3.1. Concave Microwell Fabrication via Water Vapor Permeation Through PDMS Membrane
The unique gas permeability of PDMS, particularly its high permeability to water vapor compared to other gases such as oxygen and nitrogen [
12], were exploited to fabricate the concave microwells without lithography. The exceptional permeability of water vapor through PDMS, with a rate of 3600 × 10⁻⁹ cm³·cm/(cm²·s·cmHg), is approximately 60 times that of oxygen (60 × 10⁻⁹ cm³·cm/(cm²·s·cmHg)) and 125 times that of nitrogen (28 × 10⁻⁹ cm³·cm/(cm²·s·cmHg)) [
12]. The permeability of carbon dioxide through PDMS is also very high, at 325 × 10⁻⁹ cm³·cm/(cm²·s·cmHg), yet this is still an order of magnitude lower than that of water vapor [
12]. This stark contrast underscores the anomalously high water vapor permeability, particularly considering the low solubility of water in PDMS (less than 1 ppm), which is far lower than that of CO₂ and other gases that rely on solubility-driven transport [
13].
The formation of microwells is driven by the water vapor-selective permeability of PDMS, which enables the controlled evaporation of water from droplets deposited on its surface, forming concave microwell structures without the need for external forces or complex fabrication processes. The high water vapor permeability of PDMS is attributed to its unique molecular properties, including long Si–O and Si–C bonds, high fractional free volume, and low glass transition temperature, which result in the formation of dynamic sub-nanometer hydrophobic pores. These nanopores facilitate the selective transport of water vapor from regions of high to low vapor pressure through hydrogen-bonded