FeCl3-Intercalated Carbon Nanotube Film for Long-Term Infrared Camouflage in Harsh Environments
Abstract
1. Introduction
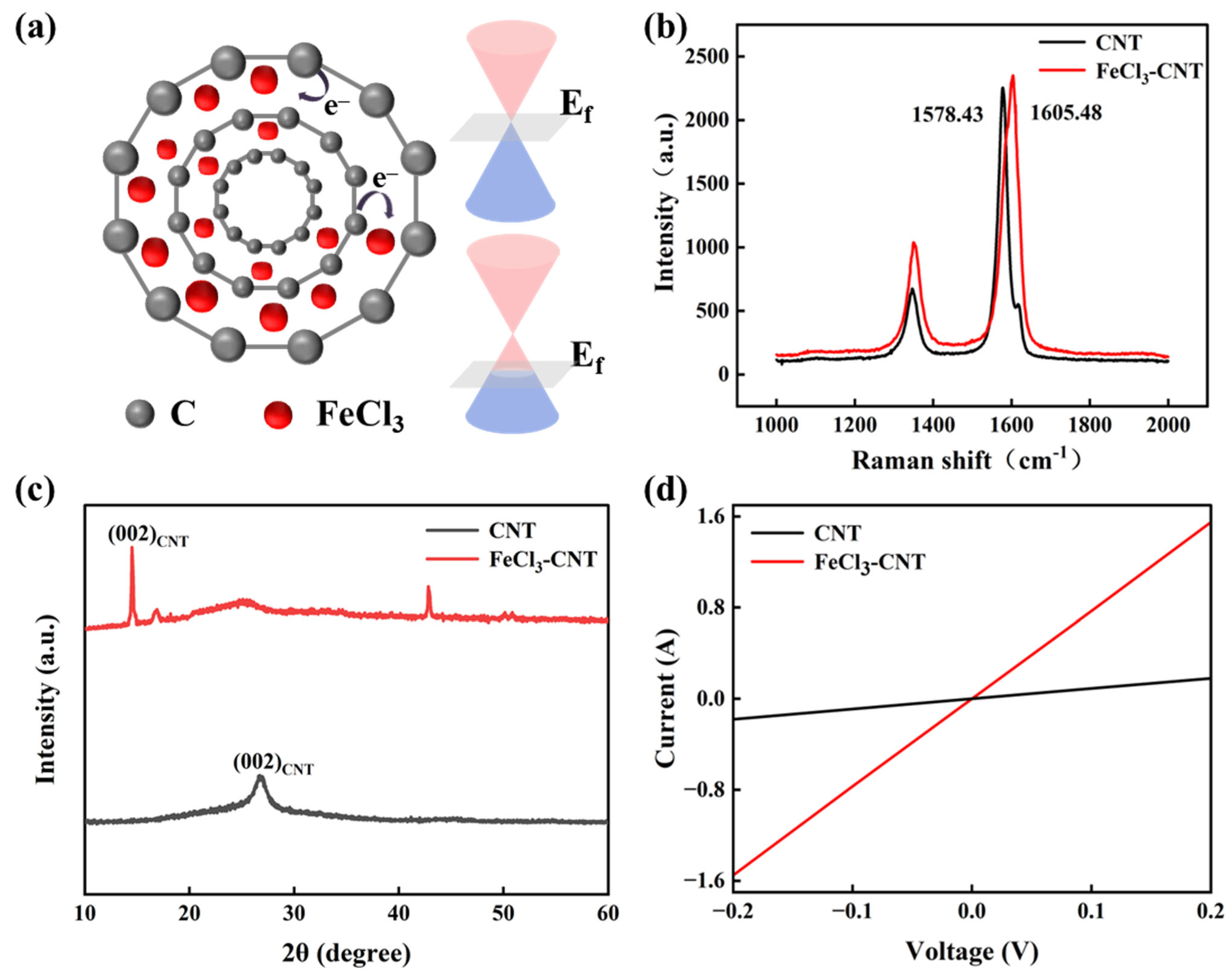
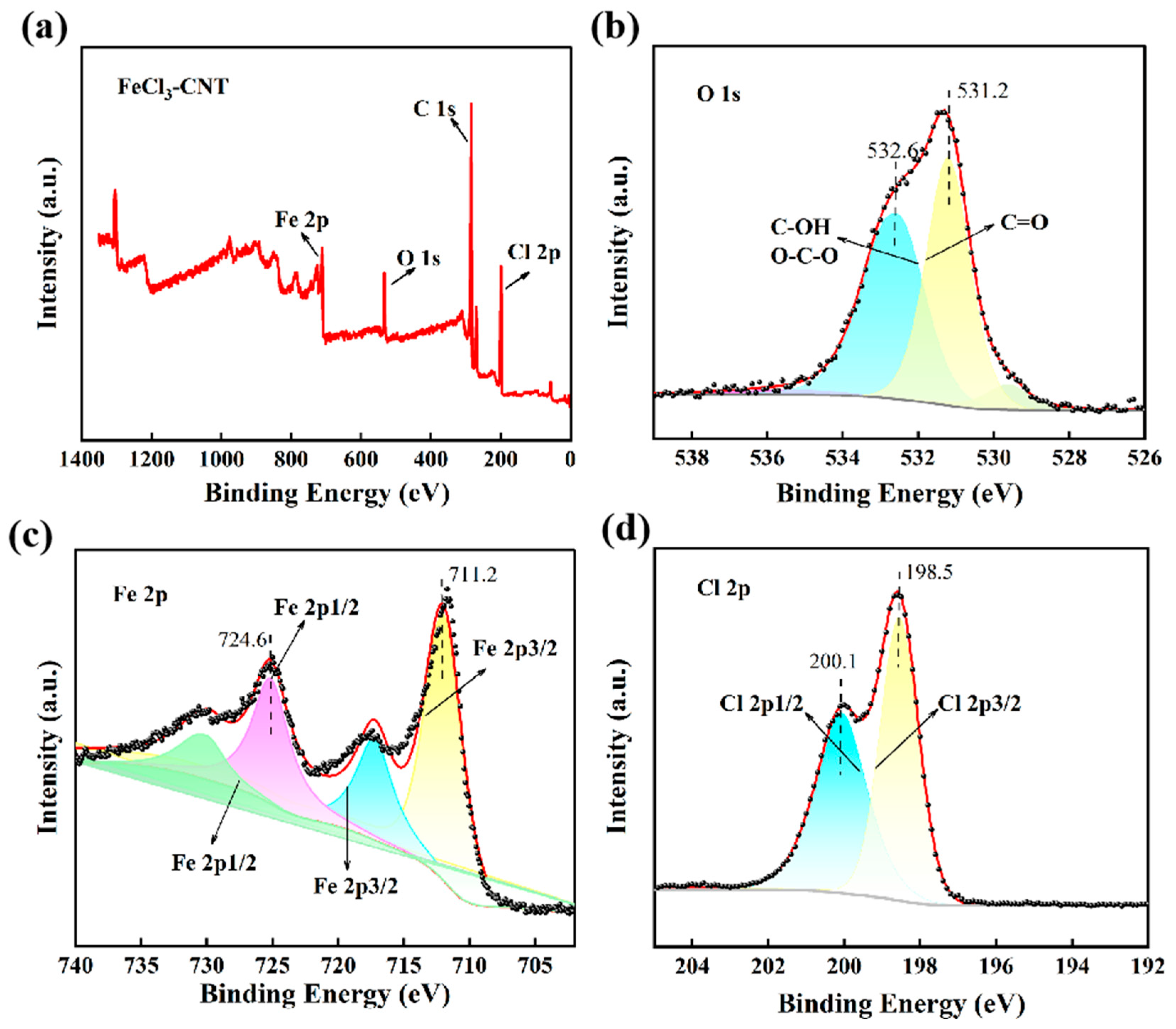
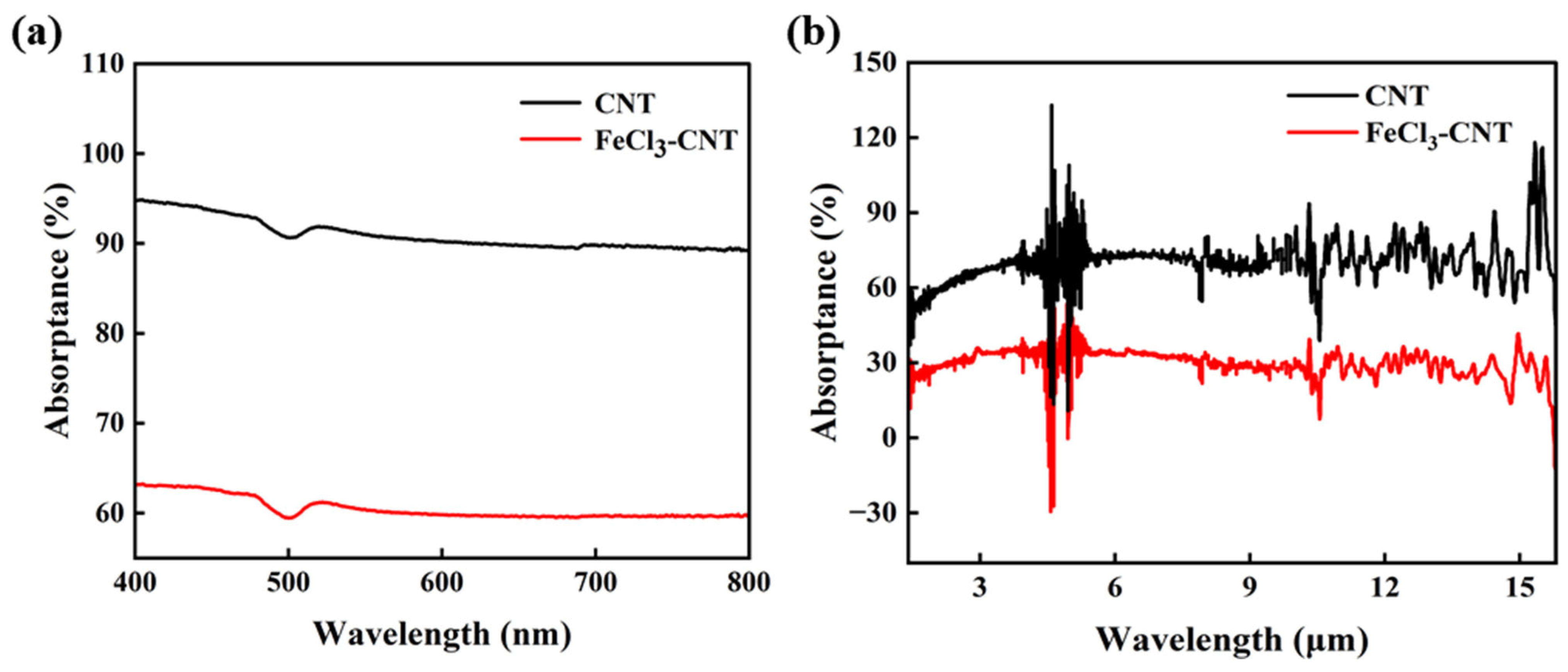
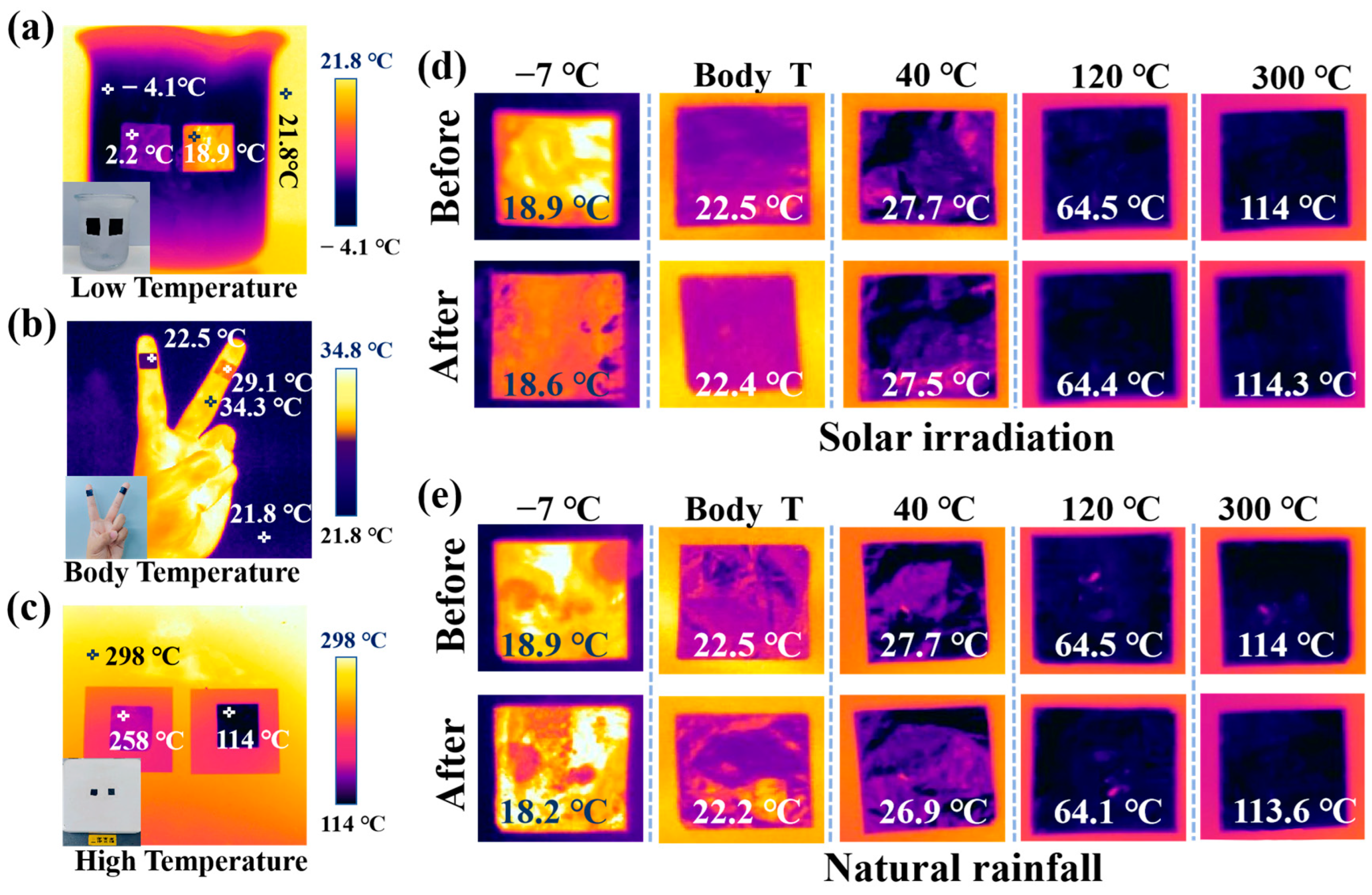
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. Material Preparation and FeCl3 Intercalation
4.2. Characterization and Measurement
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, B.X.; Luo, Z.; Yang, W.G.; Sun, H.; Ding, Y.; Yu, Z.Z.; Yang, D. Adaptive and adjustable MXene/reduced graphene oxide hybrid aerogel composites integrated with phase-change material and thermochromic coating for synchronous visible/infrared camouflages. ACS Nano 2023, 17, 6875–6885. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, Q.; Tao, C.; Hong, Y.; Xu, Z.; Shen, W.; Kaur, S.; Ghosh, P.; Qiu, M. Multispectral camouflage for infrared, visible, lasers and microwave with radiative cooling. Nat. Commun. 2021, 12, 1805. [Google Scholar] [CrossRef]
- Li, B.X.; Luo, Z.; Sun, H.; Quan, Q.; Zhou, S.; Yang, W.G.; Yu, Z.Z.; Yang, D. Spectral-selective and adjustable patterned polydimethylsiloxane/MXene/nanoporous polytetrafluoroethylene metafabric for dynamic infrared camouflage and thermal regulation. Adv. Funct. Mater. 2024, 34, 2407644. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, G.; Liu, C.; Tan, S.; Xu, C.; Zhang, Y.; Zhang, J. Low infrared emissivity of the Cr39Ni7C/inorganic silicate coatings with excellent heat-resistant. Infrared Phys. Techn 2018, 92, 234–239. [Google Scholar] [CrossRef]
- Gu, S.; Quan, C.; Liu, P.; Zhu, Z.; Zhang, J. Laser-compatible infrared stealth metamaterial based on high-temperature resistant metal. Infrared Phys. Techn. 2024, 136, 105072. [Google Scholar] [CrossRef]
