CoWO4/Reduced Graphene Oxide Nanocomposite-Modified Screen-Printed Carbon Electrode for Enhanced Voltammetric Determination of 2,4-Dichlorophenol in Water Samples
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Instrumentation
2.2. Synthesis of CoWO4/rGO Nanocomposite
2.3. SPCE Modification Using CoWO4/rGO Nanocomposite
3. Results and Discussion
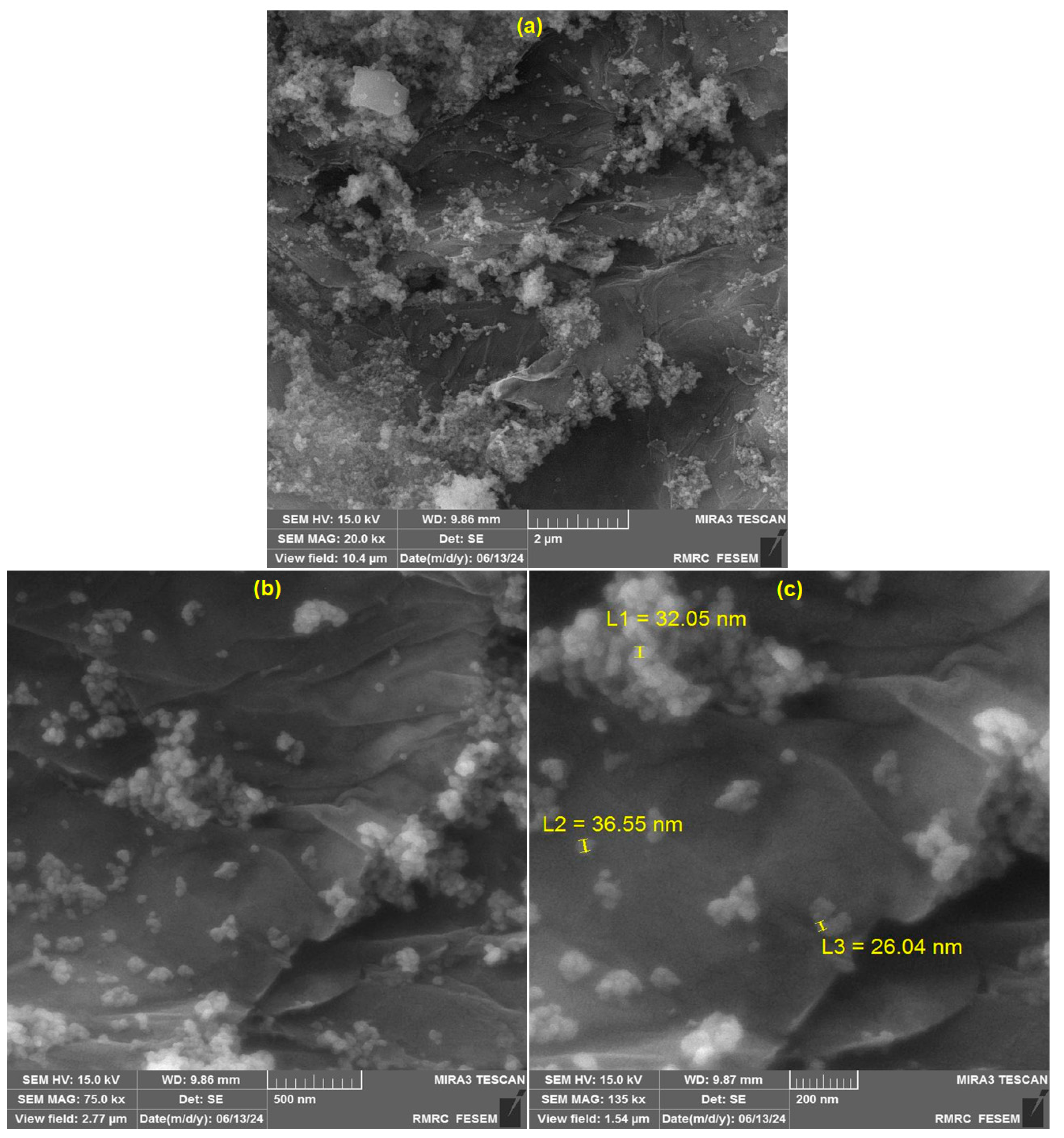
3.1. Characterization of CoWO4/rGO Nanocomposite
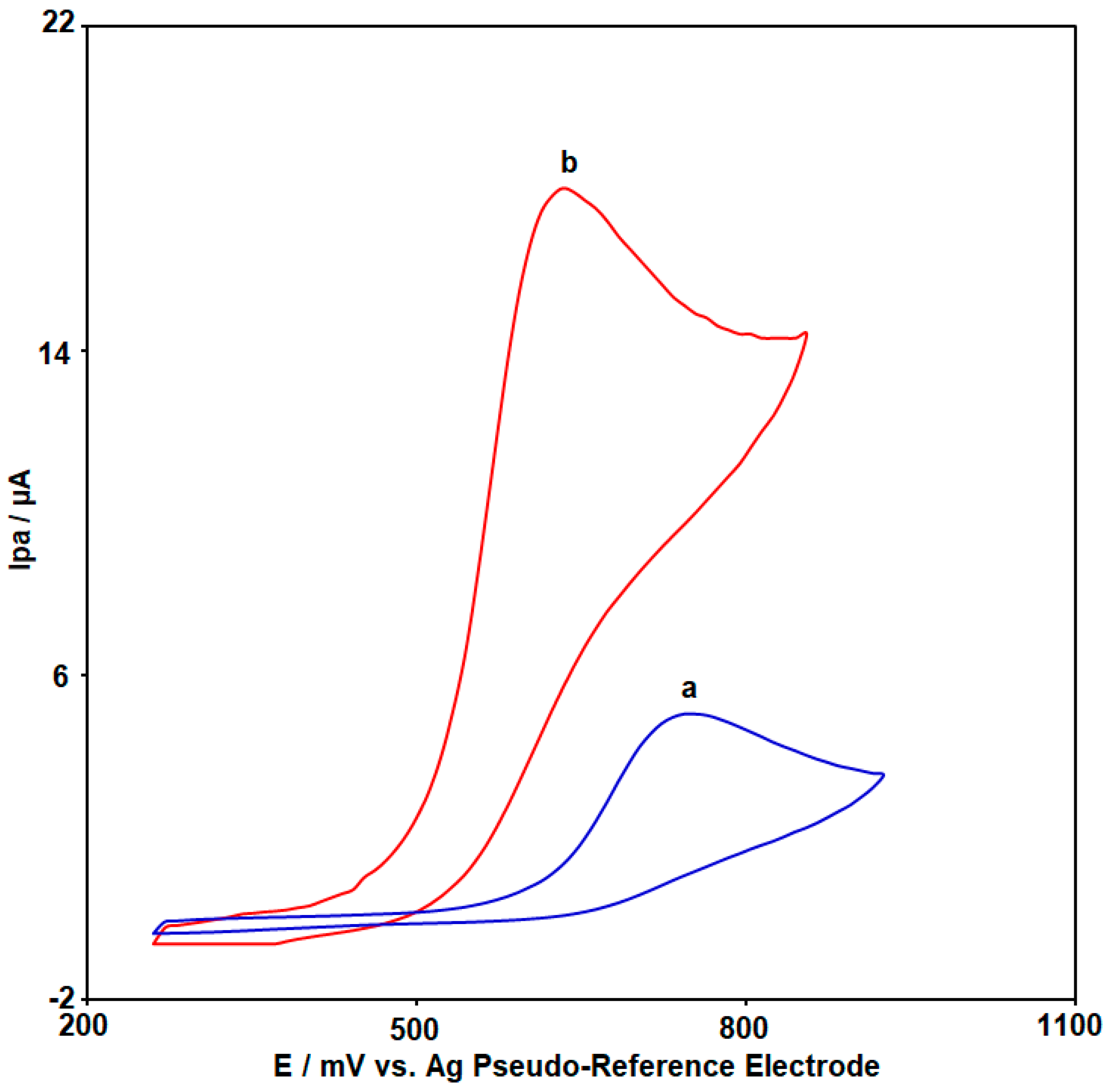
3.2. Electrochemical Response of CoWO4/rGO Modified SPCE Compared to Unmodified SPCE as Electrochemical Sensors for 2,4-DCP Determination
3.3. Effect of Scan Rate on the Electrochemical Response of CoWO4/rGO/SPCE Towards the Oxidation of 2,4-DCP
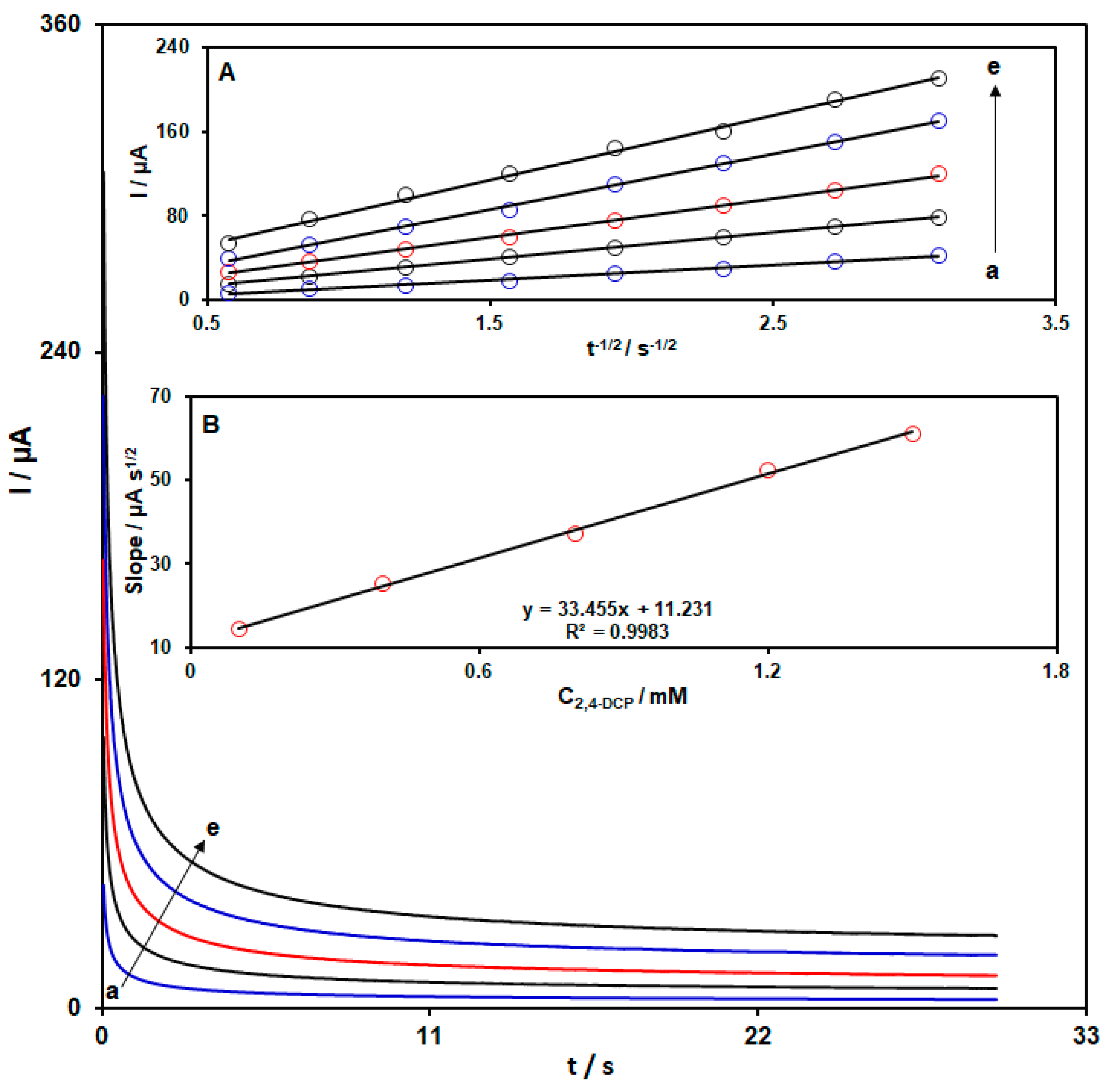
3.4. Chronoamperometric Investigations of 2,4-DCP at CoWO4/rGO/SPCE
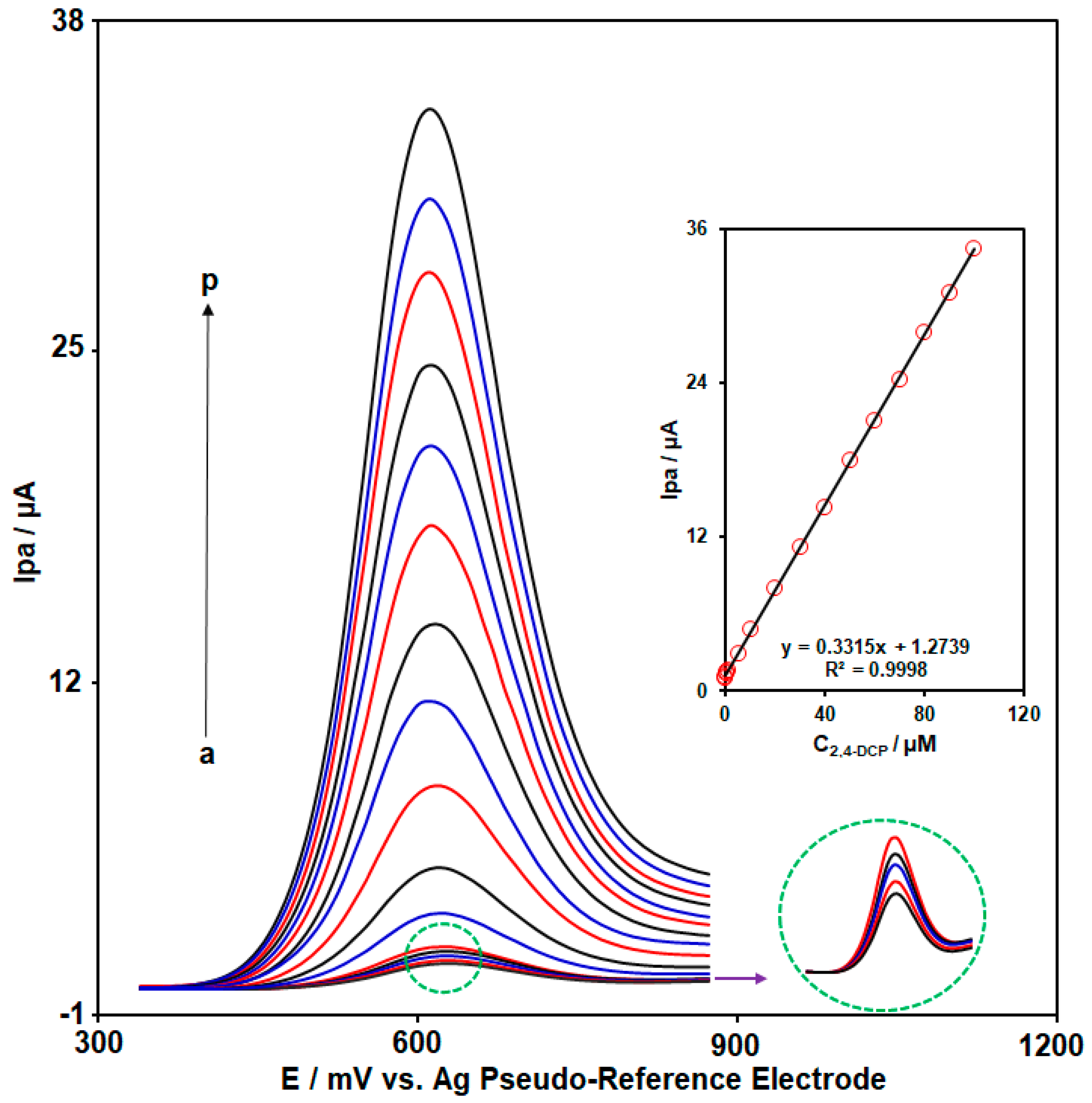
3.5. Electroanalysis Performance of CoWO4/rGO/SPCE for 2,4-DCP
3.6. Stability, Reproducibility, and Repeatability Studies of CoWO4/rGO Modified SPCE
3.7. Effects of Interfering Species
3.8. Real Sample Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galvani, A.P.; Bauch, C.T.; Anand, M.; Singer, B.H.; Levin, S.A. Human–environment interactions in population and ecosystem health. Proc. Natl. Acad. Sci. USA 2016, 113, 14502–14506. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Zhu, Y.; Zhang, Y.; Zhang, M.; Zhang, Y.; Qiao, L.; Wang, D. Recent advances in carbon nanomaterials-based electrochemical sensors for phenolic compounds detection. Microchem. J. 2021, 171, 106776. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Sun, C. Occurrence, ecological and human health risks, and seasonal variations of phenolic compounds in surface water and sediment of a potential polluted river basin in China. Int. J. Environ. Res. Public Health 2017, 14, 1140. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Yang, R.; Li, J.; Qu, L. A highly sensitive and selective electrochemical sensor based on polydopamine functionalized graphene and molecularly imprinted polymer for the 2,4-dichlorophenol recognition and detection. Talanta 2019, 195, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yue, X.; Yang, R.; Jing, S.; Qu, L. A sensitive and low toxicity electrochemical sensor for 2,4-dichlorophenol based on the nanocomposite of carbon dots, hexadecyltrimethyl ammonium bromide and chitosan. Sens. Actuators B Chem. 2016, 224, 241–247. [Google Scholar] [CrossRef]
