MXene-Based Gas Sensors for NH3 Detection: Recent Developments and Applications
Abstract
1. Introduction
2. Sensitivity Mechanism of the Gas Sensor
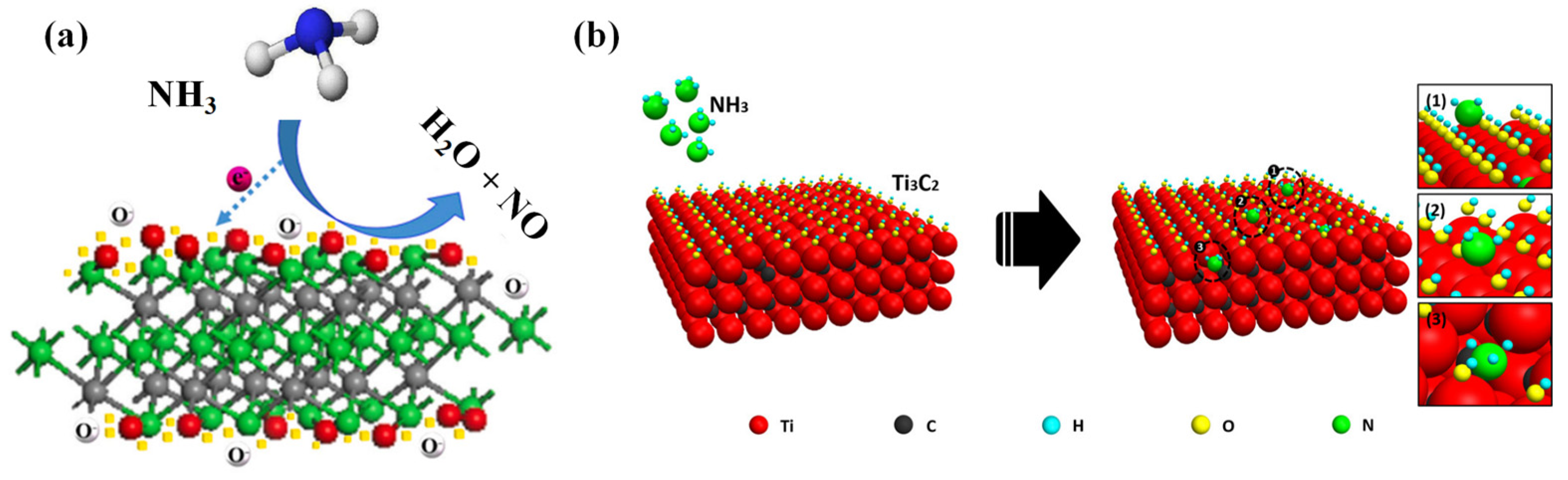
2.1. Adsorbed Oxygen Model
2.2. Terminal Functional Groups React Directly
3. Optimizing the Performance of MXene for NH3
3.1. The Introduction of Metal Oxides

3.2. The Introduction of Two-Dimensional Materials

3.3. The Introduction of Polymers

3.4. The Introduction of Precious Metals
4. Applications of MXene-Based Ammonia Gas Sensors
4.1. Industrial Production and Safety Protection
4.2. Food Safety Monitoring
4.3. Precise Management of Agriculture and Animal Husbandry
4.4. Medical Diagnosis and Health Monitoring
5. Current Challenges and Future Perspectives
5.1. Persistent Challenges
5.2. Promising Future Research Directions
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tallaksen, J.; Bauer, F.; Hulteberg, C.; Reese, M.; Ahlgren, S. Nitrogen fertilizers manufactured using wind power: Greenhouse gas and energy balance of community-scale ammonia production. J. Clean. Prod. 2015, 107, 626–635. [Google Scholar] [CrossRef]
- Luo, M.; Huang, X.; Xiong, D.; Cai, S.; Li, S.; Jia, Z.; Gao, Z. Fast response/recovery and sub-ppm ammonia gas sensors based on a novel V2CTx@MoS2 composite. J. Mater. Chem. A 2024, 12, 12225–12236. [Google Scholar] [CrossRef]
- He, K.; Liu, Y.; Tian, L.; He, W.; Cheng, Q. Review in anaerobic digestion of food waste. Heliyon 2024, 10, e28200. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Lee, S.R.; Kim, S.; Choi, Y. Characteristics of ammonia and hydrogen sulfide emitted from swine farms during process of aerobically treatment liquid swine manure. J. Anim. Environ. Sci. 2024, 26, 129–135. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Yin, G.W.; Li, W.; Tan, R.; Hou, W.L.; Liu, X.Q.; Zhao, Y.Z.; Zhang, H.Q. Highly sensitive colorimetric detection of ammonia and respiratory ammonia based on ammonia induced perylene diimide anion radical π-dimer dissociation. Sens. Actuators B Chem. 2025, 433, 137555. [Google Scholar] [CrossRef]
- Beale, D.J.; Jones, O.A.H.; Karpe, A.V.; Dayalan, S.; Oh, D.Y.; Kouremenos, K.A.; Ahmed, W.; Palombo, E.A. A Review of Analytical Techniques and Their Application in Disease Diagnosis in Breathomics and Salivaomics Research. Int. J. Mol. Sci. 2017, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Chung, J.-H.; Yoon, J.-W.; Lee, J.-H. Highly selective, sensitive, and rapidly responding acetone sensor using ferroelectric ε-WO3 spheres doped with Nb for monitoring ketogenic diet efficiency. Sens. Actuators B Chem. 2021, 338, 129823. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Zegebreal, L.T.; Tegegne, N.A.; Hone, F.G. Recent progress in hybrid conducting polymers and metal oxide nanocomposite for room-temperature gas sensor applications: A review. Sens. Actuators A Phys. 2023, 359, 114472. [Google Scholar] [CrossRef]
