Structural and Gas-Sensitive Characteristics of In2O3: Effect of Hydrothermal/Solvothermal Synthesis Conditions
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Structural and Morphological Studies
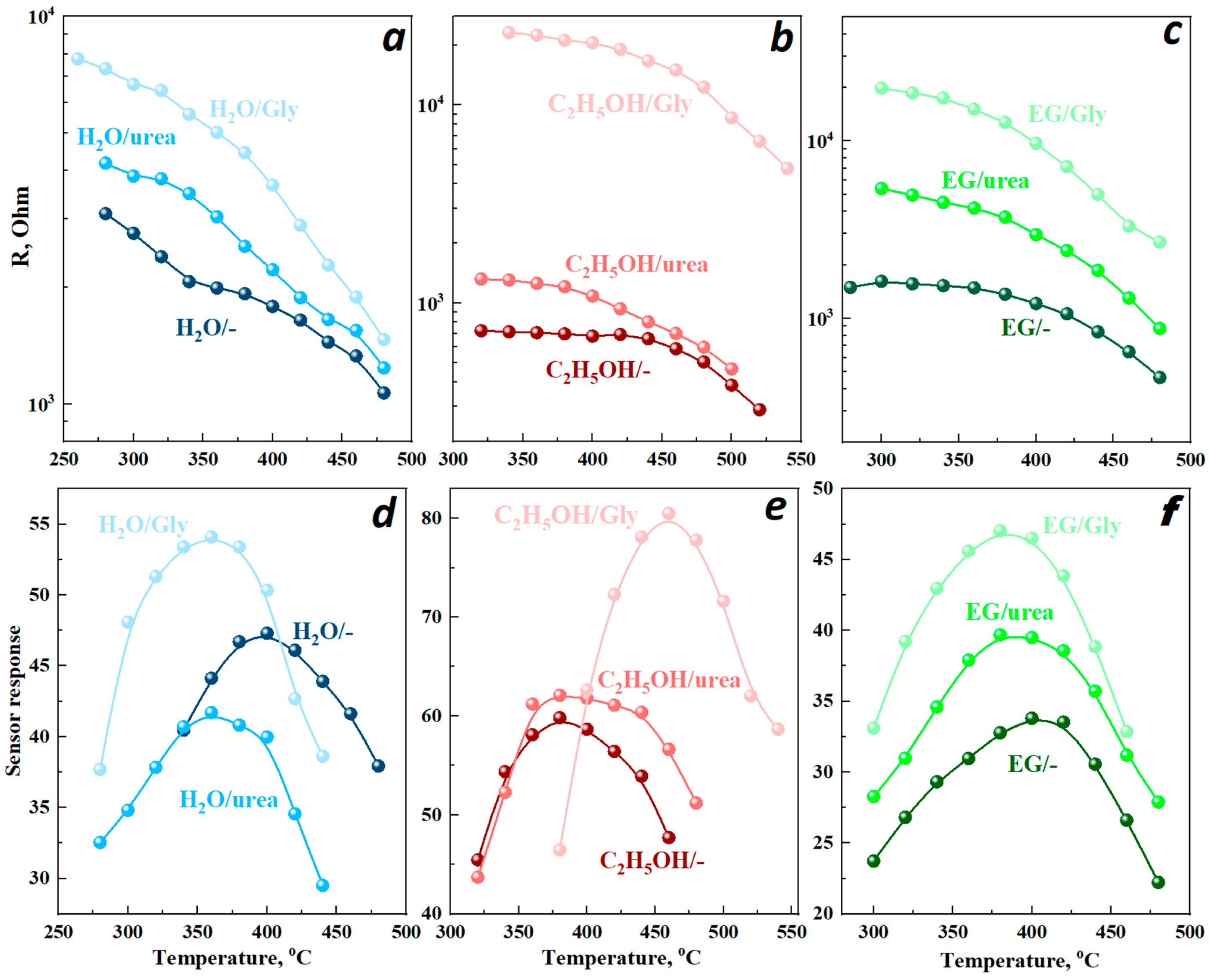
3.2. Conductivity and Sensor Response to Hydrogen
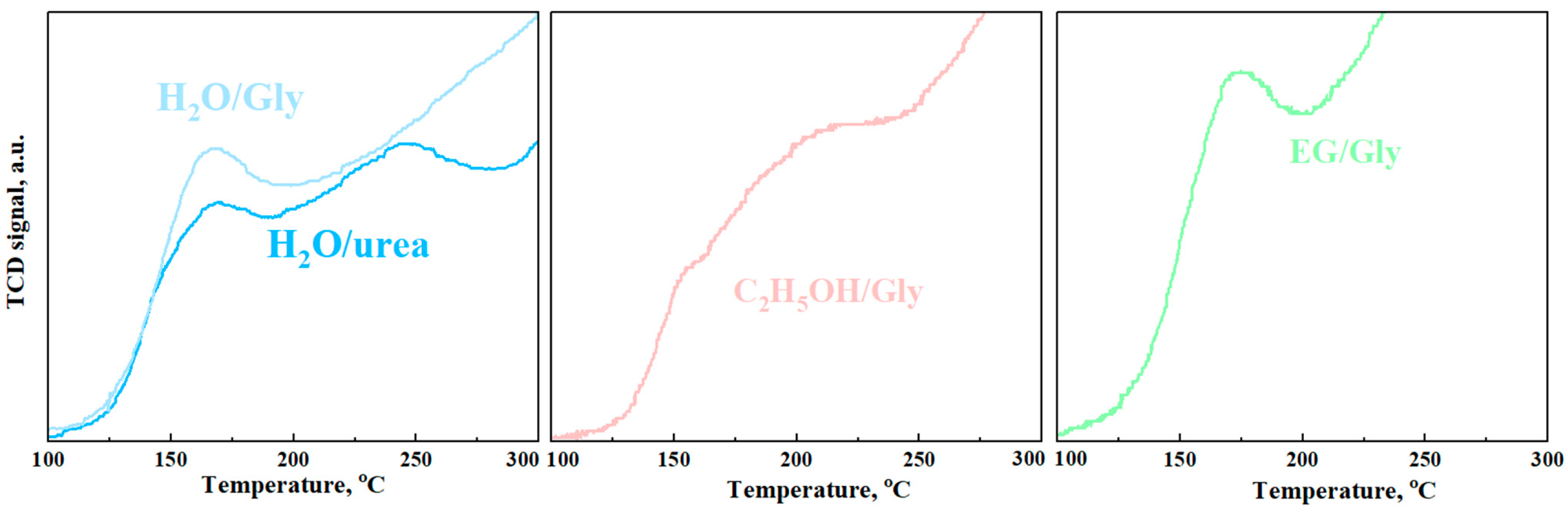
3.3. Catalytic Activity of Nanopowders
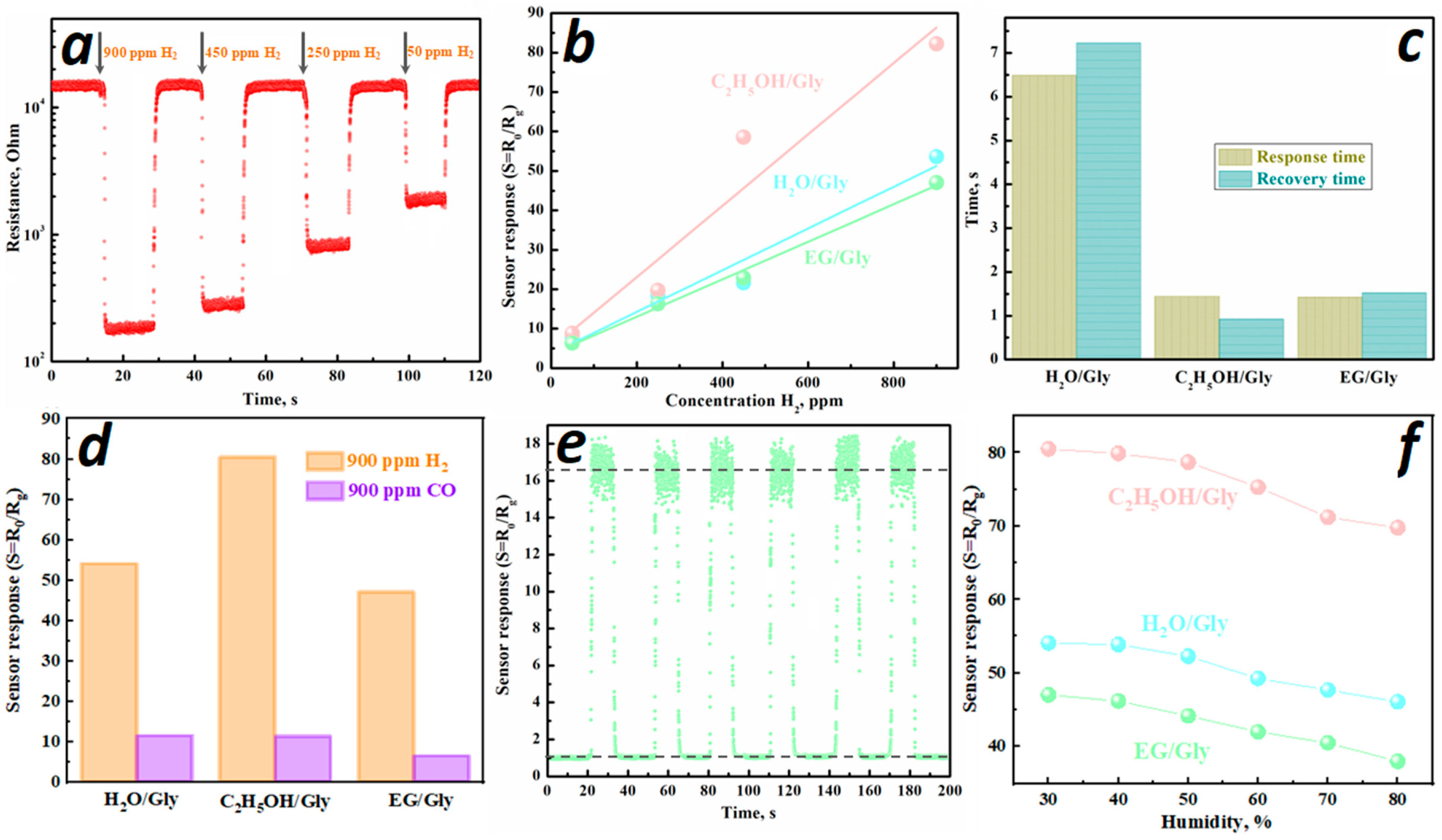
3.4. Sensor Characteristics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shah, S.; Hussain, S.; Din, S.T.U.; Shahid, A.; Amu-Darko, J.N.O.; Wang, M.; Qiao, G. A review on In2O3 nanostructures for gas sensing applications. J. Environ. Chem. Eng. 2024, 12, 112538. [Google Scholar] [CrossRef]
- Morulane, K.L.; Swart, H.C.; Motaung, D.E. A review on topical advancement and challenges of indium oxide based gas sensors: Future outlooks. J. Environ. Chem. Eng. 2024, 12, 112144. [Google Scholar] [CrossRef]
- Shi, Y.; Li, X.; Sun, X.F.; Shao, X.; Wang, H.Y. Strategies for improving the sensing performance of In2O3-based gas sensors for ethanol detection. J. Alloys Compd. 2023, 963, 171190. [Google Scholar] [CrossRef]
- Kumar, V.; Majhi, S.M.; Kim, K.H.; Kim, H.W.; Kwon, E.E. Advances in In2O3-based materials for the development of hydrogen sulfide sensors. Chem. Eng. J. 2021, 404, 126472. [Google Scholar] [CrossRef]
- Ikim, M.I.; Gerasimov, G.N.; Gromov, V.F.; Ilegbusi, O.J.; Trakhtenberg, L.I. Synthesis, structural and sensor properties of nanosized mixed oxides based on In2O3 particles. Int. J. Mol. Sci. 2023, 24, 1570. [Google Scholar] [CrossRef]
- Ma, X.; Chen, D.; Li, W.; He, L.; Jin, L.; Zhang, J.; Zhang, K. PEI doped In2O3 nanospheres based gas sensor for high-performance formaldehyde detection. Sens. Actuators B Chem. 2025, 431, 137374. [Google Scholar] [CrossRef]
- Patil, S.B.; More, M.A.; Patil, D.Y.; Ezema, F.I.; Patil, G.E. Studies on a chemiresistive gas sensor based on sol-gel grown nanocrystalline In2O3 thin film for fast carbon monoxide detection. Mater. Lett. 2025, 382, 137882. [Google Scholar] [CrossRef]