chains of water molecules. Unlike the solubility-driven transport of other permeable gases, water transport is driven by the structural flexibility and compressibility of PDMS, which operates similarly to the transport mechanisms observed in carbon nanotubes and aquaporins [
13].
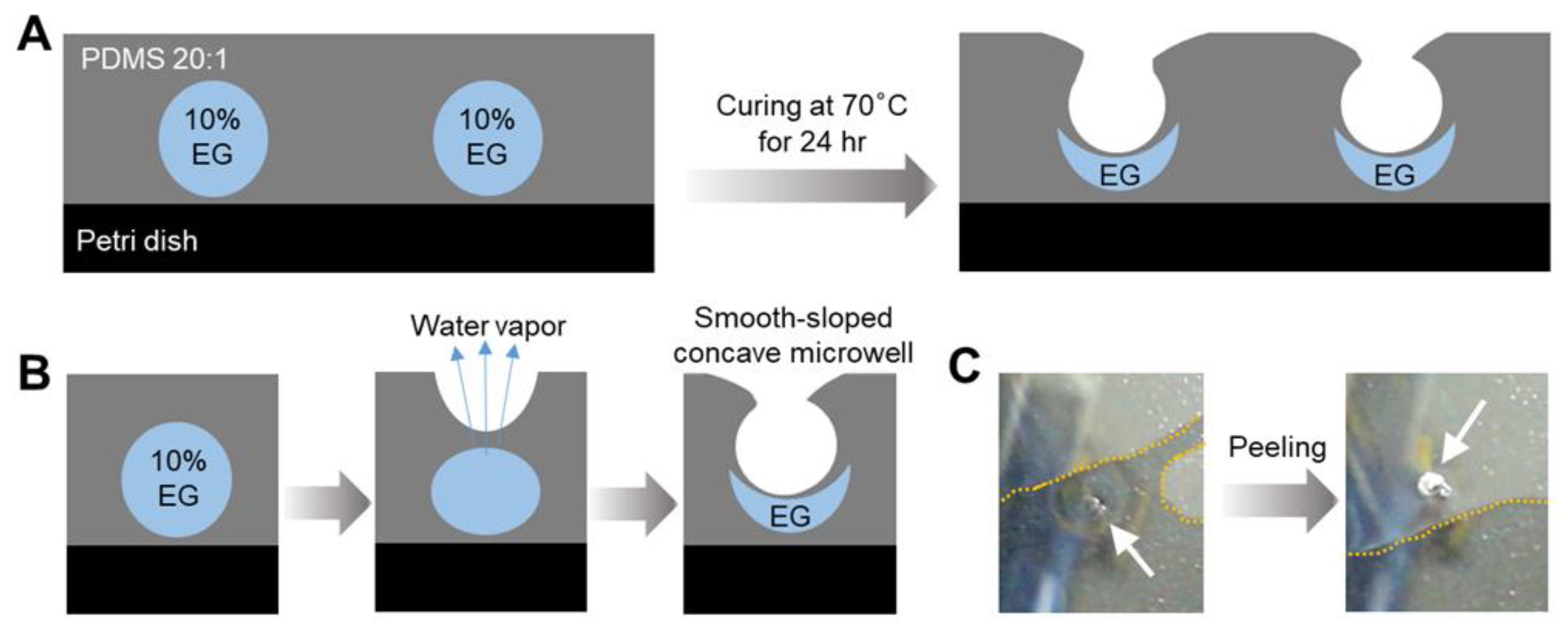
Droplets containing a mixture of EG and water were deposited onto a PDMS substrate (
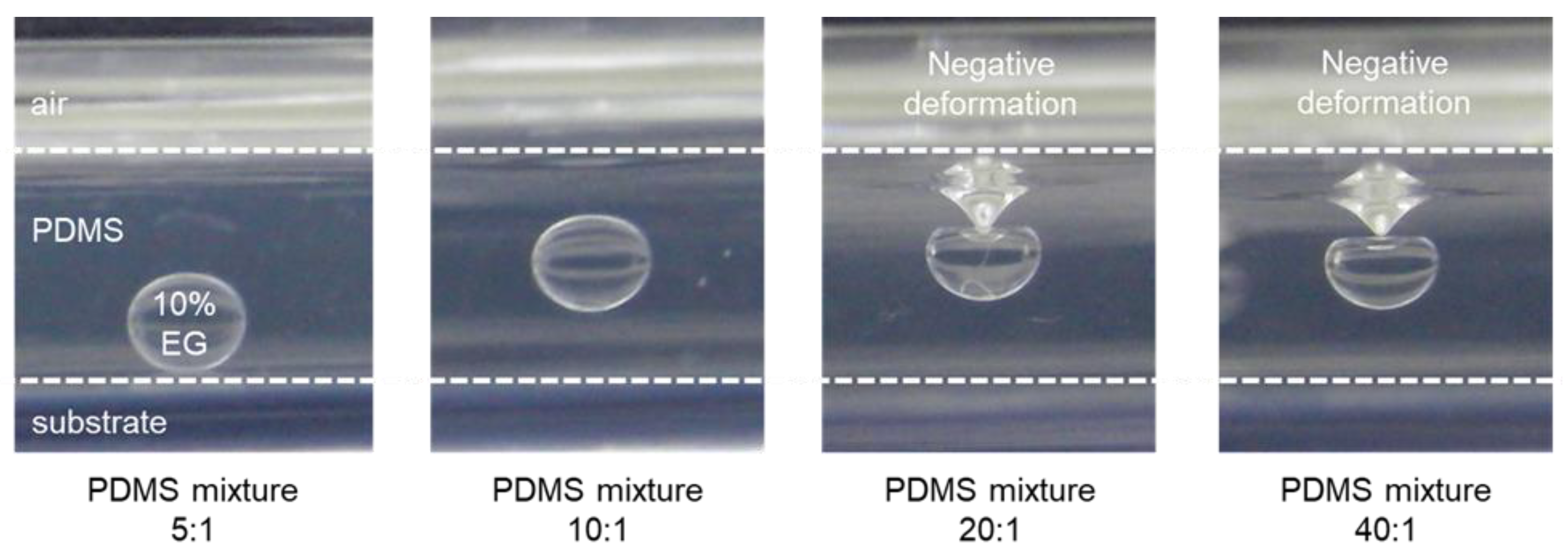
Figure 1). During curing of the PDMS at 70 °C, the water evaporates through the PDMS membrane owing to its high water vapor permeability, while the EG remains trapped. This process eventually forms concave microwells. The selective permeation of water vapor through the PDMS surface is the driving mechanism for the formation of the microwells, enabling precise control over the morphology of the structure by changing the droplet size and composition. Both the microwell formation process and the mechanical properties of the substrate are influenced by the PDMS-to-curing agent ratio [
14]. Substrates derived from PDMS mixtures with higher curing agent ratios (e.g., 5:1 or 10:1) exhibited a higher stiffness, resulting in minimal deformation of the PDMS surface. In these cases, the EG droplets maintained a stable position and did not undergo significant structural changes, as evidenced by the flat or slightly concave geometry of the surface after curing. Conversely, at lower curing agent ratios (e.g., 20:1 or 40:1), the increased elasticity of the PDMS substrate enabled greater deformation under the weight of the droplets, resulting in pronounced negative deformation (concavity) beneath the droplets (
Figure 2).
