Interlayer-Spacing-Modification of MoS2 via Inserted PANI with Fast Kinetics for Highly Reversible Aqueous Zinc-Ion Batteries
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of PANI–MoOx Nanocomposite
2.3. Preparation of PANI–MoS2 Nanocomposite
2.4. Material Characterizations
2.5. Electrochemical Measurements
3. Results
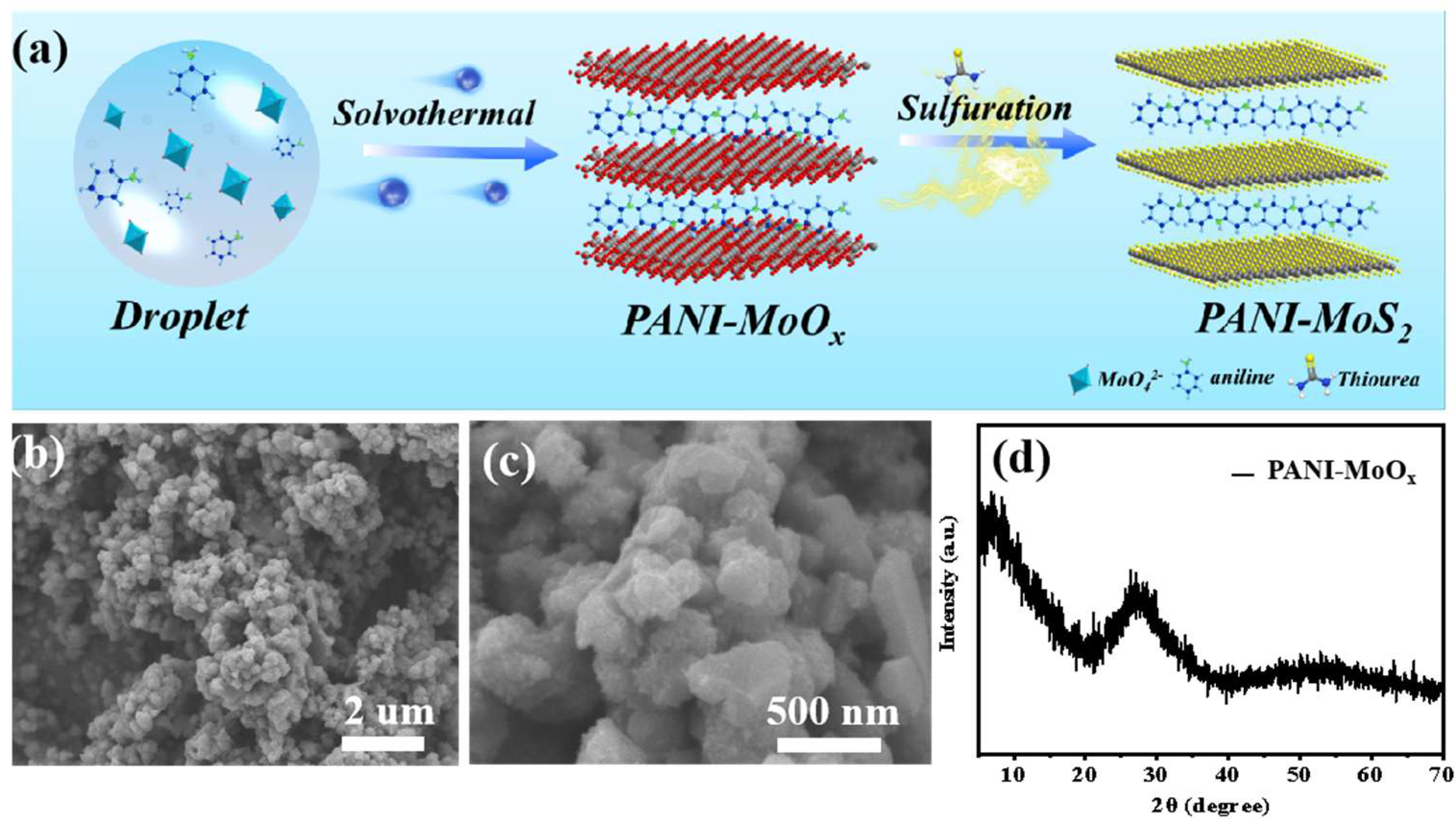
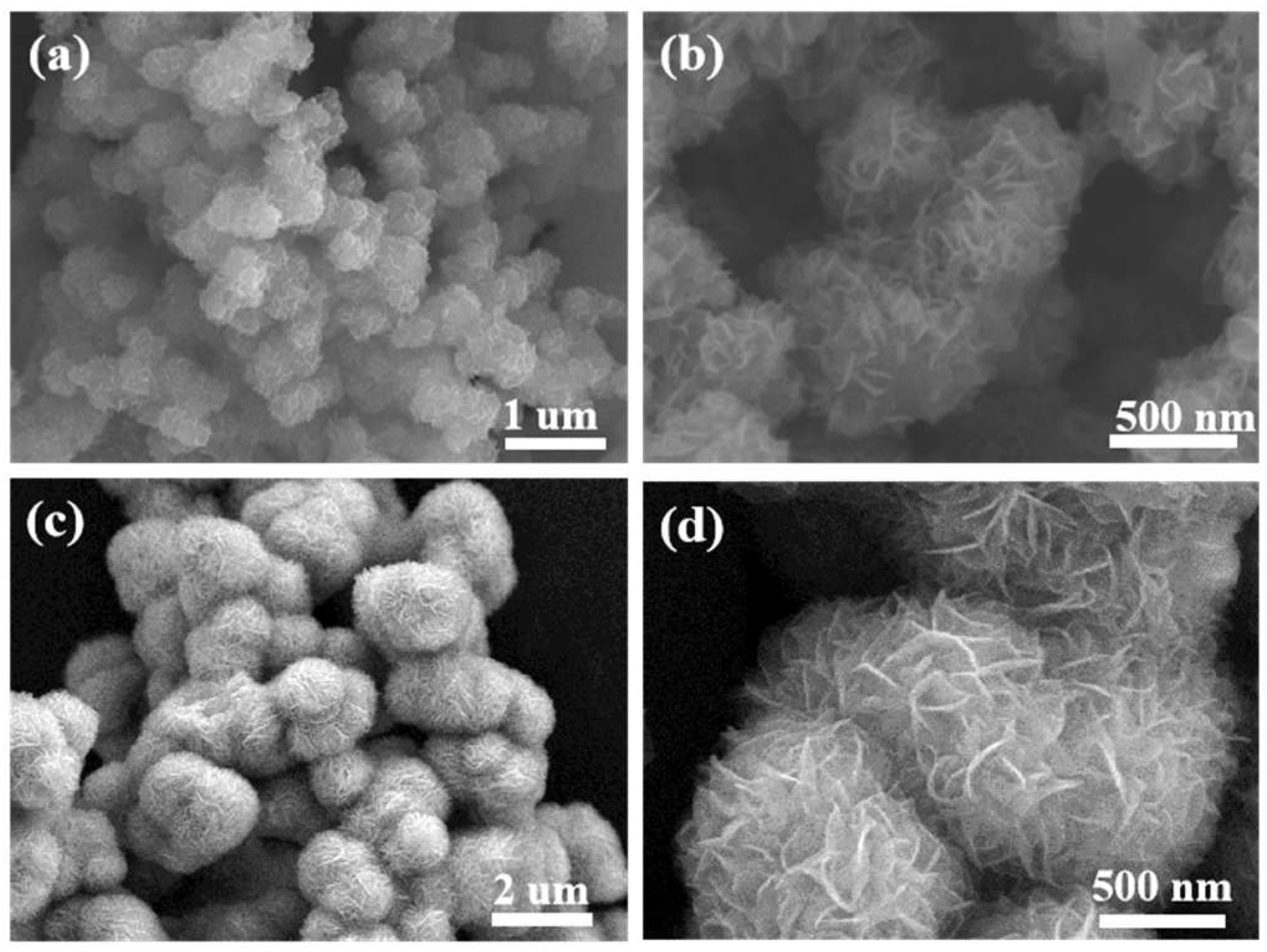
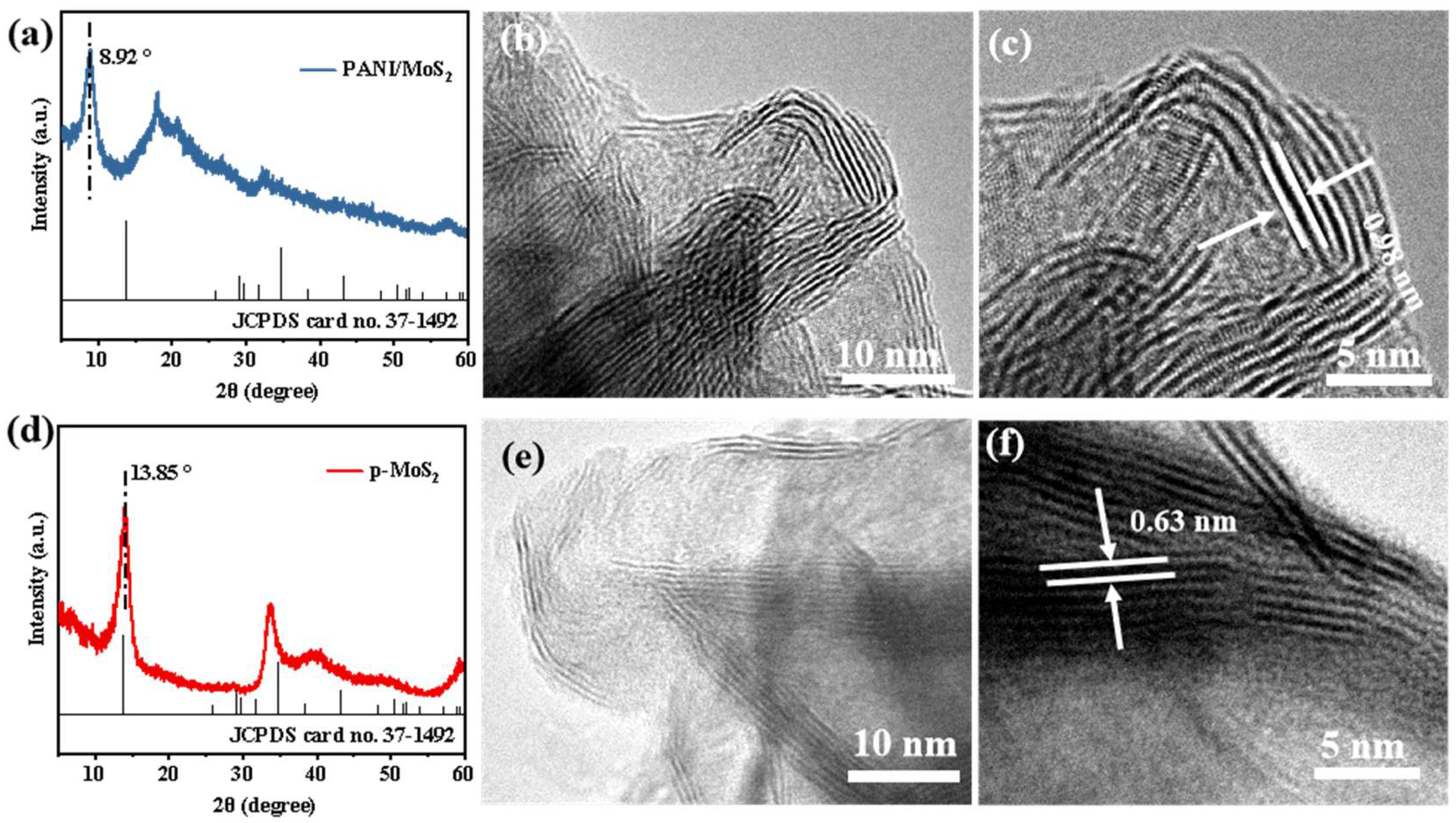
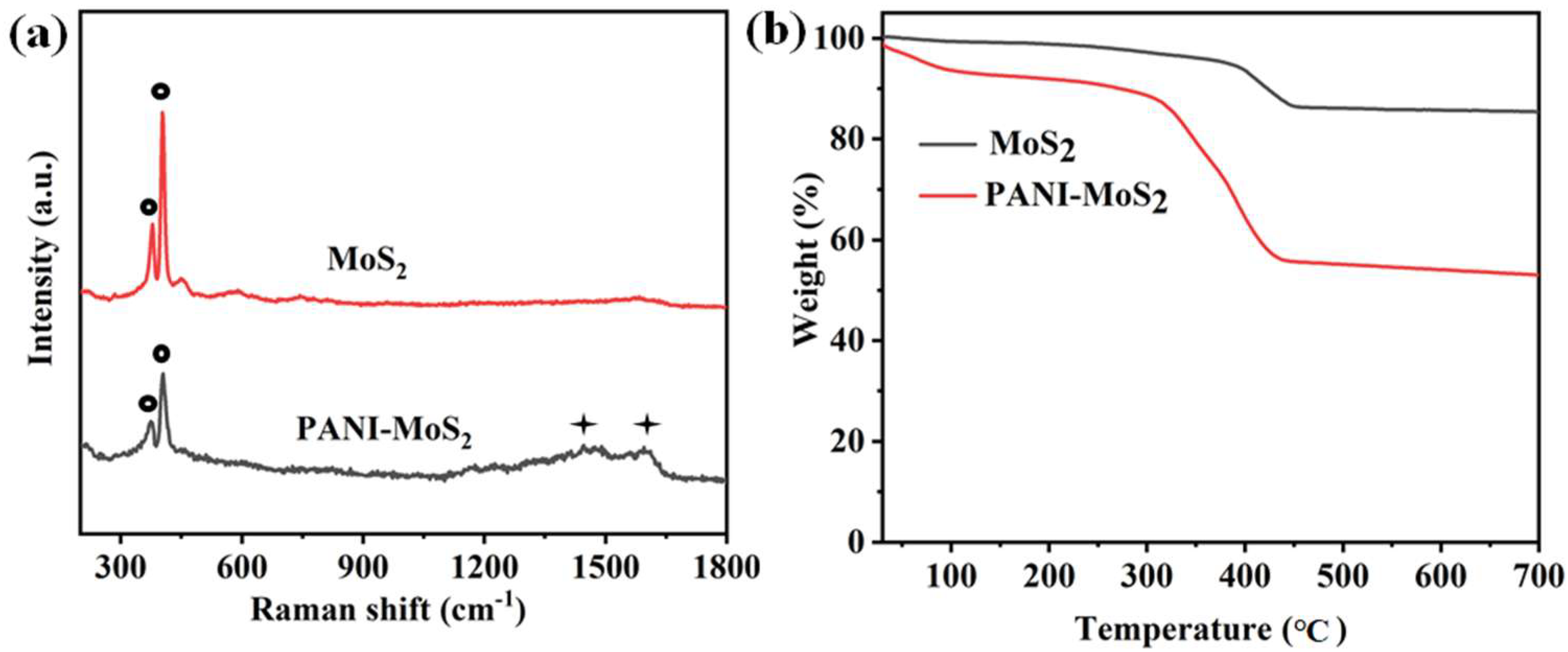
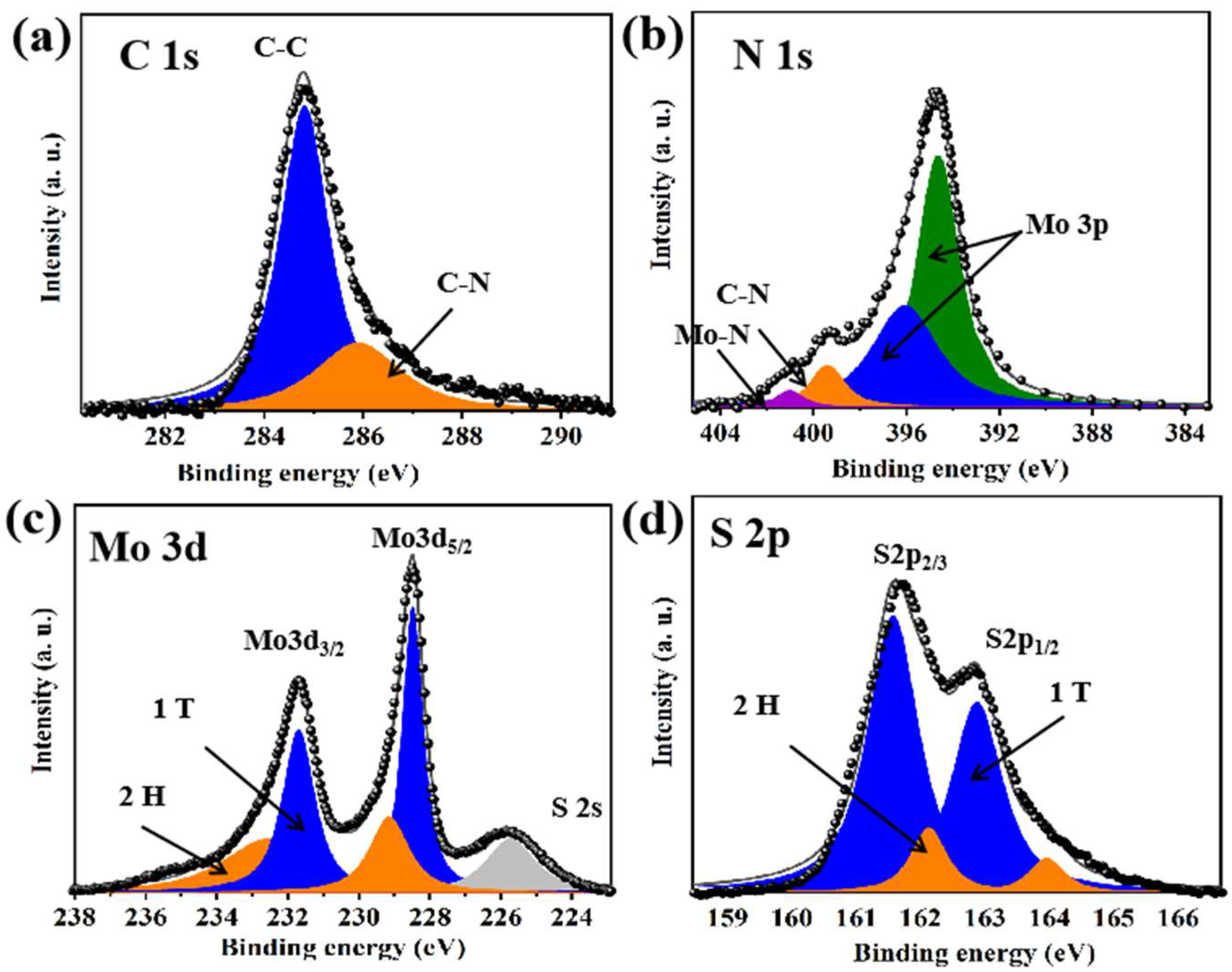
3.1. Structure and Morphology of PANI–MoS2 Composites
- (i)
- Ion exchange and coordination:3MoO6− + 11H+ + 12HO + 14C6H7N ⇌ 7 [Mo3O10(C6H8N)2]·2H2O
- (ii)
- Oxidative polymerization:7 [Mo3O10(C6H8N)2]·2H2O + (NH4)2S2O8 → PANI-MoOx
- (iii)
- Vulcanization (sulfurization) reaction:PANI-MoOx + CH4N2S → PANI-MoS2
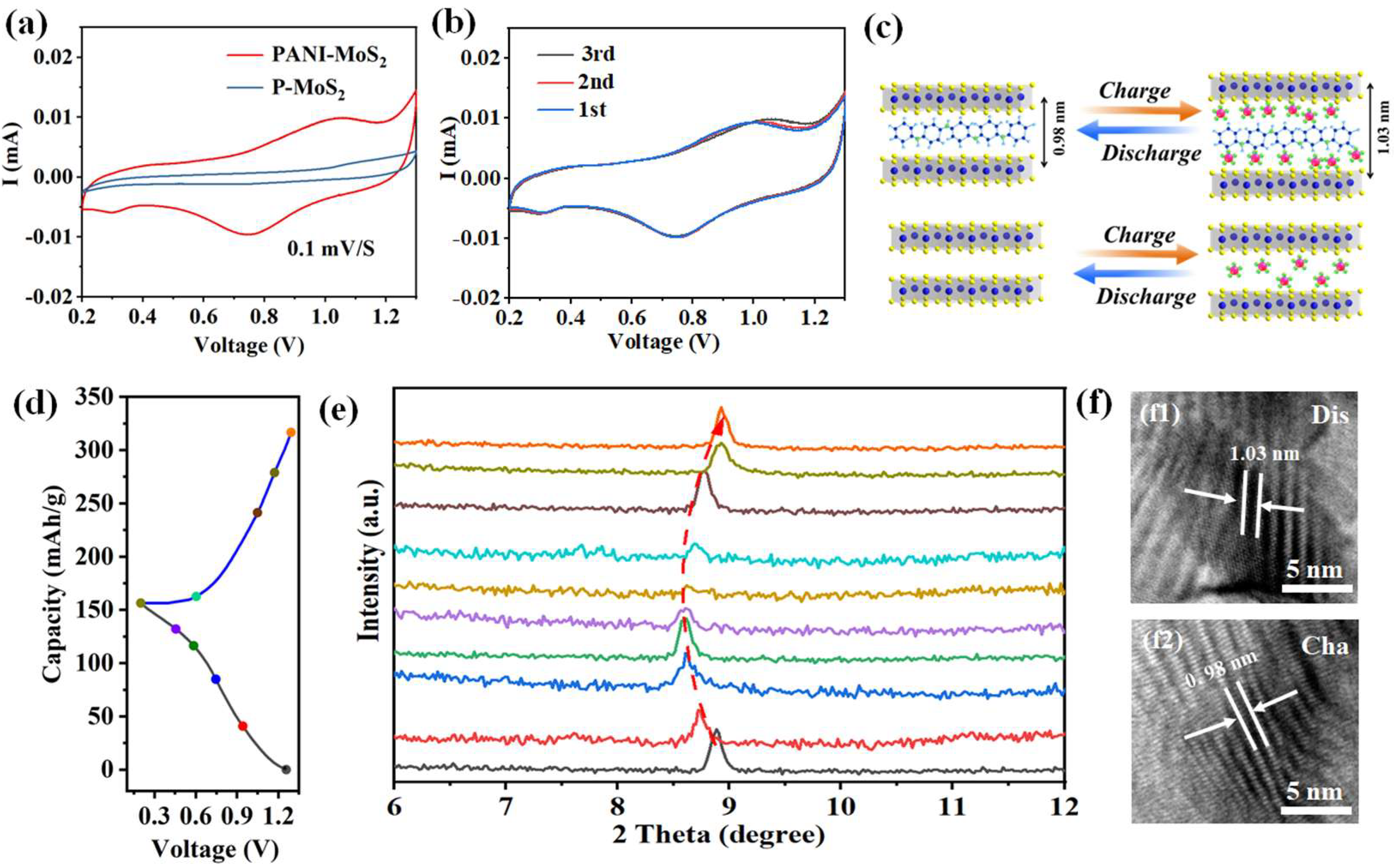
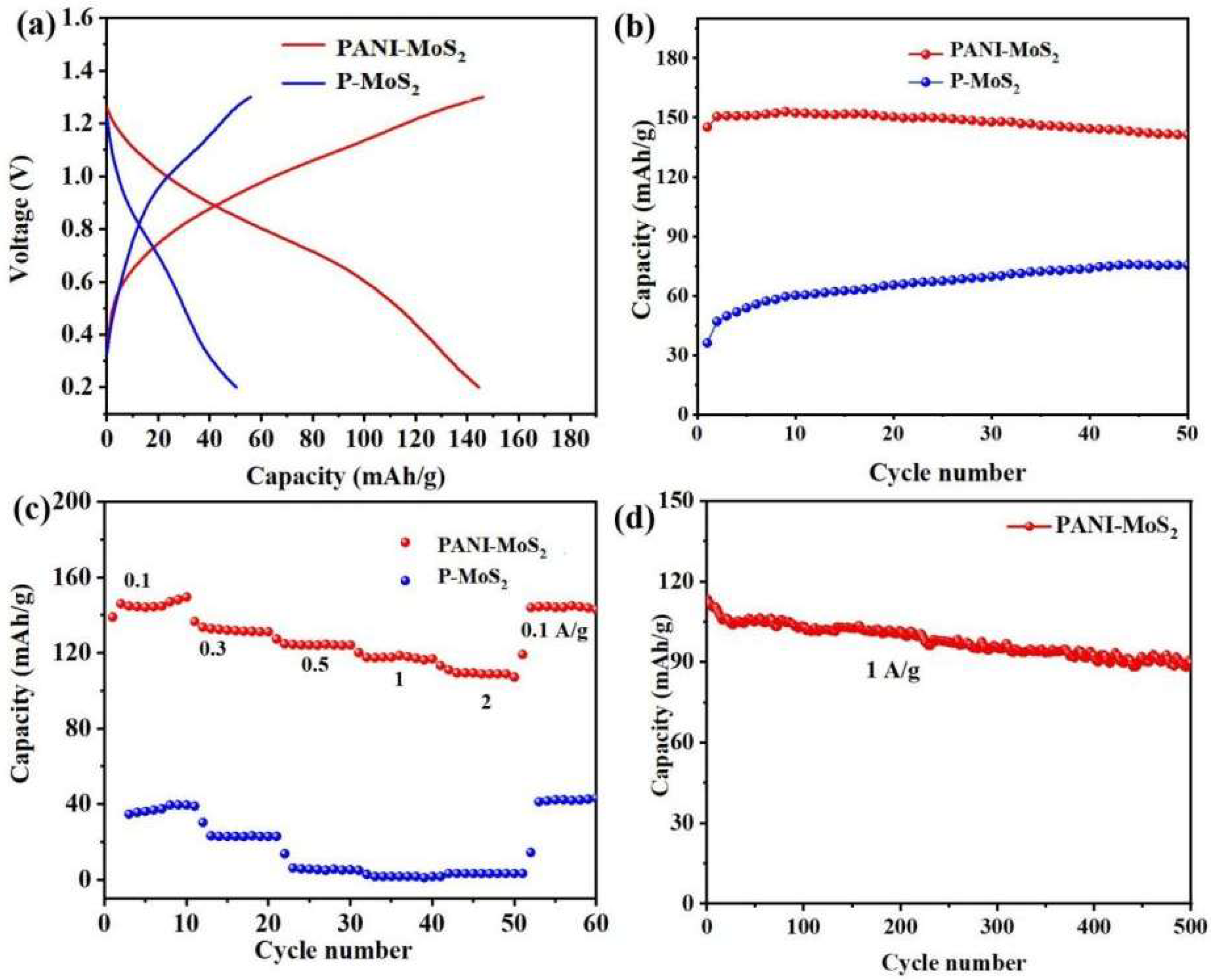
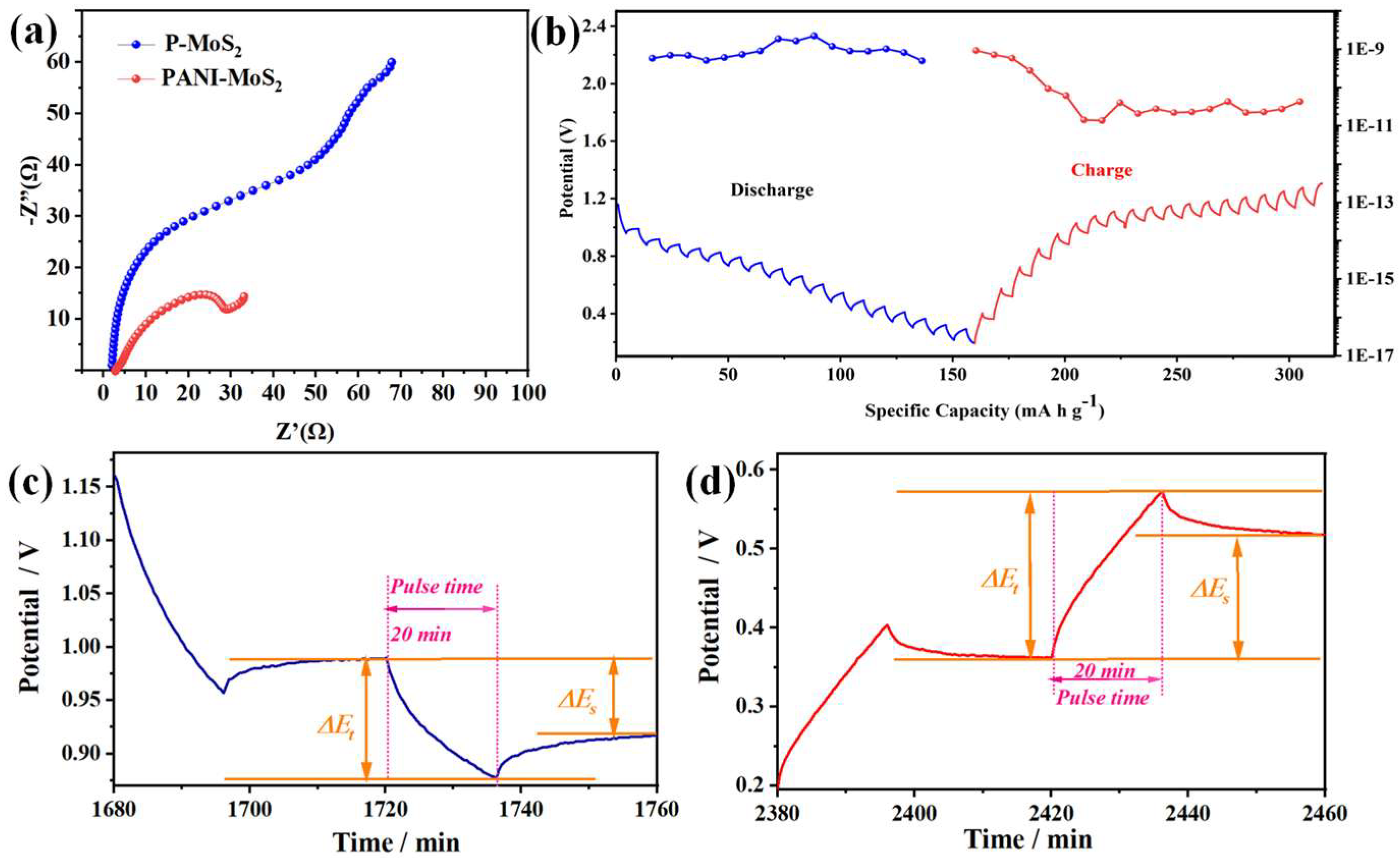
3.2. Zinc Storage Properties of PANI–MoS2 Composites
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Du, H.; Wang, Y.; Kang, Y.; Zhao, Y.; Tian, Y.; Wang, X.; Tan, Y.; Liang, Z.; Wozny, J.; Li, T.; et al. Side Reactions/Changes in Lithium-Ion Batteries: Mechanisms and Strategies for Creating Safer and Better Batteries. Adv. Mater. 2024, 36, 2401482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Du, Z.; Wang, L.; He, G.; Parkin, I.P.; Zhang, Y.; Yue, Y. Disordered materials for high-performance lithium-ion batteries: A review. Nano Energy 2024, 121, 109250. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Ding, Z.; Zhang, L.; Deng, L.; Yao, L.; Yang, H.Y. Cationic Defect-Modulated Li-Ion Migration in High-Voltage Li-Metal Batteries. ACS Nano 2023, 17, 25519–25531. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, Y.; Pan, Y.; Wang, W.; Yeoh, G.H. Review and Future Perspectives on Lithium Battery Fire Safety: Focusing on Design of Organic Components. Energy Environ. Mater. 2025, e12892. [Google Scholar] [CrossRef]
- Luo, Y.; Rao, Z.; Yang, X.; Wang, C.; Sun, X.; Li, X. Safety concerns in solid-state lithium batteries: From materials to devices. Energy Environ. Sci. 2024, 17, 7543–7565. [Google Scholar] [CrossRef]
- Kimura, K.; Marangon, V.; Fukuda, T.; Suzuki, M.; Soontornnon, N.; Tominaga, Y.; Hassoun, J. TEMPO-oxidized cellulose nanofiber hydrogel electrolyte for rechargeable Zn-ion batteries. Chem. Commun. 2024, 60, 13698–13701. [Google Scholar] [CrossRef]
- Takeda, H.; Shibasaki, M.; Murakami, K.; Tanaka, M.; Makino, K.; Tanibata, N.; Maeda, H.; Nakayama, M. Effect of synthesis process on the Li-ion conductivity of LiTa2 PO8 solid electrolyte materials for all-solid-state batteries. Energy Adv. 2024, 3, 2238–2244. [Google Scholar] [CrossRef]
- Aimi, A.; Onodera, H.; Shimonishi, Y.; Fujimoto, K.; Yoshida, S. High Li-Ion Conductivity in Pyrochlore-Type Solid Electrolyte Li2–x La (1+x)/3 M2 O6 F (M = Nb, Ta). Chem. Mater. 2024, 36, 3717–3725. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, S.; Li, Y.; Ren, X.; Zhang, P.; Sun, L.; Yang, H.Y. In Situ Grown Hierarchical Electrospun Nanofiber Skeletons with Embedded Vanadium Nitride Nanograins for Ultra-Fast and Super-Long Cycle Life Aqueous Zn-Ion Batteries. Adv. Energy Mater. 2023, 13, 2202826. [Google Scholar] [CrossRef]
