1. Introduction
DNA chemical reaction networks can perform elaborate information processing by appropriately designing the kinetics of the reaction system. The kinetics of the reaction system is defined by the reaction network structure, the reaction rate constants, and the initial concentrations, which are required to design the base sequences of DNA strands involved in the reaction system, based on DNA complementarity [
1]. Toehold-mediated strand displacement reactions (TMSDRs) are widely recognized as versatile reaction mechanisms for fabricating DNA circuits [
2]. They offer an entire practical framework of the “rational design”, where the entire system is constructed by incorporating individually pre-designed TMSDRs.
Currently, various computer-aided design tools for DNA reaction systems such as VisualDSD [
3], NUPACK [
4], etc., are available; hence, we can efficiently design the information processing mechanism rationally by assembling the reaction network structure, tuning the reaction rate constants, and determining initial concentrations. Notably, due to the availability of reliable estimates for TMSDR rate constants [
5], the predicted reaction dynamics in silico under “ideal” experimental conditions closely align with experimental observations. Here, “dynamics” refers to two aspects: transient characteristics, which describe the time evolution profile of concentration, and steady-state characteristics, which represent the concentration balance after the reaction. Meanwhile, under some circumstances, simulation results fail to predict the experimental results qualitatively or quantitatively, even if a DNA reaction system in which base sequences of all DNA strands are designed perfectly is carefully experimented with under target temperature and appropriate buffer conditions. Among various factors possibly behind this mismatch issue, the most common factor encountered is the change in reaction dynamics due to modification of reporter molecules [
6]. Recently, Li et al. evaluated the effect of fluorophore dyes and quenchers such as FAM, Cy5, and BHQ1 on the reaction dynamics of irreversible TMSDRs, indicating that these modifications significantly modulate the dynamics by almost two orders of magnitude [
7]. Furthermore, the effect on dynamics was complex wherein the transient characteristics changed depending on the modification location of the fluorophore dyes and quenchers. Importantly, measuring the dynamics of reaction systems using these reporter molecules (or probes) is a common method widely utilized in DNA reaction system design. Even if a DNA reaction system is designed to possess the desired dynamics, verifying the validity of the design becomes challenging when the modification of reporter molecules alters the reaction rate constant during the verification experiment.
In this study, we investigated the effect of modification of the reporter molecules on the reaction dynamics for mainly reversible TMSDRs (or toehold-mediated strand exchange reactions), which were not addressed in the previous study. For the irreversible TMSDRs in the previous study [
7], the effect of the reporter modification was only on transient characteristics. In contrast, for the reversible version, it appeared in both transient and steady-state characteristics. In the design of DNA circuits that perform complex information processing in the field of DNA computing, identifying ahead how the reporter modifications for measurements affect the reaction dynamics is crucial because both reversible and irreversible TMSDRs are integrated in the design of reaction systems [
8,
9,
10,
11,
12]. Here, we demonstrate that a reporter modification in which the fluorophore dye and quencher are modified an appropriate distance apart is a suitable method that can reduce the detrimental effect on the dynamics of the DNA reaction system while simultaneously expecting the effectiveness of FRET (fluorescence resonance energy transfer).
4. Discussion
Reporter molecules modified at toehold domains of TMSDRs have complexly acted on the reaction systems [
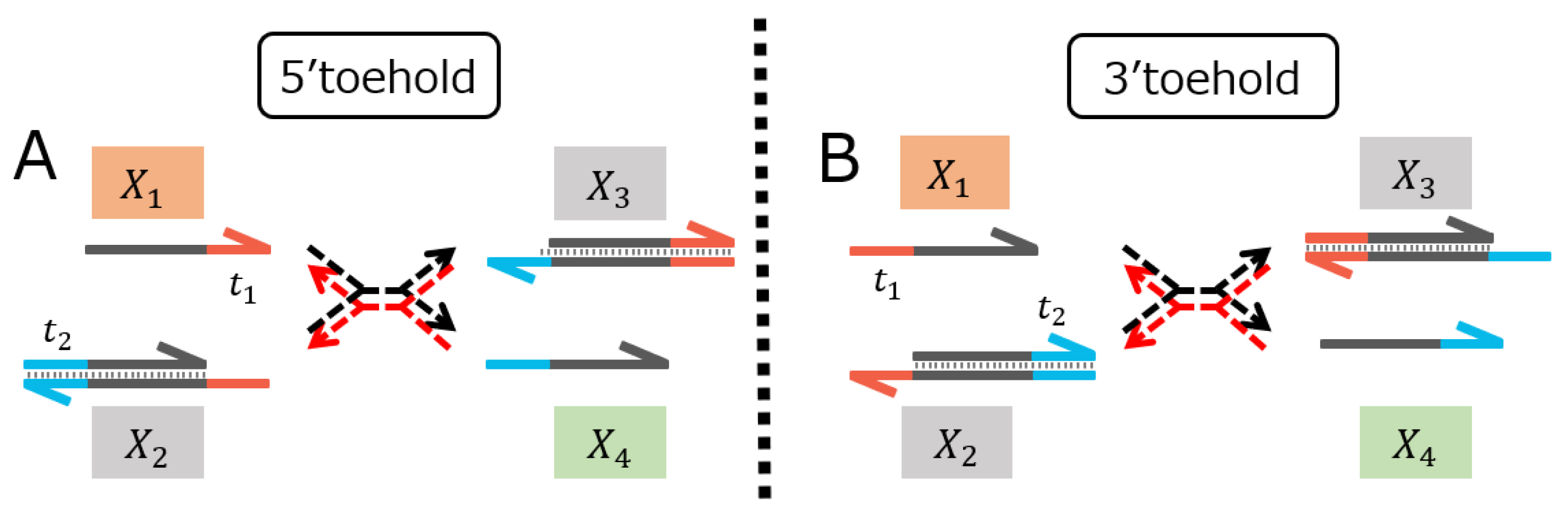
7]. In our study, the four variations of reversible TMSDRs were investigated, where the symmetric and asymmetric modifications of the reporter molecules at 5′ and 3′ toehold were designed.