- Zhang, D.Z.; Pan, W.J.; Tang, M.C.; Wang, D.Y.; Yu, S.J.; Mi, Q.; Pan, Q.N.; Hu, Y.Q. Diversiform gas sensors based on two-dimensional nanomaterials. Nano Res. 2023, 16, 11959–11991. [Google Scholar] [CrossRef]
- Yu, K.; Ren, J.; Liao, W.; Hu, B.; Bai, C.; Li, Z.; Zhang, X.; Chhattal, M.; Li, N.; Qiang, L. Maintaining the 2D Structure of MXene via Self-Assembled Monolayers for Efficient Lubrication in High Humidity. Small 2024, 20, e2402143. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Yang, Y.X.; Zou, B.S.; Zhang, Y.B. MXene-enabled gas sensors for wearable breath monitoring. Chem. Eng. J. 2025, 510, 161414. [Google Scholar] [CrossRef]
- Mirzaei, A.; Lee, M.H.; Safaeian, H.; Kim, T.U.; Kim, J.Y.; Kim, H.W.; Kim, S.S. Room Temperature Chemiresistive Gas Sensors Based on 2D MXenes. Sensors 2023, 23, 8829. [Google Scholar] [CrossRef] [PubMed]
- Cheng, K.X.; Tian, X.; Yuan, S.R.; Feng, Q.Y.; Wang, Y.D. Research Progress on Ammonia Sensors Based on Ti3C2Tx MXene at Room Temperature: A Review. Sensors 2024, 24, 4465. [Google Scholar] [CrossRef] [PubMed]
- Ahmadian, Z.; Mohammadi, S.; Mortazavi, Y.; Khodadadi, A.A. Stable N-doped Ti3C2Tx gas sensors for recoverable detection of ammonia at room temperature. Ceram. Int. 2023, 49, 38635–38643. [Google Scholar] [CrossRef]
- Lee, E.; Mohammadi, A.V.; Prorok, B.C.; Yoon, Y.S.; Beidaghi, M.; Kim, D.J. Room Temperature Gas Sensing of Two-Dimensional Titanium Carbide (MXene). ACS Appl. Mater. Interfaces 2017, 9, 37184–37190. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, Y.H.; Jing, Y.; Ma, J.M.; Du, C.F.; Yan, Q.Y. Surface Modified MXene-Based Nanocomposites for Electrochemical Energy Conversion and Storage. Small 2019, 15, e1901503. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, H.J.; Woo, B.-C.; Kim, K.J.; Kim, G.T.; Yun, W.S. Electric Property Evolution of Structurally Defected Multilayer Graphene. Nano Lett. 2008, 8, 3092–3096. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Ocola, L.E.; Chen, J. Reduced graphene oxide for room-temperature gas sensors. Nanotechnology 2009, 20, 445502. [Google Scholar] [CrossRef] [PubMed]
- Volodin, A.M.; Cherkashin, A.E. Observation conditions and thermal stability of O2− on SnO2. React. Kinet. Catal. Lett. 1981, 17, 329–332. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of tin oxide surface with O2, H2O AND H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Bannov, A.G.; Popov, M.V.; Brester, A.E.; Kurmashov, P.B. Recent Advances in Ammonia Gas Sensors Based on Carbon Nanomaterials. Micromachines 2021, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Aarya, S.; Kumar, Y.; Chahota, R.K. Recent Advances in Materials, Parameters, Performance and Technology in Ammonia Sensors: A Review. J. Inorg. Organomet. Polym. Mater. 2020, 30, 269–290. [Google Scholar] [CrossRef]
