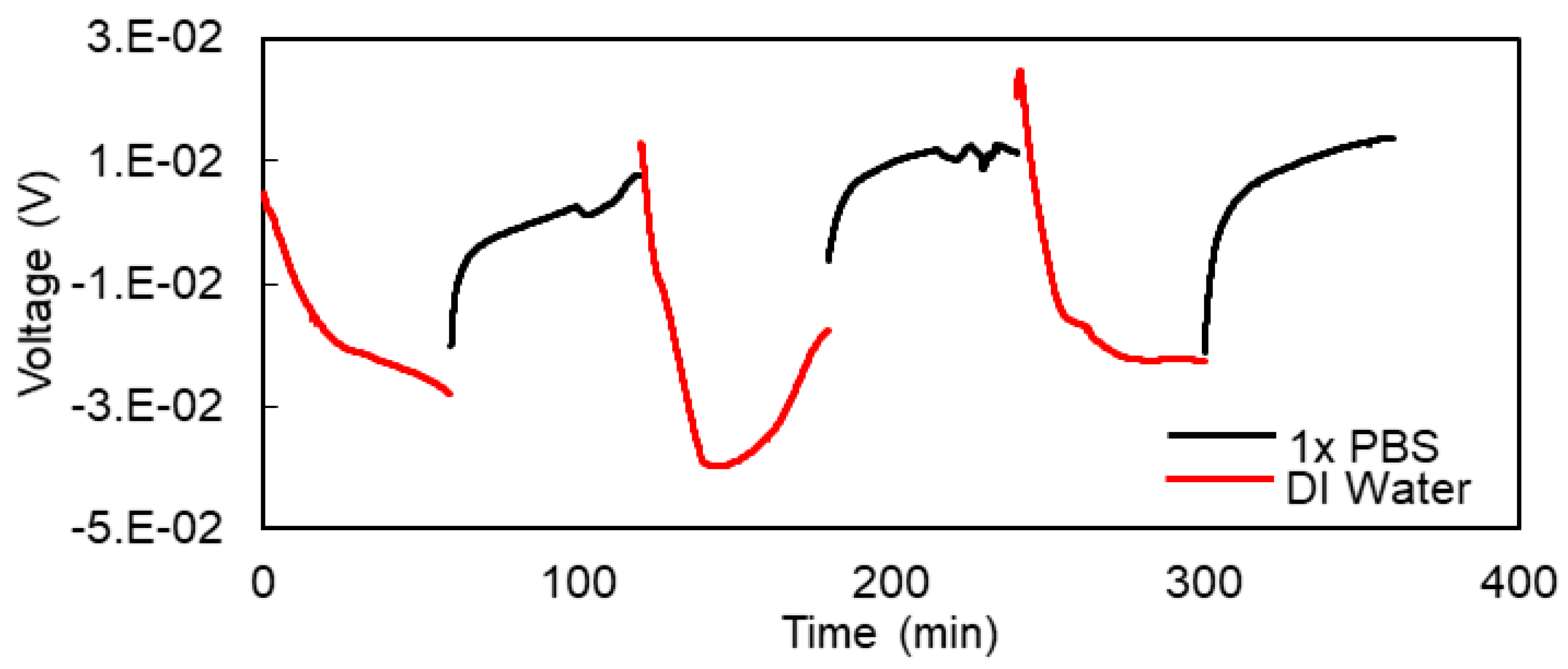
3.4. Potentiometric Method for Monitoring Hydrogel Response
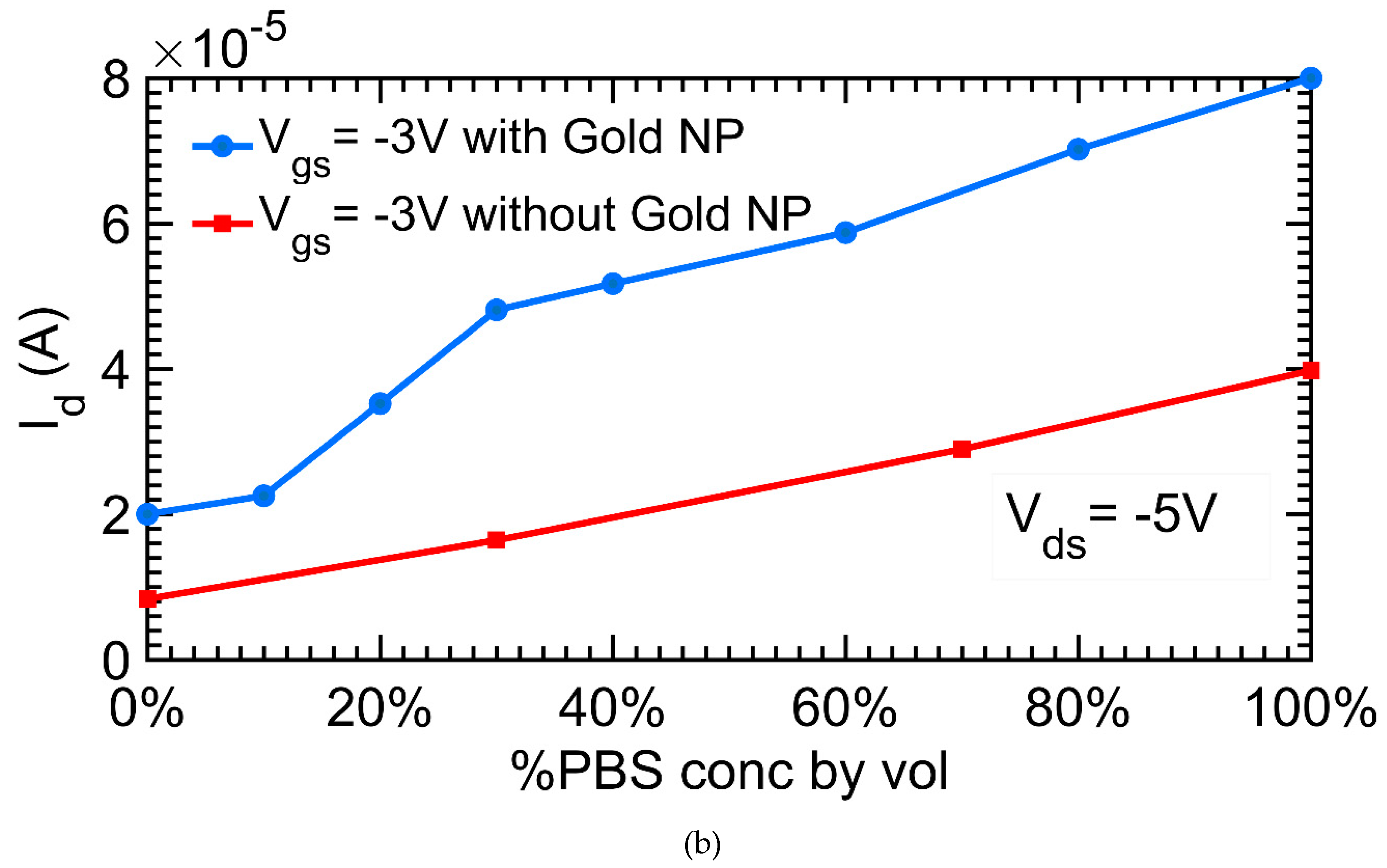
3.5. Montioring Hydrogel Response with Metal-Oxide-Hydrogel Field-Effect Transistor (MOHFET) Current-Voltage (IV) Characteristics
4. Discussion
4.1. Microwave Monitoring of Hydrogel Response
4.2. Montioring Hydrogel Response with MOHFET IV Characteristics
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Guenther, M.; Gerlach, G. Hydrogels for Chemical Sensors. Available online: https://link.springer.com/chapter/10.1007/978-3-540-75645-3_5 (accessed on 11 August 2009).
- Lin, G.; Chang, S.; Hao, H.; Tathireddy, P.; Orthner, M.; Magda, J.; Solzbacher, F. Osmotic Swelling Pressure Response of Smart Hydrogels Suitable for Chronically-Implantable Glucose Sensors. Sens. Actuators B Chem. 2010, 144, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.F.; Adler, H.P. Review on Hydrogel-based pH Sensors and microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Cong, J.; Zhang, X.; Chen, K.; Xu, J. Fiber optic Bragg grating sensor based on hydrogels for measuring salinity. Sens. Actuators B Chem. 2002, 87, 487–490. [Google Scholar] [CrossRef]
- Kuzimenkova, M.V.; Ivanov, A.E.; Thammakhet, C.; Mikhalovska, L.I.; Galaev, I.Y.; Thavarungkul, P.; Kanatharana, P.; Mattiasson, B. Optical responses, permeability and diol-specific reactivity of thin polyacrylamide gels containing immobilized phenylboronic acid. Polymer 2008, 49, 1444–1454. [Google Scholar] [CrossRef]
- Tierney, S.; Volden, S.; Stokke, B.T. Glucose sensors based on a responsive gel incorporated as a Fabry-Perot cavity on a fiber-optic readout platform. Biosens. Bioelectron. 2009, 24, 2034–2039. [Google Scholar] [CrossRef] [PubMed]
- Tierney, S.; Hjelme, D.R.; Stokke, B.T. Determination of swelling of responsive gels with nanometer resolution. Fiber-optic based platform for hydrogels as signal transducers. Anal. Chem. 2008, 80, 5086–5093. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, N.F., Jr.; Salehi-Had, S.; Tucker, R.C. Design of a conductimetric microsensor based on pH-sensitive polymer hydrogels. Proc. Annu. Conf. Eng. Med. Biol. 1991, 13, 1581–1582. [Google Scholar]
- Huang, H.M.; Liu, C.H.; Lee, V.; Lee, C.; Wang, M.J. Highly sensitive glucose biosensor based on CF4-plasma-modified interdigital transducer array (IDA) microelectrode. Sens. Actuators B Chem. 2010, 149, 59–66. [Google Scholar] [CrossRef]
