Design of a Low-Frequency Dielectrophoresis-Based Arc Microfluidic Chip for Multigroup Cell Sorting
Abstract
1. Introduction
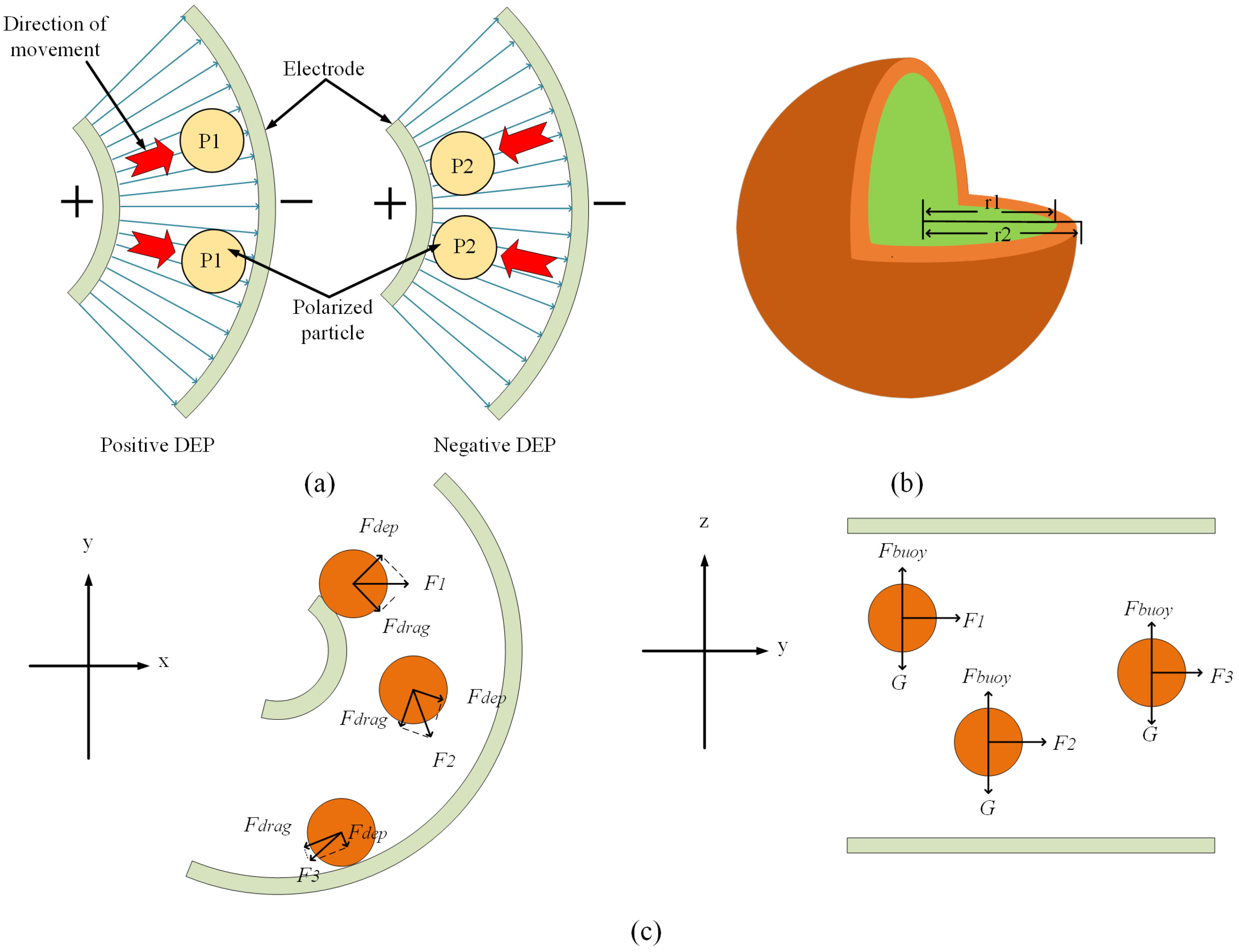
2. Theoretical Concept
3. Design and Simulation
