Single-Layered Microfluidic Network-Based Combinatorial Dilution for Standard Simplex Lattice Design
Abstract
:1. Introduction
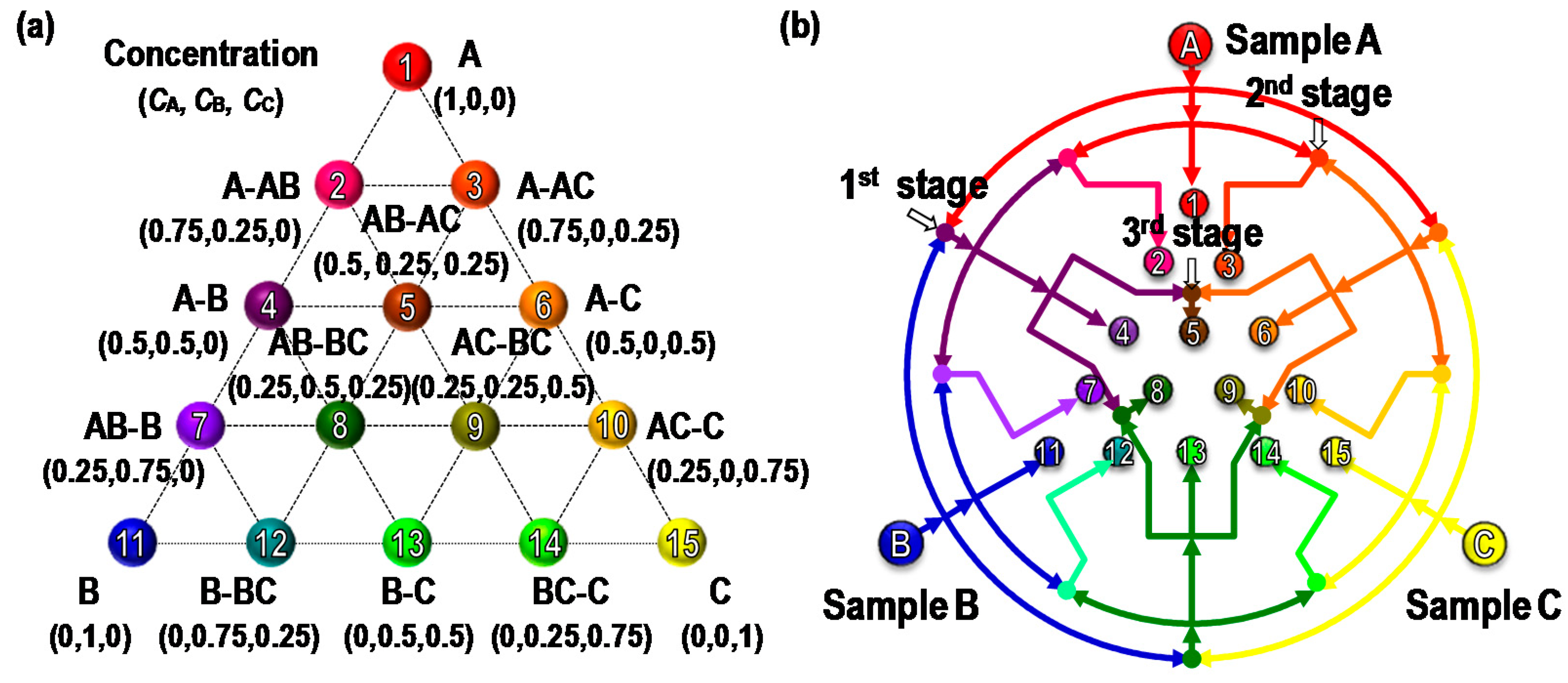
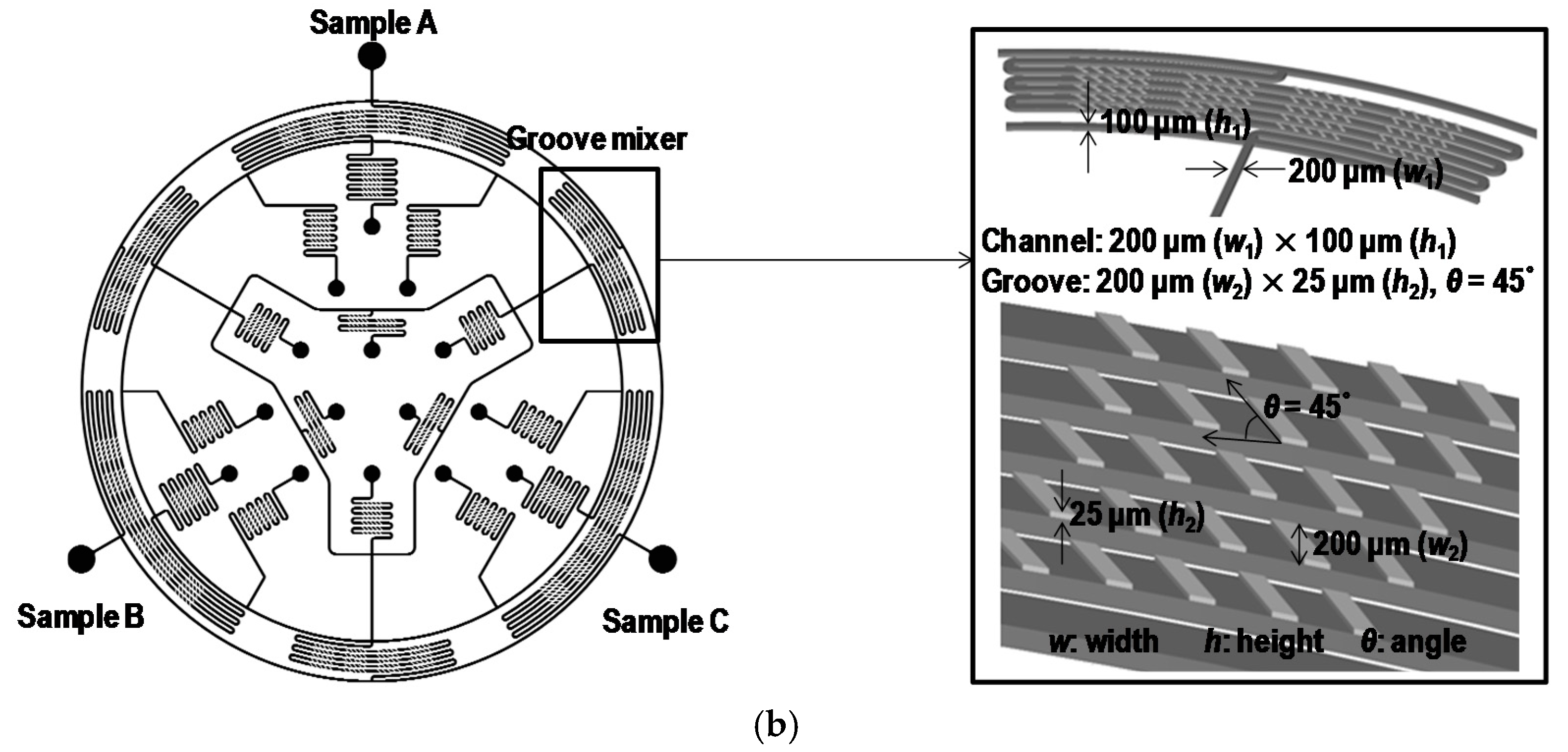
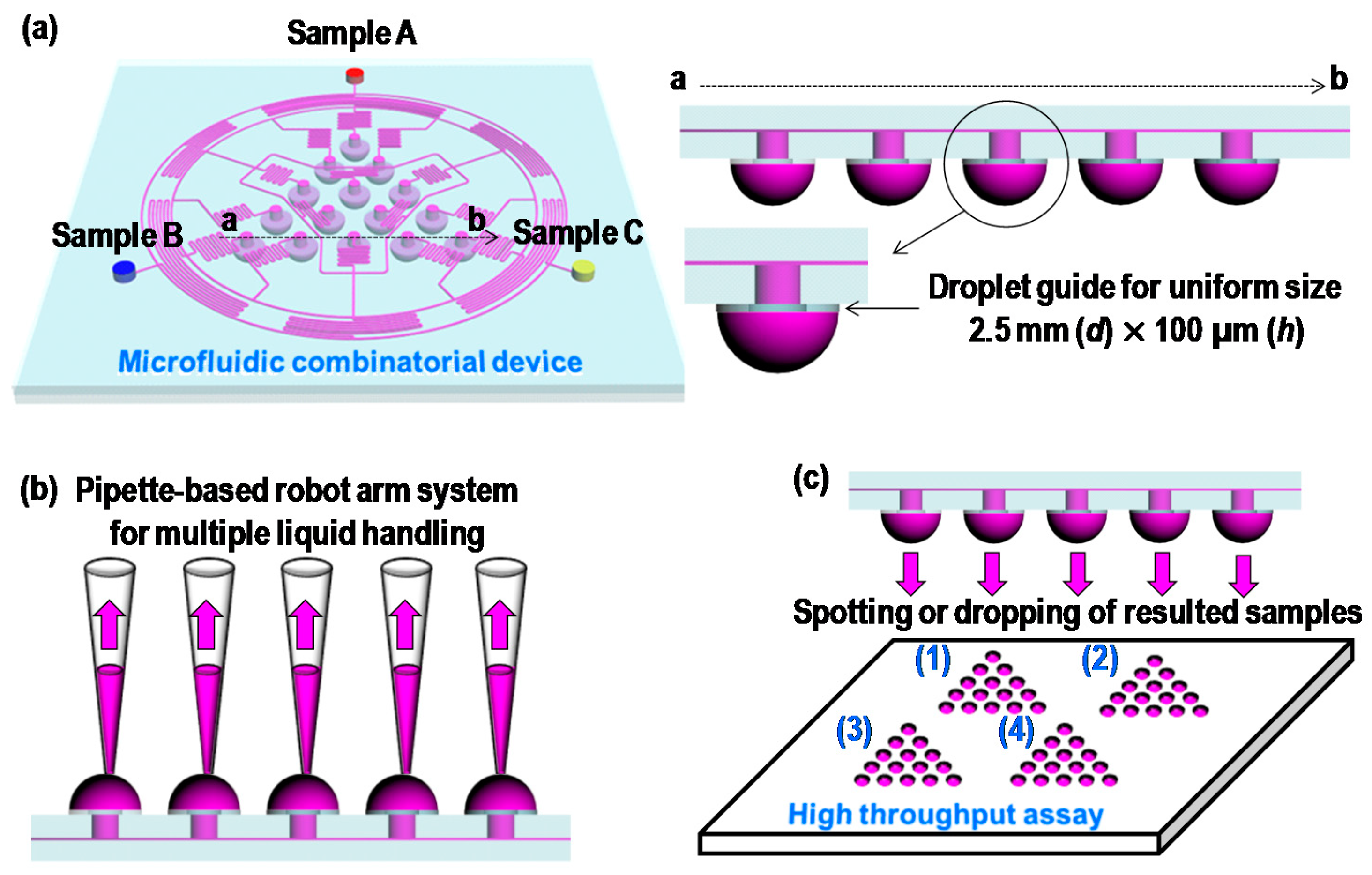
2. Concept and Design
3. Experimental
3.1. Microfabrication and Fluorescence Measurement
3.2. Computational Modeling of Concentration Profiles
4. Results and Discussion
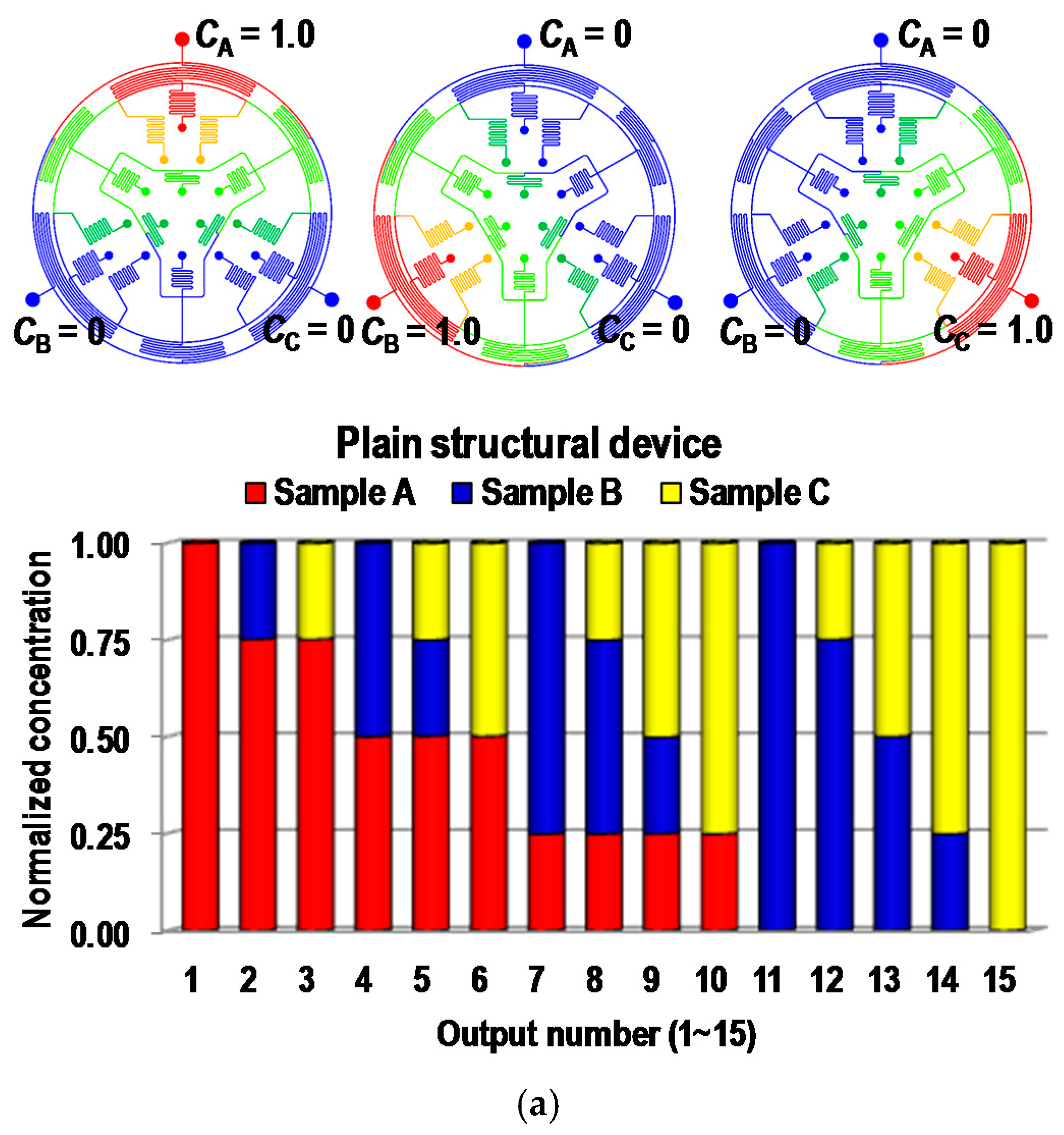
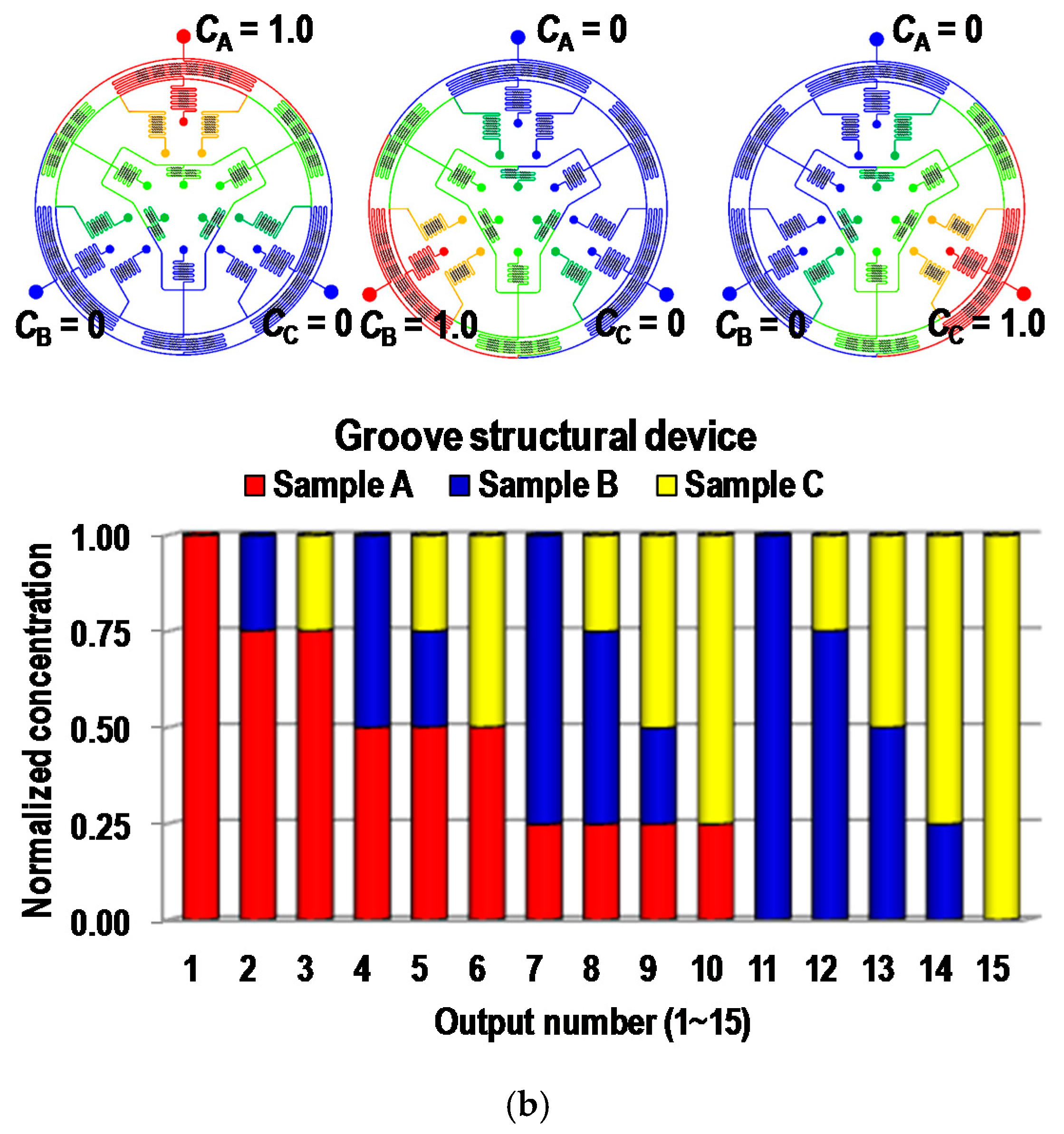
4.1. Computational Fluid Dynamic (CFD) Simulation Results
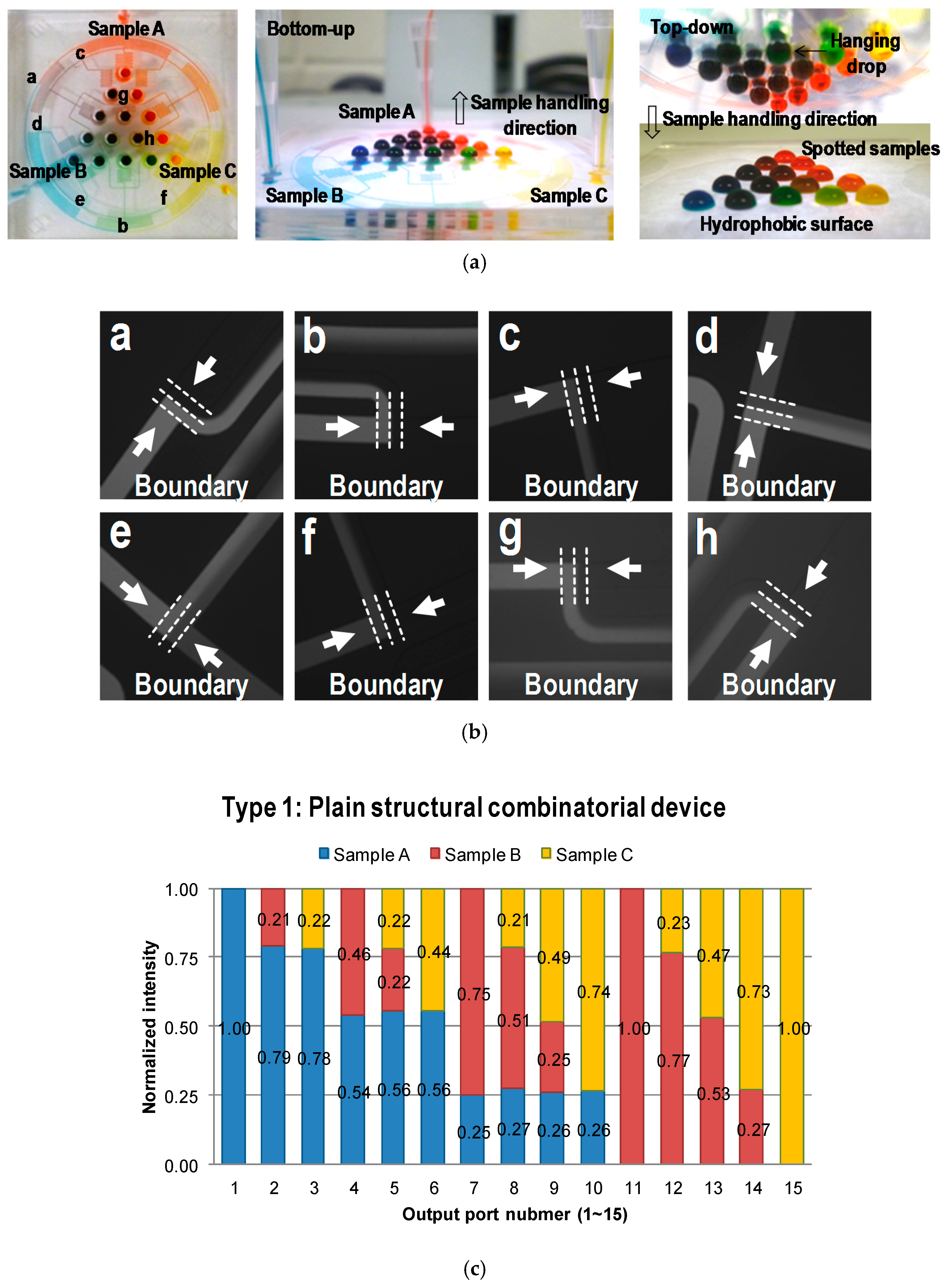
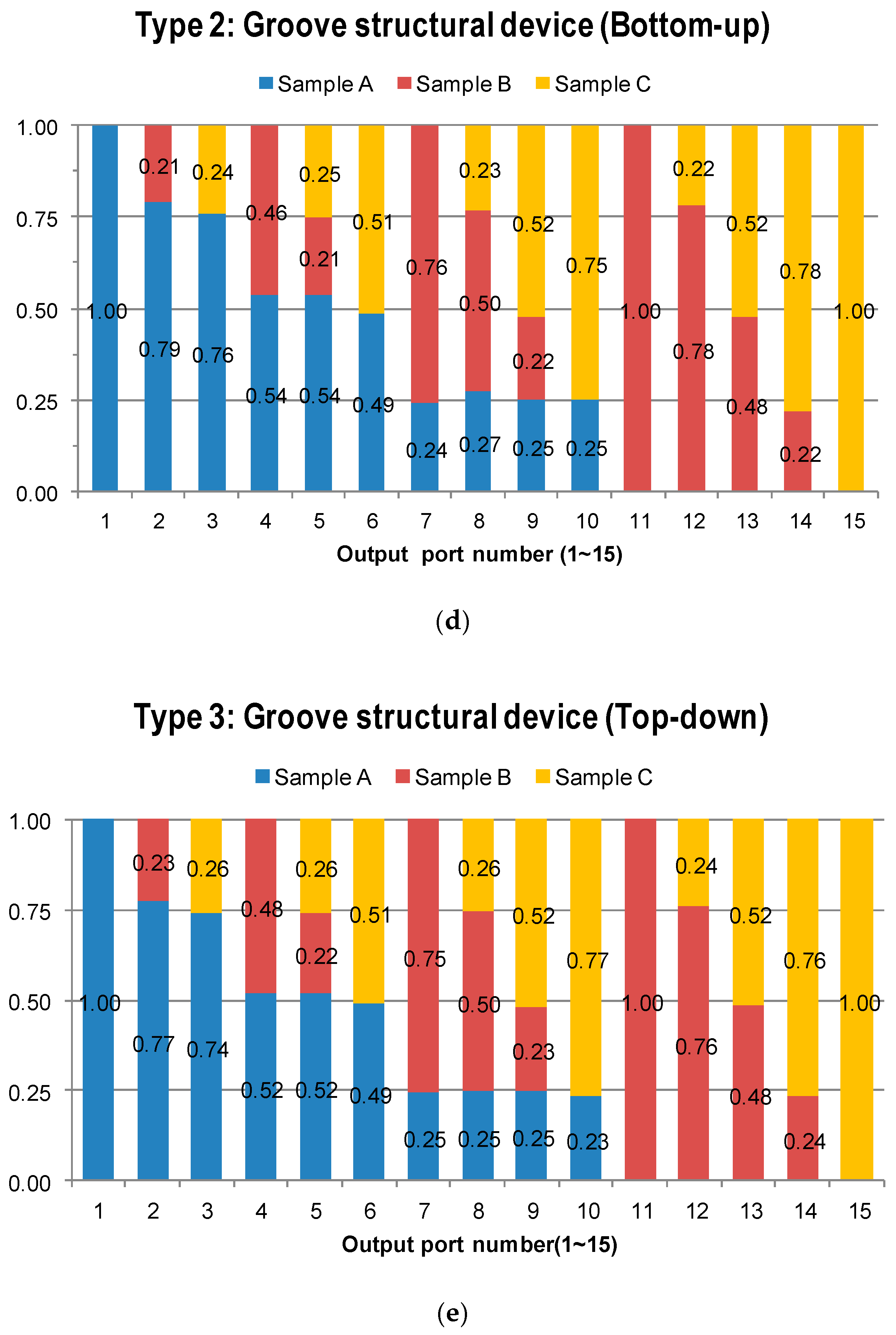
4.2. Fluorescent Experimental Results
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fisher, J.W.; Mayne, J.W. Some views on the statistical design of experiments. Med. Serv. J. Can. 1960, 16, 397–414. [Google Scholar] [PubMed]
- Anderson, M.J.; Whitcomb, P.J. DOE Simplified: Practical Tools for Effective Experimentation, 3rd ed.; CRC Press, Taylor and Francis Group: Boca Raton, FL, USA, 2015. [Google Scholar]
- Pyzdek, T.; Keller, P.A. The Six Sigma Handbook, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Maier, W.F.; Stoewe, K.; Sieg, S. Combinatorial and high-throughput materials science. Angew. Chem. Int. Ed. 2007, 46, 6016–6067. [Google Scholar] [CrossRef] [PubMed]