- Blasco-Zarzoso, S.; Beltrán-Mir, H.; Cordoncillo, E. Sustainable inorganic pigments with high near-infra-red reflectance based on Fe3+ doped YAlO3 for high temperature applications. J. Alloys Compd. 2023, 960, 170695. [Google Scholar] [CrossRef]
- Burmatova, A.; Khannanov, A.; Zubaidullina, L.; Emelianov, D.; Mostovaya, O.; Stoikov, I.; Ulakhovich, N.; Kutyreva, M. Towards controlling the morphology of cobalt loaded nanocomposites in polyol process with polyethylene glycol. Chim. Techno. Acta 2023, 10, 13. [Google Scholar] [CrossRef]
- Ma, Z.; Jiang, R.; Jing, J.; Kang, S.; Ma, L.; Zhang, K.; Li, J.; Zhang, Y.; Qin, J.; Yun, S.; et al. Lightweight dual-functional segregated nanocomposite foams for integrated infrared stealth and absorption-dominant electromagnetic interference shielding. Nano-Micro Lett. 2024, 16, 223. [Google Scholar] [CrossRef]
- Han, Y.; Ruan, K.; He, X.; Tang, Y.; Guo, H.; Guo, Y.; Qiu, H.; Gu, J. Highly thermally conductive aramid nanofiber composite films with synchronous visible/infrared camouflages and information encryption. Angew. Chem. Int. Ed. Engl. 2024, 63, e202401538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, Z.; Ruan, K.; Gu, J. Multifunctional Ti3C2Tx-(Fe3O4/polyimide) composite films with Janus structure for outstanding electromagnetic interference shielding and superior visual thermal management. Nano Res. 2022, 15, 5601–5609. [Google Scholar] [CrossRef]
- Bai, J.; Gu, W.; Bai, Y.; Li, Y.; Yang, L.; Fu, L.; Li, S.; Li, T.; Zhang, T. Multifunctional flexible sensor based on PU-TA@MXene janus architecture for selective direction recognition. Adv. Mater. 2023, 35, e2302847. [Google Scholar] [CrossRef]
- Avouris, P.; Freitag, M.; Perebeinos, V. Carbon-nanotube photonics and optoelectronics. Nat. Photonics 2008, 2, 341–350. [Google Scholar] [CrossRef]