- Feng, Q.; Li, H.; Zhang, Z.; Lin, J.M. Gold nanoparticles for enhanced chemiluminescence and determination of 2,4-dichlorophenol in environmental water samples. Analyst 2011, 136, 2156–2160. [Google Scholar] [CrossRef]
- Wang, B.; Liu, P.; Hu, Y.; Zhao, H.; Zheng, L.; Cao, Q. A Cu (II) MOF with laccase-like activity for colorimetric detection of 2,4-dichlorophenol and p-nitrophenol. Dalton Trans. 2023, 52, 2309–2316. [Google Scholar] [CrossRef]
- Li, Y.; Jiao, Y.; Guo, Y.; Yang, Y. Determination of bisphenol-A, 2,4-dichlorophenol, bisphenol-AF and tetrabromobisphenol-A in liquid foods and their packaging materials by vortex-assisted supramolecular solvent microextraction/high-performance liquid chromatography. Anal. Methods 2013, 5, 5037–5043. [Google Scholar] [CrossRef]
- Tsukagoshi, K.; Kameda, T.; Yamamoto, M.; Nakajima, R. Separation and determination of phenolic compounds by capillary electrophoresis with chemiluminescence detection. J. Chromatogr. A 2002, 978, 213–220. [Google Scholar] [CrossRef]
- Pokryshkin, S.A.; Kosyakov, D.S.; Kozhevnikov, A.Y.; Lakhmanov, D.E.; Ul’yanovskii, N.V. Highly sensitive determination of chlorophenols in sea water by gas chromatography−tandem mass spectrometry. J. Anal. Chem. 2018, 73, 991–998. [Google Scholar] [CrossRef]
- Nguyen, M.B.; Nhung, V.T.H.; Thu, V.T.; Nga, D.T.N.; Truong, T.N.P.; Giang, H.T.; Ha, V.T.T. An electrochemical sensor based on copper-based metal–organic framework-reduced graphene oxide composites for determination of 2,4-dichlorophenol in water. RSC Adv. 2020, 10, 42212–42220. [Google Scholar] [CrossRef] [PubMed]
- Ha, V.T.T.; Nguyen, M.B.; Tam, T.N.; Thu, V.T.; Yen, P.T.H.; Phong, P.H.; Hai, T.Q. Highly sensitive electrochemical sensor based on zirconium oxide-decorated gold nanoflakes nanocomposite 2,4-dichlorophenol detection. J. Appl. Electrochem. 2022, 52, 607–616. [Google Scholar] [CrossRef]
- Akond, U.S.; Mahanta, A.; Jasimuddin, S. CuO nanoleaf and β-cyclodextrin functionalized reduced graphene oxide: A highly selective and sensitive electrochemical sensor for the simultaneous detection of 2-chlorophenol and 2,4-dichlorophenol. Anal. Methods 2023, 15, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Suo, G.; Li, N.; Chen, Z.; Peng, L.; Fu, Y.; Huang, T. A simple strategy to fabricate high sensitive 2,4-dichlorophenol electrochemical sensor based on metal organic framework Cu3(BTC)2. Sens. Actuators B Chem. 2016, 222, 972–979. [Google Scholar] [CrossRef]