- Potyrailo, R.; Rajan, K.; Stoewe, K.; Takeuchi, I.; Chisholm, B.; Lam, H. Combinatorial and high-throughput screening of materials libraries: Review of state of the art. ACS Comb. Sci. 2011, 13, 579–633. [Google Scholar] [CrossRef] [PubMed]
- Lehár, J.; Krueger, A.S.; Avery, W.; Heilbut, A.M.; Johansen, L.M.; Price, E.R.; Rickles, R.J.; Short, G.F.; Staunton, J.E.; Jin, X.W.; et al. Synergistic drug combinations tend to improve therapeutically relevant selectivity. Nat. Biotechnol. 2009, 27, 864. [Google Scholar] [CrossRef]
- Agrawal, N.; Pallos, J.; Slepko, N.; Apostol, B.L.; Bodai, L.; Chang, L.W.; Chiang, A.S.; Thompson, L.M.; Marsh, J.L. Identification of combinatorial drug regimens for treatment of Huntington’s disease using Drosophila. Proc. Natl. Acad. Sci. USA 2005, 102, 3777–3781. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.T.; Zhang, S.; Adams, T.; DiPaolo, B.; Dally, J. Establishment and validation of a microfluidic capillary gel electrophoresis platform method for purity analysis of therapeutic monoclonal antibodies. Electrophoresis 2017, 38, 1353–1365. [Google Scholar] [CrossRef] [PubMed]