- Sood, T.; Thundiyil, R.; Chattopadhyay, S.; Poornesh, P. Optimized nanostructured In2O3 gas sensors: Harnessing annealing-induced defects and oxygen vacancies for ultra-sensitive and selective H2S detection at trace levels. RSC Adv. 2025, 15, 16555–16569. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Jin, X.; Sun, G.; Cao, J.; Wang, Y. Enhanced gas sensing performance of hierarchical porous In2O3 nanocubes modified with PdO and Pd for ppb-level H2S detection. Vacuum 2025, 240, 114594. [Google Scholar] [CrossRef]
- Du, Y.; Ren, L.; Yuan, J.; Zhang, R.; Xie, M.; Xu, H. In-situ synthesized Sn-doped In2O3 nanorods for low-temperature NO2 sensing with high response. Sens. Actuators B Chem. 2025, 445, 138564. [Google Scholar] [CrossRef]
- Liang, T.T.; Kim, D.S.; Yoon, J.W.; Yu, Y.T. Rapid synthesis of rhombohedral In2O3 nanoparticles via a microwave-assisted hydrothermal pathway and their application for conductometric ethanol sensing. Sens. Actuators B Chem. 2021, 346, 130578. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, L.; Gai, L.; Ma, L.; Ma, Y.; Li, M. Hierarchical rh-In2O3 crystals derived from InOOH counterparts and their sensitivity to ammonia gas. CrystEngComm 2013, 15, 7003–7009. [Google Scholar] [CrossRef]
- Ikim, M.I.; Gerasimov, G.N.; Erofeeva, A.R.; Gromov, V.F.; Ilegbusi, O.J.; Trakhtenberg, L.I. Cobalt doped cubic and rhombohedral In2O3: The role of crystalline phase of indium oxide in sensor response to hydrogen. Chem. Phys. Lett. 2024, 845, 141321. [Google Scholar] [CrossRef]
- Zhang, W.H.; Ding, S.J.; Zhang, Q.S.; Yi, H.; Liu, Z.X.; Shi, M.L.; Yue, L. Rare earth element-doped porous In2O3 nanosheets for enhanced gas-sensing performance. Rare Met. 2021, 40, 1662–1668. [Google Scholar] [CrossRef]