The observed deformation was attributed to the interplay between the mechanical properties of the PDMS and the evaporation of water. At lower curing agent ratios, the elastic properties of PDMS improve surface flexibility, enabling the droplets to exert sufficient pressure to create a concave shape; however, at excessively low curing agent ratios (e.g., above 40:1), the substrate becomes overly flexible and is therefore unable to maintain a stable concave geometry during curing. Accordingly, the z-axis position of the EG droplets and the resulting concave structure are governed by the viscoelastic properties of PDMS, which can be tailored by adjusting the mixture ratio. Thus, the optimal range of PDMS mixture ratios must balance elasticity and structural integrity to ensure the formation of uniform concave microwells. This tunability offers a simple yet effective approach for controlling the morphology of PDMS microwells, providing flexibility for various biomedical applications requiring precise structural features [
15,
16].
A PDMS ratio of 20:1 was used for microwell fabrication in this study. This ratio provides a more flexible and elastic surface than other PDMS mixtures, including the common 10:1 ratio. This 20:1 mixture affords increased elasticity that enhances the water vapor permeability of the material while maintaining its structural integrity. Softer PDMS matrixes allowed the formation of smooth microwell edges as the droplets contracted during the water evaporation process, forming a concave structure with a smooth-sloped profile. The elasticity of the PDMS is critical in preventing cracking or deformation during microwell formation, which is a common challenge for rigid materials. In addition to gas permeability, the shape and formation of the microwells are affected by the surface properties of PDMS, including its hydrophobicity. Water droplets exhibit a high contact angle on the hydrophobic surface of PDMS, which contributes to the smooth-sloped concave geometry of the microwells [
17]. As water evaporates, the EG component within the preformed droplets is left behind, resulting in the formation of concave microwells with well-defined and reproducible geometries that can be controlled by simply adjusting the droplet volume and curing conditions. Microwell fabrication via water vapor permeation through PDMS highlights the importance of the gas permeability, elasticity, and surface characteristics of the material. By optimizing the fabrication process, smooth-sloped concave microwells were obtained using a PDMS ratio of 20:1.
3.2. Impact of Droplet Volume on Microwell Formation Yield
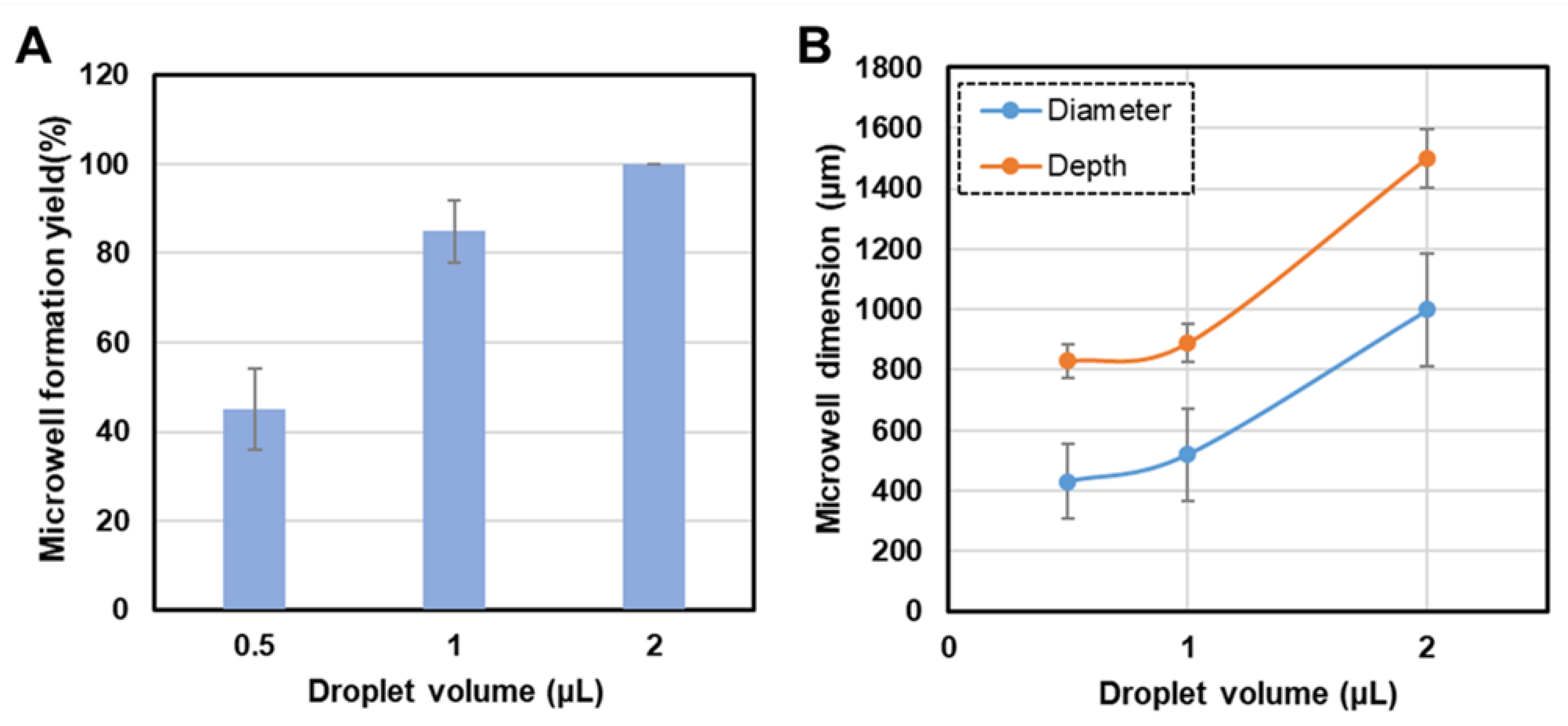
The volume of the 10% EG droplets deposited on the PDMS substrate is critical in determining the overall microwell formation yield. Therefore, the droplet volume was systematically varied to investigate its effect on the formation efficiency of concave microwells. A clear correlation between the droplet volume and microwell formation yield was observed, with the formation efficiency increasing with droplet volume (