3.1. Chip Structure Design
3.2. CM Factor Simulation
3.3. Electric Field Simulation
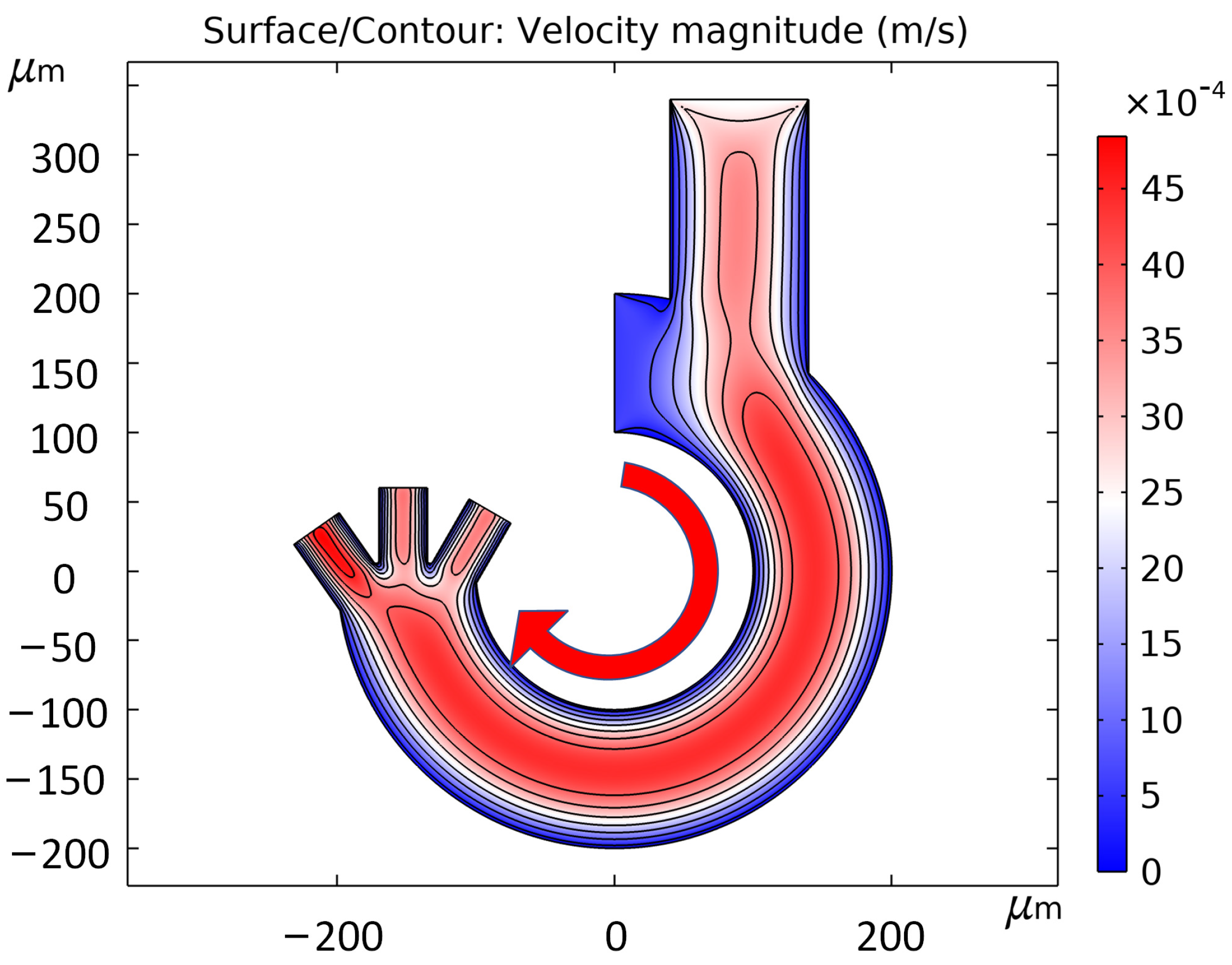
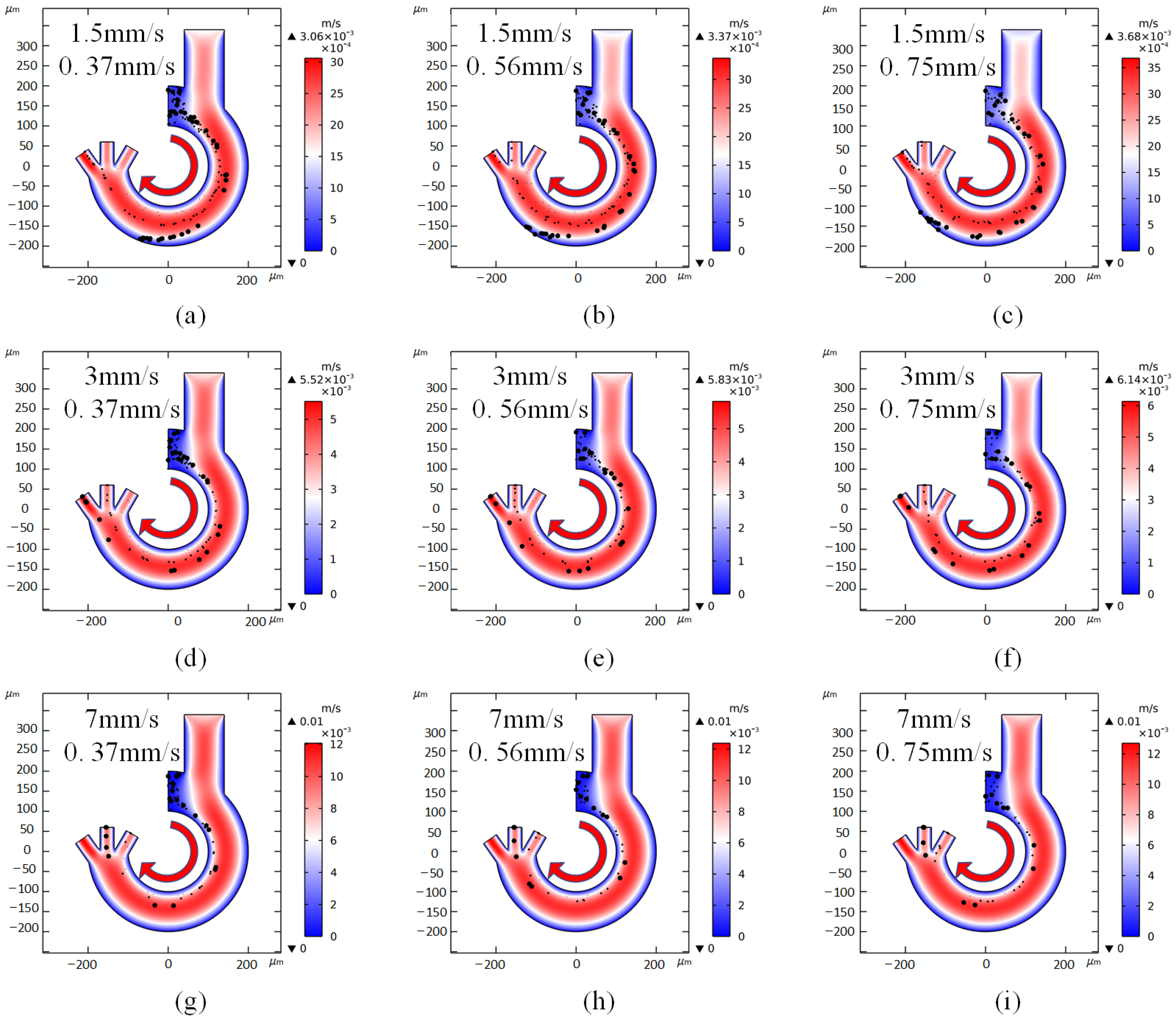
3.4. Flow Rate Simulation
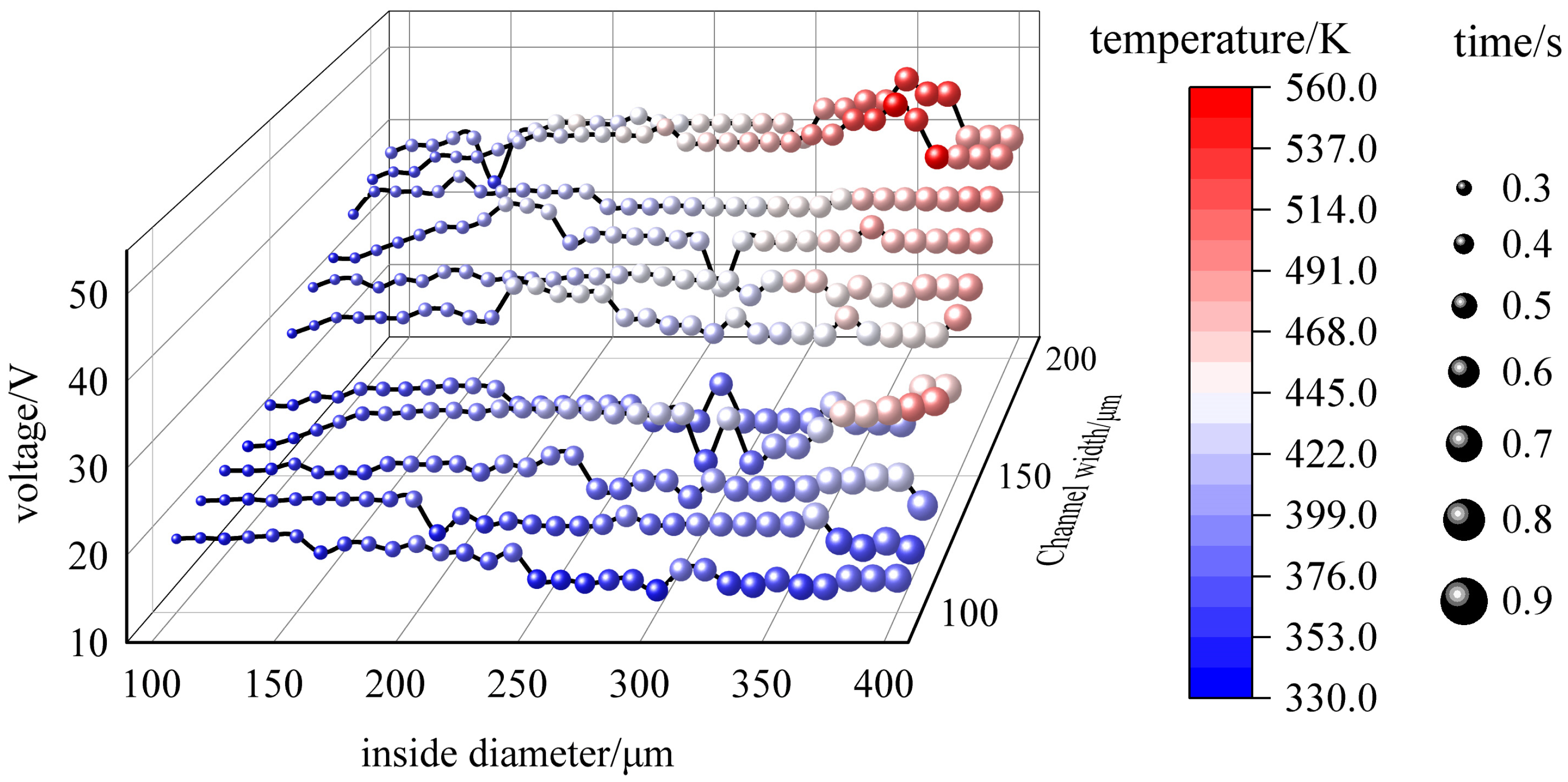
3.5. Joule Heat Simulation
- The buffer conductivity is 55 mS/m, and low conductivity is the most fundamental measure to reduce joule heat generation;
- The use of small amplitude low frequency AC at 19 V and 40 kHz;
- The minimum channel length (inner sidewall length) is 472 um, the maximum length (outer sidewall length) is 942 um and the two inlet flow rates are 0.56 mm/s and 2.4 mm/s, so that the cell stays in the channel for only 0.26~0.29 s.
