Migration Study of Dielectrophoretically Manipulated Red Blood Cells in Tapered Aluminium Microelectrode Array: A Pilot Study
Abstract
1. Introduction
2. Theory
3. Materials and Methods
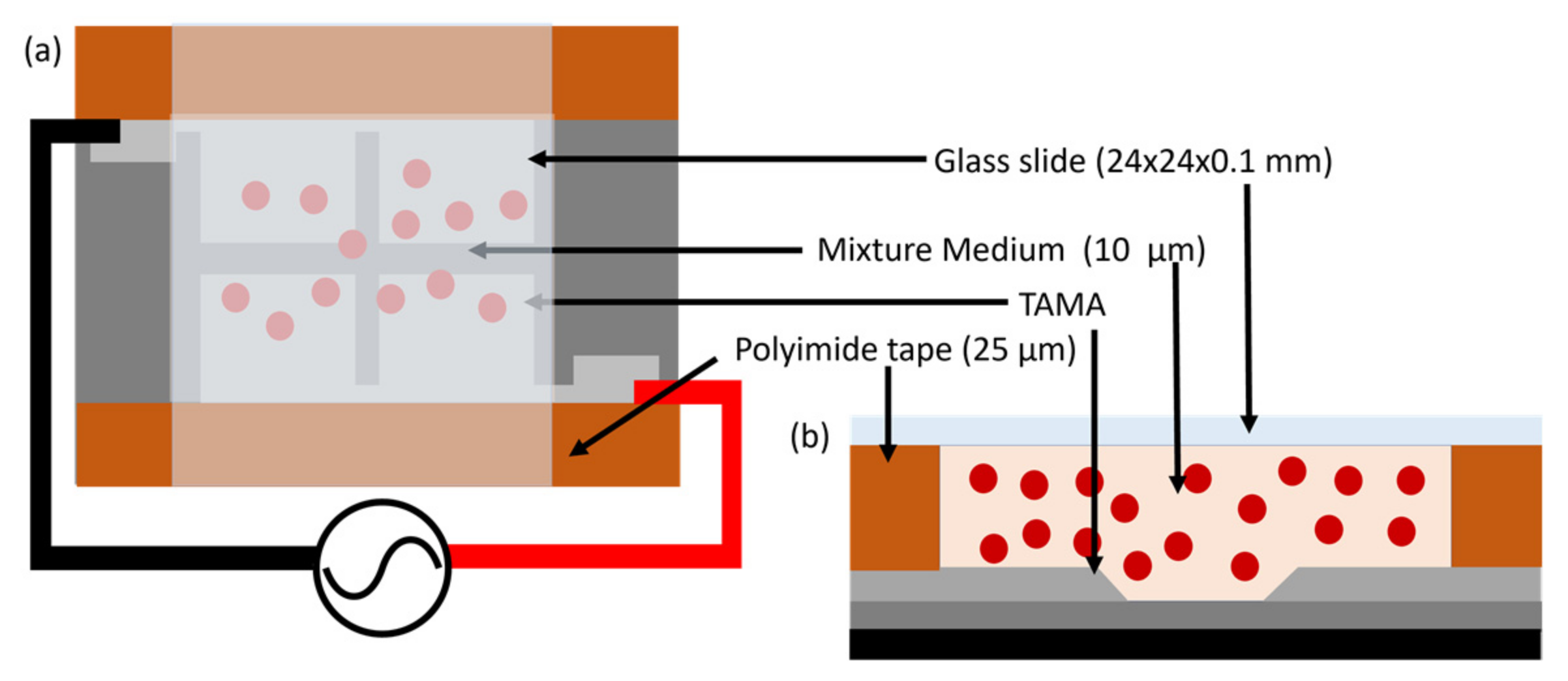
3.1. Fabrication of TAMA
3.2. Sample Preparation
3.3. Physiological Inspection of RBC
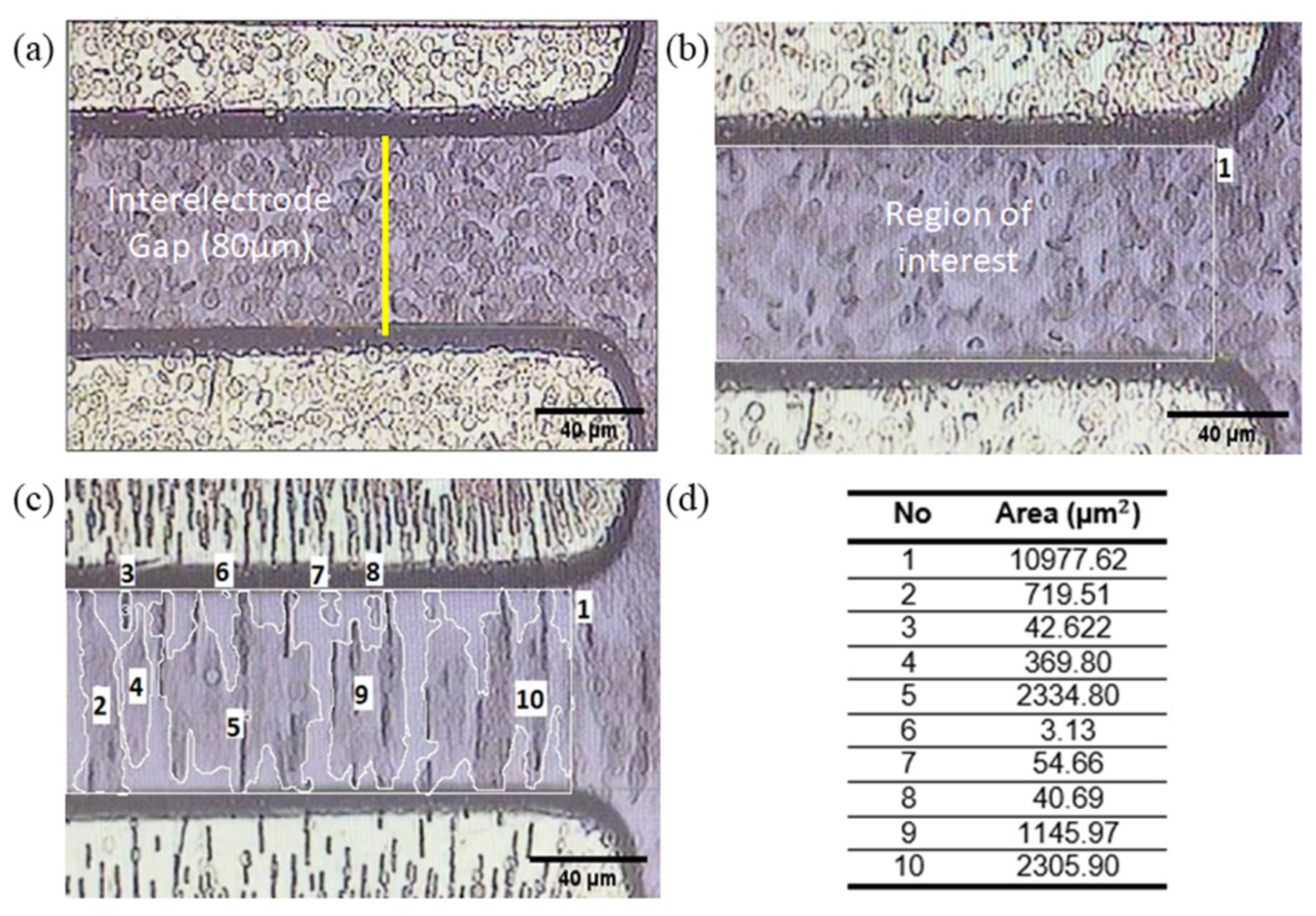
3.4. RBC Dielectrophoresis Measurement and Quantification
4. Results and Discussions
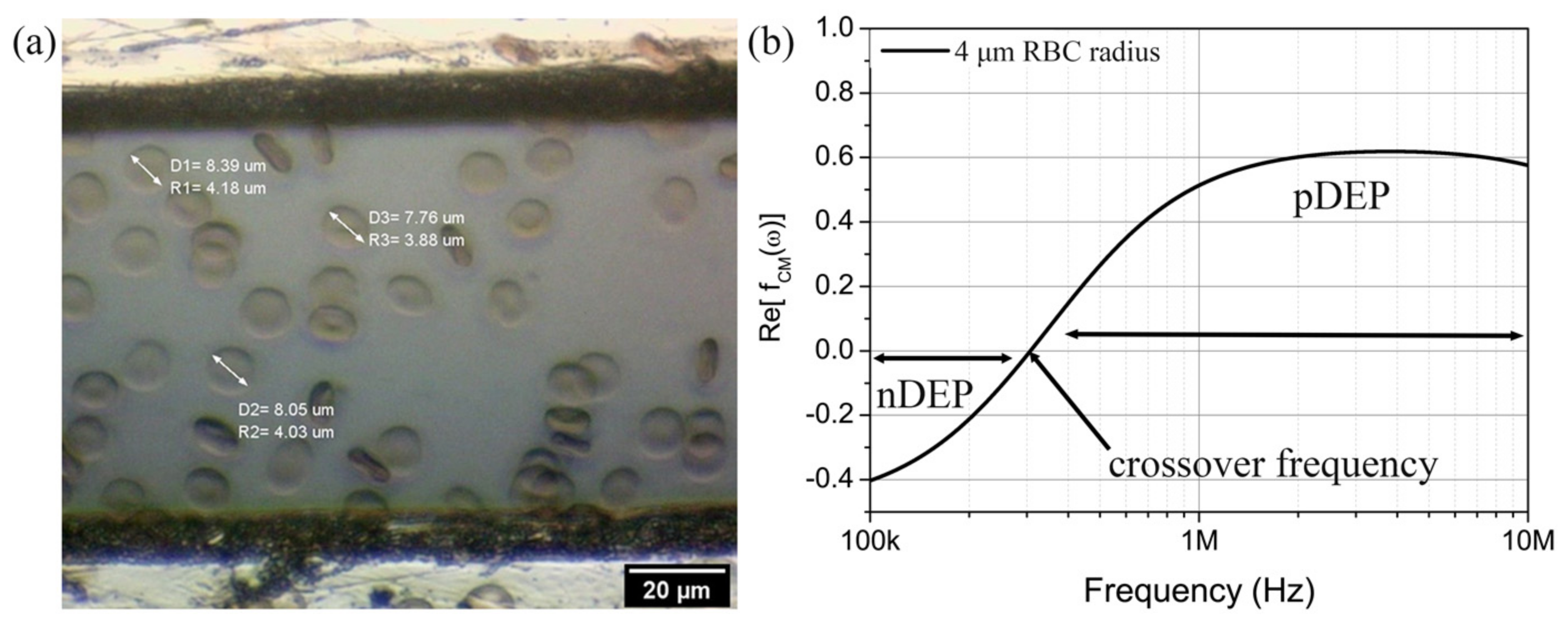
4.1. Electrophysiological Properties of RBC
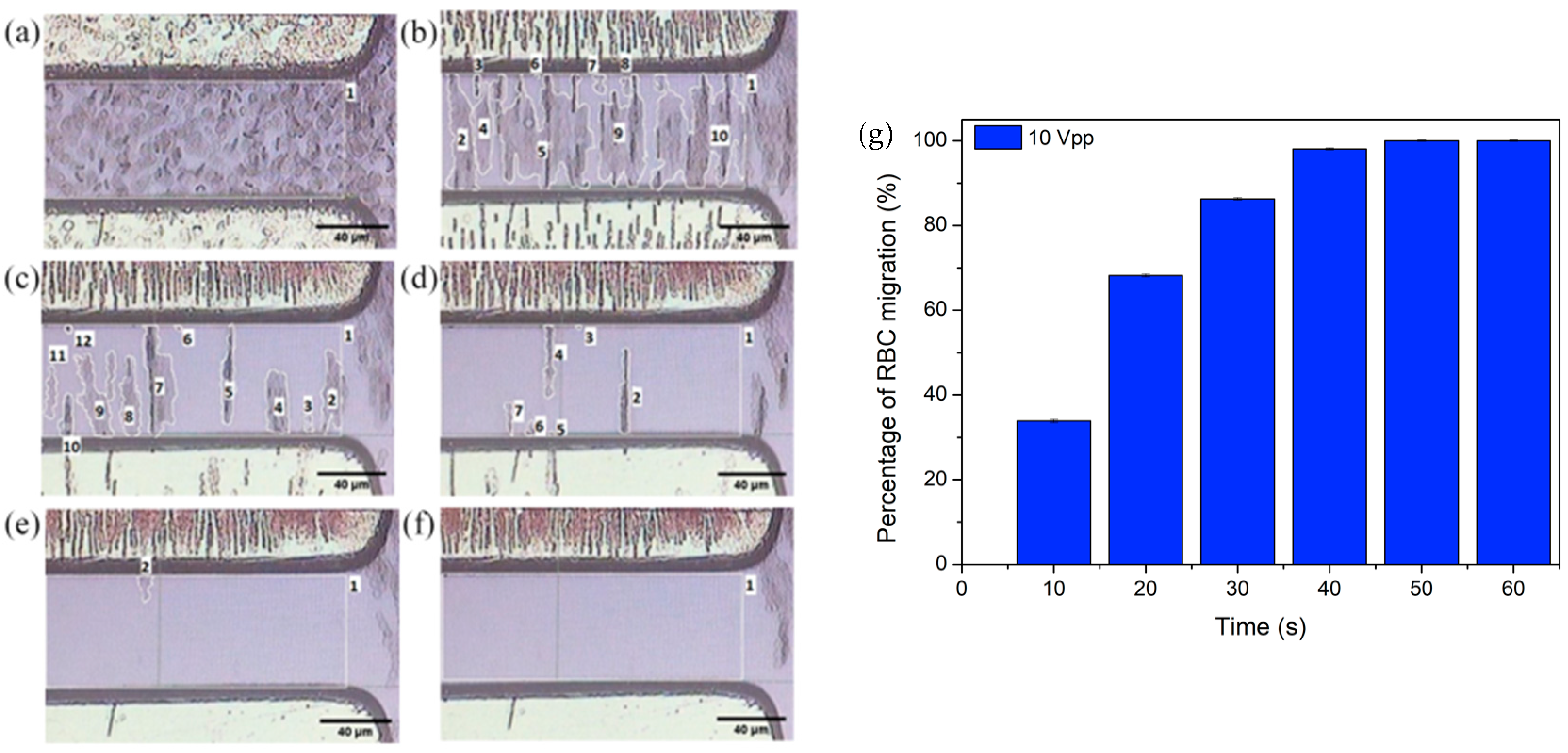
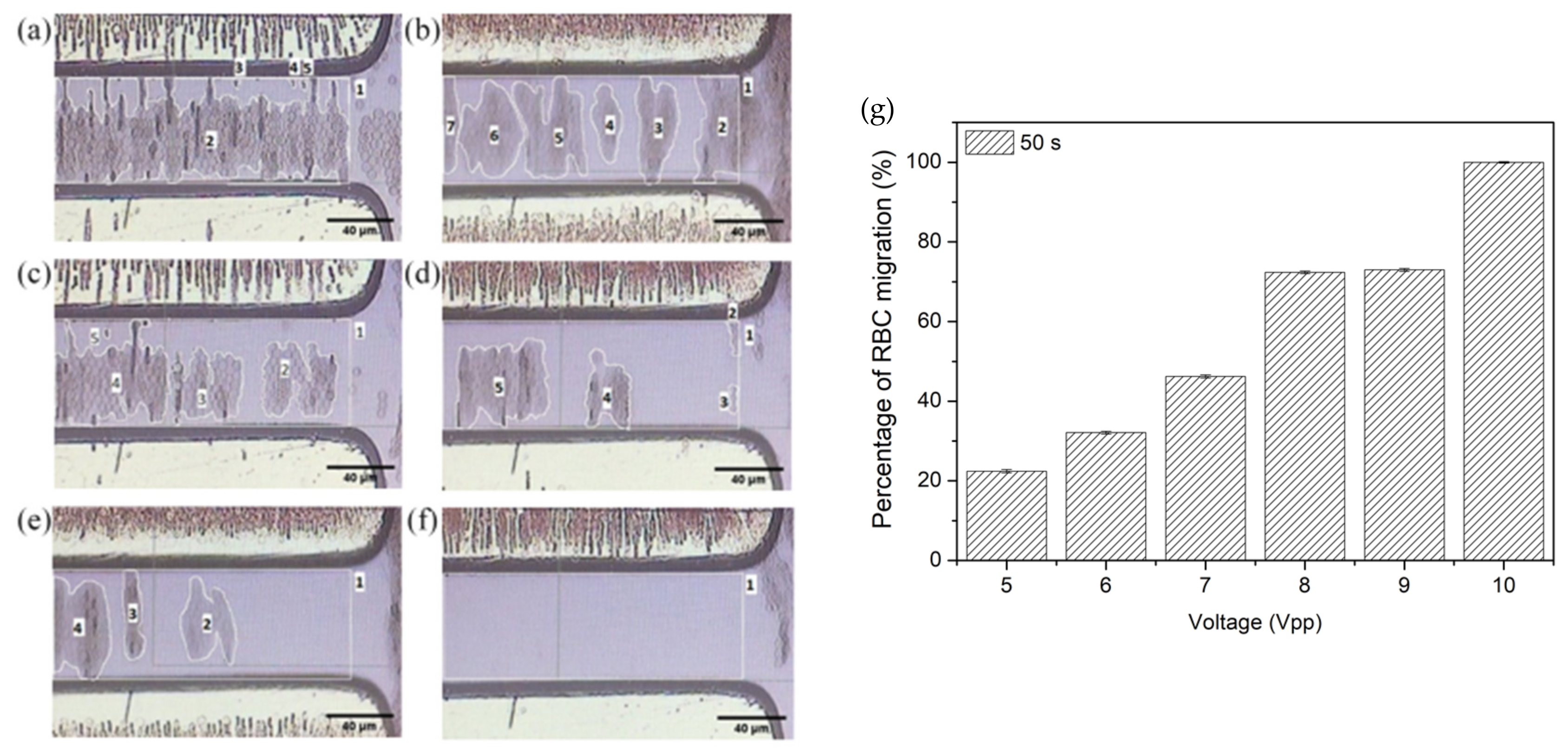
4.2. pDEP Manipulation of RBC
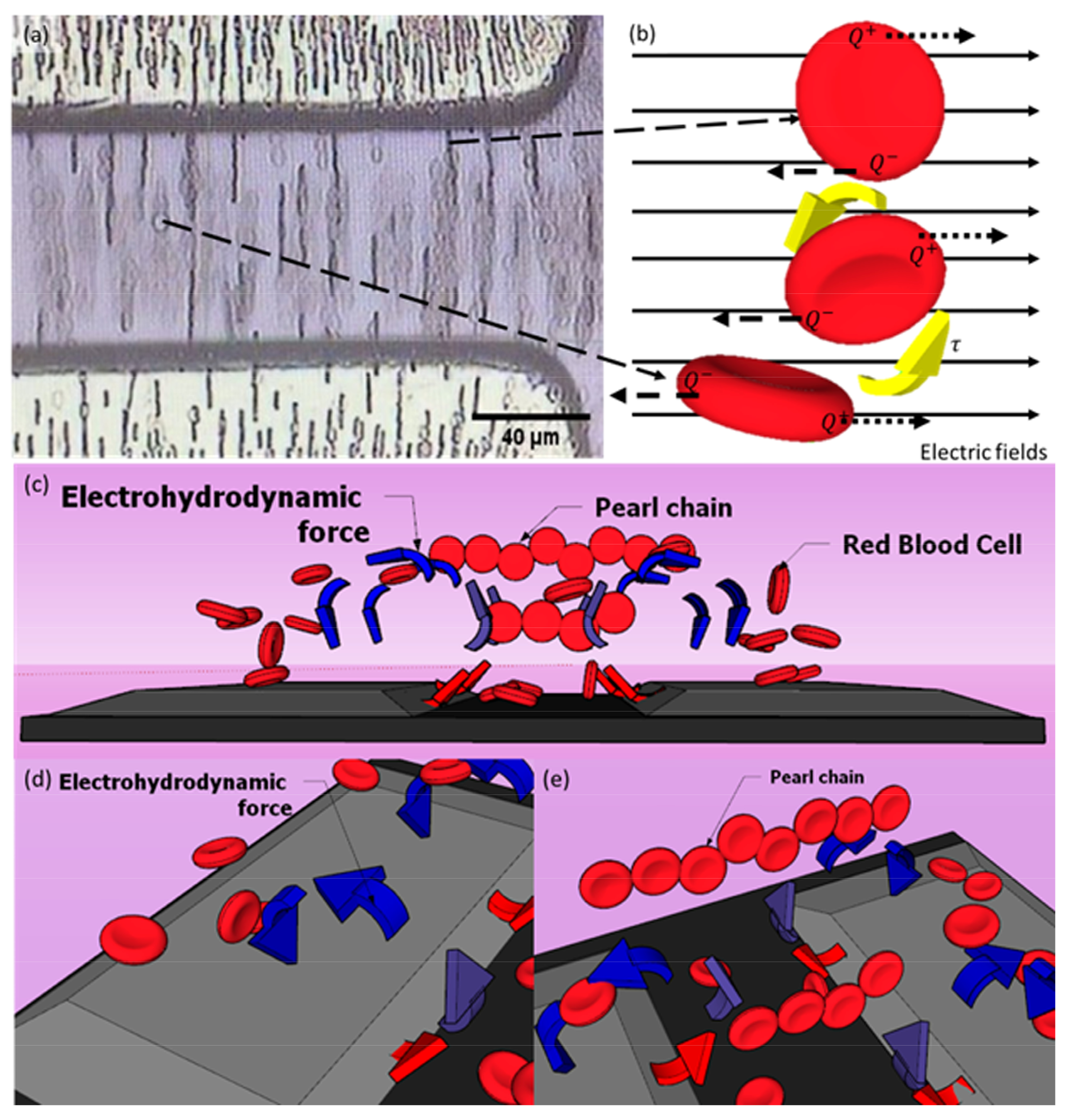
4.3. The Electrokinetic Mechanism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cheng, G. Circulating miRNAs: Roles in cancer diagnosis, prognosis and therapy. Drug Deliv. Rev. 2015, 81, 75–93. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Lautner, R.; Andreasson, U.; Öhrfelt, A.; Portelius, E.; Bjerke, M.; Hölttä, M.; Rosén, C.; Olsson, C.; Strobel, G.; et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: A systematic review and meta-analysis. Lancet Neurol. 2016, 15, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Neugebauer, S.; Giamarellos-Bourboulis, E.J.; Pelekanou, A.; Marioli, A.; Baziaka, F.; Tsangaris, I.; Bauer, M.; Kiehntopf, M. Metabolite profiles in sepsis: Developing prognostic tools based on the type of infection. Crit. Care Med. 2016, 44, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Meany, D.L.; Chan, D.W. Comparability of tumor marker immunoassays: Still an important issue for clinical diagnostics? Clin. Chem. Lab. Med. 2008, 46, 575–576. [Google Scholar] [CrossRef]