Figure 3A). The microwell formation yield, expressed as a percentage, is defined as the number of successfully formed microwells relative to the total number of droplets on the PDMS substrate. Droplets with volumes of 0.5 µL, 1.0 µL, and 2.0 µL were tested to evaluate the impact of droplet volume on the formation yield. A notable increase in microwell formation yield was observed with increasing droplet volume, with the 2.0 µL droplets producing the highest yield.
This trend can be explained by the contraction of the droplets during the evaporation of water. Smaller droplets (e.g., 0.5 µL) undergo less pronounced contraction owing to their limited volume of water, resulting in lower microwell formation efficiency. A lower water volume may result in incomplete contraction, thereby forming irregular or incomplete microwells. Furthermore, smaller droplets may increase the probability of premature drying or deformation before a microwell is fully formed. By contrast, larger droplets contain more water and therefore undergo more significant contraction during evaporation. As water vapor escapes through the PDMS membrane, the remaining EG undergoes a more uniform contraction to form well-defined microwells. A larger volume of water also ensures that evaporation occurs more slowly, allowing for the consistent, controlled, and stable formation of well-structured microwells, resulting in higher formation yields.
Additionally, larger droplets generated microwells with more uniform geometries, owing to the increased volume of EG remaining after water evaporation. This excess EG maintains the structural integrity of the microwell and prevents its collapse during the curing process. By contrast, smaller droplets may not contain sufficient EG to maintain the microwell structure, resulting in lower yields and less consistent geometries.
Although larger droplet volumes (e.g., 2.0 µL) continue to increase the overall microwell size, the formation yield plateaus at higher droplet volumes because the impact of volume on the microwell creation process is limited. Upon reaching a critical droplet size, further increases in volume primarily affected the dimensions of the microwell (diameter and depth) rather than its formation efficiency, suggesting that microwell formation yield can be effectively optimized by adjusting the droplet volume. An appropriate droplet size could maximize the formation efficiency and tailor the dimensions of the microwells for specific applications. We found that a droplet volume of 2 μL provided a good balance between formation yield and microwell uniformity and is therefore an ideal choice for applications requiring larger or deeper microwells.