- Seekaew, Y.; Kamlue, S.; Wongchoosuk, C. Room-Temperature Ammonia Gas Sensor Based on Ti3C2Tx MXene/Graphene Oxide/CuO/ZnO Nanocomposite. ACS Appl. Nano Mater. 2023, 6, 9008–9020. [Google Scholar] [CrossRef]
- Choi, Y.R.; Yoon, Y.-G.; Choi, K.S.; Kang, J.H.; Shim, Y.-S.; Kim, Y.H.; Chang, H.J.; Lee, J.-H.; Park, C.R.; Kim, S.Y.; et al. Role of oxygen functional groups in graphene oxide for reversible room-temperature NO2 sensing. Carbon 2015, 91, 178–187. [Google Scholar] [CrossRef]
- Gautam, M.; Jayatissa, A.H. Ammonia gas sensing behavior of graphene surface decorated with gold nanoparticles. Solid-State Electron. 2012, 78, 159–165. [Google Scholar] [CrossRef]
- Ali, A.-M.S. Reactions of Urea Phosphate in Calcareous and Alkaline Soils: Ammonia Volatilization and Effects on Soil Sodium and Salinity; The University of Arizona: Tucson, AZ, USA, 1989. [Google Scholar]
- Zhu, S.; Chen, M.; Wang, S.; Yue, Y.; Jiang, S.; Wu, Q.; Xiao, H.; He, S.; Han, J. 3D oxidation-resistant MXene electrode supported by softened wood toward high-performance flexible supercapacitors. Chem. Eng. J. 2024, 496, 153739. [Google Scholar] [CrossRef]
- Quan, W.; Shi, J.; Zeng, M.; Li, B.; Liu, Z.; Lv, W.; Fan, C.; Wu, J.; Liu, X.; Yang, J.; et al. Quantum Confinement and End-Sealing Effects for Highly Sensitive and Stable Nitrogen Dioxide Detection: Homogeneous Integration of Ti3C2Tx-Based Flexible Gas Sensors. ACS Sens. 2024, 9, 4578–4590. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Unnikrishnan, B.; Chen, I.W.P.; Harroun, S.G.; Chang, H.T.; Huang, C.C. Excellent oxidation resistive MXene aqueous ink for micro-supercapacitor application. Energy Storage Mater. 2020, 25, 563–571. [Google Scholar] [CrossRef]
- Gasso, S.; Mahajan, A. Development of Highly Sensitive and Humidity Independent Room Temeprature NO2 Gas Sensor Using Two Dimensional Ti3C2TX Nanosheets and One Dimensional WO3 Nanorods Nanocomposite br. ACS Sens. 2022, 7, 2454–2464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.F.J.; Pinilla, S.; McEyoy, N.; Cullen, C.P.; Anasori, B.; Long, E.; Park, S.H.; Seral-Ascaso, A.; Shmeliov, A.; Krishnan, D.; et al. Oxidation Stability of Colloidal Two-Dimensional Titanium Carbides (MXenes). Chem. Mater. 2017, 29, 4848–4856. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Xu, J.; Lei, X.; Sun, H.; Ma, F.; Ai, T.; Chu, P.K. CeO2 and Nb2CTx heterojunction for efficient room-temperature NH3 detection. Chem. Eng. J. 2025, 512, 162687. [Google Scholar] [CrossRef]
- Bi, H.; Zhang, L.-X.; Xing, Y.; Zhang, P.; Chen, J.-J.; Yin, J.; Bie, L.-J. Morphology-controlled synthesis of CeO2 nanocrystals and their facet-dependent gas sensing properties. Sens. Actuators B Chem. 2021, 330, 129374. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, C.; Zhang, J.; Huang, Y.; Xu, H.; Lu, H.; Xu, K.; Ma, F. Enhanced NO2-sensing performances of CeO2 nanoparticles on MoS2 at room temperature. Appl. Surf. Sci. 2022, 600, 154157. [Google Scholar] [CrossRef]