- Lin, C.; Zhang, Y.; Lieu, W.Y.; Xu, Y.; Li, D.-S.; Sliva, A.; Yang, H.Y. Boosting Zinc-Ion Storage Capability in Longitudinally Aligned MXene Arrays with Microchannel Architecture. Adv. Funct. Mater. 2025, 35, 2413613. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, J.; Hong, L.; Zhang, J.; Chen, C.; Xu, A.; Huang, M.; Ren, X.; Bai, J.; Wang, H.; et al. Customizing H2 O-Poor Electric Double Layer and Boosting Texture Exposure of Zn (101) Plane towards Super-High Areal Capacity Zinc Metal Batteries. Angew. Chem. 2025, 137, e202414757. [Google Scholar] [CrossRef]
- Wu, B.; Liu, J.; Rao, S.; Zheng, C.; Song, W.; Ma, Q.; Niu, J.; Wang, F. Transforming Undesired Corrosion Products into a Nanoflake-Array Functional Layer: A Gelatin-Assistant Modification Strategy for High Performance Zn Battery Anodes. Small 2024, 20, 2400926. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Huang, S.; Fan, S.; Sun, L.; Tian, B.; Chen, F.; Wang, Y.; Shi, Y.; Yang, H.Y. Rechargeable Aqueous Zinc-Ion Batteries in MgSO4/ZnSO4 Hybrid Electrolytes. Nano-Micro Lett. 2020, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Peng, H.; Kang, J.; Chen, H.; Zhang, M.; Liu, Y.; Kim, D.H.; Liu, Y.; Lin, Z. Zn-based batteries for sustainable energy storage: Strategies and mechanisms. Chem. Soc. Rev. 2024, 53, 4877–4925. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Tao, J.; Tan, Y.; Song, H.; Huang, D.; Liu, P.; Chen, Z.; Yin, X.; Zhu, J.; Xu, J.; et al. Comprehensive Understanding of Steric-Hindrance Effect on the Trade-Off Between Zinc Ions Transfer and Reduction Kinetics to Enable Highly Reversible and Stable Zn Anodes. Adv. Energy Mater. 2024, 14, 2404018. [Google Scholar] [CrossRef]