- Green, S.F. The cost of poor blood specimen quality and errors in preanalytical processes. Clin. Biochem. 2013, 46, 1175–1179. [Google Scholar] [CrossRef]
- Plebani, M. Exploring the iceberg of errors in laboratory medicine. Clin. Chim. Acta 2009, 404, 16–23. [Google Scholar] [CrossRef]
- Sang, S.; Feng, Q.; Jian, A.; Li, H.; Ji, J.; Duan, Q.; Zhang, W.; Wang, T. Portable microsystem integrates multifunctional dielectrophoresis manipulations and a surface stress biosensor to detect red blood cells for hemolytic anemia. Sci. Rep. 2016, 6, 33626. [Google Scholar] [CrossRef]
- Lu, M.; Lezzar, D.L.; Vörös, E.; Shevkoplyas, S.S. Traditional and emerging technologies for washing and volume reducing blood products. J. Blood Med. 2019, 10, 37–46. [Google Scholar] [CrossRef]
- Mielczarek, W.S.; Obaje, E.A.; Bachmann, T.T.; Kersaudy-Kerhoas, M. Microfluidic blood plasma separation for medical diagnostics: Is it worth it? Lab Chip 2016, 16, 3441–3448. [Google Scholar] [CrossRef]
- Brune, T.; Fill, S.; Heim, G.; Rabsilber, A.; Wohlfarth, K.; Garritsen, H.S.P. Quality and stability of red cells derived from gravity-separated placental blood with a hollow-fiber system. Transfusion 2007, 47, 2271–2275. [Google Scholar] [CrossRef]