- Liu, Z.; He, T.; Sun, H.; Huang, B.; Li, X. Layered MXene heterostructured with In2O3 nanoparticles for ammonia sensors at room temperature. Sens. Actuators B Chem. 2022, 365, 131918. [Google Scholar] [CrossRef]
- Gu, D.; Li, X.; Wang, H.; Li, M.; Xi, Y.; Chen, Y.; Wang, J.; Rumyntseva, M.N.; Gaskov, A.M. Light enhanced VOCs sensing of WS2 microflakes based chemiresistive sensors powered by triboelectronic nangenerators. Sens. Actuators B Chem. 2018, 256, 992–1000. [Google Scholar] [CrossRef]
- Tai, H.; Duan, Z.; He, Z.; Li, X.; Xu, J.; Liu, B.; Jiang, Y. Enhanced ammonia response of Ti3C2Tx nanosheets supported by TiO2 nanoparticles at room temperature. Sens. Actuators B Chem. 2019, 298, 126874. [Google Scholar] [CrossRef]
- Li, Y.; Deng, X.; Tian, J.; Liang, Z.; Cui, H. Ti3C2 MXene-derived Ti3C2/TiO2 nanoflowers for noble-metal-free photocatalytic overall water splitting. Appl. Mater. Today 2018, 13, 217–227. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, C.; Li, P.; Sun, Y.e. Layer-by-Layer Self-assembly of Co3O4 Nanorod-Decorated MoS2 Nanosheet-Based Nanocomposite toward High-Performance Ammonia Detection. ACS Appl. Mater. Interfaces 2017, 9, 6462–6471. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.S.; Luo, M.Y.; He, Q.; Huang, X.P.; Cai, S.J.; Li, S.; Jia, Z.H.; Gao, Z.X. Nb2CTx/MoSe2 composites for a highly sensitive NH3 gas sensor at room temperature. Talanta 2025, 286, 127446. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.S.; Kim, J.-Y.; Mirzaei, A.; Lee, M.H.; Kim, H.W.; Kim, S.S. Au- and Pt-decorated Ti3C2Tx MXenes for preparing self-heated and flexible NH3 gas sensors. Sens. Actuators B Chem. 2024, 403, 135112. [Google Scholar] [CrossRef]
- Guo, Z.Z.; Zhang, H.R.; Zhang, J.M.; Alwadie, N.; Duan, L.Y.; Li, Y.L.; Hou, Z.Y.; Dao, V.; Irshad, M.S. Tailoring MoS2 nanoflakes over MXenes nanobelts for efficient ammonia detection at room temperature. J. Alloys Compd. 2025, 1010, 177710. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Zhang, H.; Gong, L.; Yang, Y.; Zhao, W.; Yu, S.; Yin, Y.; Sun, D. Polymerized polyaniline/MXene (V2C) as building blocks of supercapacitor and ammonia sensor self-powered by electromagnetic-triboelectric hybrid generator. Nano Energy 2021, 88, 106242. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, S.; Bai, S.; Shen, L.; Peng, J.; Zhang, Y.; Cui, X.; Yu, C.; Zhang, R.; Li, Y.; et al. Sub-ppt NH3 detection by MoS2@sulfur nanosheets. J. Alloys Compd. 2024, 985, 174070. [Google Scholar] [CrossRef]