- Vealan, S.M.; Chinnathambi, S. Simultaneous determination of environmental endocrine disruptors bisphenol A and 4-nitrophenol bleached from food-contacting materials using the spin-ladder compound La2Cu2O5 modified glassy carbon electrode. Chemosphere 2024, 353, 141559. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Mohammadi, S.Z.; Azimzadeh, M.; Zhang, K.; Van Le, Q.; Shokouhimehr, M. Recent developments in electrochemical sensors for detecting hydrazine with different modified electrodes. RSC Adv. 2020, 10, 30481–30498. [Google Scholar] [CrossRef]
- Rasen, N.D.; Gomaa, E.A.; Salem, S.E.; Abd El-Hady, M.N.; El-Defrawy, A.M. Voltammetric Analysis of Lead Nitrate with Various Ligands in Aqueous Solutions at 302.15 K. Chem. Methodol. 2023, 7, 761–775. [Google Scholar]
- Pushpanjali, P.A.; Manjunatha, J.G.; Amrutha, B.M.; Hareesha, N. Development of carbon nanotube-based polymer-modified electrochemical sensor for the voltammetric study of Curcumin. Mater. Res. Innov. 2021, 25, 412–420. [Google Scholar] [CrossRef]
- Mahmoudi Moghaddam, H.; Beitollahi, H.; Tajik, S.; Jahani, S.; Khabazzadeh, H.; Alizadeh, R. Voltammetric determination of droxidopa in the presence of carbidopa using a nanostructured base electrochemical sensor. Russ. J. Electrochem. 2017, 53, 452–460. [Google Scholar] [CrossRef]
- Al-Azzawi, M.A.; Saleh, W.R. Fabrication of environmental monitoring amperometric biosensor based on alkaloids compound derived from catharanthus roseus extract nanoparticles for detection of cadmium pollution of water. Chem. Methodol. 2023, 7, 358–371. [Google Scholar]
- Hareesha, N.; Manjunatha, J.G.; Alothman, Z.A.; Sillanpää, M. Simple and affordable graphene nano-platelets and carbon nanocomposite surface decorated with cetrimonium bromide as a highly responsive electrochemical sensor for rutin detection. J. Electroanal. Chem. 2022, 917, 116388. [Google Scholar] [CrossRef]
- Tajik, S.; Lohrasbi-Nejad, A.; Mohammadzadeh Jahani, P.; Askari, M.B.; Salarizadeh, P.; Beitollahi, H. Co-detection of carmoisine and tartrazine by carbon paste electrode modified with ionic liquid and MoO3/WO3 nanocomposite. J. Food Meas. Charact. 2022, 16, 722–730. [Google Scholar] [CrossRef]
- Manjunatha, J.G. A promising enhanced polymer modified voltammetric sensor for the quantification of catechol and phloroglucinol. Anal. Bioanal. Electrochem. 2020, 12, 893–903. [Google Scholar]
- Roshanfekr, H. A simple specific dopamine aptasensor based on partially reduced graphene oxide–AuNPs composite. Prog. Chem. Biochem. Res. 2023, 6, 61–70. [Google Scholar]