- Cao, E.; Wu, L.; Zhang, Y.; Sun, L.; Yu, Z.; Nie, Z. Hydrothermal synthesis of cubic-rhombohedral-In2O3 microspheres with superior acetone sensing performance. Appl. Surf. Sci. 2023, 613, 156045. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Liu, J.; Luo, T.; Qian, Y. Shape-and phase-controlled synthesis of In2O3 with various morphologies and their gas-sensing properties. Sens. Actuators B Chem. 2009, 137, 103–110. [Google Scholar] [CrossRef]
- Yang, G.; Park, S.J. Conventional and microwave hydrothermal synthesis and application of functional materials: A review. Materials 2019, 12, 1177. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A.; Vollebregt, S. Effect of temperature and humidity on the sensing performance of TiO2 nanowire-based ethanol vapor sensors. Nanotechnology 2021, 32, 325501. [Google Scholar] [CrossRef]
- Pandey, G.; Lawaniya, S.D.; Kumar, S.; Dwivedi, P.K.; Awasthi, K. A highly selective, efficient hydrogen gas sensor based on bimetallic (Pd–Au) alloy nanoparticle (NP)-decorated SnO2 nanorods. J. Mater. Chem. A 2023, 11, 26687–26697. [Google Scholar] [CrossRef]
- Ding, J.; Wang, Y.; Qu, Y.; Chen, H.; Fu, H. Hydrothermal synthesis of ZnO/In2O3 nanocomposites derived from dual MOFs to enhance the gas sensing performance for ethanolamine. Sens. Actuators A Phys. 2025, 397, 117198. [Google Scholar] [CrossRef]
- Ikim, M.I.; Gerasimov, G.N.; Gromov, V.F.; Ilegbusi, O.J.; Trakhtenberg, L.I. Phase composition, conductivity, and sensor properties of cerium-doped indium oxide. Nano Mater. Sci. 2024, 6, 193–200. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, H.; Xu, M.; Cao, S.; Hu, R.; Peng, L.; Zhang, D. pH-mediated hydrothermal synthesis of SnO2 for enhanced ethanol sensing: Structural regulation and performance optimization. J. Alloys Compd. 2025, 1025, 180334. [Google Scholar] [CrossRef]
- Li, P.; Cai, C.; Cheng, T.; Huang, Y. Hydrothermal synthesis and Cl2 sensing performance of porous-sheets-like In2O3 structures with phase transformation. RSC Adv. 2017, 7, 50760–50771. [Google Scholar] [CrossRef]