- You, C.-W.; Fu, T.; Li, C.-B.; Song, X.; Tang, B.; Song, X.; Yang, Y.; Deng, Z.-P.; Wang, Y.-Z.; Song, F. A Latent-Fire-Detecting Olfactory System Enabled by Ultra-Fast and Sub-ppm Ammonia-Responsive Ti3C2Tx MXene/MoS2 Sensors. Adv. Funct. Mater. 2022, 32, 2208131. [Google Scholar] [CrossRef]
- Gasso, S.; Carrier, J.; Radu, D.; Lai, C.Y. Novel Gas Sensing Approach: ReS2/Ti3C2Tx Heterostructures for NH3 Detection in Humid Environments. ACS Sens. 2024, 9, 4788–4802. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.Y.; Zhang, J.N.; Liu, J.; Li, G.; Qiao, Y.; Zhang, X.Y.; Gao, J.Z.; Lu, H.B. PANI/Ti3C2Tx composite nanofiber-based flexible conductometric sensor for the detection of NH3 at room temperature. Sens. Actuators B Chem. 2023, 392, 134128. [Google Scholar] [CrossRef]
- Li, X.; Xu, J.; Jiang, Y.; He, Z.; Liu, B.; Xie, H.; Li, H.; Li, Z.; Wang, Y.; Tai, H. Toward agricultural ammonia volatilization monitoring: A flexible polyaniline/Ti3C2Tx hybrid sensitive films based gas sensor. Sens. Actuators B Chem. 2020, 316, 128144. [Google Scholar] [CrossRef]
- Liu, J.; Cui, N.; Xu, Q.; Wang, Z.; Gu, L.; Dou, W. High-Performance PANI-Based Ammonia Gas Sensor Promoted by Surface Nanostructuralization. ECS J. Solid State Sci. Technol. 2021, 10, 027007. [Google Scholar] [CrossRef]
- Wang, H.; Nie, S.; Li, H.; Ali, R.; Fu, J.; Xiong, H.; Li, J.; Wu, Z.; Lau, W.-M.; Mahmood, N.; et al. 3D Hollow Quasi-Graphite Capsules/Polyaniline Hybrid with a High Performance for Room-Temperature Ammonia Gas Sensors. ACS Sens. 2019, 4, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sens. Actuators B Chem. 2020, 318, 128104. [Google Scholar] [CrossRef]
- Lv, D.; Shen, W.; Chen, W.; Tan, R.; Xu, L.; Song, W. PSS-PANI/PVDF composite based flexible NH3 sensors with sub-ppm detection at room temperature. Sens. Actuators B Chem. 2021, 328, 129085. [Google Scholar] [CrossRef]
- Li, S.; Liu, A.; Yang, Z.; Zhao, L.; Wang, J.; Liu, F.; You, R.; He, J.; Wang, C.; Yan, X.; et al. Design and preparation of the WO3 hollow spheres@ PANI conducting films for room temperature flexible NH3 sensing device. Sens. Actuators B Chem. 2019, 289, 252–259. [Google Scholar] [CrossRef]
- Liu, C.; Tai, H.; Zhang, P.; Ye, Z.; Su, Y.; Jiang, Y. Enhanced ammonia-sensing properties of PANI-TiO2-Au ternary self-assembly nanocomposite thin film at room temperature. Sens. Actuators B Chem. 2017, 246, 85–95. [Google Scholar] [CrossRef]
- Qiu, J.Y.; Xia, X.L.; Hu, Z.H.; Zhou, S.; Wang, Y.J.; Wang, Y.H.; Zhang, R.J.; Li, J.; Zhou, Y. Molecular ammonia sensing of PEDOT:PSS/nitrogen doped MXene Ti3C2Tx composite film at room temperature. Nanotechnology 2022, 33, 065501. [Google Scholar] [CrossRef] [PubMed]
- Rasool, A.; Amiruddin, R.; Kossar, S.; Santhosh Kumar, M.C. Realization of In:ZnO/PEDOT:PSS based multifunctional device for ultraviolet (UV) light detection and resistive switching memory applications. J. Appl. Phys. 2020, 128, 044503. [Google Scholar] [CrossRef]