- Manjunatha, J.G. Highly sensitive polymer based sensor for determination of the drug mitoxantrone. J. Surf. Sci. Technol. 2018, 34, 74–80. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Aflatoonian, M.R.; Makarem, A. Glutathione detection at carbon paste electrode modified with ethyl 2-(4-ferrocenyl-[1,2,3] triazol-1-yl) acetate, ZnFe2O4 nano-particles and ionic liquid. J. Electrochem. Sci. Eng. 2022, 12, 209–217. [Google Scholar] [CrossRef]
- Soltani Nejad, H.; Garkani Nejad, F.; Beitollahi, H. Development of a highly sensitive voltammetric sensor for the detection of folic acid by using MoS2 and ionic liquid-modified carbon paste electrode. ADMET DMPK 2023, 11, 361–371. [Google Scholar] [CrossRef]
- Rajab, N.; Ibrahim, H.; Hassan, R.Y.; Youssef, A.F. Selective determination of nitrite in water and food samples using zirconium oxide (ZrO2)@MWCNTs modified screen printed electrode. RSC Adv. 2023, 13, 21259–21270. [Google Scholar] [CrossRef]
- Saisree, S.; Shamili, C.; Sandhya, K.Y.; Peethambharan, S.K.; Chandran, A. Nanomolar level electrochemical detection of glycine on a miniaturized modified screen-printed carbon-based electrode: A comparison of performance with glassy carbon electrode system. J. Mater. Chem. B 2024, 12, 7557–7563. [Google Scholar]
- Ferreira, J.H.; Medeiros, A.M.A.; Peres, R.M.; Canevari, T.C. Ultra-sensitive determination of fenitrothion pesticide in orange juice by gold-printed electrode modified with AgNP/carbon Dot/MWCNT nanoarchitecture employing electrochemical impedance spectroscopy. Food Anal. Methods 2024, 17, 825–833. [Google Scholar] [CrossRef]
- Charithra, M.M.; Manjunatha, J.G. Electrochemical sensing of adrenaline using surface modified carbon nanotube paste electrode. Mater. Chem. Phys. 2021, 262, 124293. [Google Scholar] [CrossRef]
- Patil, N.A.; Udgire, S.; Shinde, D.R.; Patil, P.D. Green synthesis of gold nanoparticles using extract of Vitis vinifera, Buchananialanzan, Juglandaceae, phoenix dactylifera plants, and evaluation of antimicrobial activity. Chem. Methodol. 2023, 7, 15–27. [Google Scholar]
- Sikkander, A.M.; Bassyouni, F.; Yasmeen, K.; Mishra, S.; Lakshmi, V. Synthesis of zinc oxide and lead nitrate nanoparticles and their applications: Comparative studies of bacterial and fungal (E. coli, A. Niger). J. Appl. Organomet. Chem. 2023, 3, 255–267. [Google Scholar]
- Pang, X.; An, B.; Zheng, S.; Wang, B. Synergistic enhancement of Li-S battery low-temperature cycling performance by nano-sized uniformly compounded FeCoNi and MnO nanoparticles. Chem. Eng. J. 2023, 458, 141445. [Google Scholar] [CrossRef]