- Li, G.; Sun, L.; Zhang, S.; Zhang, C.; Jin, H.; Davey, K.; Liang, G.; Liu, S.; Mao, J.; Guo, Z. Developing Cathode Materials for Aqueous Zinc Ion Batteries: Challenges and Practical Prospects. Adv. Funct. Mater. 2024, 34, 2301291. [Google Scholar] [CrossRef]
- Mao, Y.; Bai, J.; Lin, S.; Wang, P.; Li, W.; Xiao, K.; Wang, S.; Zhu, X.; Zhao, B.; Sun, Y. Two Birds with One Stone: V4 C3 MXene Synergistically Promoted VS2 Cathode and Zinc Anode for High-Performance Aqueous Zinc-Ion Batteries. Small 2024, 20, 2306615. [Google Scholar] [CrossRef]
- Wang, W.; Ran, L.; Hu, R.; Zhang, C.; Huang, R.; Li, Y.; Ouyang, Y.; Yan, J. Boosting the Zn-storage performance of layered VSe2 cathodes via an in situ electrochemical oxidation strategy. J. Alloys Compd. 2024, 981, 173692. [Google Scholar] [CrossRef]
- Zhao, D.; Pu, X.; Wang, C.; Pan, Z.; Ding, M.; Cao, Y.; Chen, Z. Low-strain layered Zn0.56VOPO4∙2H2O as a high-voltage and long-lifespan cathode material for Zn-ion batteries. Energy Storage Mater. 2024, 66, 103239. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, Y.; Fan, Z.; Cui, Y.; Liu, X.; Wang, X. Two-dimension amorphous VOPO4/graphene heterostructure for high-voltage aqueous Zn-ion battery. Chem. Commun. 2025, 61, 6190–6193. [Google Scholar] [CrossRef]
- Guo, A.; Wang, Z.; Chen, L.; Liu, W.; Zhang, K.; Cao, L.; Liang, B.; Luo, D. A Comprehensive Review of the Mechanism and Modification Strategies of V2 O5 Cathodes for Aqueous Zinc-Ion Batteries. ACS Nano 2024, 18, 27261–27286. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Gao, G. V2O5-based cathodes for aqueous zinc ion batteries: Mechanisms, preparations, modifications, and electrochemistry. Nano Energy 2024, 127, 109691. [Google Scholar] [CrossRef]