- Hackstein, H.; Möller, A.; Gerlach, M.; Sachs, U.; Bein, G. Prospective quality control study of a novel gravity-driven whole blood separation system suitable for humanitarian crises. Vox Sang. 2017, 112, 806–809. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Kwok, M.; Marks, D.C. Preparation of red blood cell concentrates and plasma units from whole blood held overnight using a hollow fibre separation system. Trans. Med. 2015, 25, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, P.; Petersson, K.; Augustsson, P.; Laurell, T. Acoustic impedance matched buffers enable separation of bacteria from blood cells at high cell concentrations. Sci. Rep. 2018, 8, 9156. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Sanders, C.K.; Marrone, B.L. Separation of Escherichia coli bacteria from peripheral blood mononuclear cells using standing surface acoustic waves. Anal. Chem. 2013, 85, 9126–9134. [Google Scholar] [CrossRef]
- Li, S.; Ma, F.; Bachman, H.; Cameron, C.E.; Zeng, X.; Huang, T.J. Acoustofluidic bacteria separation. J. Micromech. Microeng. 2016, 27, 015031. [Google Scholar] [CrossRef]
- Szydzik, C.; Khoshmanesh, K.; Mitchell, A.; Karnutsch, C. Microfluidic platform for separation and extraction of plasma from whole blood using dielectrophoresis. Biomicrofluidics 2015, 9, 064120. [Google Scholar] [CrossRef]
- Liao, S.H.; Chang, C.Y.; Chang, H.C. A capillary dielectrophoretic chip for real-time blood cell separation from a drop of whole blood. Biomicrofluidics 2013, 7, 024110. [Google Scholar] [CrossRef]