- Flaim, C.J.; Chien, S.; Bhatia, S.N. An extracellular matrix microarray for probing cellular differentiation. Nat. Methods 2005, 2, 119. [Google Scholar] [CrossRef] [PubMed]
- Tavana, H.; Jovic, A.; Mosadegh, B.; Lee, Q.Y.; Liu, X.; Luker, K.E.; Luker, G.D.; Weiss, S.J.; Takayama, S. Nanolitre liquid patterning in aqueous environments for spatially defined reagent delivery to mammalian cells. Nat. Mater. 2009, 8, 736. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.G.; Levenberg, S.; Langer, R. Nanoliter-scale synthesis of arrayed biomaterials and application to human embryonic stem cells. Nat. Biotechnol. 2004, 22, 863. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Tao, S.; Chen, C.F.; Liu, J.; DeVoe, D.L. Interfacing microfluidics to LDI-MS by automatic robotic spotting. Microfluid. Nanofluid. 2010, 8, 777–787. [Google Scholar] [CrossRef]
- Ferreira, M.V.; Jahnen-Dechent, W.; Neuss, S. Standardization of automated cell-based protocols for toxicity testing of biomaterials. J. Biomol. Screen. 2011, 16, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Meyvantsson, I.; Warrick, J.W.; Hayes, S.; Skoien, A.; Beebe, D.J. Automated cell culture in high density tubeless microfluidic device arrays. Lab Chip 2008, 8, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Kamei, K.I.; Takahashi, H.; Shu, C.J.; Wang, X.; He, G.W.; Silverman, R.; Radu, C.G.; Witte, O.N.; Lee, K.B. Tseng, H.R. Integrated