- He, X.; Gao, W.; Xie, L.; Li, B.; Zhang, Q.; Lei, S.; Robinson, J.M.; Haroz, E.H.; Doorn, S.K.; Wang, W.; et al. Wafer-scale monodomain films of spontaneously aligned single-walled carbon nanotubes. Nat. Nano Tech. 2016, 11, 633–638. [Google Scholar] [CrossRef]
- Lei, S.; Paulus, B.; Li, S.; Schmidt, B. Curvature-dependent adsorption of water inside and outside armchair carbon nanotubes. J. Comput. Chem. 2016, 37, 1313–1320. [Google Scholar] [CrossRef]
- Sevik, C.; Sevincli, H.; Cuniberti, G.; Cagin, T. Phonon engineering in carbon nanotubes by controlling defect concentration. Nano Lett. 2011, 11, 4971–4977. [Google Scholar] [CrossRef] [PubMed]
- Geier, M.L.; Moudgil, K.; Barlow, S.; Marder, S.R.; Hersam, M.C. Controlled n-type doping of carbon nanotube transistors by an organorhodium dimer. Nano Lett. 2016, 16, 4329–4334. [Google Scholar] [CrossRef]
- Lin, Y.; Cao, Y.; Ding, S.; Zhang, P.; Xu, L.; Liu, C.; Hu, Q.; Jin, C.; Peng, L.-M.; Zhang, Z. Scaling aligned carbon nanotube transistors to a sub-10 nm node. Nat. Electron. 2023, 6, 506–515. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Gong, C.; Zhang, D.; Zhang, H.; Zhuang, Q.; Yu, X.; Gong, S.; Chen, X.; Yang, J.; et al. 2D/3D heterojunction engineering at the buried interface towards high-performance inverted methylammonium-free perovskite solar cells. Nat. Energy 2023, 8, 946–955. [Google Scholar] [CrossRef]
- Americo, S.; Pakdel, S.; Thygesen, K.S. Enhancing metallicity and basal plane reactivity of 2D materials via self-intercalation. ACS Nano 2024, 18, 4746–4755. [Google Scholar] [CrossRef] [PubMed]
- Shakir, H.M.; Suleiman, A.A.; Pehlivanoğlu, D.; Kalkan, K.N.; Parsi, A.; Başçı, U.; Durmuş, M.A.; Ölçer, A.O.; Korkut, H.; Sevik, C.; et al. Photoluminescence enhancement in two-dimensional semiconductors via spacer-free metallic screening. npj 2D Mater. Appl. 2025, 9, 54. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Peng, L. Doping-free carbon nanotube optoelectronic devices. Chin. Sci. Bull. 2011, 57, 149–156. [Google Scholar] [CrossRef]
