Decoration of Poly-3-methyl Aniline with As(III) Oxide and Hydroxide as an Effective Photoelectrode for Electroanalytical Photon Sensing with Photodiode-like Behavior
Abstract
1. Introduction
2. Experimental Section
2.1. Materials and Characterization
2.2. P3MA Preparation
2.3. As2O3–As(OH)3/P3MA Optoelectronic and Photodiode Preparation
2.4. The Electrical Study
3. Results and Discussion
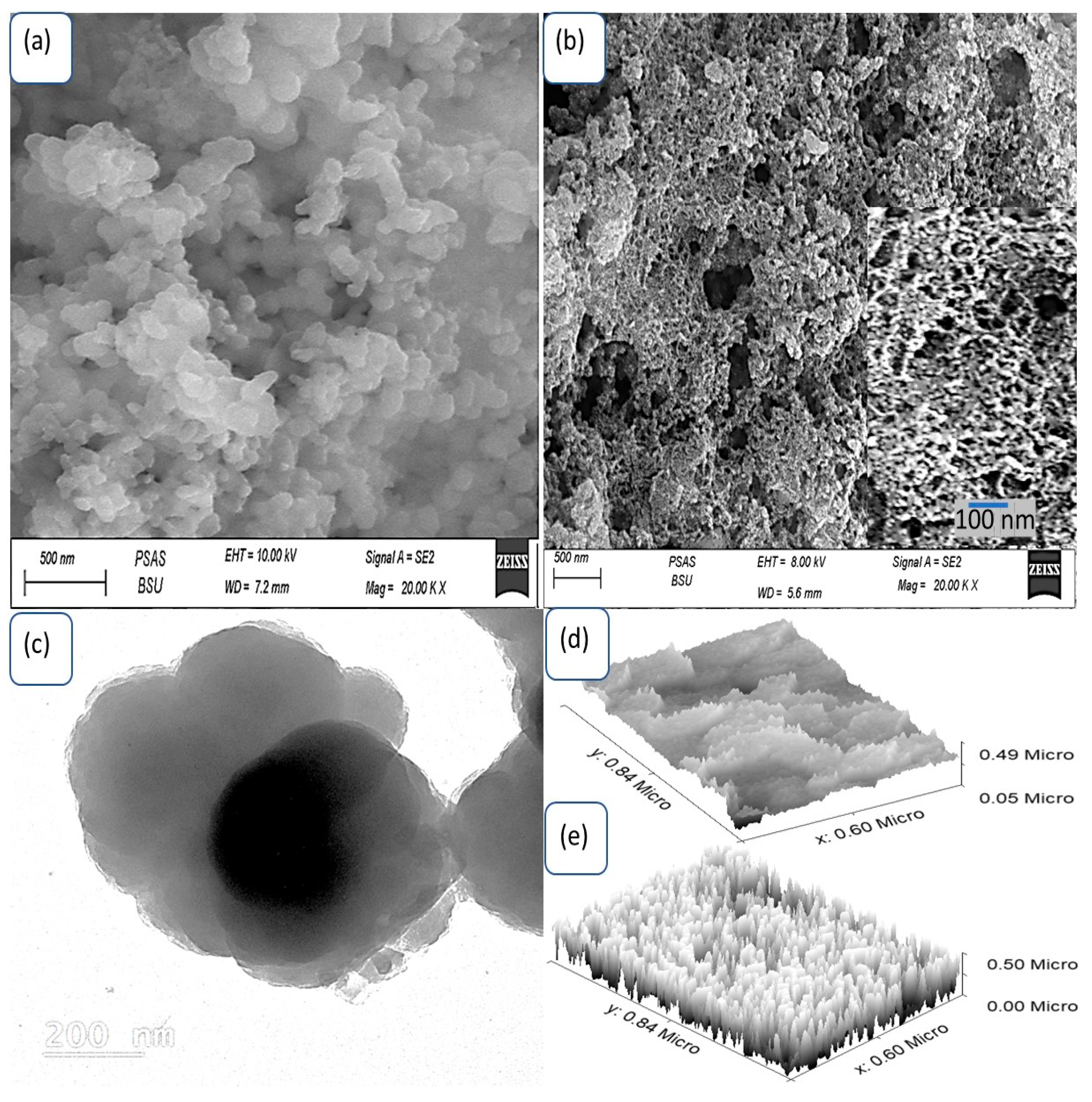
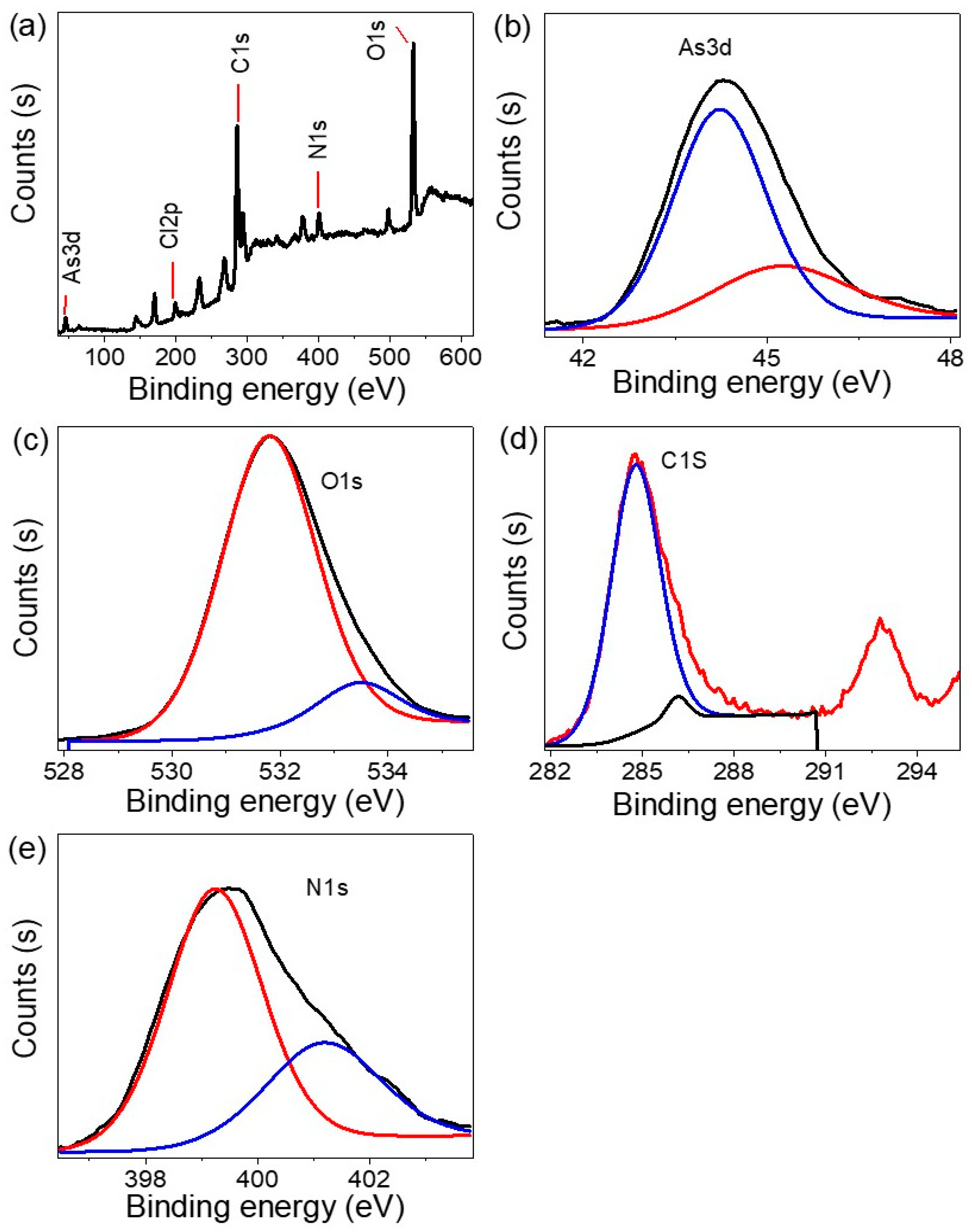
3.1. Analyses
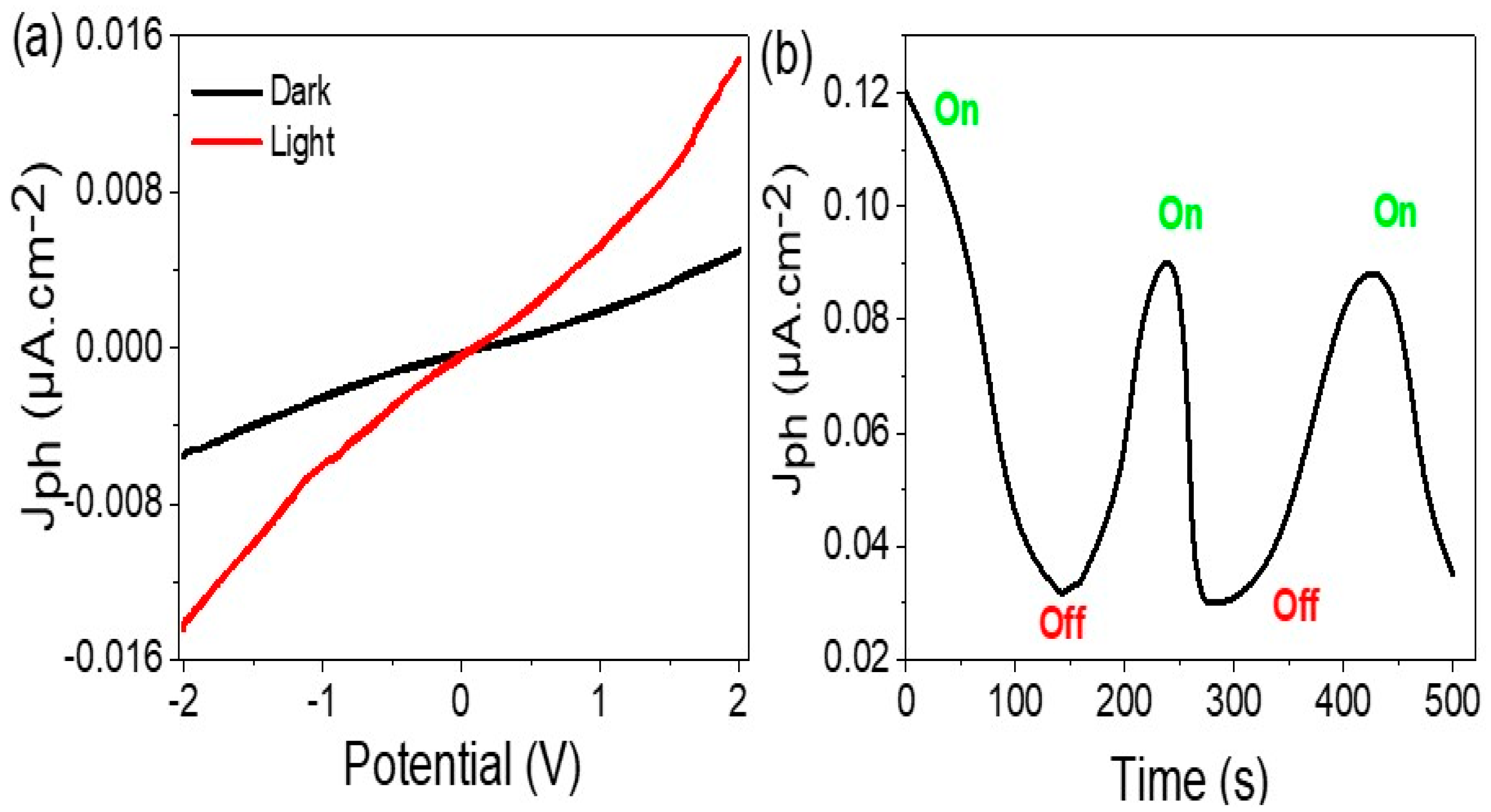
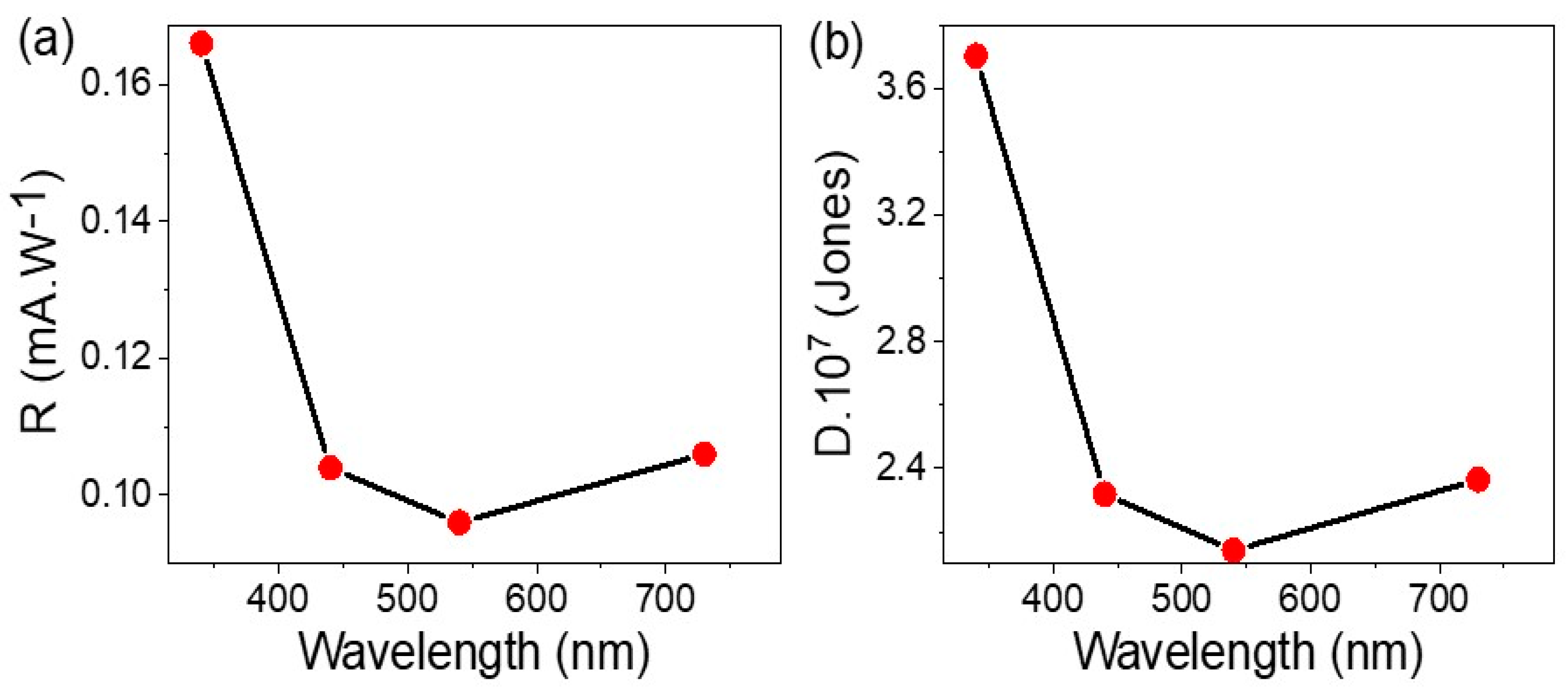
3.2. Electrical Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qin, Z.; She, Z.; Chen, C.; Wu, W.; Lau, J.K.Y.; Ip, N.Y.; Qu, J.Y. Deep Tissue Multi-Photon Imaging Using Adaptive Optics with Direct Focus Sensing and Shaping. Nat. Biotechnol. 2022, 40, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Shafique, S.; Yang, S.; Wang, Y.; Woldu, Y.T.; Cheng, B.; Ji, P. High-Performance Photodetector Using Urchin-like Hollow Spheres of Vanadium Pentoxide Network Device. Sens. Actuators A Phys. 2019, 296, 38–44. [Google Scholar] [CrossRef]
- Proppe, A.H.; Berkinsky, D.B.; Zhu, H.; Šverko, T.; Kaplan, A.E.K.; Horowitz, J.R.; Kim, T.; Chung, H.; Jun, S.; Bawendi, M.G. Highly Stable and Pure Single-Photon Emission with 250 Ps Optical Coherence Times in InP Colloidal Quantum Dots. Nat. Nanotechnol. 2023, 2023, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Akter, A.; Kum, H.S.; Baek, Y.; Ra, Y.H.; Yoo, G.; Lee, K.; Mi, Z.; Heo, J. Mid-Infrared Photon Sensing Using InGaN/GaN Nanodisks via Intersubband Absorption. Sci. Rep. 2022, 12, 4301. [Google Scholar] [CrossRef]