microfluidic devices for combinatorial cell-based assays. Biomed. Microdevices 2009, 11, 547–555. [Google Scholar]
- Schudel, B.R.; Choi, C.J.; Cunningham, B.T.; Kenis, P.J. Microfluidic chip for combinatorial mixing and screening of assays. Lab Chip 2009, 9, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.C.; Stockton, A.M.; Chiesl, T.N.; Kim, J.; Bera, A.; Mathies, R.A. Digitally programmable microfluidic automaton for multiscale combinatorial mixing and sample processing. Lab Chip 2013, 13, 288–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, Y.H.; Hancock, M.J.; Kim, S.B.; Selimović, Š.; Sim, W.Y.; Bae, H.; Khademhosseini, A. An integrated microfluidic device for two-dimensional combinatorial dilution. Lab Chip 2011, 11, 3277–3286. [Google Scholar] [CrossRef] [PubMed]
- Kikutani, Y.; Horiuchi, T.; Uchiyama, K.; Hisamoto, H.; Tokeshi, M.; Kitamori, T. Glass microchip with three-dimensional microchannel network for 2 × 2 parallel synthesis. Lab Chip 2002, 2, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Neils, C.; Tyree, Z.; Finlayson, B.; Folch, A. Combinatorial mixing of microfluidic streams. Lab Chip 2004, 4, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Cooksey, G.A.; Sip, C.G.; Folch, A. multi-purpose microfluidic perfusion system with combinatorial choice of inputs, mixtures, gradient patterns, and flow rates. Lab Chip 2009, 9, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Ho, D.; Tai, Y.C. Monolithic fabrication of three-dimensional microfluidic networks for constructing cell culture array with an integrated combinatorial mixer. Sens. Actuators B Chem. 2008, 129, 826–833. [Google Scholar] [CrossRef]
- Yang, C.G.; Wu, Y.F.; Xu, Z.R.; Wang, J.H. A radial microfluidic concentration gradient generator with high-density channels for cell apoptosis assay. Lab Chip 2011, 11, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, C.; Kim, Y.; Ahn, B.; Bang, J.; Kim, J.; Panchapakesan, R.; Yoon, Y.-K.; Kang, J.Y.; Oh, K.W. Microfluidic concentration-on-demand combinatorial dilutions. Microfluid. Nanofluid. 2011, 11, 75–86. [Google Scholar] [CrossRef]
- Lee, K.; Kim, C.; Jung, G.; Kim, T.S.; Kang, J.Y.; Oh, K.W. Microfluidic network-based combinatorial dilution device for high throughput screening and optimization. Microfluid. Nanofluid. 2010, 8, 677–685. [Google Scholar] [CrossRef]