3.6. Different Size Simulation
4. Results
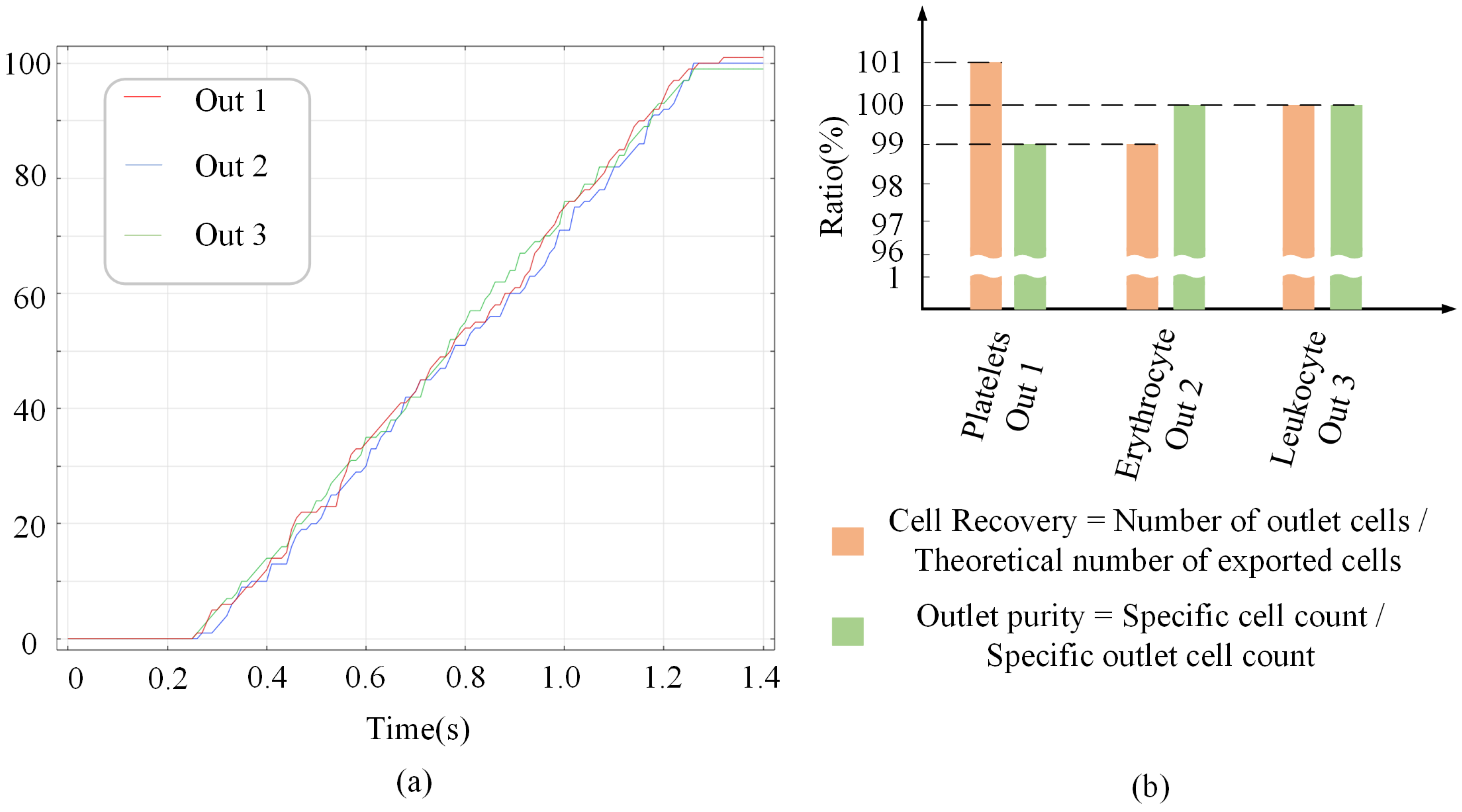
4.1. Feasibility Verification
4.2. Selectivity Verification
5. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, B.; Huang, J.; Tao, C.; Li, Z.; Zhang, D.; Yamaguchi, Y. Detection of Periodontal Pathogens Based on an Integrated Continuous Flow PCR and Capillary Electrophoresis Microfluidic Chip. Separations 2023, 10, 271. [Google Scholar] [CrossRef]
- Rozaini, A.Z.A.; Abdulhameed, A.; Deivasigamani, R.; Nadzreen, N.; Zin, N.M.; Kayani, A.A.; Buyong, M.R. Dielectrophoresis Microbial Characterization and Isolation of Staphylococcus aureus Based on Optimum Crossover Frequency. Electrophoresis 2023, 44, 1–14. [Google Scholar] [CrossRef]
- Malekanfard, A.; Beladi-Behbahani, S.; Tzeng, T.-R.; Zhao, H.; Xuan, X. AC Insulator-Based Dielectrophoretic Focusing of Particles and Cells in an “Infinite” Microchannel. Anal. Chem. 2021, 93, 5947–5953. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ding, H.; Zhong, X.; Yin, B.; Liu, Z.; Zhou, T. Mixing Mechanism of Microfluidic Mixer with Staggered Virtual Electrode Based on Light-Actuated AC Electroosmosis. Micromachines 2021, 12, 744. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, Z.; Zhang, D.; Yamaguchi, Y.; Xiao, W. Direct Count of Fluorescent Microspheres in a Microfluidic Chip Based on the Capillary Electrophoresis Method. Anal. Methods 2023, 15, 3014–3018. [Google Scholar] [CrossRef] [PubMed]
- Prüfer, M.; Stanke, S.; Bier, F.F.; Hölzel, R. Catalytic Activity of Glucose Oxidase after Dielectrophoretic Immobilization on Nanoelectrodes. Electrophoresis 2023, 44, 956–967. [Google Scholar] [CrossRef]
- Yin, B.; Qian, C.; Wang, S.; Wan, X.; Zhou, T. A Microfluidic Chip-Based MRS Immunosensor for Biomarker Detection via Enzyme-Mediated Nanoparticle Assembly. Front. Chem. 2021, 9, 688442. [Google Scholar] [CrossRef]
- Olsen, T.R.; Tapia-Alveal, C.; Wen, K.; Worgall, T.S.; Stojanovic, M.N.; Lin, Q. Microfluidic Isolation of Aptamers with Affinity towards Multiple Myeloma Monoclonal Immunoglobulins. Biomed. Microdevices 2023, 25, 3. [Google Scholar] [CrossRef]
- Pillay, E.; Coetzer, T.L. A Novel Filtering Tool for Detecting Sickle Cell Disease in the Pediatric Population. Blood 2018, 132 (Suppl. S1), 2238. [Google Scholar] [CrossRef]
- MacDonald, M.P.; Spalding, G.C.; Dholakia, K. Microfluidic Sorting in an Optical Lattice. Nature 2003, 426, 421–424. [Google Scholar] [CrossRef]