- Abdullah, M.; Younis, M.; Sohail, M.T.; Wu, S.; Zhang, X.; Khan, K.; Asif, M.; Yan, P. Recent progress of 2D materials-based photodetectors from UV to THz waves: Principles, materials, and applications. Small 2024, 20, e2402668. [Google Scholar] [CrossRef]
- Liu, S.; Teng, Y.; Zhang, Z.; Lai, J.; Hu, Z.; Zhang, W.; Zhang, W.; Zhu, J.; Wang, X.; Li, Y.; et al. Interlayer charge transfer induced electrical behavior transition in 1D AgI@sSWCNT van der waals heterostructures. Nano Lett. 2024, 24, 741–747. [Google Scholar] [CrossRef]
- Bayat, R.; Yildizay, H.D.; Şen, F. Thermopower energy waves propagation in novel generation carbon fibers/fuel composite. Fuel 2025, 385, 134112. [Google Scholar] [CrossRef]
- Wang, L.G.; Yao, M.W.; Zhu, C.M.; Yu, G.B.; Zhou, H.B.; Huang, R.T. Investigation on microstructure transition and electrical behavior in (1−x)Bi4Ti3O12/xBi4.5K0.5Ti4O15 lead-free composites. J. Alloys Compd. 2022, 905, 164166. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Luo, F.; Zhang, X.; Chen, X.; Ru, Y.; Song, B.; Cui, Z.; Zhang, K. High performance THz metasurface sensor based on modified-SWCNTs film for femtomolar protein detection. Carbon 2024, 227, 119273. [Google Scholar] [CrossRef]
- Li, C.; Guan, M.; Hong, H.; Chen, K.; Wang, X.; Ma, H.; Wang, A.; Li, Z.; Hu, H.; Xiao, J.; et al. Coherent ultrafast photoemission from a single quantized state of a one-dimensional emitter. Sci. Adv. 2023, 9, eadf4170. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, S.; Bini, M.; Capsoni, D.; Galinetto, P.; Grandi, M.S.; Griebner, U.; Steinmeyer, G.; Agnesi, A.; Pirzio, F.; Ugolotti, E.; et al. Optimizing single-walled-carbon-nanotube-based saturable absorbers for ultrafast lasers. Adv. Funct. Mater. 2012, 22, 4369–4375. [Google Scholar] [CrossRef]