- Wu, L.; Cao, E.; Zhang, Y.; Sun, L.; Sun, B.; Yu, Z. La and Fe co-doped walnut-like cubic-rhombohedral-In2O3 for highly sensitive and selective detection of acetone vapor. Mater. Lett. 2023, 336, 133869. [Google Scholar] [CrossRef]
- Tseng, T.T.; Tseng, W.J. Effect of polyvinylpyrrolidone on morphology and structure of In2O3 nanorods by hydrothermal synthesis. Ceram. Int. 2009, 35, 2837–2844. [Google Scholar] [CrossRef]
- Jia, P.; Zhang, D.; Shao, X.; Zhai, J.; Guo, J.; Wang, Z.; Ji, X. High-efficient and selective hydrogen gas sensor based on bimetallic Ag/Cu nanoparticles decorated on In2O3: Experimental and DFT calculation. Int. J. Hydrogen Energy 2025, 101, 15–25. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Xu, X.; Wang, X.; Sun, G.; Zhang, B.; Cao, J. Chemiresistive detection of H2 at near room temperature by porous In2O3 nanotubes co-sensitized with La-dopant and Pd-modifier. Sens. Actuators B Chem. 2025, 426, 137110. [Google Scholar] [CrossRef]
- Nath, D.; Singh, F.; Das, R. X-ray diffraction analysis by Williamson-Hall, Halder-Wagner and size-strain plot methods of CdSe nanoparticles-a comparative study. Mater. Chem. Phys. 2020, 239, 122021. [Google Scholar] [CrossRef]
- Shinde, D.V.; Jadhav, V.V.; Lee, D.Y.; Shrestha, N.K.; Lee, J.K.; Lee, H.Y.; Han, S.H. A coordination chemistry approach for shape controlled synthesis of indium oxide nanostructures and their photoelectrochemical properties. J. Mater. Chem. A 2014, 2, 5490–5498. [Google Scholar] [CrossRef]
- Hirano, M.; Kato, E. Hydrothermal synthesis of nanocrystalline cerium (IV) oxide powders. J. Am. Ceram. Soc. 1999, 82, 786–788. [Google Scholar] [CrossRef]
- Shukla, R.; Dutta, D.P.; Ramkumar, J.; Mandal, B.P.; Tyagi, A.K. Nanocrystalline Functional Oxide Materials. In Springer Handbook of Nanomaterials; Springer: Berlin/Heidelberg, Germany, 2013; p. 517. [Google Scholar] [CrossRef]
- Nielsen, I.G.; Sommer, S.; Iversen, B.B. Phase control for indium oxide nanoparticles. Nanoscale 2021, 13, 4038–4050. [Google Scholar] [CrossRef] [PubMed]