- Al Taee, M.B.; Al Shabander, B.M. Study the effect of ZnO concentrations on the photocatalytic ctivity of TiO2/cement nanocomposites. Chem. Methodol. 2022, 6, 831–841. [Google Scholar]
- Manjunatha Charithra, M.; Manjunatha, J.G. Electrochemical sensing of paracetamol using electropolymerised and sodium lauryl sulfate modified carbon nanotube paste electrode. ChemistrySelect 2020, 5, 9323–9329. [Google Scholar] [CrossRef]
- Emmanuel Ibukun, A.; Ariffuddin Bin Abd Hamid, M.; Husaini Mohamed, A.; Nadhirah Mohamad Zain, N.; Kamaruddin, M.A.; Hong Loh, S.; Kamaruzaman, S.; Yahaya, N. A review on the toxicity and properties of organochlorine pesticides, and their adsorption/removal studies from aqueous media using graphene-based sorbents. J. Chem. Rev. 2024, 6, 237–303. [Google Scholar]
- Ibrar, S.; Ali, N.Z.; Ojegu, E.O.; Odia, O.B.; Ikhioya, L.; Ahmad, I. Assessing high-performance energy storage of the synthesized ZIF-8 and ZIF-67. J. Appl. Organomet. Chem. 2023, 3, 294–307. [Google Scholar]
- Fajri Alif, M.; Zainul, R.; Mulyani, A.; Syakirah, S.; Zikri, A.; Iqbal, A.; Abdullah, M.; Adekanmi Adeyi, A. Comprehensive review of functionalized multi-walled carbon nanotubes: Emerging trends and applications in simultaneous detection of uric acid, dopamine and ascorbic acid. Adv. J. Chem. A 2024, 7, 319–337. [Google Scholar]
- Raril, C.; Manjunatha, J.G.; Ravishankar, D.K.; Fattepur, S.; Siddaraju, G.; Nanjundaswamy, L. Validated electrochemical method for simultaneous resolution of tyrosine, uric acid, and ascorbic acid at polymer modified nano-composite paste electrode. Surf. Eng. Appl. Electrochem. 2020, 56, 415–426. [Google Scholar] [CrossRef]
- Beitollahi, H.; Shahsavari, M.; Sheikhshoaie, I.; Tajik, S.; Mohammadzadeh Jahani, P.; Mohammadi, S.Z.; Afshar, A.A. Amplified electrochemical sensor employing screen-printed electrode modified with Ni-ZIF-67 nanocomposite for high sensitive analysis of Sudan I in present bisphenol A. Food Chem. Toxicol. 2022, 161, 112824. [Google Scholar] [CrossRef] [PubMed]
- Sharifi Pour, E.; Ebrahimi, M.; Beitollai, H. Electrochemical sensing of theophylline using modified glassy carbon electrode. Chem. Methodol. 2022, 7, 560–568. [Google Scholar]