- Unger, M.A.; Chou, H.P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.C.; Sui, G.; Elizarov, A.; Shu, C.J.; Shin, Y.S.; Dooley, A.N.; Dooley, J.; Huang, A.; Daridon, P.; Wyatt, D.; et al. Multistep synthesis of a radiolabeled imaging probe using integrated microfluidics. Science 2005, 310, 1793–1796. [Google Scholar] [CrossRef] [PubMed]
- Dertinger, S.K.; Chiu, D.T.; Jeon, N.L.; Whitesides, G.M. Generation of gradients having complex shapes using microfluidic networks. Anal. Chem. 2001, 73, 1240. [Google Scholar] [CrossRef]
- Oh, K.W.; Lee, K.; Ahn, B.; Furlani, E.P. Design of pressure-driven microfluidic networks using electric circuit analogy. Lab Chip 2012, 12, 515–545. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Kim, C.; Ahn, B.; Panchapakesan, R.; Full, A.R.; Nordee, L.; Kang, J.Y.; Oh, K.W. Generalized serial dilution module for monotonic and arbitrary microfluidic gradient generators. Lab Chip 2009, 9, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Bhattacharya, B.B.; Chakrabarty, K. Optimization of dilution and mixing of biochemical samples using digital microfluidic biochips. IEEE Trans. Comput.-Aid. Des. Integr. Circuits Syst. 2010, 29, 1696–1708. [Google Scholar] [CrossRef]