- Schlicker, L.; Bekheet, M.F.; Gurlo, A. Scaled-up solvothermal synthesis of nanosized metastable indium oxyhydroxide (InOOH) and corundum-type rhombohedral indium oxide (rh-In2O3). Cryst. Mater. 2017, 232, 129–140. [Google Scholar] [CrossRef]
- Yan, T.; Wang, X.; Long, J.; Lin, H.; Yuan, R.; Dai, W.; Fu, X. Controlled preparation of In2O3, InOOH and In(OH)3 via a one-pot aqueous solvothermal route. New J. Chem. 2008, 32, 1843–1846. [Google Scholar] [CrossRef]
- Choi, K.I.; Lee, J.H. Preparation and dispersion of metal oxide nanostructures using amino acid-assisted chemical routes: An overview. Sci. Adv. Mater. 2011, 3, 811–820. [Google Scholar] [CrossRef]
- Bekheet, M.F.; Schwarz, M.R.; Lauterbach, S.; Kleebe, H.J.; Kroll, P.; Riedel, R.; Gurlo, A. Orthorhombic In2O3: A metastable polymorph of indium sesquioxide. Angew. Chem. Int. Ed. 2013, 52, 6531–6535. [Google Scholar] [CrossRef]
- Ikim, M.I.; Gromov, V.F.; Gerasimov, G.N.; Spiridonova, E.Y.; Erofeeva, A.R.; Kurmangaleev, K.S.; Trakhtenberg, L.I. Structure, conductivity, and sensor properties of nanosized ZnO-In2O3 composites: Influence of synthesis method. Micromachines 2023, 14, 1685. [Google Scholar] [CrossRef]
- Shen, H.; Li, L.; Xu, D. Preparation of one-dimensional SnO2–In2O3 nano-heterostructures and their gas-sensing property. RSC Adv. 2017, 7, 33098. [Google Scholar] [CrossRef]
- Han, G.; Lu, Q.; Liu, G.; Ye, X.; Lin, S.; Song, Y.; Li, G. Enhanced ethanol sensing properties based on α-Fe2O3/In2O3 hollow microspheres. J. Mater. Sci. Mater. Electron. 2012, 23, 1616–1620. [Google Scholar] [CrossRef]
- Trakhtenberg, L.I.; Ikim, M.I.; Ilegbusi, O.J.; Gromov, V.F.; Gerasimov, G.N. Effect of nanoparticle interaction on structural, conducting and sensing properties of mixed metal oxides. Chemosensors 2023, 11, 320. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U.D.O. Conduction model of metal oxide gas sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Chen, F.; Yang, M.; Wang, X.; Song, Y.; Guo, L.; Xie, N.; Lu, G. Template-free synthesis of cubic-rhombohedral-In2O3 flower for ppb level acetone detection. Sens. Actuators B Chem. 2019, 290, 459–466. [Google Scholar] [CrossRef]