- Li, Y.; Zhang, L.; Wu, M.; Ma, G.; Motlak, M.; Mahdi, A. Novel electrochemical strategy for determination of anticancer drug flutamide based on MXene/MOF composite. Inorg. Chem. Commun. 2023, 155, 111061. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Garkani Nejad, F.; Beitollahi, H.; Jahani, S.; Rezapour, M.; Larijani, B. Highly sensitive voltammetric sensor for determination of ascorbic acid using graphite screen printed electrode modified with ZnO/Al2O3 nanocomposite. Int. J. Electrochem. Sci. 2017, 12, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Beitollahi, H.; Tajik, S.; Dourandish, Z.; Garkani Nejad, F. Simple preparation and characterization of hierarchical flower-like NiCo2O4 nanoplates: Applications for sunset yellow electrochemical analysis. Biosensors 2022, 12, 912. [Google Scholar] [CrossRef]
- Nandagopal, T.; Balaji, G.; Vadivel, S. Tuning the morphology and size of NiMoO4 nanoparticles anchored on reduced graphene oxide (rGO) nanosheets: The optimized hybrid electrodes for high energy density asymmetric supercapacitors. J. Electroanal. Chem. 2023, 928, 116944. [Google Scholar] [CrossRef]
- Bai, L.W.; Shi, Y.F.; Zhang, X.; Liu, X.B.; Wu, F.; Liu, C.; Lu, W.B. A two-dimensional NiMoO4 nanowire electrode for the sensitive determination of hydroquinone in four types of actual water samples. J. Anal. Test. 2022, 6, 382–392. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Vilian, A.E.; Ghoreishian, S.M.; Umapathi, R.; Hwang, S.K.; Oh, C.W.; Han, Y.K. Hybridized 1D–2D MnMoO4–MXene nanocomposites as high-performing electrochemical sensing platform for the sensitive detection of dihydroxybenzene isomers in wastewater samples. J. Hazard. Mater. 2022, 421, 126775. [Google Scholar] [CrossRef]
- Ling, C.; Zhou, L.; Jia, H. First-principles study of crystalline CoWO4 as oxygen evolution reaction catalyst. RSC Adv. 2014, 4, 24692–24697. [Google Scholar] [CrossRef]
- Jia, H.; Stark, J.; Zhou, L.; Ling, C.; Sekito, T.; Markin, Z. Different catalytic behavior of amorphous and crystalline cobalt tungstate for electrochemical water oxidation. RSC Adv. 2012, 2, 10874–10881. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, J.; Yan, J.; Liu, Y.; Guo, M.; Zhang, Y.; Meng, C. La-doped NiWO4 coupled with reduced graphene oxide for effective electrochemical determination of diphenylamine. Dalton Trans. 2023, 52, 12808–12818. [Google Scholar] [CrossRef] [PubMed]
- Sriram, B.; Gouthaman, S.; Wang, S.F.; Hsu, Y.F. Cobalt molybdate hollow spheres decorated graphitic carbon nitride sheets for electrochemical sensing of dimetridazole. Food Chem. 2024, 430, 136853. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneswari, C.; Elangovan, A.; Sharmila, C.; Sudha, K.; Arivazhagan, G. Fabrication of cobalt tungstate/N-rGO nanocomposite: Application towards the detection of antibiotic drug-Furazolidone. Colloids Surf. A Physicochem. Eng. Asp. 2023, 656, 130299. [Google Scholar] [CrossRef]