- Nardes, A.M.; Kemerink, M.; de Kok, M.M.; Vinken, E.; Maturova, K.; Janssen, R.A.J. Conductivity, work function, and environmental stability of PEDOT:PSS thin films treated with sorbitol. Org. Electron. 2008, 9, 727–734. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Wang, Y.; Li, B.; Chen, P.; Jin, G.X.; Shen, X.Q.; Shao, Z.G.; Wu, L. Multifunctional and Flexible Sensor Based on PU-Supported Ti3C2Tx/TiO2/PPy Yarns for Ammonia Sensing and Human Motion Monitoring. ACS Appl. Electron. Mater. 2024, 6, 6226–6237. [Google Scholar] [CrossRef]
- Boota, M.; Anasori, B.; Voigt, C.; Zhao, M.-Q.; Barsoum, M.W.; Gogotsi, Y. Pseudocapacitive Electrodes Produced by Oxidant-Free Polymerization of Pyrrole between the Layers of 2D Titanium Carbide (MXene). Adv. Mater. 2016, 28, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Wang, Y.; Chen, W.; Jin, G.X.; Shi, Q.S.; Zhou, B.C.; Pan, Y.F.; Wu, L.; Shao, Z.G. Room temperature ammonia sensor based on Ag NPs loaded Ti3C2Tx nanocomposites. Surf. Interfaces 2024, 51, 104605. [Google Scholar] [CrossRef]
- Liu, D.; Pan, J.; Tang, J.; Liu, W.; Bai, S.; Luo, R. Ag decorated SnO2 nanoparticles to enhance formaldehyde sensing properties. J. Phys. Chem. Solids 2019, 124, 36–43. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Wang, Z.K.; Zhang, S.W.; Wu, L. TPU-Supported Ti3C2Tx/TiO2/Ru electrospun mat towards flexible and multi-functional sensors for human motion and NH3 detection. J. Environ. Chem. Eng. 2024, 12, 114962. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, S.; Qiu, L.; Rasaki, S.A.; Qu, F.; Thomas, T.; Liu, Y.; Yang, M. Ru-decorated WO3 nanosheets for efficient xylene gas sensing application. J. Alloys Compd. 2020, 826, 154196. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Song, P.; Ji, J.; Wang, Q. Metal-organic frameworks-derived In2O3 microtubes/Ti3C2Tx MXene composites for NH3 detection at room temperature. Sens. Actuators B Chem. 2022, 361, 131755. [Google Scholar] [CrossRef]
- Yan, H.; Song, P.; Zhang, S.; Zhang, J.; Yang, Z.; Wang, Q. A low temperature gas sensor based on Au-loaded MoS2 hierarchical nanostructures for detecting ammonia. Ceram. Int. 2016, 42, 9327–9331. [Google Scholar] [CrossRef]
- Wang, Y.-D.; Wu, X.-H.; Su, Q.; Li, Y.-F.; Zhou, Z.-L. Ammonia-sensing characteristics of Pt and SiO2 doped SnO2 materials. Solid-State Electron. 2001, 45, 347–350. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Cui, X.; Gao, Y.; Ma, J.; Sun, Y.; Sun, P.; Liu, F.; Liang, X.; Zhang, T.; et al. NH3 gas sensing performance enhanced by Pt-loaded on mesoporous WO3. Sens. Actuators B Chem. 2017, 238, 473–481. [Google Scholar] [CrossRef]
- Liu, M.; Ji, J.; Song, P.; Wang, J.X.; Wang, Q. Sensing performance of α-Fe2O3/Ti3C2Tx MXene nanocomposites to NH3 at room temperature. J. Alloys Compd. 2022, 898, 162812. [Google Scholar] [CrossRef]