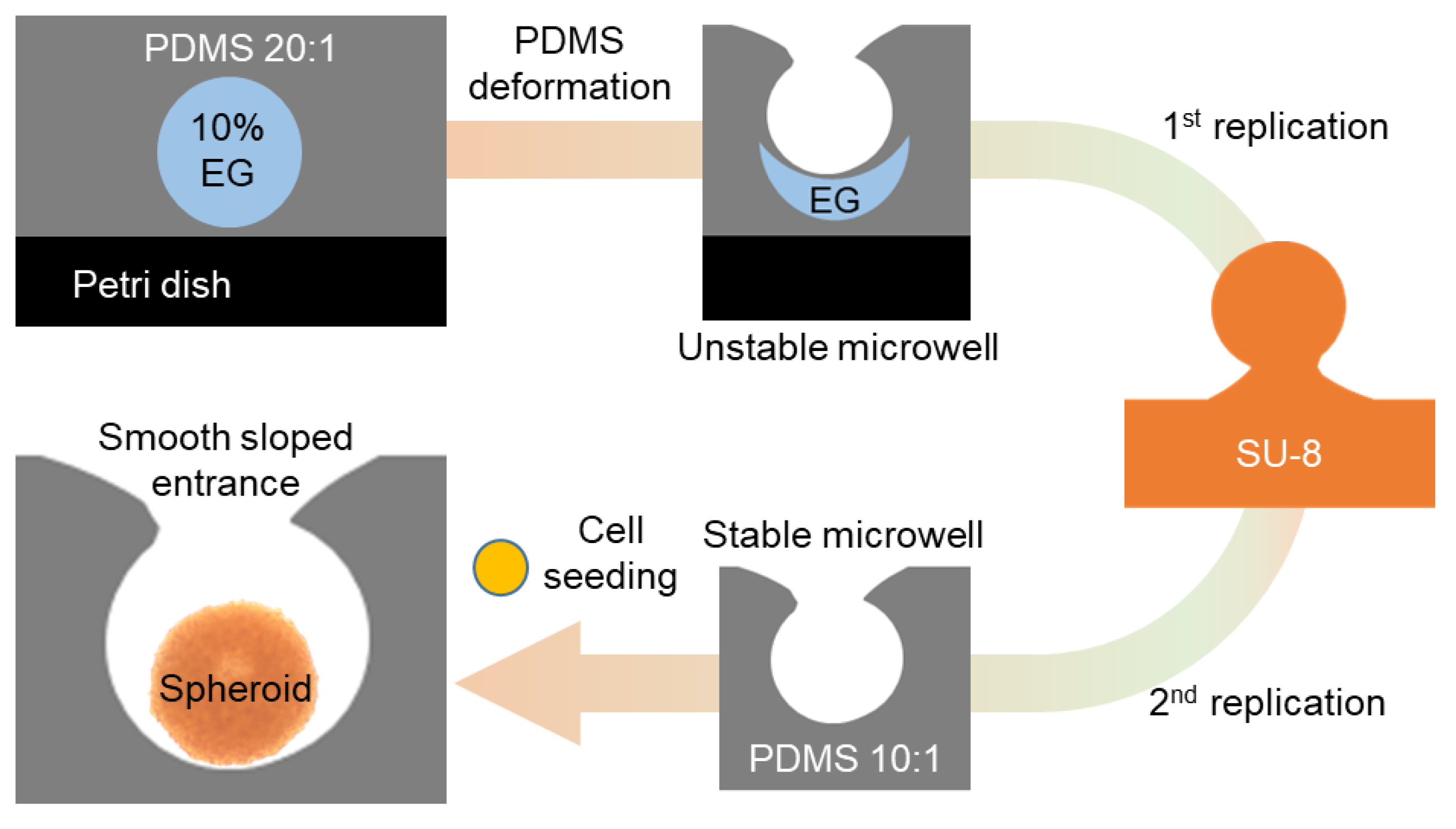
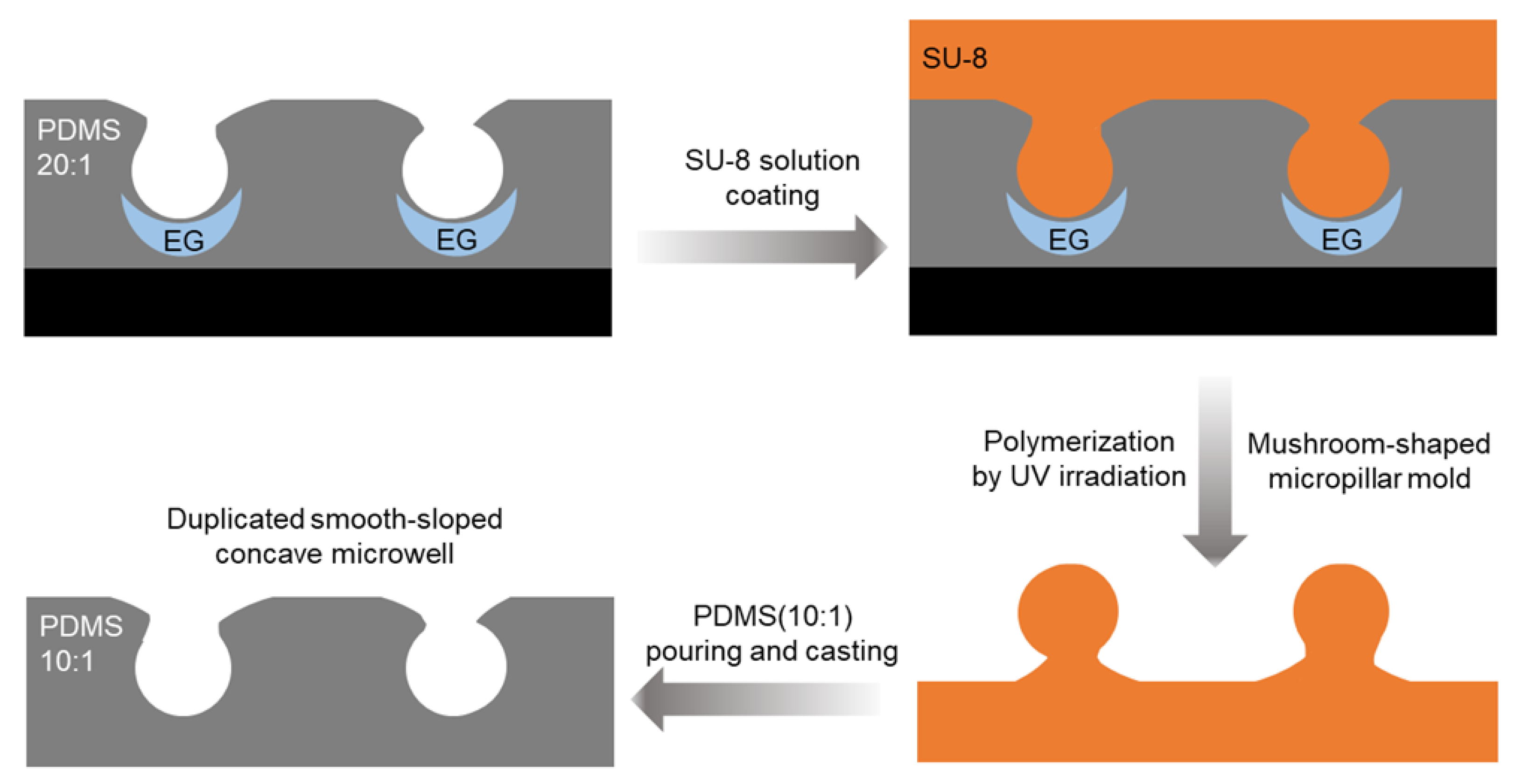
3.3. Microwell Replication Using SU-8 Mold and Dimensional Characterization
The smooth-sloped concave microwells were replicated using an SU-8 mold, and the dimensional characteristics of the microwells replicates was evaluated. An SU-8 photoresist was selected as the molding material because of its high resolution and stability during microfabrication. The SU-8 molds enabled the accurate replication of microwell geometries, preserving the concave structure with high fidelity. The initial step in the replication process is the formation of a mushroom-shaped micropillar mold using SU-8 photoresist. The mold was polymerized over the original microwells created on a 20:1 PDMS substrate using UV irradiation. The low structural stability of the microwells formed directly on the 20:1 PDMS substrate necessitated the replication of the microwells using SU-8 models. Although the PDMS microwells exhibited smooth-sloped concave structures (
Figure 1C), significant deformation occurred during mechanical manipulation, such as peeling or handling. The dotted yellow line in the optical image shows the interface where the PDMS base adhered to the surface of the petri dish, illustrating the structural deformation that occurs during mechanical manipulation. This instability was a consequence of the high elasticity of the 20:1 PDMS substrate, which lacked the mechanical rigidity required to maintain the integrity of the concave microwell structures under stress. To overcome this limitation, robust and dimensionally stable templates consisting of SU-8 molds were fabricated to ensure accurate replication of microwell geometries. The SU-8 mold also ensures high fidelity in preserving the smooth concave morphology and facilitates applications that require high mechanical stability.
The polymerization process ensured that the SU-8 mold precisely retained the structural features of the microwells, including a smooth-sloped entrance and a concave profile. The resulting mushroom-shaped micropillars were then used as negative molds to replicate the microwells during the subsequent PDMS casting. Thus, the 10:1 PDMS mixture, which provides a balance between mechanical strength and elasticity which ensures accurate replication and facilitates extraction from the mold, was poured onto the SU-8 mold and cured. After curing, the replicated microwells were removed from the SU-8 mold and the dimensions of the replicated structures were compared with those of the original microwells (
Figure 4). The replicated PDMS (10:1) microwells retained the smooth-sloped concave structure of the original PDMS (20:1) microwells. The diameter and depth of the microwells, measured using SEM, were consistent with those of the original mold, demonstrating that the SU-8-based replication method produced high-fidelity replicas of the initial microwells (
Figure 5B,C).