- Kumar, R.; Bhuvana, T.; Sharma, A. Nickel tungstate–graphene nanocomposite for simultaneous electrochemical detection of heavy metal ions with application to complex aqueous media. RSC Adv. 2017, 7, 42146–42158. [Google Scholar] [CrossRef]
- Xu, X.; Shen, J.; Li, N.; Ye, M. Facile synthesis of reduced graphene oxide/CoWO4 nanocomposites with enhanced electrochemical performances for supercapacitors. Electrochim. Acta 2014, 150, 23–34. [Google Scholar] [CrossRef]
- Maurya, K.K.; Singh, K.; Malviya, M. Fabrication of dopamine sensor based on a carbon paste electrode modified with tetracyanoquinodimethane and cobalt tungstate nanoparticles. J. Mater. Sci. Mater. Electron. 2024, 35, 13. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, J.; Li, J.; Xiahou, M.; Sun, Z.; Cao, A.; Chen, Y. Delicate construction of Z-scheme heterojunction photocatalysts by ZnS quantum dots wrapped CoWO4 nanoparticles for highly efficient environmental remediation. ACS Appl. Nano Mater. 2024, 7, 20101–20113. [Google Scholar] [CrossRef]
- Ramanathan, S.; Thamilselvan, A.; Radhika, N.; Padmanabhan, D.; Durairaj, A.; Obadiah, A.; Vasanthkumar, S. Development of rutin-rGO/TiO2 nanocomposite for electrochemical detection and photocatalytic removal of 2,4-DCP. J. Iran. Chem. Soc. 2021, 18, 2457–2472. [Google Scholar] [CrossRef]
- Peleyeju, M.G.; Idris, A.O.; Umukoro, E.H.; Babalola, J.O.; Arotiba, O.A. Electrochemical detection of 2,4-dichlorophenol on a ternary composite electrode of diamond, graphene, and polyaniline. ChemElectroChem 2017, 4, 1074–1080. [Google Scholar] [CrossRef]
- Huang, H.; Wang, M.; Wang, Y.; Li, X.; Niu, Z.; Wang, X.; Song, J. Electrochemical determination of 2,4-dichlorophenol by using a glassy carbon electrode modified with molybdenum disulfide, ionic liquid and gold/silver nanorods. Microchim. Acta 2018, 185, 292. [Google Scholar] [CrossRef]
- Rasdi, F.L.M.; Mohamad, S.; Manan, N.S.A.; Nodeh, H.R. Electrochemical determination of 2,4-dichlorophenol at β-cyclodextrin functionalized ionic liquid modified chemical sensor: Voltammetric and amperometric studies. RSC Adv. 2016, 6, 100186–100194. [Google Scholar] [CrossRef]


| Modified Electrode | Detection Method | Linear Range | LOD | Reference |
|---|---|---|---|---|
| Carbon dots–hexadecyltrimethyl ammonium bromide–chitosan (CDs-CTAB-CS) modified glassy carbon electrode (GCE) | DPV | 0.04 µM to 8 µM | 0.01 µM | [5] |