- Hughes, M.P. Nanoelectromechanics in Engineering and Biology; CRC Press LLC: Boca Raton, FL, USA, 2003. [Google Scholar]
- Minerick, A.R.; Zhou, R.; Takhistov, P.; Chang, H.C. Manipulation and characterization of red blood cells with alternating current fields in microdevices. Electrophoresis 2003, 24, 3703–3717. [Google Scholar] [CrossRef]
- Kua, C.H.; Lam, Y.C.; Rodriguez, I.; Yang, C.; Youcef-Toumi, K. Dynamic Cell Fractionation and Transportation Using Moving Dielectrophoresis. Anal. Chem. 2007, 79, 6975–6987. [Google Scholar] [CrossRef]
- Zaman, M.A.; Padhy, P.; Wu, M.; Ren, W.; Jensen, M.A.; Davis, R.W.; Hesselink, L. Controlled transport of individual microparticles using dielectrophoresis. Langmuir 2023, 39, 101–110. [Google Scholar] [CrossRef]
- Chan, J.Y.; Ahmad Kayani, A.B.; Md Ali, M.A.; Kok, C.K.; Ramdzan Buyong, M.; Hoe SL, L.; Marzuki, M.; Soo-Beng Khoo, A.; Sriram, S.; Ostrikov, K.K. Dielectrophoretic deformation of breast cancer cells for lab on a chip applications. Electrophoresis 2019, 40, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Al-Ahdal, S.A.; Ahmad Kayani, A.B.; Md Ali, M.A.; Chan, J.Y.; Ali, T.; Adnan, N.; Buyong, M.R.; Mhd Noor, E.E.; Majlis, B.Y.; Sriram, S. Dielectrophoresis of amyloid-beta proteins as a microfluidic template for Alzheimer’s research. Int. J. Mol. Sci. 2019, 20, 3595. [Google Scholar] [CrossRef] [PubMed]