The use of the SU-8 mold enabled the replication of concave microwells with a high degree of structural fidelity and preserved the smooth-sloped profiles of the microwells, as confirmed by SEM (
Figure 5C), which is critical for applications that require uniform cell growth or deposition of materials in a controlled environment, such as cell culture and drug testing platforms [
11]. The replication process was also repeatable, allowing the production of multiple PDMS microwell arrays from the same SU-8 mold. This scalability enables the mass production of microwells without the need to repeatedly fabricate new molds and is one of the key advantages of the SU-8 mold. The SU-8 mold is also sufficiently robust to withstand multiple casting cycles without degradation or loss of dimensional accuracy [
18,
19].
Figure 3B shows the relationship between the initial droplet volume of EG and the dimensions (diameter and depth) of the microwells fabricated on 10:1 PDMS after replication. Both the diameter and depth of the microwells increased from approximately 400 μm to 1000 μm as the droplet volume increased from 0.5 µL to 2.0 µL. This significant increase reflects the growth of the contact area between the droplet and the PDMS substrate as the droplet volume increases. Similarly, the depth of the microwells increased from approximately 800 µm to 1500 µm with the same increase in droplet size. The increase in depth is correlated with the increased evaporation-driven deformation caused by the higher volume of the liquid and prolonged interaction with the substrate. These results underscore the tunability of the microwell dimensions by precisely controlling the droplet volume. The ability to modulate both the diameter and depth offers significant flexibility in fabricating microwells tailored specific applications such as cell culture and tissue engineering, in which the geometry of the microwell can directly impact biological outcomes. In addition, the use of SU-8 molds to replicate smooth-sloped concave microwells provides a reliable and scalable method for producing high-fidelity PDMS microwell arrays. Dimensional characterization confirmed that the replication process preserved the structures of the original microwells. Thus, this technique is suitable for applications requiring precise and consistent microscale features.
3.4. Cell Spheroid Formation on the Microwell Platform
The fabricated smooth-sloped concave microwells serve as an innovative platform for the formation of cell spheroids, which are critical components in 3D cell culture systems. Spheroids are clusters of cells grown in a 3D environment that more closely resembles the physiological conditions of tissues than conventional 2D monolayer cultures. The concave geometry of the microwells facilitates the formation of stable spheroids by promoting natural cell aggregation and enhancing cell–cell interactions.
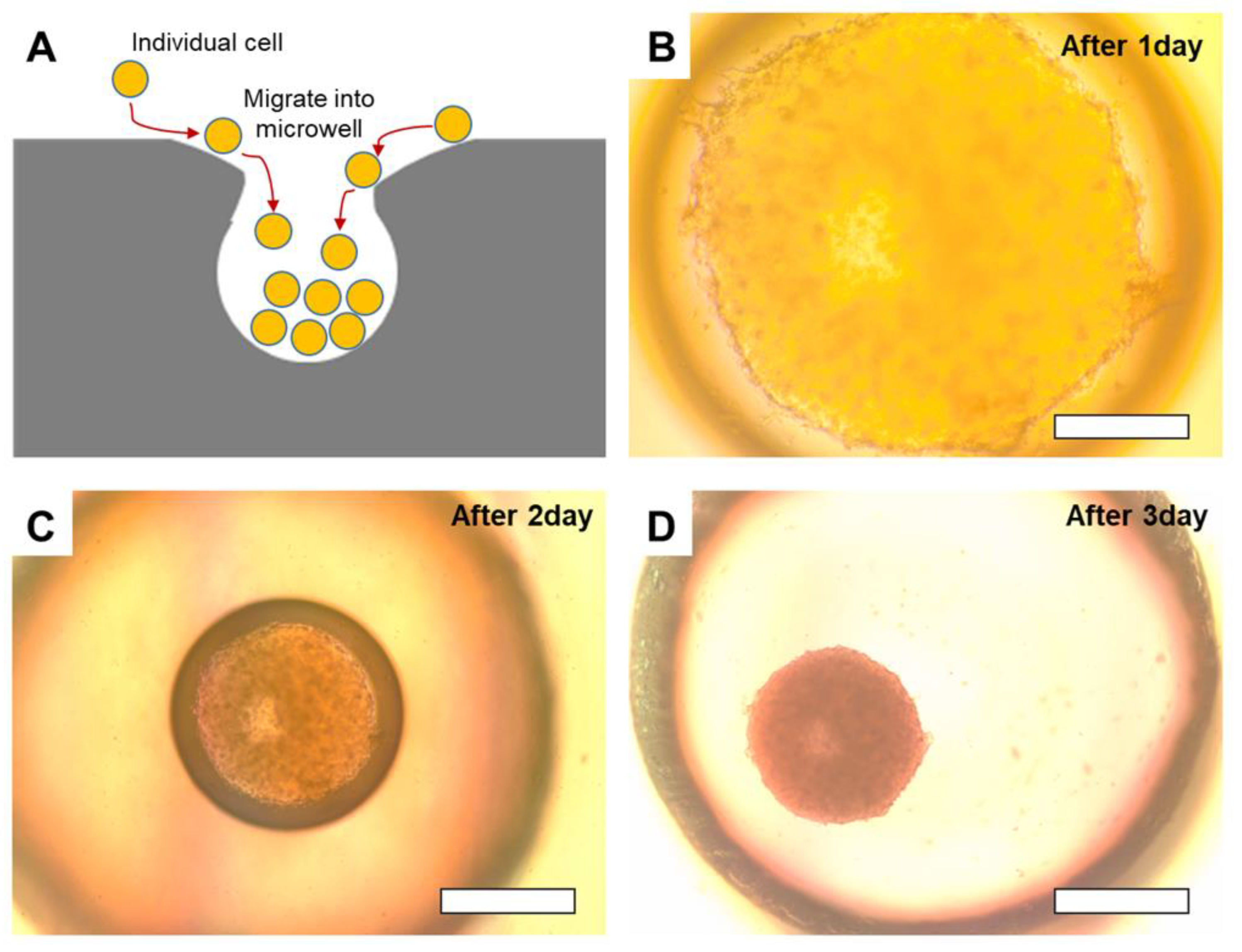
To evaluate the efficacy of the microwell platform, microwells created using water vapor permeation were seeded with SW1222 cells. The cells migrated into the concave microwells over several days, gradually forming aggregates at the bottom of the microwell owing to the smooth-sloped entrance, which facilitated efficient cell migration. Optical microscopy observations at various times revealed the progression of spheroid formation: on day 1, the cells began clustering at the base of the microwell; by day 2, compact aggregates were formed; and by day 3, fully formed, well-defined spheroids were observed (