- Xiao, K.; Wu, J.-F.; Yan, H.; Mo, Y.; Zhou, W.; Peng, Y.; Chen, S.; Cui, X.; Chen, L.; Xu, C.; et al. Intercalation-deposition mechanism induced by aligned carbon fiber toward dendrite-free metallic potassium batteries. Energy Storage Mater. 2022, 51, 122–129. [Google Scholar] [CrossRef]
- Zhang, H.; Lerner, M.M. Preparation of graphite intercalation compounds containing crown ethers. Inorg. Chem. 2016, 55, 8281–8284. [Google Scholar] [CrossRef]
- Wang, X.; Xiang, M.; Abulimiti, B.; Ma, J.; Hu, W. Rational design of zero-dimensional gold cluster/two-dimensional TMD heterostructures for enhanced photocatalytic and optoelectronic performance. Nano Lett. 2025, 25, 9801–9808. [Google Scholar] [CrossRef]
- Zhukova, M.O.; Hogan, B.T.; Oparin, E.N.; Shaban, P.S.; Grachev, Y.V.; Kovalska, E.; Walsh, K.K.; Craciun, M.F.; Baldycheva, A.; Tcypkin, A.N. Transmission properties of FeCl(3)-intercalated graphene and WS(2) thin films for terahertz time-domain spectroscopy applications. Nanoscale Res. Lett. 2019, 14, 225. [Google Scholar] [CrossRef]
- Yan, Z.; Zhuxia, Z.; Tianbao, L.; Xuguang, L.; Bingshe, X. XPS and XRD study of FeCl3-graphite intercalation compounds prepared by arc discharge in aqueous solution. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2008, 70, 1060–1064. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Han, F.; Zhang, C.; Zhang, F.; Zhou, D.; Liu, H.; Fan, C.; Li, X.; Liu, J. FeCl3 intercalated microcrystalline graphite enables high volumetric capacity and good cycle stability for lithium-ion batteries. Energy Technol. 2019, 7, 1801091. [Google Scholar] [CrossRef]
- Zhao, W.; Tan, P.H.; Liu, J.; Ferrari, A.C. Intercalation of few-layer graphite flakes with FeCl3: Raman determination of Fermi level, layer by layer decoupling, and stability. J. Am. Chem. Soc. 2011, 133, 5941–5946. [Google Scholar] [CrossRef]