- Guan, T.; Ceyssens, F.; Puers, R. Fabrication and testing of a MEMS platform for characterization of stimuli-sensitive hydrogels. J. Micromech. Microeng. 2012, 22, 087001. [Google Scholar] [CrossRef]
- Kikuchi, A.; Suzuki, K.; Okabayashi, O.; Hoshino, H.; Kataoka, K.; Sakurai, Y.; Okano, T. Glucose-sensing electrode coated with polymer complex gel containing phenylboronic acid. Anal. Chem. 1996, 68, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Mac Kenna, N.; Calvert, P.; Morrin, A. Impedimetric transduction of swelling in pH-responsive hydrogels. Analyst 2015, 140, 3003–3011. [Google Scholar] [CrossRef] [PubMed]
- Tabib-Azar, M.; Leclair, S.R. Applications of evanescent microwave probes in gas and chemical sensors. Sens. Actuators B Chem. 2000, 67, 112–121. [Google Scholar] [CrossRef]
- Tabib-Azar, M.; Wang, R. Planar evanescent microwave imaging probes for nondestructive evaluation of materials with very high spatial resolutions and scan rates. AIP Conf. Proc. 2001, 557, 446. [Google Scholar]
- Tabib-Azar, M. Microwave microscopy and its applications. AIP Conf. Proc. 2001, 557, 400–413. [Google Scholar]
- Tabib-Azar, M. Evanescent Microwave Microscope: A New Nondestructive Material Evaluation Tool with Very High Resolutions-Part 2. CSNDT J. 2001, 22, 14–21. [Google Scholar]
- Sinha, K.; Fawole, O.C.; Tabib-Azar, M. Non-invasive monitoring of electrical parameters of Schefflera arboricola leaf. In Proceedings of the 2015 IEEE SENSORS, Busan, South Korea, 1–4 November 2015. [Google Scholar]
- García-Valenzuela, A.; Tabib-Azar, M. Evanescent microwave probes and microscopy. Rev. Mex. Fis. 1999, 45, 539–550. [Google Scholar]
- Jones, T.R.; Member, G.S.; Zarifi, M.H.; Member, S. Miniaturized Quarter-Mode Substrate Integrated Cavity Resonators for Humidity Sensing Miniaturized Quarter-Mode Substrate Integrated Cavity Resonators for Humidity Sensing. IEEE Microw. Wireless Compon. Lett. 2017, 27, 612–614. [Google Scholar] [CrossRef]
- Bogner, A.; Steiner, C.; Walter, S.; Kita, J.; Hagen, G.; Sensors, R.M. Planar Microstrip Ring Resonators for Microwave-Based Gas Sensing: Design Aspects and Initial Transducers for Humidity and Ammonia Sensing. Sensors 2017, 17, 2422. [Google Scholar] [CrossRef] [PubMed]