- Li, Y.; Xin, H.; Liu, X.; Zhang, Y.; Lei, H.; Li, B. Trapping and Detection of Nanoparticles and Cells Using a Parallel Photonic Nanojet Array. ACS Nano 2016, 10, 5800–5808. [Google Scholar] [CrossRef] [PubMed]
- Legut, M.; Sanjana, N.E. Immunomagnetic Cell Sorting. Nat. Biomed. Eng. 2019, 3, 759–760. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, X.; He, Z.; Hou, L.; Ge, C.; Wang, L.; Li, S.; Xu, Y. Detection of Prostate Specific Antigen in Whole Blood by Microfluidic Chip Integrated with Dielectrophoretic Separation and Electrochemical Sensing. Biosens. Bioelectron. 2022, 204, 114057. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, Y.; Wang, Z.; Duan, X. Microscale Acoustic Streaming for Biomedical and Bioanalytical Applications. TrAC Trends Anal. Chem. 2023, 160, 116958. [Google Scholar] [CrossRef]
- Han, S.-I.; Soo Kim, H.; Han, A. In-Droplet Cell Concentration Using Dielectrophoresis. Biosens. Bioelectron. 2017, 97, 41–45. [Google Scholar] [CrossRef]
- Barik, A.; Zhang, Y.; Grassi, R.; Nadappuram, B.P.; Edel, J.B.; Low, T.; Koester, S.J.; Oh, S.-H. Graphene-Edge Dielectrophoretic Tweezers for Trapping of Biomolecules. Nat. Commun. 2017, 8, 1867. [Google Scholar] [CrossRef]
- Hata, M.; Suzuki, M.; Yasukawa, T. Selective Retrieval of Antibody-Secreting Hybridomas in Cell Arrays Based on the Dielectrophoresis. Biosens. Bioelectron. 2022, 209, 114250. [Google Scholar] [CrossRef]
- Nieuwelink, A.-E.; Vollenbroek, J.C.; Tiggelaar, R.M.; Bomer, J.G.; Van Den Berg, A.; Odijk, M.; Weckhuysen, B.M. High-Throughput Activity Screening and Sorting of Single Catalyst Particles with a Droplet Microreactor Using Dielectrophoresis. Nat. Catal. 2021, 4, 1070–1079. [Google Scholar] [CrossRef]
- Gao, T.; Zhao, K.; Zhang, J.; Zhang, K. DC-Dielectrophoretic Manipulation and Isolation of Microplastic Particle-Treated Microalgae Cells in Asymmetric-Orifice-Based Microfluidic Chip. Micromachines 2023, 14, 229. [Google Scholar] [CrossRef]
- Sharbati, P.; Sadaghiani, A.K.; Koşar, A. New Generation Dielectrophoretic-Based Microfluidic Device for Multi-Type Cell Separation. Biosensors 2023, 13, 418. [Google Scholar] [CrossRef]
- Vu-Dinh, H.; Quang, L.D.; Lin, Y.; Jen, C. A Dielectrophoresis-based Platform of Cancerous Cell Capture Using Aptamer-functionalized Gold Nanoparticles in a Microfluidic Channel. Electrophoresis 2023, 44, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, N.; Li, F.; Zhao, K.; Wang, D.; Niu, Y.; Xu, F.; Cheng, J.; Wang, J. Trapping of a Single Microparticle Using AC Dielectrophoresis Forces in a Microfluidic Chip. Micromachines 2023, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, X. Blood Cells Separation Microfluidic Chip Based on Dielectrophoretic Force. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 206. [Google Scholar] [CrossRef]