- Meng, Y.; Feng, J.; Han, S.; Xu, Z.; Mao, W.; Zhang, T.; Kim, J.S.; Roh, I.; Zhao, Y.; Kim, D.H.; et al. Photonic van Der Waals Integration from 2D Materials to 3D Nanomembranes. Nat. Rev. Mater. 2023, 2023, 1–20. [Google Scholar] [CrossRef]
- Essig, S.; Allebé, C.; Remo, T.; Geisz, J.F.; Steiner, M.A.; Horowitz, K.; Barraud, L.; Ward, J.S.; Schnabel, M.; Descoeudres, A.; et al. Raising the One-Sun Conversion Efficiency of III–V/Si Solar Cells to 32.8% for Two Junctions and 35.9% for Three Junctions. Nat. Energy 2017, 2, 17144. [Google Scholar] [CrossRef]
- Lin, T.N.; Santiago, S.R.M.S.; Zheng, J.A.; Chao, Y.C.; Yuan, C.T.; Shen, J.L.; Wu, C.H.; Lin, C.A.J.; Liu, W.R.; Cheng, M.C.; et al. Enhanced Conversion Efficiency of III–V Triple-Junction Solar Cells with Graphene Quantum Dots. Sci. Rep. 2016, 6, 39163. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Rabia, M.; Shaban, M.; Aly, A.H.; Ahmed, A.M. Preparation of Hexagonal Nanoporous Al2O3/TiO2/TiN as a Novel Photodetector with High Efficiency. Sci. Rep. 2021, 11, 17572. [Google Scholar] [CrossRef]
- Hadia, N.M.A.; Shaban, M.; Mohamed, S.H.; Al-Ghamdi, A.F.; Alzaid, M.; Elsayed, A.M.; Mourad, A.H.I.; Amin, M.A.; Boukherroub, R.; Abdelazeez, A.A.A.; et al. Highly Crystalline Hexagonal PbI2 Sheets on Polyaniline/Antimony Tin Oxide Surface as a Novel and Highly Efficient Photodetector in UV, Vis, and near IR Regions. Polym. Adv. Technol. 2022, 33, 3977–3987. [Google Scholar] [CrossRef]
- Bakulin, A.A.; Neutzner, S.; Bakker, H.J.; Ottaviani, L.; Barakel, D.; Chen, Z. Charge Trapping Dynamics in PbS Colloidal Quantum Dot Photovoltaic Devices. ACS Nano 2013, 7, 8771–8779. [Google Scholar] [CrossRef]