- Rydosz, A.; Maciak, E.; Wincza, K.; Gruszczynski, S. Microwave-based sensors with phthalocyanine films for acetone, ethanol and methanol detection. Sens. Actuators B Chem. 2016, 237, 876–886. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Gholidoust, A.; Abdolrazzaghi, M.; Shariaty, P.; Hashisho, Z.; Daneshmand, M. Sensitivity enhancement in planar microwave active-resonator using metal organic framework for CO2 detection. Sens. Actuators B Chem. 2018, 255, 1561–1568. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Borodinov, N.; Luzinov, L.; Yu, G.J.; Wang, P.S. Multi-frequency measurement of volatile organic compounds with a radio-frequency interferometer. IEEE Sens. J. 2017, 17, 3323–3331. [Google Scholar] [CrossRef]
- Fawole, O.; Sinha, K.; Tabib-Azar, M. Monitoring yeast activation with sugar and zero-calorie sweetener using terahertz waves. In Proceedings of the 2015 IEEE SENSORS, Busan, South Korea, 1–4 November 2015. [Google Scholar]
- Fawole, O.C.; Tabib-Azar, M. Terahertz Near-Field Imaging of Biological Samples with Horn Antenna-Excited Probes. IEEE Sens. J. 2016, 16, 8752–8760. [Google Scholar] [CrossRef]
- Wu, H.; Yu, G.; Pan, L.; Liu, N.; McDowell, M.T.; Bao, Z.; Cui, Y. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 2013, 4, 1943. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Zhao, W.; Li, S.; Yang, G. Self-Powered Hydrogel Induced by Ion Transport. Nanoscale 2017, 9, 17080–17090. [Google Scholar] [CrossRef] [PubMed]
- Dolai, S.; Leu, H.Y.; Magda, J.; Tabib-Azar, M. Hydrogel Gold Nanoparticle Switch. IEEE Electron Device Lett. 2018, 39, 1421–1424. [Google Scholar] [CrossRef]
- Dolai, S.; Leu, H.Y.; Magda, J.; Tabib-Azar, M. Metal-Oxide-Hydrogel Field-Effect Sensor. IEEE Electron Device Lett. 2018, 39, 1421–1424. [Google Scholar] [CrossRef]
- Pai, P.; Chowdhury, F.K.; Dang-Tran, T.V.; Tabib-Azar, M. TiOx memristors with variable turn-on voltage using field-effect for non-volatile memory. In Proceedings of the 2013 IEEE SENSORS, Baltimore, MD, USA, 3–6 November 2013. [Google Scholar]
- Mou, N.I.; Zhang, Y.; Pai, P.; Tabib-Azar, M. Steep sub-threshold current slope (∼2 mV/dec) Pt/Cu2S/Pt gated memristor with lon/Ioff> 100. Solid State Electron. 2017, 127, 20–25. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, A.; Sato, N.; Sakata, T.; Yoshida, R.; Kataoka, K.; Miyahara, Y. Chemical-to-electrical-signal transduction synchronized with smart gel volume phase transition. Adv. Mater. 2009, 21, 4372–4378. [Google Scholar] [CrossRef] [PubMed]
- Orthner, M.P.; Lin, G.; Avula, M.; Buetefisch, S.; Magda, J.; Rieth, L.W.; Solzbacher, F. Hydrogel based sensor arrays (2 × 2) with perforated piezoresistive diaphragms for metabolic monitoring (in vitro). Sens. Actuators B Chem. 2010, 145, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Principles of Polymer Chemistry; Cornell University Press: New York, NY, USA, 1953. [Google Scholar]