- Lu, L.; Liu, M.Y.; Sui, Q.L.; Zhang, C.H.; Zou, Y.J.; Xu, F.; Sun, L.X.; Xiang, C.L. MXene/MoS2 nanosheet/polypyrrole for high-sensitivity detection of ammonia gas at room temperature. Mater. Today Commun. 2023, 35, 106239. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Wang, Y.; Chen, P.; Wu, L. Fabrication of PU-supported PPy/Ti3C2Tx yarns for flexible and multi-functional sensors. Surf. Interfaces 2024, 53, 105004. [Google Scholar] [CrossRef]
- He, T.T.; Sun, S.P.; Huang, B.Y.; Li, X.G. MXene/SnS2 Heterojunction for Detecting Sub-ppm NH3 at Room Temperature. ACS Appl. Mater. Interfaces 2023, 15, 4194–4207. [Google Scholar] [CrossRef] [PubMed]
- Sardana, S.; Debnath, A.K.; Aswal, D.K.; Mahajan, A. WS2 nanosheets decorated multi-layered MXene based chemiresistive sensor for efficient detection and discrimination of NH3 and NO2. Sens. Actuators B Chem. 2023, 394, 134352. [Google Scholar] [CrossRef]
- Liu, M.; Sun, R.Y.; Ding, Y.L.; Wang, Q.; Song, P. Au/α-Fe2O3/Ti3C2Tx MXene Nanosheet Heterojunctions for High-Performance NH3 Gas Detection at Room Temperature. ACS Appl. Nano Mater. 2023, 6, 11856–11867. [Google Scholar] [CrossRef]
- Liang, J.R.; Han, Y.; Chen, H.; Zhang, Y.X.; Gao, X.P. Layered Ti3C2Tx MXene heterostructured with V2O5 nanoparticles for enhanced room temperature ammonia sensing. J. Alloys Compd. 2025, 1010, 177798. [Google Scholar] [CrossRef]
- Wang, X.W.; Gong, L.K.; Liu, H.S.; Zhou, X.H. Intelligent triboelectric sliding bearing for gas leak self-sensing and mechanical fault self-diagnosis in green ammonia production. Nano Energy 2025, 140, 111060. [Google Scholar] [CrossRef]
- Huang, J.; Huang, Y.; Li, T.; Xu, H.; Wu, H.; Su, Z. Respiration-Driven Ammonia Sensing Mask for Multifunctional Self-powered Monitoring Application. Chem. Eng. J. 2025, 507, 160598. [Google Scholar] [CrossRef]
- Elancheziyan, M.; Singh, M.; Bhuvanendran, N.; Won, K. Ni-doped MnO2/Ti3C2Tx MXene nanocomposite for highly sensitive electrochemical ammonia gas sensing at room temperature. J. Alloys Compd. 2025, 1022, 179941. [Google Scholar] [CrossRef]
- Yao, Y.; Han, Y.; Wang, Z.; Yang, Y.; Shi, Q.; Wu, D.; Zhu, Z. Transient MBene surpassing MXene for high-selectivity NH3 sensing in meat spoilage detection. Chem. Eng. J. 2025, 503, 158516. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, P.; Yu, Z.; Tao, M.; Zhou, D.; Yang, B.; Zhang, T. Light-driven, ultra-sensitive and multifunctional ammonia wireless sensing system by plasmonic-functionalized Nb2CTx MXenes towards smart agriculture. Nano Energy 2023, 108, 108216. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, J.; Hu, N.; Wu, S.; Lu, Y. Machine Learning-Assisted Pd-Au/MXene Sensor Array for Smart Gas Identification. Small Struct. 2025, 6, 2400619. [Google Scholar] [CrossRef]