Figure 6). This gradual formation process demonstrates the effectiveness of microwell geometry in supporting natural cell aggregation without external chemical or mechanical stimulation. The concave structure of the microwells facilitated cell entry and aggregation while maintaining spheroid integrity by preventing the premature dissociation of cells. This unique design mimics the physical constraints of in vivo conditions and provides a physiologically relevant 3D cell culture environment.
The ability of the microwell platform to control spheroid size by varying the dimensions of the microwells offers a significant advantage over existing methods. The diameter and depth of the microwells, which are influenced by the droplet volume during fabrication, directly affect the size of the spheroids. Larger microwells, produced using larger droplets (e.g., 2.0 µL), will produce larger spheroids, while smaller microwells (e.g., 0.5 µL droplets) will generate smaller spheroids. This tunability affords precise control over the dimensions of the spheroids, which is crucial for applications in which spheroid size influences biological outcomes. For example, limited oxygen and nutrient diffusion in the core of larger spheroids may create a hypoxic environment that mimics those of solid tumors, making larger spheroids suitable for tumor model studies [
20,
21]. Conversely, high-throughput drug screening and testing applications where uniformity and rapid growth are essential are better served by smaller spheroids [
22,
23].
The ability to reproducibly form cell spheroids in microwells developed in this study has significant implications for a range of biomedical and biotechnological applications. The spheroids generated in these microwells can serve as building blocks for larger tissue constructs, enabling the precise engineering of tissues with desired properties and thus providing a promising tool for regenerative medicine [
4,
24]. The platform is also highly suited to the creation of tumor spheroids that replicate the structure and microenvironment of solid tumors, facilitating the evaluation of anticancer drug penetration and efficacy under physiologically relevant conditions [
2]. In addition, the microwell platform provides a scalable and efficient method for producing uniform muscle cell spheroids that can differentiate into muscle fibers with a consistent structure and quality, supporting the mass production of cultured meat [
8,
25]. Furthermore, microwells provide a controlled environment ideal for studying stem cell behavior and differentiation, enabling the growth of stem cell spheroids that offer insights into tissue regeneration and developmental biology [
26].
The scalability and reproducibility of the microwell platform further enhance its utility. Multiple arrays of microwells with consistent dimensions were produced using the same SU-8 mold to ensure uniform spheroid formation. This consistency reduces the variability in experimental results and supports high-throughput applications such as drug screening or cultured meat production. The platform offers a versatile, scalable, and reproducible system for the formation of cell spheroids and provides new opportunities for biomedical and biotechnological innovation.