- Li, Y.; An, N.; Lu, Z.; Wang, Y.; Chang, B.; Tan, T.; Guo, X.; Xu, X.; He, J.; Xia, H.; et al. Nonlinear Co-Generation of Graphene Plasmons for Optoelectronic Logic Operations. Nat. Commun. 2022, 13, 3138. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Primavera, B.A.; Chiles, J.; McCaughan, A.N.; Buckley, S.M.; Tait, A.N.; Lita, A.; Biesecker, J.; Fox, A.; Olaya, D.; et al. Superconducting Optoelectronic Single-Photon Synapses. Nat. Electron. 2022, 5, 650–659. [Google Scholar] [CrossRef]
- Asahi, S.; Teranishi, H.; Kusaki, K.; Kaizu, T.; Kita, T. Two-Step Photon up-Conversion Solar Cells. Nat. Commun. 2017, 8, 14962. [Google Scholar] [CrossRef]
- Fu, J.; Fan, Z.; Nakabayashi, M.; Ju, H.; Pastukhova, N.; Xiao, Y.; Feng, C.; Shibata, N.; Domen, K.; Li, Y. Interface Engineering of Ta3N5 Thin Film Photoanode for Highly Efficient Photoelectrochemical Water Splitting. Nat. Commun. 2022, 13, 729. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; El-Sayed, A.M.A.; Ahmed, A.; Sayed, S. Photocatalytic Properties of PbS/Graphene Oxide/Polyaniline Electrode for Hydrogen Generation. Sci. Rep. 2017, 7, 14100. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, P.; Zhang, Z.; Teng, F.; Zheng, L.; Hu, K.; Fang, X.; Chen, H.Y.; Yu, P.P.; Teng, F.; et al. Ultrasensitive Self-Powered Solar-Blind Deep-Ultraviolet Photodetector Based on All-Solid-State Polyaniline/MgZnO Bilayer. Small 2016, 12, 5809–5816. [Google Scholar] [CrossRef] [PubMed]
- Acikgoz, S.; Yungevis, H. Controlled Electrochemical Growth of Micro-Scaled As2O3 and Ga2O3 Oxide Structures on p-Type Gallium Arsenide. Appl. Phys. A Mater. Sci. Process. 2022, 128, 824. [Google Scholar] [CrossRef]