- Valijam, S.; Salehi, A.; Andersson, M. Design of a Low-Voltage Dielectrophoresis Lab-on-the Chip to Separate Tumor and Blood Cells. Microfluid. Nanofluid 2023, 27, 22. [Google Scholar] [CrossRef]
- Varmazyari, V.; Habibiyan, H.; Ghafoorifard, H.; Ebrahimi, M.; Ghafouri-Fard, S. A Dielectrophoresis-Based Microfluidic System Having Double-Sided Optimized 3D Electrodes for Label-Free Cancer Cell Separation with Preserving Cell Viability. Sci. Rep. 2022, 12, 12100. [Google Scholar] [CrossRef]
- Oshiro, K.; Wakizaka, Y.; Takano, M.; Itoi, T.; Ohge, H.; Koba, K.; Yarimizu, K.; Fujiyoshi, S.; Maruyama, F. Fabrication of a New All-in-One Microfluidic Dielectrophoresis Integrated Chip and Living Cell Separation. iScience 2022, 25, 103776. [Google Scholar] [CrossRef]
- Ur Rehman, A.; Zabibah, R.S.; Kharratian, S.; Mustafa, A. Microfluidic Device for the Separation of Non-Metastatic (MCF-7) and Non-Tumor (MCF-10A) Breast Cancer Cells Using AC Dielectrophoresis. J. Vis. Exp. 2022, 186, 63850. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, P.; Dong, J.; Wei, Y.; Chen, B.; Wang, Y.; Pan, X.; Wang, J. Implementation of an Integrated Dielectrophoretic and Magnetophoretic Microfluidic Chip for CTC Isolation. Biosensors 2022, 12, 757. [Google Scholar] [CrossRef]
- Tada, S.; Seki, Y. Analysis of Temperature Field in the Dielectrophoresis-Based Microfluidic Cell Separation Device. Fluids 2022, 7, 263. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Pesch, G.R.; Baune, M.; Du, F.; Liu, X. Rational Design and Numerical Analysis of a Hybrid Floating CIDE Separator for Continuous Dielectrophoretic Separation of Microparticles at High Throughput. Micromachines 2022, 13, 582. [Google Scholar] [CrossRef] [PubMed]
- Cottet, J.; Fabregue, O.; Berger, C.; Buret, F.; Renaud, P.; Frénéa-Robin, M. MyDEP: A New Computational Tool for Dielectric Modeling of Particles and Cells. Biophys. J. 2019, 116, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Pakhira, W.; Kumar, R.; Ibrahimi, K.M.; Bhattacharjee, R. Design and Analysis of a Microfluidic Lab-on-Chip Utilizing Dielectrophoresis Mechanism for Medical Diagnosis and Liquid Biopsy. J Braz. Soc. Mech. Sci. Eng. 2022, 44, 482. [Google Scholar] [CrossRef]
- Nguyen, N.-V.; Van Manh, H.; Van Hieu, N. Numerical Simulation-Based Performance Improvement of the Separation of Circulating Tumor Cells from Bloodstream in a Microfluidic Platform by Dielectrophoresis. Korea-Aust. Rheol. J. 2022, 34, 335–347. [Google Scholar] [CrossRef]