- Pozar, D.M. Microwave Engineering; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Meaney, P.M.; Gregory, A.P.; Epstein, N.R.; Paulsen, K.D. Microwave open-ended coaxial dielectric probe: Interpretation of the sensing volume re-visited. BMC Med. Phys. 2014, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Garnett, J.M. VII. Colours in metal glasses, in metallic films, and in metallic solutions.—II. Phil. Trans. R. Soc. Lond. A 1906, 205, 237–288. [Google Scholar] [CrossRef]
- Jilani, M.; Wen, W.; Cheong, L.Y.; Zakariya, M.A.; Rehman, M.Z. Equivalent circuit modeling of the dielectric loaded microwave biosensor. Radioengineering 2014, 23, 1038–1047. [Google Scholar]
- Kataoka, H.; Saito, Y.; Sakai, T.; Quartarone, E.; Mustarelli, P. Conduction Mechanisms of PVDF-Type Gel Polymer Electrolytes of Lithium Prepared by a Phase Inversion Process. J. Phys. Chem. B 2000, 104, 11460–11464. [Google Scholar] [CrossRef]
- Salsbery, G.; Tabib-Azar, M. Wireless sensor for determining the impedance of human skin. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar]
- Salsbery, G.; Tabib-Azar, M. Optical sensor for determining concentration of glucose in water. In Proceedings of the 2016 IEEE SENSORS, Orlando, FL, USA, 30 October–3 November 2016. [Google Scholar]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fawole, O.C.; Dolai, S.; Leu, H.-Y.; Magda, J.; Tabib-Azar, M. Remote Microwave and Field-Effect Sensing Techniques for Monitoring Hydrogel Sensor Response. Micromachines 2018, 9, 526. https://doi.org/10.3390/mi9100526
Fawole OC, Dolai S, Leu H-Y, Magda J, Tabib-Azar M. Remote Microwave and Field-Effect Sensing Techniques for Monitoring Hydrogel Sensor Response. Micromachines. 2018; 9(10):526. https://doi.org/10.3390/mi9100526
Chicago/Turabian StyleFawole, Olutosin Charles, Subhashish Dolai, Hsuan-Yu Leu, Jules Magda, and Massood Tabib-Azar. 2018. "Remote Microwave and Field-Effect Sensing Techniques for Monitoring Hydrogel Sensor Response" Micromachines 9, no. 10: 526. https://doi.org/10.3390/mi9100526
APA StyleFawole, O. C., Dolai, S., Leu, H.-Y., Magda, J., & Tabib-Azar, M. (2018). Remote Microwave and Field-Effect Sensing Techniques for Monitoring Hydrogel Sensor Response. Micromachines, 9(10), 526. https://doi.org/10.3390/mi9100526