- Abdullahi, Y.Z.; Caglayan, R.; Mogulkoc, A.; Mogulkoc, Y.; Ersan, F. New Stable Ultrawide Bandgap As2O3 Semiconductor Materials. J. Phys. Mater. 2023, 6, 025003. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y. Self-Powered UV–Visible Photodetectors Based on ZnO/Cu2O Nanowire/Electrolyte Heterojunctions. J. Alloys Compd. 2016, 675, 325–330. [Google Scholar] [CrossRef]
- Costas, A.; Florica, C.; Preda, N.; Apostol, N.; Kuncser, A.; Nitescu, A.; Enculescu, I. Radial Heterojunction Based on Single ZnO-CuxO Core-Shell Nanowire for Photodetector Applications. Sci. Rep. 2019, 9, 5553. [Google Scholar] [CrossRef]
- Tan, W.C.; Shih, W.H.; Chen, Y.F. A Highly Sensitive Graphene-Organic Hybrid Photodetector with a Piezoelectric Substrate. Adv. Funct. Mater. 2014, 24, 6818–6825. [Google Scholar] [CrossRef]
- Hamid, M.M.A.; Alruqi, M.; Elsayed, A.M.; Atta, M.M.; Hanafi, H.A.; Rabia, M. Testing the Photo-Electrocatalytic Hydrogen Production of Polypyrrole Quantum Dot by Combining with Graphene Oxide Sheets on Glass Slide. J. Mater. Sci. Mater. Electron. 2023, 34, 831. [Google Scholar] [CrossRef]
- Bioud, Y.A.; Boucherif, A.; Belarouci, A.; Paradis, E.; Drouin, D.; Arès, R. Chemical Composition of Nanoporous Layer Formed by Electrochemical Etching of P-Type GaAs. Nanoscale Res. Lett. 2016, 11, 446. [Google Scholar] [CrossRef] [PubMed]
- Balayeva, O.O.; Azizov, A.A.; Muradov, M.B.; Alosmanov, R.M.; Eyvazova, G.M.; Mammadyarova, S.J. Cobalt Chromium-Layered Double Hydroxide, α- and β- Co(OH)2 and Amorphous Cr(OH)3: Synthesis, Modification and Characterization. Heliyon 2019, 5, e02725. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, Y.; Li, F.; Song, G.; Shao, Y.; Ouyang, R.; Xu, L.; Li, W.; Miao, Y. A Newly Prepared Ni(OH)2@Cr(OH)3 Nanohybrid for Its Bioelectrocatalysis. Sens. Actuators B Chem. 2014, 198, 350–359. [Google Scholar] [CrossRef]
- Linganathan, P.; Sundararajan, J.; Samuel, J.M. Synthesis, Characterization, and Photoconductivity Studies on Poly(2-Chloroaniline) and Poly(2-Chloroaniline)/CuO Nanocomposites. J. Compos. 2014, 2014, 838975. [Google Scholar] [CrossRef]
- Trabelsi, A.B.G.; Essam, D.H.; Alkallas, F.M.; Ahmed, A.; Rabia, M. Petal-like NiS-NiO/G-C3N4 Nanocomposite for High-Performance Symmetric Supercapacitor. Micromachines 2022, 13, 2134. [Google Scholar] [CrossRef]
- Ben Gouider Trabelsi, A.; MElsayed, A.; HAlkallas, F.; Al-Noaimi, M.; Kusmartsev, F.V.; Rabia, M. A Fractal, Flower Petal-like CuS-CuO/G-C3N4 Nanocomposite for High Efficiency Supercapacitors. Coatings 2022, 12, 1834. [Google Scholar] [CrossRef]
- Rabia, M.; Essam, D.; Alkallas, F.H.; Shaban, M.; Elaissi, S.; Ben Gouider Trabelsi, A. Flower-Shaped CoS-Co2O3/G-C3N4 Nanocomposite for Two-Symmetric-Electrodes Supercapacitor of High Capacitance Efficiency Examined in Basic and Acidic Mediums. Micromachines 2022, 13, 2234. [Google Scholar] [CrossRef]
- Khalafalla, M.A.H.; Hadia, N.M.A.; Elsayed, A.M.; Alruqi, M.; El Malti, W.; Shaban, M.; Rabia, M. ATO/Polyaniline/PbS Nanocomposite as Highly Efficient Photoelectrode for Hydrogen Production from Wastewater with Theoretical Study for the Water Splitting. Adsorpt. Sci. Technol. 2022, 2022, 5628032. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Alkallas, F.H.; Trabelsi, A.B.G.; AlFaify, S.; Shkir, M.; Alrebdi, T.A.; Almugren, K.S.; Kusmatsev, F.V.; Rabia, M. Photodetection Enhancement via Graphene Oxide Deposition on Poly 3-Methyl Aniline. Micromachines 2023, 14, 606. [Google Scholar] [CrossRef] [PubMed]