- Faraghat, S.A.; Hoettges, K.F.; Steinbach, M.K.; van der Veen, D.R.; Brackenbury, W.J.; Henslee, E.A.; Labeed, F.H.; Hughes, M.P. High-throughput, low-loss, low-cost, and label-free cell separation using electrophysiology-activated cell enrichment. Proc. Natl. Acad. Sci. USA 2017, 114, 4591–4596. [Google Scholar] [CrossRef]
- Buyong, M.R.; Larki, F.; Faiz, M.S.; Hamzah, A.A.; Yunas, J.; Yeop Majlis, B. A tapered aluminium microelectrode array for improvement of dielectrophoresis-based particle manipulation. Sensors 2015, 15, 10973–10990. [Google Scholar] [CrossRef]
- Abd Samad, M.I.; Buyong, M.R.; Kim, S.S.; Yeop Majlis, B. Dielectrophoresis velocities response on tapered electrode profile: Simulation and experimental. Microelectron. Int. 2019, 6, 45–53. [Google Scholar] [CrossRef]
- Abd Samad, M.I.; Buyong, M.R.; Yunus, F.W.; Kim, S.S.; Hamzah, A.A.; Yeop Majlis, B. Voltage Characterization on Dielectrophoretic Force Response to Hematologic Cell Manipulation. In Proceedings of the 2018 IEEE International Conference on Semiconductor Electronics (ICSE), Kuala Lumpur, Malaysia, 15–17 August 2018; pp. 13–16. [Google Scholar]
- Abdul Rahim, M.K.; Jamaludin NM, A.; Santhanam, J.; Hamzah, A.A.; Buyong, M.R. Rapid ESKAPE Pathogens Detection Method using Tapered Dielectrophoresis Electrodes via Crossover Frequency Analysis. Sains Malays. 2020, 12, 2913–2925. [Google Scholar] [CrossRef]
- Jamaludin NM, A.; Abdul Rahim, K.M.; Hamzah, A.A.; Abu, N.; Buyong, M.R. Rapid Manipulation of Extracellular Vesicles using Dielectrophoretic Mechanism. Sains Malays. 2020, 49, 2901–2912. [Google Scholar] [CrossRef]