- Nouchi, R.; Saito, T.; Tanigaki, K. Determination of carrier type doped from metal contacts to graphene by channel-length-dependent shift of charge neutrality points. Appl. Phys. Express 2011, 4, 35101. [Google Scholar] [CrossRef]
- Stosser, R.; Herrmann, W. Physical and chemical response of FeCl3/FeCl4(-) spin probes on the functionalizing of ionic liquids. J. Phys. Chem. A 2013, 117, 3960–3971. [Google Scholar] [CrossRef]
- Lin, Z.; Shao, G.; Liu, W.; Wang, Y.; Wang, H.; Wang, H.; Fan, B.; Lu, H.; Xu, H.; Zhang, R. In-situ TEM observations of the structural stability in carbon nanotubes, nanodiamonds and carbon nano-onions under electron irradiation. Carbon 2022, 192, 356–365. [Google Scholar] [CrossRef]
- Lau, C.; Srimani, T.; Bishop, M.D.; Hills, G.; Shulaker, M.M. Tunable n-type doping of carbon nanotubes through engineered atomic layer deposition HfO(X) films. ACS Nano 2018, 12, 10924–10931. [Google Scholar] [CrossRef]
- Khrapach, I.; Withers, F.; Bointon, T.H.; Polyushkin, D.K.; Barnes, W.L.; Russo, S.; Craciun, M.F. Novel highly conductive and transparent graphene-based conductors. Adv. Mater. 2012, 24, 2844–2849. [Google Scholar] [CrossRef]
- Zinni, J.; Camerano, L.; Speyer, L.; Cahen, S.; El Hajj, I.; Bolmont, M.; Medjahdi, G.; Lagrange, P.; Lamura, G.; Profeta, G.; et al. Charge transfer in alkaline-earth metal graphite intercalation compounds. Carbon 2024, 230, 119652. [Google Scholar] [CrossRef]
- Mordkovich, V.Z.; Baxendale, M.; Chang, R.P.H.; Yoshimura, S. Intercalation into carbon nanotubes without breaking the tubular structure. Synth. Met. 1997, 86, 2049–2050. [Google Scholar] [CrossRef]
- Skowroński, J.M.; Morawski, A.W. Synthesis and structure of FeCl3-CrO3-Graphite Bi-int7ercalation compound. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 2006, 310, 63–68. [Google Scholar] [CrossRef]