- Hwang, D.K.; Lee, Y.T.; Lee, H.S.; Lee, Y.J.; Shokouh, S.H.; Kyhm, J.H.; Lee, J.; Kim, H.H.; Yoo, T.H.; Nam, S.H.; et al. Ultrasensitive PbS Quantum-Dot-Sensitized InGaZnO Hybrid Photoinverter for near-Infrared Detection and Imaging with High Photogain. NPG Asia Mater. 2016, 8, e233. [Google Scholar] [CrossRef]
- Ding, H.; Lv, G.; Cai, X.; Chen, J.; Cheng, Z.; Peng, Y.; Tang, G.; Shi, Z.; Xie, Y.; Fu, X.; et al. An Optoelectronic Thermometer Based on Microscale Infrared-to-Visible Conversion Devices. Light Sci. Appl. 2022, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Shaban, M.; Ahmed, A.M.; Abdel-Rahman, E.; Hamdy, H. Tunability and Sensing Properties of Plasmonic/1D Photonic Crystal. Sci. Rep. 2017, 7, 41983. [Google Scholar] [CrossRef]
- Sulaman, M.; Yang, S.; Imran, A.; Zhang, Z.; Bukhtiar, A.; Ge, Z.; Song, Y.; Sun, F.; Jiang, Y.; Tang, L.; et al. Two Bulk-Heterojunctions Made of Blended Hybrid Nanocomposites for High-Performance Broadband, Self-Driven Photodetectors. ACS Appl. Mater. Interfaces 2023, 15, 25671–25683. [Google Scholar] [CrossRef]
- Noothongkaew, S.; Thumthan, O.; An, K.S. Minimal Layer Graphene/TiO2 Nanotube Membranes Used for Enhancement of UV Photodetectors. Mater. Lett. 2018, 218, 274–279. [Google Scholar] [CrossRef]
- Hong, Q.; Cao, Y.; Xu, J.; Lu, H.; He, J.; Sun, J.L. Self-Powered Ultrafast Broadband Photodetector Based on p-n Heterojunctions of CuO/Si Nanowire Array. ACS Appl. Mater. Interfaces 2014, 6, 20887–20894. [Google Scholar] [CrossRef]