- Choi, K.; Ng, A.H.; Fobel, R.; Chang-Yen, D.A.; Yarnell, L.E.; Pearson, E.L.; Oleksak, C.M.; Fischer, A.T.; Luoma, R.P.; Robinson, J.M.; et al. Automated digital microfluidic platform for magnetic-particle-based immunoassays with optimization by design of experiments. Anal. Chem. 2013, 85, 9638–9646. [Google Scholar] [CrossRef] [PubMed]
- Lafrenière, N.M.; Mudrik, J.M.; Ng, A.H.; Seale, B.; Spooner, N.; Wheeler, A.R. Attractive design: An elution solvent optimization platform for magnetic-bead-based fractionation using digital microfluidics and design of experiments. Anal. Chem. 2015, 87, 3902–3910. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Seki, M. Hydrodynamic filtration for on-chip particle concentration and classification utilizing microfluidics. Lab Chip 2005, 5, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.M.; Monteiro-Riviere, N.; Rouse, J.; O’Neill, A.T. A linear dilution microfluidic device for cytotoxicity assays. Lab Chip 2007, 7, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.; Fleischman, A.J.; Roy, S. Fabrication of multi-layer SU-8 microstructures. J. Micromech. Microeng. 2006, 16, 276. [Google Scholar] [CrossRef]
- Tseng, L.Y.; Yang, A.S.; Lee, C.Y.; Hsieh, C.Y. CFD-based optimization of a diamond-obstacles inserted micromixer with boundary protrusions. Eng. Appl. Comput. Fluid Mech. 2011, 5, 210–222. [Google Scholar] [CrossRef]