- Deivasigamani, R.; Maidin NN, M.; Wee MF, M.R.; Mohamed, M.A.; Buyong, M.R. Dielectrophoresis Prototypic Polystyrene Particle Synchronization toward Alive Keratinocyte Cells for Rapid Chronic Wound Healing. Sensors 2021, 21, 3007. [Google Scholar] [CrossRef]
- Hoettges, K.F.; Henslee, E.A.; Torcal Serrano, R.M.; Jabr, R.I.; Abdallat, R.G.; Beale, A.D.; Waheed, A.; Camelliti, P.; Fry, C.H.; Van der Veen, D.R.; et al. Ten–Second Electrophysiology: Evaluation of the 3DEP Platform for high-speed, high-accuracy cell analysis. Sci. Rep. 2019, 9, 19153. [Google Scholar] [CrossRef]
- Elitas, M.; Islam, M.; Korvink, J.G.; Sengul, E.; Sharbati, P.; Ozogul, B.; Kaymaz, S.V. Quantifying Deformation and Migration Properties of U87 Glioma Cells Using Dielectrophoretic Forces. Biosensors 2022, 12, 946. [Google Scholar] [CrossRef]
- Kang, D.H.; Kim, K.; Kim, Y.J. An anti-clogging method for improving the performance and lifespan of blood plasma separation devices in real-time and continuous microfluidic systems. Sci. Rep. 2018, 8, 8066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Le, M.Q.; Nguyen, V.C.; Mogniotte, J.F.; Capsal, J.F.; Grinberg, D.; Cottinet, P.J.; Petit, L. Characterization of micro-ZnO/PDMS composite structured via dielectrophoresis–toward medical application. Mater. Des. 2021, 208, 109912. [Google Scholar] [CrossRef]
- Morgan, H.; Green, N.G.; Ramos, A. Nano-scale AC electrokinetics and electrohydrodynamics. J. Phys. D Appl. Phys. 2017, 50, 011001. [Google Scholar] [CrossRef][Green Version]