Regarding the reversible TMSDR in the 5′ toehold case along with the symmetric modification (
Figure 2A), the forward reaction
was hardly affected by the reporter molecules because no modifications were observed at approximately the 5′ toehold as a binding site. According to [
7], the BHQ1-modified lower strand of the gate
, which includes the binding site for the backward reaction, can accelerate the displacement reaction, therefore possibly affecting the increase of the reaction rate constant
. This speculation considerably agreed with our experimental results (first row in
Table 1).
Regarding the reversible TMSDR in the 5′ toehold case along with the asymmetric modification (
Figure 2B), the backward reaction
is hardly affected by the reporter molecules because no modifications were observed at approximately the 3′ toehold as a binding site. Meanwhile, the BHQ1-modified upper and the FAM-modified lower strands of the gate
, which includes the binding site for the forward reaction, can decelerate and accelerate the displacement reaction, respectively, according to [
7]. Although these conflicting effects of the modification of reporter molecules were complex to predict, the estimated rate constants implied an effect to slightly decrease the reaction rate constant
while
was reasonably close to the theoretical value (second row in
Table 1). Collectively, the asymmetric modification of the reporter molecules might be preferable to the symmetric modification.
Regarding the reversible TMSDR in the 3′ toehold case along with the asymmetric modification (
Figure 3A), the FAM-modified upper and the BHQ1-modified lower strands of the gate
, which includes the binding site for the forward reaction, can accelerate the displacement reaction according to [
7]. In contrast, the backward reaction
was anticipated to be hardly affected by the reporter molecules because no modifications were observed at approximately the 5′ toehold as a binding site. However, the estimated
was unexpectedly shifted from the theoretical value. In contrast, the estimated rate constants implied an effect to slightly increase the reaction rate constant
as a result of conflicting effects of the modification of reporter molecules (third row in
Table 1).
Finally, regarding the reversible TMSDR in the 3′ toehold case along with the symmetric modification (
Figure 3B), the forward reaction
is hardly affected by the reporter molecules because of no modifications at approximately the 3′ toehold as a binding site. However, as in the asymmetric case above, the estimated
unexpectedly shifted from the theoretical values. The FAM-modified lower strand of the gate X3, which includes the binding site for the backward reaction, can accelerate the displacement reaction and, therefore, possibly affect the increase of the reaction rate constant
. This speculation considerably agreed with our experimental results (fourth row in
Table 1).
In conclusion, the reversible TMSDR in the 5′ toehold case, along with the asymmetric modification shown in
Figure 2B, might be preferred for the design of the DNA reaction system and its validation experiments in terms of less modification effect while guaranteeing the FRET efficiency. On the other hand, the mechanism by which the reaction rates
and
shift from the theoretical values is unknown and requires further study. In this study, we focused on a specific toehold length (6 mers) to determine if the position of fluorophore labels affects the TMSDR. By concentrating on a specific toehold length, we aimed to isolate and analyze the behaviors and interactions within the TMSDR mechanism without introducing the complexity of varying toehold lengths. This controlled approach allowed us to measure the influence of label positions precisely. Future research should focus on increasing the diversity of toehold lengths and different base compositions with the use of other dyes and quenchers, like Alexa and Cy5, to comprehensively understand their influence on reaction kinetics.
5. Conclusions
In this study, we investigated the effects of reporter molecule modifications on the reaction dynamics of reversible toehold-mediated strand displacement reactions, which are integral to DNA chemical reaction networks. While previous studies have demonstrated that such modifications influence the transient characteristics of irreversible TMSDRs, our results show that in reversible systems, the impact extends to both transient and steady-state characteristics. This finding is crucial for the rational design of DNA circuits, as these circuits often incorporate both reversible and irreversible reactions.
We demonstrated that the modification of fluorophore dye and quencher can significantly tune reaction dynamics. However, by modifying the fluorescent dye and quencher an appropriate distance apart, we can mitigate adverse effects on the system while still enabling effective fluorescence resonance energy transfer measurements. These insights are vital for the accurate design and verification of DNA-based circuits, specifically in scenarios where complex information processing is required.
In summary, our results highlight the importance of careful consideration of reporter molecule modifications during the design phase of DNA reaction systems. Future work should focus on further quantifying these effects across a broader range of reaction conditions and exploring potential optimization strategies for minimizing disruption to reaction kinetics.