- Chen, Z.; Ci, H.; Tan, Z.; Dou, Z.; Chen, X.-D.; Liu, B.; Liu, R.; Lin, L.; Cui, L.; Gao, P.; et al. Growth of 12-Inch Uniform Monolayer Graphene Film on Molten Glass and Its Application in PbI 2-Based Photodetector. Nano Res. 2019, 12, 1888–1893. [Google Scholar] [CrossRef]
- Ismail, R.A.; Mousa, A.M.; Shaker, S.S. Visible-Enhanced Silver-Doped PbI2 Nanostructure/Si Heterojunction Photodetector: Effect of Doping Concentration on Photodetector Parameters. Opt. Quantum Electron. 2019, 51, 362. [Google Scholar] [CrossRef]
- Lan, T.; Fallatah, A.; Suiter, E.; Padalkar, S. Size Controlled Copper (I) Oxide Nanoparticles Influence Sensitivity of Glucose Biosensor. Sensors 2017, 17, 1944. [Google Scholar] [CrossRef]
- Liu, K.; Sakurai, M.; Liao, M.; Aono, M. Giant Improvement of the Performance of ZnO Nanowire Photodetectors by Au Nanoparticles. J. Phys. Chem. C 2010, 114, 19835–19839. [Google Scholar] [CrossRef]
- Naldoni, A.; Guler, U.; Wang, Z.; Marelli, M.; Malara, F.; Meng, X.; Besteiro, L.V.; Govorov, A.O.; Kildishev, A.V.; Boltasseva, A.; et al. Broadband Hot-Electron Collection for Solar Water Splitting with Plasmonic Titanium Nitride. Adv. Opt. Mater. 2017, 5, 1601031. [Google Scholar] [CrossRef]
- Wang, S.B.; Hsiao, C.H.; Chang, S.J.; Lam, K.T.; Wen, K.H.; Hung, S.C.; Young, S.J.; Huang, B.R. A CuO Nanowire Infrared Photodetector. Sens. Actuators A Phys. 2011, 171, 207–211. [Google Scholar] [CrossRef]


| Structure | Wavelength (nm) | Bais (V) | R (mA·W−1) |
|---|---|---|---|
| Graphene/P3HT [21] | 325 | 1 | NA |
| Polyaniline/MgZnO [16] | 250 | 5 | 0.1 |