- Castellanos, A.; Ramos, A.; González, A.; Green, N.G.; Morgan, H. Electrohydrodynamics and dielectrophoresis in microsystems: Scaling laws. J. Phys. D Appl. Phys. 2003, 36, 2584–2597. [Google Scholar] [CrossRef]
- Hughes, M.P.; Morgan, H. AC electrokinetics: A review of forces in microelectrode structures. J. Phys. D Appl. Phys. 1998, 31, 2205–2210. [Google Scholar] [CrossRef]
- Zhao, Y.; Brcka, J.; Faguet, J.; Zhang, G. Elucidating the mechanisms of two unique phenomena governed by particle-particle interaction under DEP: Tumbling motion of pearl chains and alignment of of ellipsoidal particles. Micromachines 2018, 9, 279. [Google Scholar] [CrossRef]
- Ho, C.T.; Lin, R.Z.; Chang, W.Y.; Chang, H.Y.; Liu, C.H. Rapid heterogeneous liver-cell on-chip patterning via the enhanced field-induced dielectrophoresis trap. Lab Chip 2006, 6, 724–734. [Google Scholar] [CrossRef]
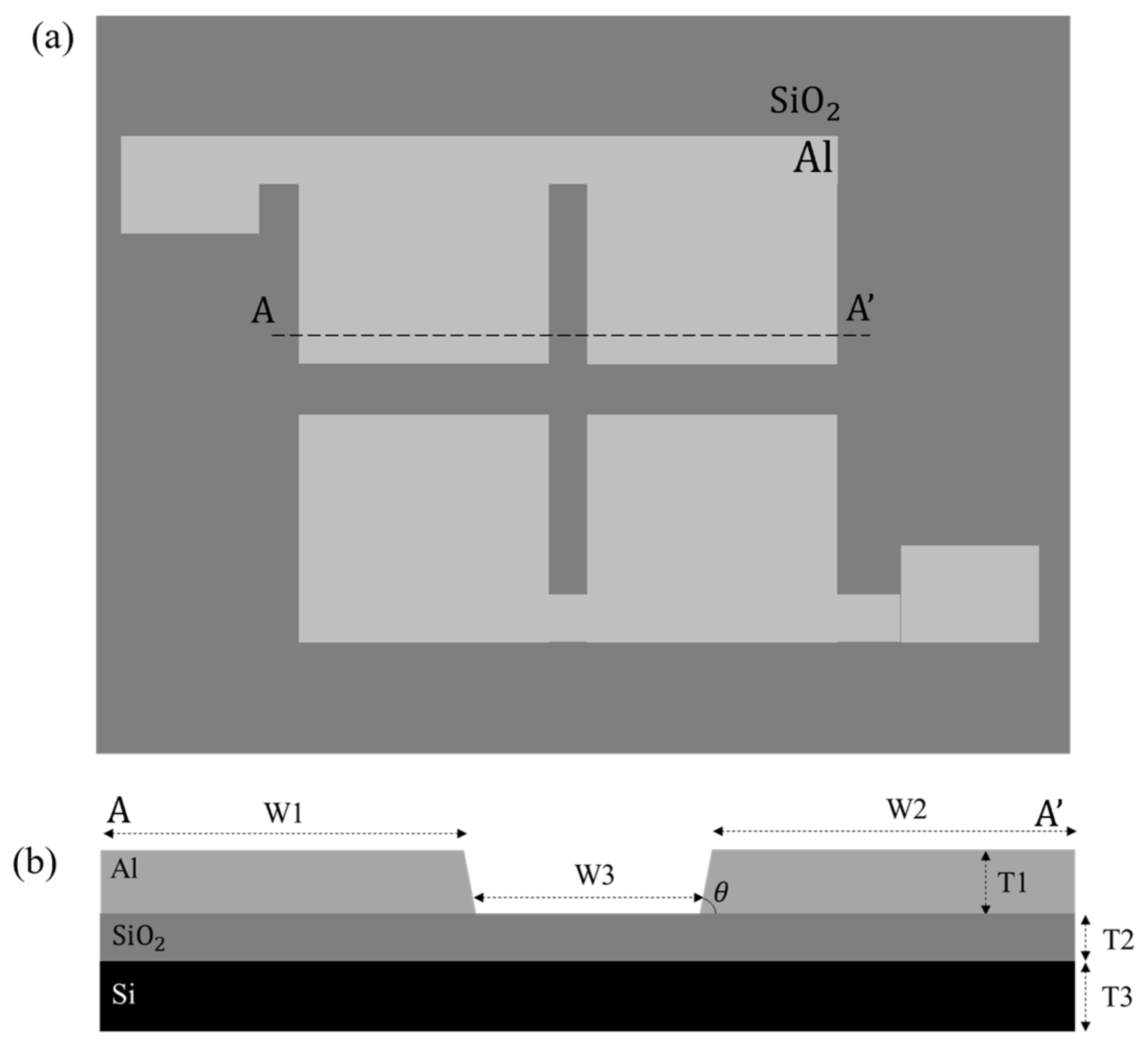

| Parameter | Dimension |
|---|---|
| Microelectrode width, W1 | 1000 μm |
| Microelectrode width, W2 | 1000 μm |
| Interelectrode spacing, W3 | 80 μm |
| Microelectrode edge slope, θ | 70° |
| Microelectrode thickness (Al), T1 | 4 μm |
| Insulation layer thickness (SiO2), T2 | 1 μm |
| Substrate thickness, T3 | 600 μm |
| Category | Parameter | Value | Reference |
|---|---|---|---|
| Medium suspension | Relative permittivity for | 80 | [7] |
| Conductivity, | 45 mS/m | [7] | |
| Living RBC | Total cell radius, | 4.00 µm | Own data |
| Cytoplasma radius, | 3.99 µm | Own data | |