- Kozhushner, M.A.; Lidskii, B.V.; Oleynik, I.I.; Posvyanskii, V.S.; Trakhtenberg, L.I. Inhomogeneous Charge Distribution in Semiconductor Nanoparticles. J. Phys. Chem. C 2015, 119, 16286–16292. [Google Scholar] [CrossRef]
- Posvyanskii, V.S.; Bodneva, V.L.; Chertkov, A.V.; Kurmangaleev, K.S.; Ikim, M.I.; Novozhilov, V.B.; Trakhtenberg, L.I. Tensor Network Modeling of Electronic Structure of Semiconductor Nanoparticles and Sensory Effect of Layers Based on Them. Mathematics 2025, 13, 3296. [Google Scholar] [CrossRef]
- Bielz, T.; Lorenz, H.; Jochum, W.; Kaindl, R.; Klauser, F.; Klötzer, B.; Penner, S. Hydrogen on In2O3: Reducibility, bonding, defect formation, and reactivity. J. Phys. Chem. C 2010, 114, 9022–9029. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.Y.; Senftle, T.P.; Zhu, J.; Zhang, G.; Guo, X.; Song, C. Variation in the In2O3 crystal phase alters catalytic performance toward the reverse water gas shift reaction. ACS Catal. 2019, 10, 3264–3273. [Google Scholar] [CrossRef]
- Liao, L.; Men, Y.; Wang, Y.; Xu, S.; Wu, S.; Wang, J.; Miao, X. Unravelling the morphology effect of Pt/In2O3 catalysts for highly efficient hydrogen production by methanol steam reforming. Fuel 2024, 372, 132221. [Google Scholar] [CrossRef]
- Analytical Methods Committee. Recommendations for the definition, estimation and use of the detection limit. Analyst 1987, 112, 199–204. [Google Scholar] [CrossRef]
- Tang, M.; Qin, C.; Sun, X.; Li, M.; Wang, Y.; Cao, J.; Wang, Y. Enhanced H2 gas sensing performances by Pd-loaded In2O3 microspheres. Appl. Phys. A 2024, 130, 741. [Google Scholar] [CrossRef]
- Deb, S.; Mondal, A.; Ajitha, B.; Reddy, Y.A.K. Evaluation of hydrogen gas-sensing performance based on polymorphic-engineered In2O3 nanostructures. Microchem. J. 2025, 219, 115914. [Google Scholar] [CrossRef]

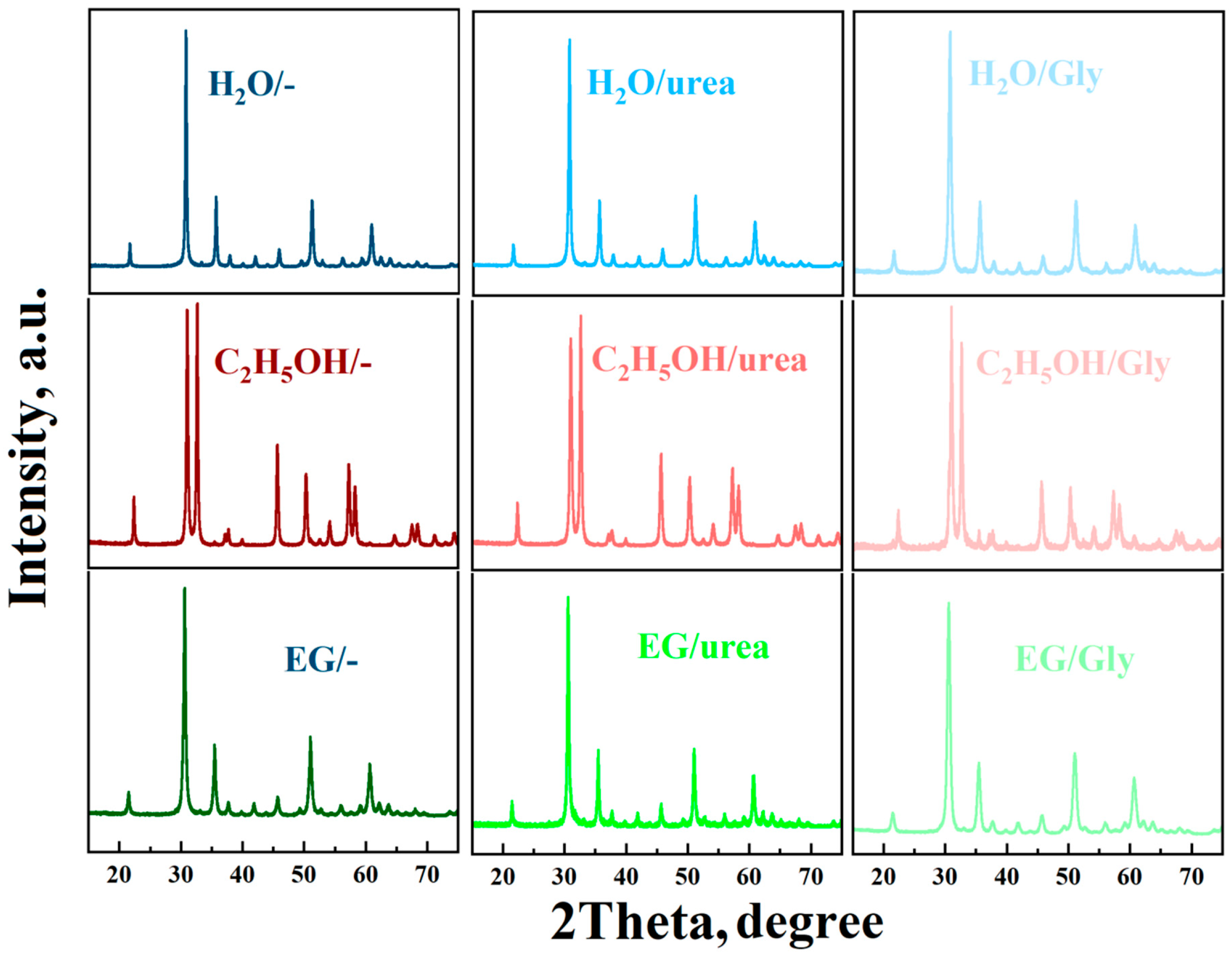