- Azmi, Z.; Senapati, K.C.; Goswami, A.K.; Mohapatra, S.R. A comprehensive review of strategies to augment the performance of MnO2 cathode by structural modifications for aqueous zinc ion battery. J. Power Sources 2024, 613, 234816. [Google Scholar] [CrossRef]
- Xiankai, F.; Kaixiong, X.; Wei, Z.; Weina, D.; Hai, Z.; Liang, C.; Han, C. A novel improvement strategy and a comprehensive mechanism insight for α-MnO2 energy storage in rechargeable aqueous zinc-ion batteries. Carbon. Energy 2024, 6, e536. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, M.; Sun, Y.; Cui, Y.; El-khodary, S.A.; Ng, D.H.L.; Lian, J.; Ma, J. Zinc-Ion and Proton as Joint Charge Carriers of S-MoO2 for High-Capacity Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2024, 34, 2308834. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, F.; Tang, Y.; Jiang, C.; Yue, C.; Shu, J. The Construction of Nanoflower-like MoO2 @C Composites via Crystal Engineering for Ultrastable Aqueous Zinc-Ion Batteries. Energy Tech. 2024, 12, 2400397. [Google Scholar] [CrossRef]
- Li, F.; Ma, H.; Sheng, H.; Wang, Z.; Qi, Y.; Wan, D.; Shao, M.; Yuan, J.; Li, W.; Wang, K.; et al. Interlayer and Phase Engineering Modifications of K-MoS2 @C Nanoflowers for High-Performance Degradable Zn-Ion Batteries. Small 2024, 20, 2306276. [Google Scholar] [CrossRef]
- Syed, W.A.; Kakarla, A.K.; Bandi, H.; Shanthappa, R.; Yu, J.S. Improved rate and cycling capability of V2O5@MoS2 nanocomposites as an advanced cathode material for rechargeable aqueous zinc-ion batteries. Sustain. Mater. Technol. 2024, 40, e00968. [Google Scholar] [CrossRef]