| Cytoplasma conductivity, | 310 mS/m | [7] | |
| Relative permittivity for | 59 | [7] | |
| Shell electrical conductivity, | 1 µS/m | [7] | |
| Shell relative permittivity for | 4.4 | [7] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samad, M.I.A.; Ponnuthurai, D.R.; Badrudin, S.I.; Ali, M.A.M.; Razak, M.A.A.; Buyong, M.R.; Latif, R. Migration Study of Dielectrophoretically Manipulated Red Blood Cells in Tapered Aluminium Microelectrode Array: A Pilot Study. Micromachines 2023, 14, 1625. https://doi.org/10.3390/mi14081625
Samad MIA, Ponnuthurai DR, Badrudin SI, Ali MAM, Razak MAA, Buyong MR, Latif R. Migration Study of Dielectrophoretically Manipulated Red Blood Cells in Tapered Aluminium Microelectrode Array: A Pilot Study. Micromachines. 2023; 14(8):1625. https://doi.org/10.3390/mi14081625
Chicago/Turabian StyleSamad, Muhammad Izzuddin Abd, Darven Raj Ponnuthurai, Syazwani Izrah Badrudin, Mohd Anuar Mohd Ali, Mohd Azhar Abdul Razak, Muhamad Ramdzan Buyong, and Rhonira Latif. 2023. "Migration Study of Dielectrophoretically Manipulated Red Blood Cells in Tapered Aluminium Microelectrode Array: A Pilot Study" Micromachines 14, no. 8: 1625. https://doi.org/10.3390/mi14081625
APA StyleSamad, M. I. A., Ponnuthurai, D. R., Badrudin, S. I., Ali, M. A. M., Razak, M. A. A., Buyong, M. R., & Latif, R. (2023). Migration Study of Dielectrophoretically Manipulated Red Blood Cells in Tapered Aluminium Microelectrode Array: A Pilot Study. Micromachines, 14(8), 1625. https://doi.org/10.3390/mi14081625