| ZnO-CuO [20] | 405 | 1 | 3 × 10−3 |
| CuO/Si Nanowire [37] | 405 | 0.2 | 3.8 × 10−3 |
| PbI2-graphene [38] | 550 | 2 | NA |
| PbI2-5%Ag [39] | 532 | 6 | NA |
| GO/Cu2O [40] | 300 | 2 | 0.5 × 10−3 |
| ZnO/RGO [41] | 350 | 5 | 1.3 × 10−3 |
| ZnO/Cu2O [19] | 350 | 2 | 4 × 10−3 |
| TiN/TiO2 [42] | 550 | 5 | - |
| CuO nanowires [43] | 390 | 5 | - |
| ZnO/RGO [41] | 350 | 5 | 1.3 × 10−3 |
| As2O3/As(OH)3/P3MA (this work) | 440 | 2 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rabia, M.; Elsayed, A.M.; Alnuwaiser, M.A. Decoration of Poly-3-methyl Aniline with As(III) Oxide and Hydroxide as an Effective Photoelectrode for Electroanalytical Photon Sensing with Photodiode-like Behavior. Micromachines 2023, 14, 1573. https://doi.org/10.3390/mi14081573
Rabia M, Elsayed AM, Alnuwaiser MA. Decoration of Poly-3-methyl Aniline with As(III) Oxide and Hydroxide as an Effective Photoelectrode for Electroanalytical Photon Sensing with Photodiode-like Behavior. Micromachines. 2023; 14(8):1573. https://doi.org/10.3390/mi14081573
Chicago/Turabian StyleRabia, Mohamed, Asmaa M. Elsayed, and Maha Abdallah Alnuwaiser. 2023. "Decoration of Poly-3-methyl Aniline with As(III) Oxide and Hydroxide as an Effective Photoelectrode for Electroanalytical Photon Sensing with Photodiode-like Behavior" Micromachines 14, no. 8: 1573. https://doi.org/10.3390/mi14081573
APA StyleRabia, M., Elsayed, A. M., & Alnuwaiser, M. A. (2023). Decoration of Poly-3-methyl Aniline with As(III) Oxide and Hydroxide as an Effective Photoelectrode for Electroanalytical Photon Sensing with Photodiode-like Behavior. Micromachines, 14(8), 1573. https://doi.org/10.3390/mi14081573