- Niu, F.; Bai, Z.; Chen, J.; Gu, Q.; Wang, X.; Wei, J.; Mao, Y.; Dou, S.X.; Wang, N. In Situ Molecular Engineering Strategy to Construct Hierarchical MoS2 Double-Layer Nanotubes for Ultralong Lifespan “Rocking-Chair” Aqueous Zinc-Ion Batteries. ACS Nano 2024, 18, 6487–6499. [Google Scholar] [CrossRef]
- Huang, M.; Mai, Y.; Zhao, L.; Liang, X.; Fang, Z.; Jie, X. Hierarchical MoS2 @CNTs Hybrid as a Long-Life and High-Rate Cathode for Aqueous Rechargeable Zn-Ion Batteries. ChemElectroChem 2020, 7, 4218–4223. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Zhao, X.; Shen, Q.; Zhao, W.; Tan, Q.; Zhang, N.; Li, P.; Jiao, L.; Qu, X. Sandwich-Like Heterostructures of MoS2 /Graphene with Enlarged Interlayer Spacing and Enhanced Hydrophilicity as High-Performance Cathodes for Aqueous Zinc-Ion Batteries. Adv. Mater. 2021, 33, 2007480. [Google Scholar] [CrossRef]
- Liang, H.; Cao, Z.; Ming, F.; Zhang, W.; Anjum, D.H.; Cui, Y.; Cavallo, L.; Alshareef, H.N. Aqueous Zinc-Ion Storage in MoS2 by Tuning the Intercalation Energy. Nano Lett. 2019, 19, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Chen, N.; Tang, W.; Liu, Y.; Xia, Y.; Wu, Z.; Li, F.; Liu, Y.; Sun, A. Template-assisted hydrothermal synthesized hydrophilic spherical 1T-MoS2 with excellent zinc storage performance. J. Alloys Compd. 2022, 898, 162854. [Google Scholar] [CrossRef]
- Sheng, Z.; Qi, P.; Lu, Y.; Liu, G.; Chen, M.; Gan, X.; Qin, Y.; Hao, K.; Tang, Y. Nitrogen-Doped Metallic MoS2 Derived from a Metal–Organic Framework for Aqueous Rechargeable Zinc-Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 34495–34506. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Zhang, W.; Ren, X.; Yin, Y.; Zhao, Y.; Ren, Z.; Sun, Y.; Lei, Q.; Wang, J.; Wang, L.; et al. A Volume Self-Regulation MoS2 Superstructure Cathode for Stable and High Mass-Loaded Zn-Ion Storage. ACS Nano 2022, 16, 12095–12106. [Google Scholar] [CrossRef]