| Cu-based metal–organic framework/electrochemically reduced graphene oxide composite (Cu-MOF/ErGO) modified GCE | DPV | 1.5 µM to 24 µM | 0.083 µM | [11] |
| Au nanoflakes/ZrO2 nanocomposite modified GCE | DPV | 1.5 µM to 24 µM | 0.053 µM | [12] |
| Rutin-rGO-TiO2 nanocomposite modified GCE | DPV | 5 µM to 150 µM | 0.02 µM | [58] |
| Diamond–graphene–polyaniline modified GCE | Square wave voltammetry (SWV) | 5 µM to 80 µM | 0.25 µM | [59] |
| Molybdenum disulfide-ionic liquid-Au-Ag nanorods (MoS2-IL-Au-Ag NRs) modified GCE | DPV | 0.01 µM to 50 µM | 2.6 nM | [60] |
| β-cyclodextrin functionalized IL modified carbon paste electrode (CPE) | Amperometry | 4 µM to 100 µM | 1.2 µM | [61] |
| CoWO4/rGO modified SPCE | DPV | 0.001 µM to 100.0 µM | 0.0007 µM | This work |
| Sample | Concentration of 2,4-DCP (µM) | Results | ||
|---|---|---|---|---|
| Added | Found | Recovery (%) | R.S.D. (%) | |
| Tap water | 0 | - | - | - |
| 5.0 | 4.9 | 98.0 | 3.4 | |
| 7.0 | 7.3 | 104.3 | 1.9 | |
| 9.0 | 9.1 | 101.1 | 2.7 | |
| 11.0 | 10.7 | 97.3 | 2.2 | |
| Well water | 0 | - | - | - |
| 5.5 | 5.6 | 101.8 | 2.5 | |
| 7.5 | 7.4 | 98.7 | 3.0 | |
| 9.5 | 9.8 | 103.2 | 2.3 | |
| 11.5 | 11.4 | 99.1 | 2.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tajik, S.; Beitollahi, H.; Garkani Nejad, F.; Zaimbashi, R. CoWO4/Reduced Graphene Oxide Nanocomposite-Modified Screen-Printed Carbon Electrode for Enhanced Voltammetric Determination of 2,4-Dichlorophenol in Water Samples. Micromachines 2024, 15, 1360. https://doi.org/10.3390/mi15111360
Tajik S, Beitollahi H, Garkani Nejad F, Zaimbashi R. CoWO4/Reduced Graphene Oxide Nanocomposite-Modified Screen-Printed Carbon Electrode for Enhanced Voltammetric Determination of 2,4-Dichlorophenol in Water Samples. Micromachines. 2024; 15(11):1360. https://doi.org/10.3390/mi15111360
Chicago/Turabian StyleTajik, Somayeh, Hadi Beitollahi, Fariba Garkani Nejad, and Reza Zaimbashi. 2024. "CoWO4/Reduced Graphene Oxide Nanocomposite-Modified Screen-Printed Carbon Electrode for Enhanced Voltammetric Determination of 2,4-Dichlorophenol in Water Samples" Micromachines 15, no. 11: 1360. https://doi.org/10.3390/mi15111360
APA StyleTajik, S., Beitollahi, H., Garkani Nejad, F., & Zaimbashi, R. (2024). CoWO4/Reduced Graphene Oxide Nanocomposite-Modified Screen-Printed Carbon Electrode for Enhanced Voltammetric Determination of 2,4-Dichlorophenol in Water Samples. Micromachines, 15(11), 1360. https://doi.org/10.3390/mi15111360