- Naruse, J.; Yokoi, T.; Ishino, K.; Hikita, Y.; Iwase, K. High-temperature electrical conductivity and thermal stability of FeCl3 doping in carbon nanotube tapes. Appl. Phys. Express 2021, 14, 95002. [Google Scholar] [CrossRef]
- Yang, X.J.; Xu, X.M.; Xu, J.; Han, Y.F. Iron oxychloride (FeOCl): An efficient fenton-like catalyst for producing hydroxyl radicals in degradation of organic contaminants. J. Am. Chem. Soc. 2013, 135, 16058–16061. [Google Scholar] [CrossRef]
- Madrona, C.; Vila, M.; Oropeza, F.E.; de la Peña O’Shea, V.A.; Vilatela, J.J. Macroscopic yarns of FeCl3-intercalated collapsed carbon nanotubes with high doping and stability. Carbon 2021, 173, 311–321. [Google Scholar] [CrossRef]
- Qi, X.; Qu, J.; Zhang, H.-B.; Yang, D.; Yu, Y.; Chi, C.; Yu, Z.-Z. FeCl3 intercalated few-layer graphene for high lithium-ion storage performance. J. Mater. Chem. A 2015, 3, 15498–15504. [Google Scholar] [CrossRef]
- Bointon, T.H.; Khrapach, I.; Yakimova, R.; Shytov, A.V.; Craciun, M.F.; Russo, S. Approaching magnetic ordering in graphene materials by FeCl3 intercalation. Nano Lett. 2014, 14, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, A.; Jones, G.F.; Wehenkel, D.J.; Bezares, F.; Koppens, F.H.L.; Craciun, M.F.; Russo, S. Extraordinary linear dynamic range in laser-defined functionalized graphene photodetectors. Sci. Adv. 2017, 3, e1602617. [Google Scholar] [CrossRef]
- Ergoktas, M.S.; Bakan, G.; Kovalska, E.; Le Fevre, L.W.; Fields, R.P.; Steiner, P.; Yu, X.; Salihoglu, O.; Balci, S.; Fal’ko, V.I.; et al. Multispectral graphene-based electro-optical surfaces with reversible tunability from visible to microwave wavelengths. Nat. Photonics 2021, 15, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Wan, J.; Han, X.; Cai, X.; Zhu, H.; Kim, D.; Ma, D.; Xu, Y.; Munday, J.N.; Drew, H.D.; et al. Approaching the limits of transparency and conductivity in graphitic materials through lithium intercalation. Nat. Commun. 2014, 5, 4224. [Google Scholar] [CrossRef] [PubMed]