| Name | Value |
|---|---|
| L1 | 100 μm |
| L2 | 30 μm |
| L3 | 40 μm |
| L4 | 35 μm |
| L5 | 35 μm |
| L6 | 60 μm |
| L7 | 200 μm |
| R1 | 200 μm |
| R2 | 100 μm |
| θ1 | 90° |
| θ2 | θ2 + θ3 = 90° |
| θ3 | θ3 = θ4 |
| θ4 | θ4 = θ3 |
| Cell Type | r2 [μm] | σ1 [S/m] | ε1 | σ2 [S/m] | ε2 | r2 − r1 [nm] | Ref |
|---|---|---|---|---|---|---|---|
| Platelets | 1.8 | 0.25 | 50 | 1.00 × 10−6 | 6 | 8 | [32] |
| Erythrocyte | 5 | 0.31 | 59 | 1.00 × 10−6 | 4.44 | 9 | [32] |
| Leukocyte | 12 | 0.65 | 60 | 2.74 × 10−5 | 6 | 7 | [33] |
| A549 | 17 | 0.78 | 52 | 2.50 × 10−5 | 11.75 | 15 | [33] |
| MCF-7 | 17 | 0.8 | 50 | 1.00 × 10−6 | 11 | 7 | [27] |
| MCF-10A | 16 | 0.6 | 100 | 1.00 × 10−6 | 11 | 7 | [27] |
| T-lymphocyte | 3.4 | 0.65 | 60 | 2.74 × 10−5 | 11.1 | 7 | [28] |
| HT-29 | 11 | 0.72 | 120 | 3.40 × 10−5 | 11.1 | 4 | [28] |
| MDA-MB-231 | 12.4 | 0.62 | 52 | 1.00 × 10−6 | 11.75 | 4 | [28] |
| Number of Cell Types | Voltage | σm | Inlet 1 Flow Rate | Out 1 | Out 2 | Out 3 |
|---|---|---|---|---|---|---|
| 4 | 12 V | 0.055 S/m | 2.4 mm/s | PLT, RBC | RBC, WBC | A549 |
| 2 | 12 V | 0.055 S/m | 60 mm/s | MCF-10A, MCF-7 | MCF-10A, MCF-7 | |
| 3 | 22 V | 0.1 S/m | 2.4 mm/s | PLT, TC | HT-29 | |
| 3 | 12 V | 0.1 S/m | 2.4 mm/s | PLT | HT-29, MDA-231 | HT-29, MDA-231 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nan, X.; Zhang, J.; Wang, X.; Kang, T.; Cao, X.; Hao, J.; Jia, Q.; Qin, B.; Mei, S.; Xu, Z. Design of a Low-Frequency Dielectrophoresis-Based Arc Microfluidic Chip for Multigroup Cell Sorting. Micromachines 2023, 14, 1561. https://doi.org/10.3390/mi14081561
Nan X, Zhang J, Wang X, Kang T, Cao X, Hao J, Jia Q, Qin B, Mei S, Xu Z. Design of a Low-Frequency Dielectrophoresis-Based Arc Microfluidic Chip for Multigroup Cell Sorting. Micromachines. 2023; 14(8):1561. https://doi.org/10.3390/mi14081561
Chicago/Turabian StyleNan, Xueli, Jiale Zhang, Xin Wang, Tongtong Kang, Xinxin Cao, Jinjin Hao, Qikun Jia, Bolin Qin, Shixuan Mei, and Zhikuan Xu. 2023. "Design of a Low-Frequency Dielectrophoresis-Based Arc Microfluidic Chip for Multigroup Cell Sorting" Micromachines 14, no. 8: 1561. https://doi.org/10.3390/mi14081561
APA StyleNan, X., Zhang, J., Wang, X., Kang, T., Cao, X., Hao, J., Jia, Q., Qin, B., Mei, S., & Xu, Z. (2023). Design of a Low-Frequency Dielectrophoresis-Based Arc Microfluidic Chip for Multigroup Cell Sorting. Micromachines, 14(8), 1561. https://doi.org/10.3390/mi14081561