- Xin, D.; Zhang, X.; Zhang, Z.; Sun, J.; Li, Q.; He, X.; Jiang, R.; Liu, Z.; Lei, Z. Pre-Intercalation of TMA Cations in MoS2 Interlayers for Fast and Stable Zinc Ion Storage. Small 2024, 20, 2403050. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Huang, C.; Gao, L.; Shen, Q.; Li, P.; Qu, X.; Jiao, L.; Liu, Y. Unveiling the “Proton Lubricant” Chemistry in Aqueous Zinc-MoS2 Batteries. Angew. Chem. 2022, 134, e202211478. [Google Scholar] [CrossRef]
- Huang, M.; Mai, Y.; Zhao, L.; Liang, X.; Fang, Z.; Jie, X. Tuning the kinetics of zinc ion in MoS2 by polyaniline intercalation. Electrochim. Acta 2021, 388, 138624. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Hu, K.; Chen, X.; Yin, H. High conductivity graphene-like MoS2/polyaniline nanocomposites and its application in supercapacitor. J. Alloys Compd. 2015, 619, 38–43. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, H.; Hu, Y.; Li, N.; Zhao, X.; Li, C. 2D MoS2 /polyaniline heterostructures with enlarged interlayer spacing for superior lithium and sodium storage. J. Mater. Chem. A 2017, 5, 5383–5389. [Google Scholar] [CrossRef]
- Li, C.; Liu, C.; Wang, Y.; Lu, Y.; Zhu, L.; Sun, T. Drastically-enlarged interlayer-spacing MoS2 nanocages by inserted carbon motifs as high performance cathodes for aqueous zinc-ion batteries. Energy Storage Mater. 2022, 49, 144–152. [Google Scholar] [CrossRef]