- Baah, D.; Vickers, D.; Hollinger, A.; Floyd-Smith, T. Patterned dispersion of nanoparticles in hydrogels using microfluidics. Mater. Lett. 2008, 62, 3833–3835. [Google Scholar] [CrossRef]
- Stroock, A.D.; Dertinger, S.K.; Ajdari, A.; Mezić, I.; Stone, H.A.; Whitesides, G.M. Whitesides, Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Wu, Z. Micromixers—A review. J. Micromech. Microeng. 2005, 15, R1. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Kim, C.; Oh, K.W. Single-Layered Microfluidic Network-Based Combinatorial Dilution for Standard Simplex Lattice Design. Micromachines 2018, 9, 489. https://doi.org/10.3390/mi9100489
Lee K, Kim C, Oh KW. Single-Layered Microfluidic Network-Based Combinatorial Dilution for Standard Simplex Lattice Design. Micromachines. 2018; 9(10):489. https://doi.org/10.3390/mi9100489
Chicago/Turabian StyleLee, Kangsun, Choong Kim, and Kwang W. Oh. 2018. "Single-Layered Microfluidic Network-Based Combinatorial Dilution for Standard Simplex Lattice Design" Micromachines 9, no. 10: 489. https://doi.org/10.3390/mi9100489
APA StyleLee, K., Kim, C., & Oh, K. W. (2018). Single-Layered Microfluidic Network-Based Combinatorial Dilution for Standard Simplex Lattice Design. Micromachines, 9(10), 489. https://doi.org/10.3390/mi9100489