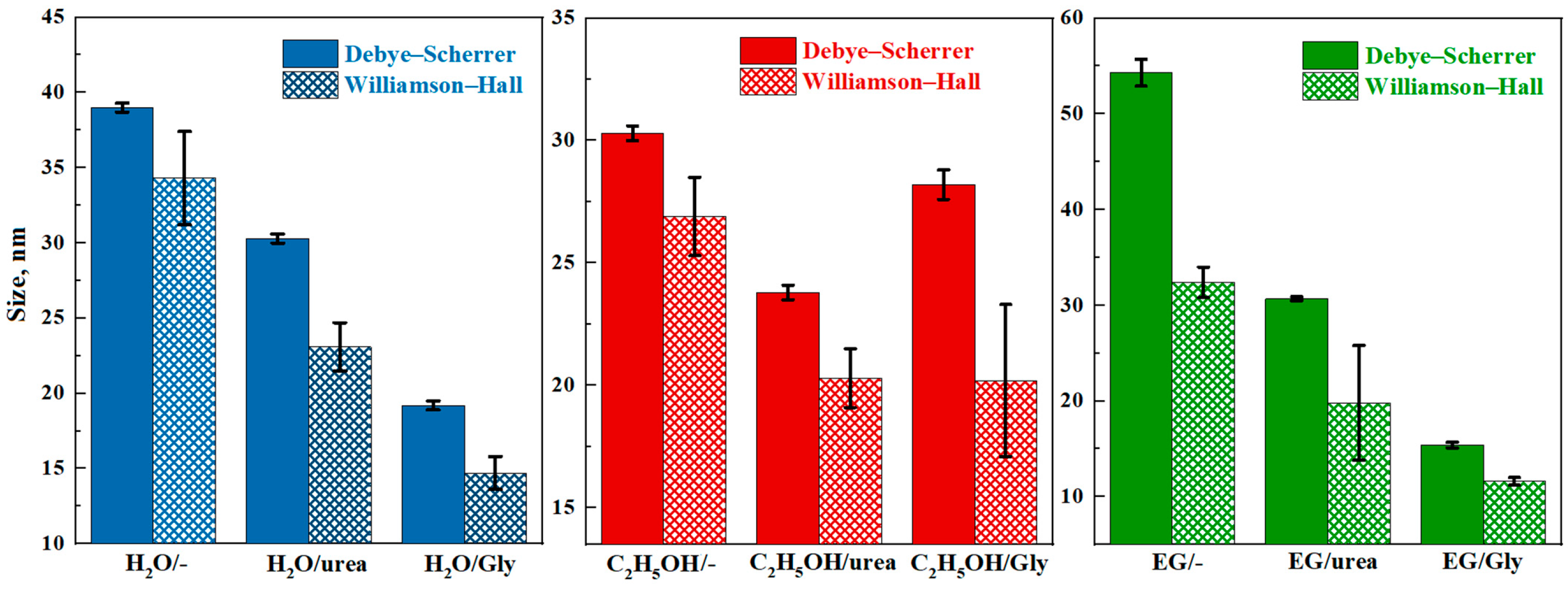
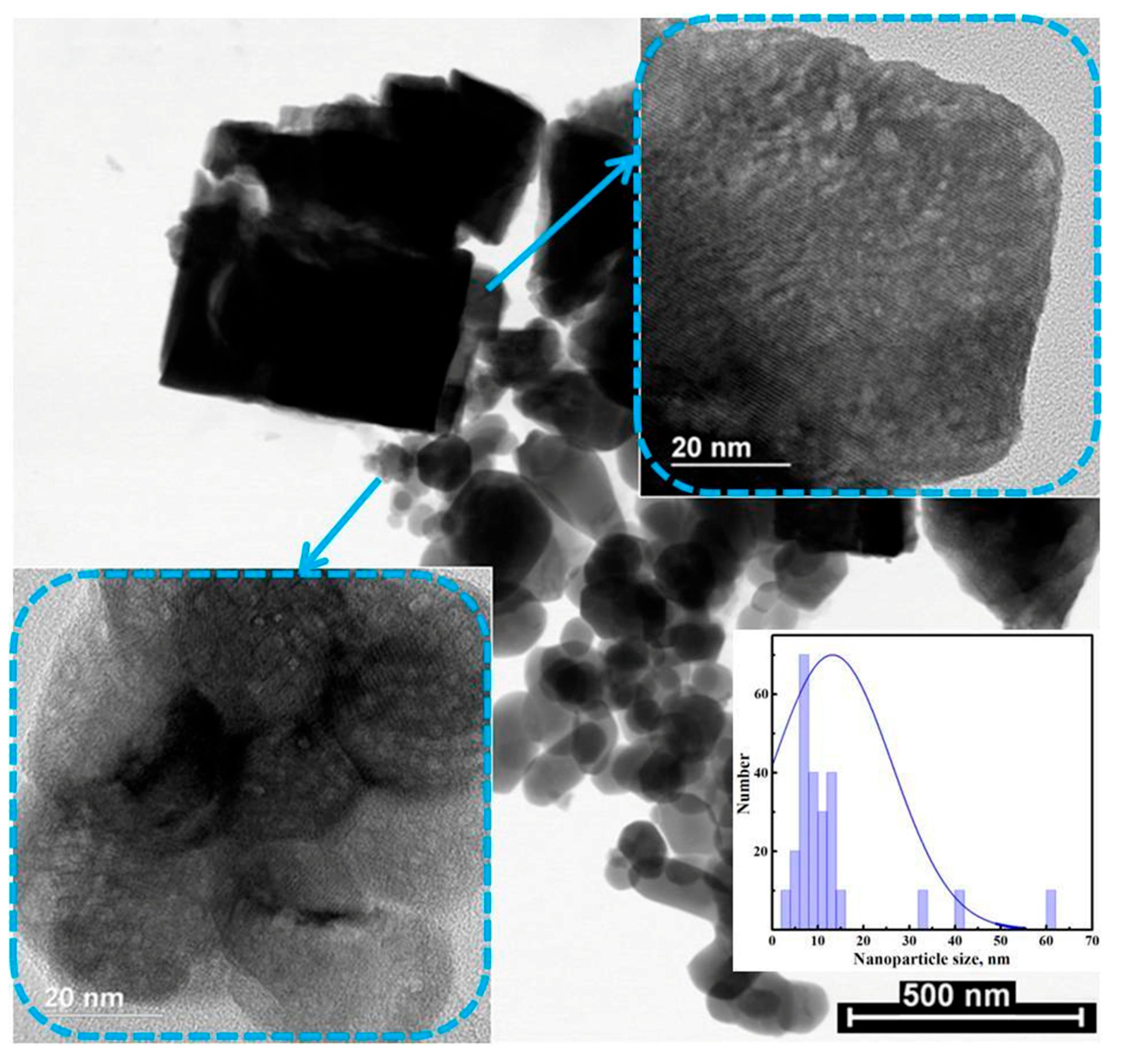
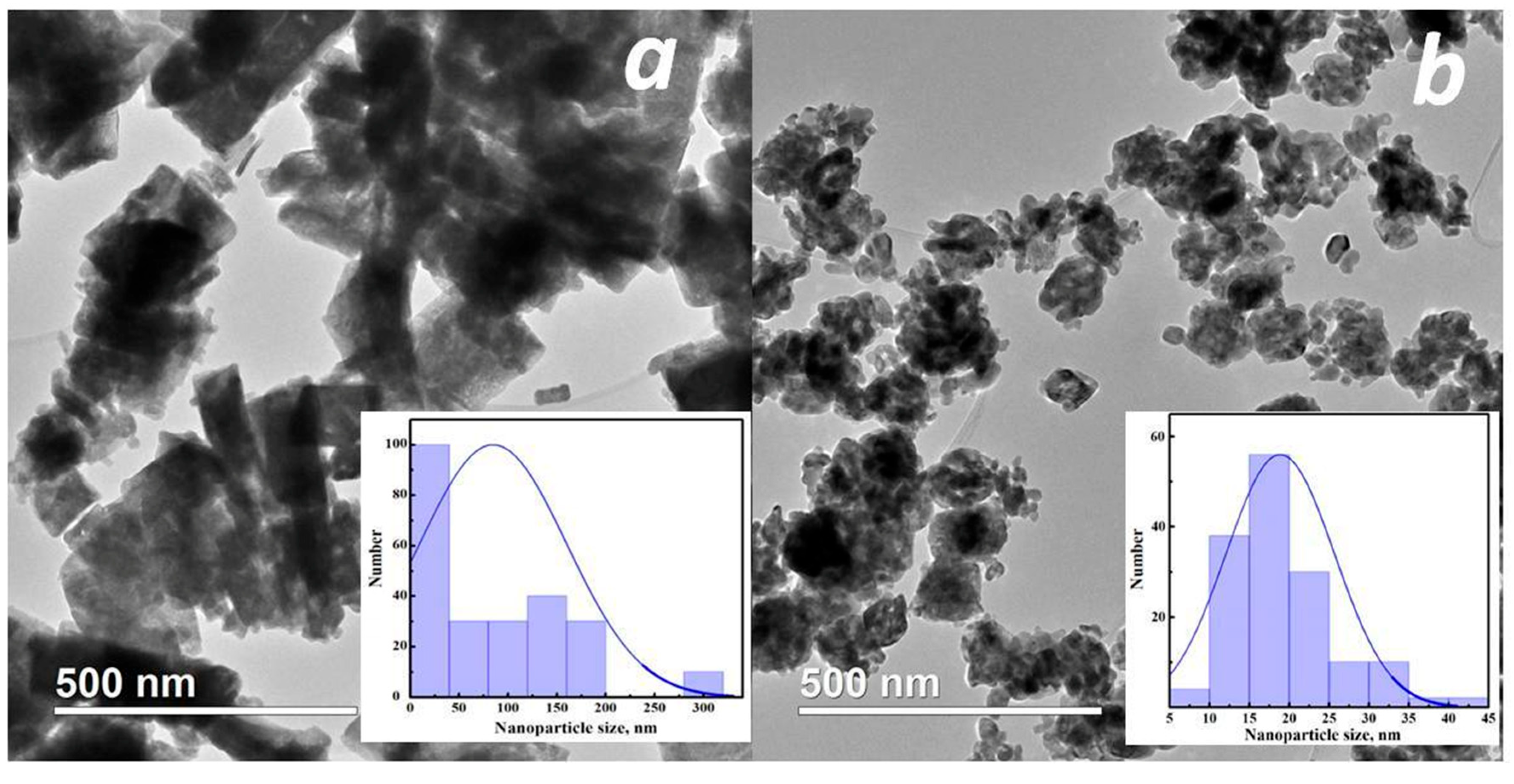
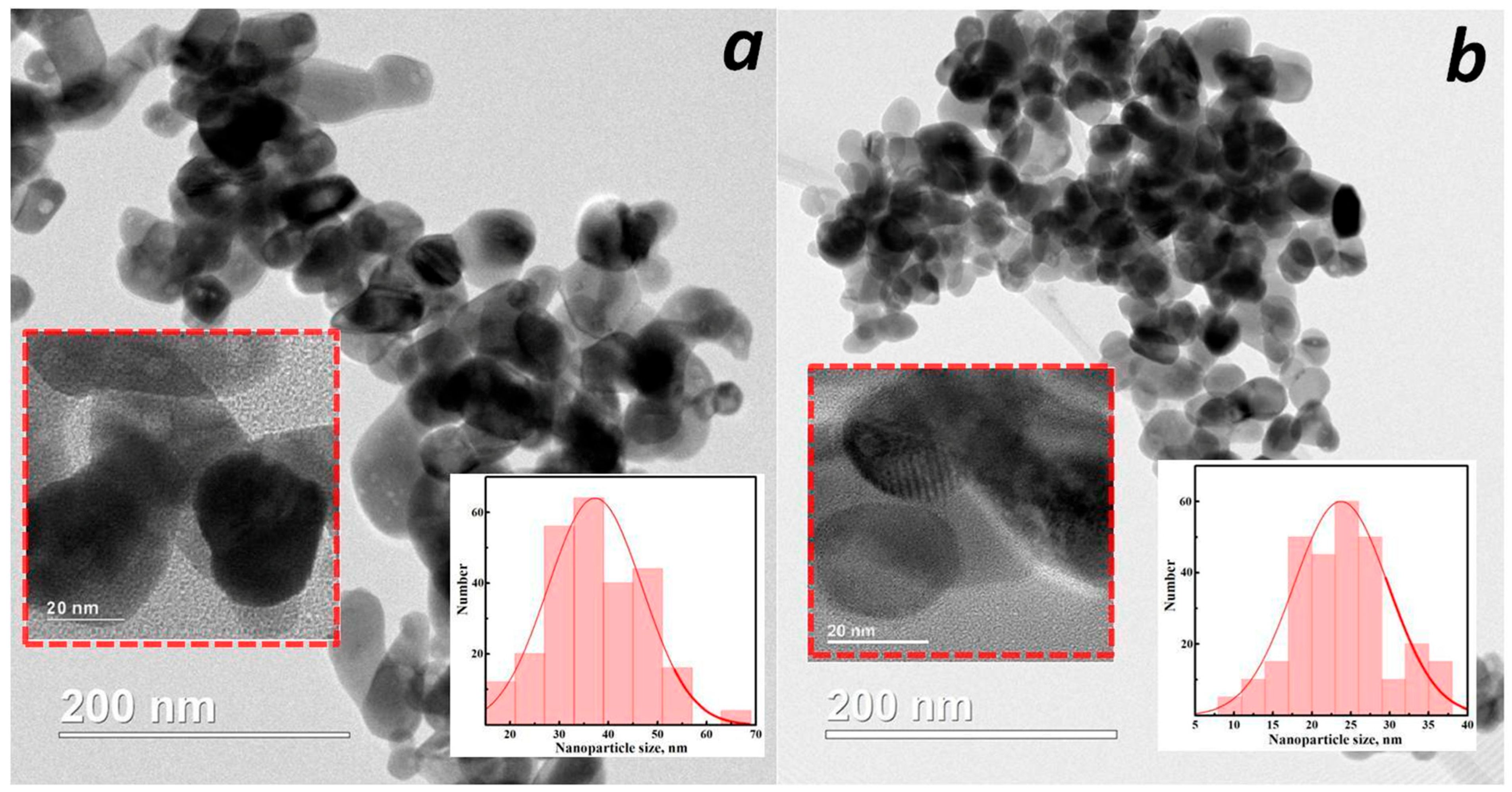
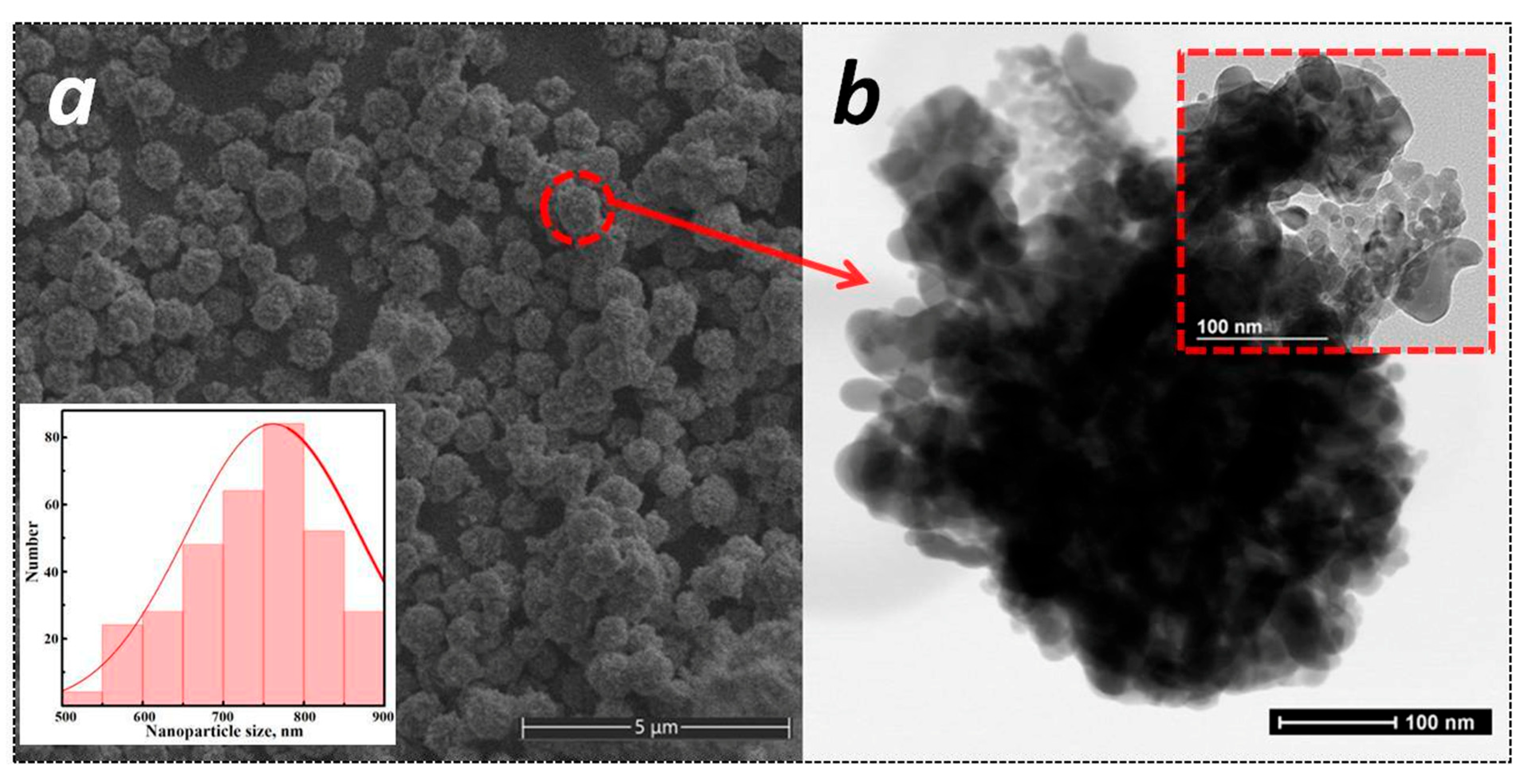
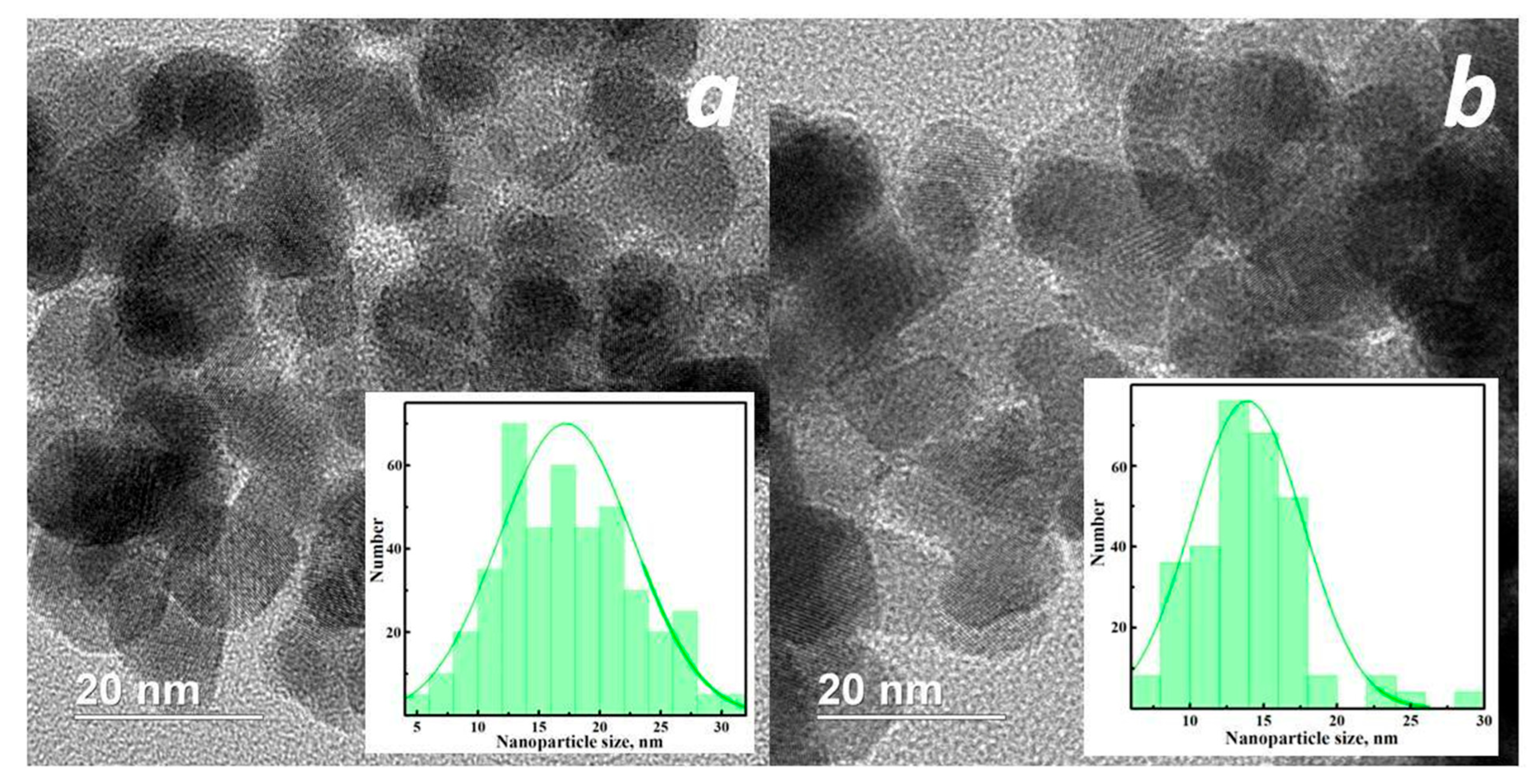
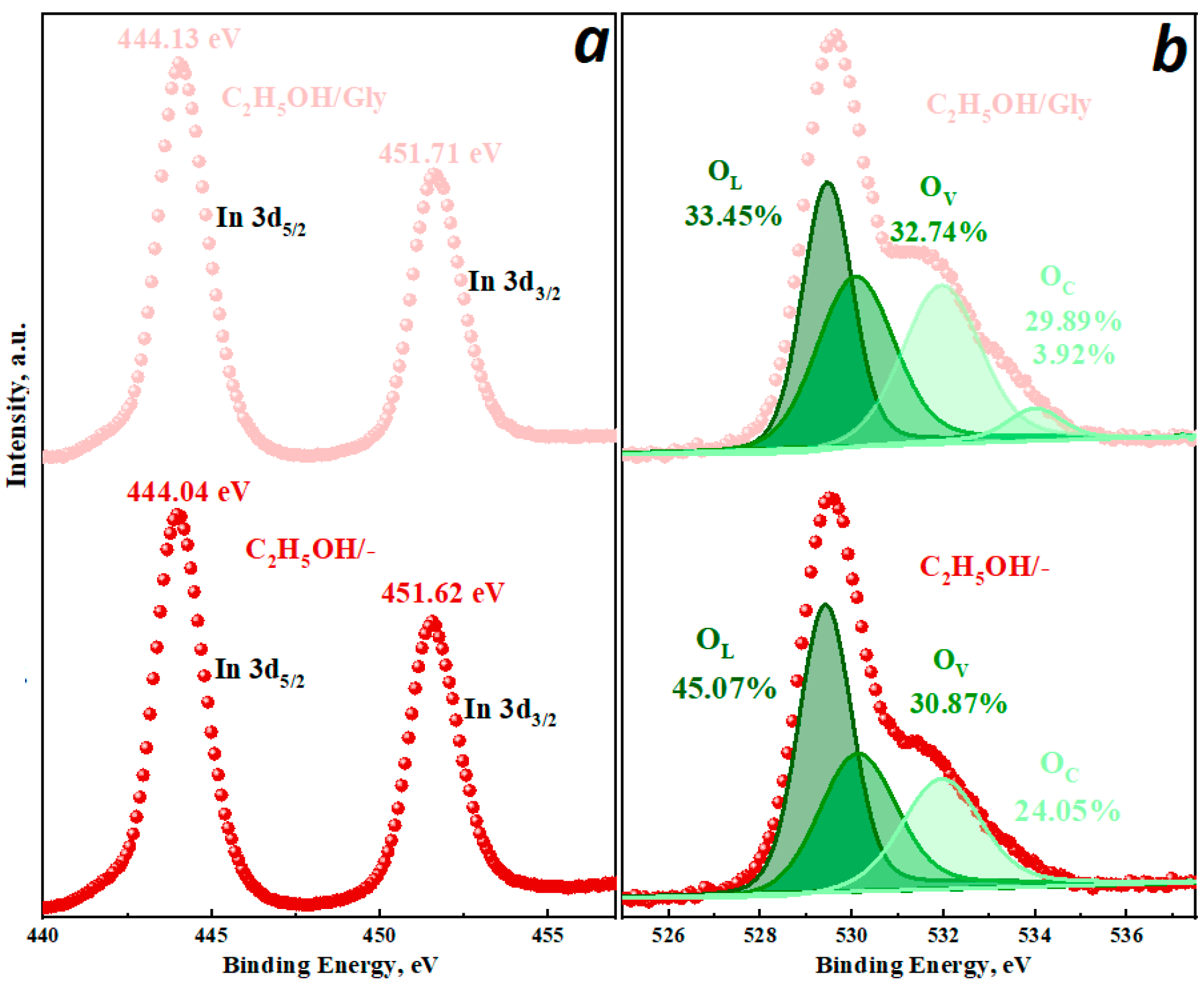

| Sample | Crystalline Phase, wt% | 2θ, ° | Lattice Parameters, nm | d-Spacing, nm |
|---|---|---|---|---|
| H2O/- | 100% c-In2O3 | 30.63 (222) | a = b = c = 1.0106 | 0.2916 (222) |
| H2O/urea | 100% c-In2O3 | 30.61 (222) | a = b = c = 1.0111 | 0.2918 (222) |
| H2O/Gly | 100% c-In2O3 | 30.59 (222) | a = b = c = 1.0116 | 0.2920 (222) |
| C2H5OH/- | 100% rh-In2O3 | 30.98 (104) 32.61 (110) | a = b = 0.5483; c = 1.4502 | 0.2885 (104) 0.2743 (110) |
| C2H5OH/urea | 100% rh-In2O3 | 31.00 (104) 32.61 (110) | a = b = 0.5486; c = 1.4507 | 0.2884 (104) 0.2744 (110) |
| C2H5OH/Gly | 89.2% rh-In2O3 | 31.00 (104) 32.64 (110) | a = b = 0.5484; c = 1.4508 | 0.2882 (104) 0.2742 (110) |
| 10.8% c-In2O3 | 30.63 (222) | a = b = c = 1.0117 | 0.2917 (222) | |
| EG/- | 100% c-In2O3 | 30.54 (222) | a = b = c = 1.0118 | 0.2924 (222) |
| EG/urea | 100% c-In2O3 | 30.58 (222) | a = b = c = 1.0122 | 0.2925 (222) |
| EG/Gly | 100% c-In2O3 | 30.54 (222) | a = b = c = 1.0119 | 0.2921 (222) |
| Sample | Low Temperature Peaks, °C | Consumption of H2, mmol/g | Maximum Sensor Response |
|---|---|---|---|
| H2O/- | 158 265 | 0.43 | 47.3 |
| H2O/urea | 156 234 | 0.42 | 41.8 |
| H2O/Gly | 164 | 0.48 | 53.6 |
| C2H5OH/- | 207 | 0.79 | 59.8 |
| C2H5OH/urea | 206 | 0.8 | 62.1 |
| C2H5OH/Gly | 210 | 1.34 | 80.5 |
| EG/- | 177 | 0.32 | 33.8 |
| EG/urea | 173 | 0.34 | 39.6 |
| EG/Gly | 170 | 0.35 | 47 |
| In2O3 Phase | Materials for Synthesis | S at [H2] | T, °C | tres/trec, s | LOD | SH2/SCO | Ref. |
|---|---|---|---|---|---|---|---|
| c-In2O3 | 0.5 g of In(NO3)3 and 9 g of C6H5Na3O7, 1.5 g of CO(NH2)2 were dissolved in 60 mL water | 2.3 * at 100 ppm, 3 at 200 ppm | 300 | 24/25 | - | 2 | [26] |