- Agassi, J. The kirchhoff-planck radiation law: Considering Kirchhoff’s law as it was initially meant may help us understand the rise of quantum theory. Science 1967, 156, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Piepho, H.P. An adjusted coefficient of determination (R2) for generalized linear mixed models in one go. Biom. J. 2023, 65, e2200290. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wu, D.; Li, J.; Wu, W. Thermal insulation characteristics of a lightweight, porous nanomaterial in high-temperature environments. Mater. Des. 2018, 140, 376–386. [Google Scholar] [CrossRef]
- Su, Y.; Deng, Z.; Qin, W.; Wang, X.; Gong, R. Adaptive infrared camouflage based on quasi-photonic crystal with Ge2Sb2Te. Opt. Commun. 2021, 497, 127203. [Google Scholar] [CrossRef]
- Singh, Y. Electrical resistivity measurements: A review. Int. J. Mod. Phys. Conf. Ser. 2013, 22, 745–756. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Li, Y.; Wang, Z.; Wang, Y.; Chen, R.; Zeng, G. FeCl3-Intercalated Carbon Nanotube Film for Long-Term Infrared Camouflage in Harsh Environments. Micromachines 2026, 17, 38. https://doi.org/10.3390/mi17010038
Li Y, Wang Z, Wang Y, Chen R, Zeng G. FeCl3-Intercalated Carbon Nanotube Film for Long-Term Infrared Camouflage in Harsh Environments. Micromachines. 2026; 17(1):38. https://doi.org/10.3390/mi17010038
Chicago/Turabian StyleLi, Yijie, Zixuan Wang, Yong Wang, Ruiyun Chen, and Ganying Zeng. 2026. "FeCl3-Intercalated Carbon Nanotube Film for Long-Term Infrared Camouflage in Harsh Environments" Micromachines 17, no. 1: 38. https://doi.org/10.3390/mi17010038
APA StyleLi, Y., Wang, Z., Wang, Y., Chen, R., & Zeng, G. (2026). FeCl3-Intercalated Carbon Nanotube Film for Long-Term Infrared Camouflage in Harsh Environments. Micromachines, 17(1), 38. https://doi.org/10.3390/mi17010038