- Pani, J.; Maru, D.; Chaudhary, P.; Gangwar, J.; Kumar, K.U.; Yadav, B.C.; Borkar, H. Improved Supercapacitor Performance with Enhanced Interlayer Spacing of Nanoflower MoS2 in Long Discharge Time in LED-Glowing Application. Energy Tech. 2023, 11, 2300193. [Google Scholar] [CrossRef]
- Rawat, S.; Bamola, P.; Karishma; Rani, C.; Dhoundiyal, H.; Sharma, N.; Dwivedi, C.; Kumar, U.; Satyarthi, P.S.; Sharma, M.; et al. Surface and Interface Investigation on MoS2-rGO Hybrids for Room Temperature Gas Sensing. IEEE Sens. J. 2024, 24, 22218–22226. [Google Scholar] [CrossRef]
- Guo, H.; Montes-García, V.; Peng, H.; Samorì, P.; Ciesielski, A. Molecular Connectors Boosting the Performance of MoS2 Cathodes in Zinc-Ion Batteries. Small 2024, 20, 2310338. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Gong, Y.; Chen, S.; Zhang, Y. Interlayer-Spacing-Modification of MoS2 via Inserted PANI with Fast Kinetics for Highly Reversible Aqueous Zinc-Ion Batteries. Micromachines 2025, 16, 754. https://doi.org/10.3390/mi16070754
Fan S, Gong Y, Chen S, Zhang Y. Interlayer-Spacing-Modification of MoS2 via Inserted PANI with Fast Kinetics for Highly Reversible Aqueous Zinc-Ion Batteries. Micromachines. 2025; 16(7):754. https://doi.org/10.3390/mi16070754
Chicago/Turabian StyleFan, Shuang, Yangyang Gong, Suliang Chen, and Yingmeng Zhang. 2025. "Interlayer-Spacing-Modification of MoS2 via Inserted PANI with Fast Kinetics for Highly Reversible Aqueous Zinc-Ion Batteries" Micromachines 16, no. 7: 754. https://doi.org/10.3390/mi16070754
APA StyleFan, S., Gong, Y., Chen, S., & Zhang, Y. (2025). Interlayer-Spacing-Modification of MoS2 via Inserted PANI with Fast Kinetics for Highly Reversible Aqueous Zinc-Ion Batteries. Micromachines, 16(7), 754. https://doi.org/10.3390/mi16070754