| c-In2O3 | 0.6 g of In(NO3)3 and 0.3 g of H2BDC were dissolved in 60 mL DMF | 1.72 * at 50 ppm | 160 | 74/259 | - | 1.6 | [27] |
| c-In2O3 | 409.12 mg In(NO3)3 and 996.98 mg urea were dissolved in 80 mL ethanol | 1.62 * at 1000 ppm | 160 | - | 5 ppm | 1.03 | [49] |
| c+rh-In2O3 | 0.5 mmol In(NO3)3 and 0.5 mmol CO(NH2)2, 0.1 mmol SDS were dissolved in 20 mL ethanol and water | 111.6 ** at 200 ppm | 175 | 5/8 | - | 13 | [50] |
| c-In2O3 | 0.5 mmol In(NO3)3 and 0.5 mmol CO(NH2)2, 0.1 mmol SDS were dissolved in 20 mL absolute and water | 10.2 ** at 200 ppm | 175 | 9/10 | - | - | [50] |
| c-In2O3 | 1.36 mmol In(NO3)3 and 16 mmol Gly were dissolved in 80 mL water | 53.6 * at 900 ppm | 360 | 6.5/7.2 | 323 ppb | 4.7 | This work |
| c+rh-In2O3 | 1.36 mmol In(NO3)3 and 16 mmol Gly were dissolved in 80 mL ethanol | 80.5 * at 900 ppm | 480 | 1.4/1 | 173 ppb | 7.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ikim, M.I.; Demina, V.A.; Spiridonova, E.Y.; Ilegbusi, O.J.; Trakhtenberg, L.I. Structural and Gas-Sensitive Characteristics of In2O3: Effect of Hydrothermal/Solvothermal Synthesis Conditions. Micromachines 2025, 16, 1299. https://doi.org/10.3390/mi16111299
Ikim MI, Demina VA, Spiridonova EY, Ilegbusi OJ, Trakhtenberg LI. Structural and Gas-Sensitive Characteristics of In2O3: Effect of Hydrothermal/Solvothermal Synthesis Conditions. Micromachines. 2025; 16(11):1299. https://doi.org/10.3390/mi16111299
Chicago/Turabian StyleIkim, Mariya I., Varvara A. Demina, Elena Y. Spiridonova, Olusegun J. Ilegbusi, and Leonid I. Trakhtenberg. 2025. "Structural and Gas-Sensitive Characteristics of In2O3: Effect of Hydrothermal/Solvothermal Synthesis Conditions" Micromachines 16, no. 11: 1299. https://doi.org/10.3390/mi16111299
APA StyleIkim, M. I., Demina, V. A., Spiridonova, E. Y., Ilegbusi, O. J., & Trakhtenberg, L. I. (2025). Structural and Gas-Sensitive Characteristics of In2O3: Effect of Hydrothermal/Solvothermal Synthesis Conditions. Micromachines, 16(11), 1299. https://doi.org/10.3390/mi16111299