- Hu, J.; Hu, N.; Pan, D.; Zhu, Y.; Jin, X.; Wu, S.; Lu, Y. Smell cancer by machine learning-assisted peptide/MXene bioelectronic array. Biosens. Bioelectron. 2024, 262, 116562. [Google Scholar] [CrossRef] [PubMed]



| Composite Material Type | Material | Sensitivity | Work Temperature | Res/Rec (s) | Detection Limit | Reference |
|---|---|---|---|---|---|---|
| Metal oxides | CeO2/Nb2CTx | 51.2% (50 ppm) | 25 °C | 70/298 | 500 ppb | [33] |
| In2O3/Ti3C2Tx | 5 (50 ppm) | 25 °C | 60/300 | 1 ppm | [36] | |
| Ti3C2Tx/TiO2 | 4.7% (10 ppb) | 31 °C | 33/277 | 500 ppb | [38] | |
| Ti3C2Tx MXene/GO/CuO/ZnO | 59.9% (100 ppm) | Room temperature | 26/25 | 25 ppm | [24] | |
| α-Fe2O3/Ti3C2Tx | 18.3% (5 ppm) | Room temperature | 2.5/2 | 5 ppm | [69] | |
| Polymers | PANI/Ti3C2Tx | 55.9% (20 ppm) | 25 °C | Feb-50 | 5 ppm | [47] |
| PEDOT:PSS/Ti3C2Tx | 20% (25 ppm) | 20 °C | 280/393 | 10 ppm | [56] | |
| Ti3C2Tx/TiO2/PPy | 28% (5 ppm) | 25 °C | 162/260 | 5 ppm | [59] | |
| MXene/MoS2/PPy | 21% (100 ppm) | Room temperature | 33/277 | 10 ppm | [70] | |
| PPy/Ti3C2Tx | 26% (100 ppm) | Room temperature | 62/451 | 5 ppm | [71] | |
| Two-dimensional materials | Nb2CTx/MoSe2 | 71% (50 ppm) | 25 °C | 15/20 | 1 ppm | [41] |
| MoS2/Ti3C2 | 10% (100 ppm) | Room temperature | 7-Oct | 1 ppm | [43] | |
| ReS2/Ti3C2Tx | 7.8% (10 ppm) | 25 °C | 40/50 | 1 ppm | [46] | |
| MXene/SnS2 | 42.9% (10 ppm) | Room temperature | 161/80 | 10 ppb | [72] | |
| WS2/MXene | 15.5% (5 ppm) | 25 °C | 160/100 | 100 ppb | [73] | |
| Precious metals | Ag@Ti3C2Tx | 64.07% (100 ppm) | 25 °C | 230/172 | 10 ppm | [61] |
| Ti3C2Tx/TiO2/Ru | 15.06% (100 ppm) | Room temperature | 113/381 | 5 ppm | [63] | |
| Au/Ti3C2Tx and Pt/MXene | 16% and 9% (50 ppm) | 25 °C | 190/650 | 10 ppm | [42] | |
| Au/α-Fe2O3/Ti3C2Tx | 16.9% (1 ppm) | Room temperature | 2-Mar | 1 ppm | [74] | |
| Pt@SnS2/Ti3C2Tx | 22.7 (10 ppm) | Room temperature | 164/38 | 23 ppb | [75] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, Y.; Lei, Z.; Wang, C.; Meng, X.; Cheng, P. MXene-Based Gas Sensors for NH3 Detection: Recent Developments and Applications. Micromachines 2025, 16, 820. https://doi.org/10.3390/mi16070820
Xu Y, Wang Y, Lei Z, Wang C, Meng X, Cheng P. MXene-Based Gas Sensors for NH3 Detection: Recent Developments and Applications. Micromachines. 2025; 16(7):820. https://doi.org/10.3390/mi16070820
Chicago/Turabian StyleXu, Yiyang, Yinglin Wang, Zhaohui Lei, Chen Wang, Xiangli Meng, and Pengfei Cheng. 2025. "MXene-Based Gas Sensors for NH3 Detection: Recent Developments and Applications" Micromachines 16, no. 7: 820. https://doi.org/10.3390/mi16070820
APA StyleXu, Y., Wang, Y., Lei, Z., Wang, C., Meng, X., & Cheng, P. (2025). MXene-Based Gas Sensors for NH3 Detection: Recent Developments and Applications. Micromachines, 16(7), 820. https://doi.org/10.3390/mi16070820








