Nmr-VSM: Non-Touch Motion-Robust Vital Sign Monitoring via UWB Radar Based on Deep Learning
Abstract
1. Introduction
1.1. Motivation and Challenges
1.2. Contributions and Paper Organization
- We design a UWB radar that can not only monitor human heart rate and respiratory rate in real time but also capture parameters such as human motion status and distance in real time, achieving the monitoring of multi-dimensional vital signs.
- We take the radar’s heart rate monitoring as an example, collect data through real experiments, and subsequently analyze the impact of human motion status on the accuracy of heart rate monitoring. At the same time, we combine variables such as angle, distance, direction, and the subject’s gender, weight, height, etc., and propose a DNN-based heart rate data correction model, which improves the robustness of the system in motion environments.
- We model the HRV of the human body and propose a CNN-based cardiac anomaly detection model. Additionally, we propose a metric, the latency value k, to evaluate the performance of this model. Verification results demonstrate that this model achieves low-latency detection of heart diseases such as ventricular tachycardia and ventricular fibrillation.
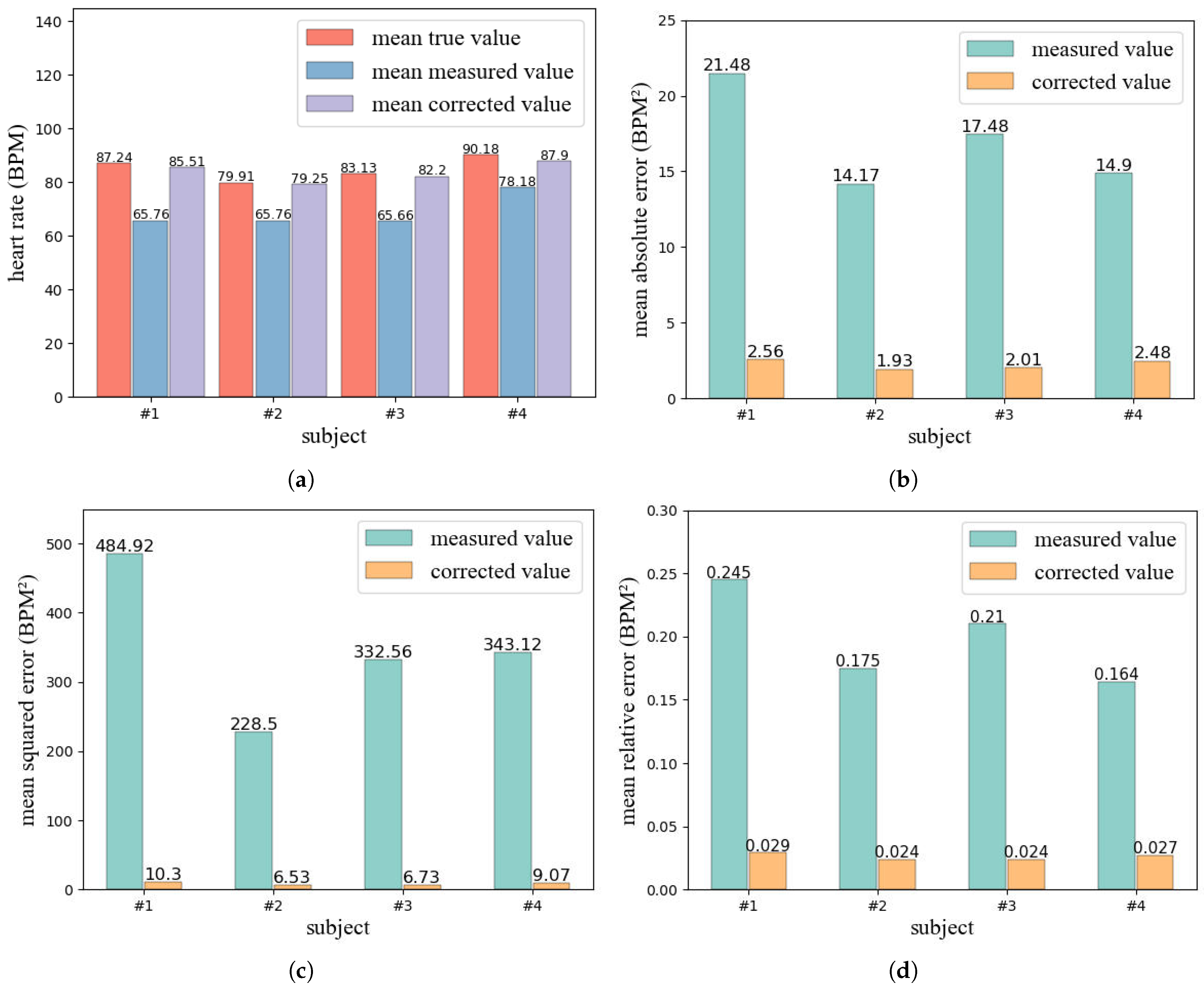
- We conduct experiments in a real environment and the results show that, after correcting the heart rate detected by UWB radar, mean absolute error (MAE), mean squared error (MSE), and mean relative error (MRE), compared with before correction, all at least decreased by more than 85%, and the heart rate change trend after correction is more consistent with the real heart rate change trend. In addition, our designed anomaly detection model achieves a high detection success rate with low latency.
2. Related Work
2.1. UWB Radar
2.2. Vital Sign Monitoring
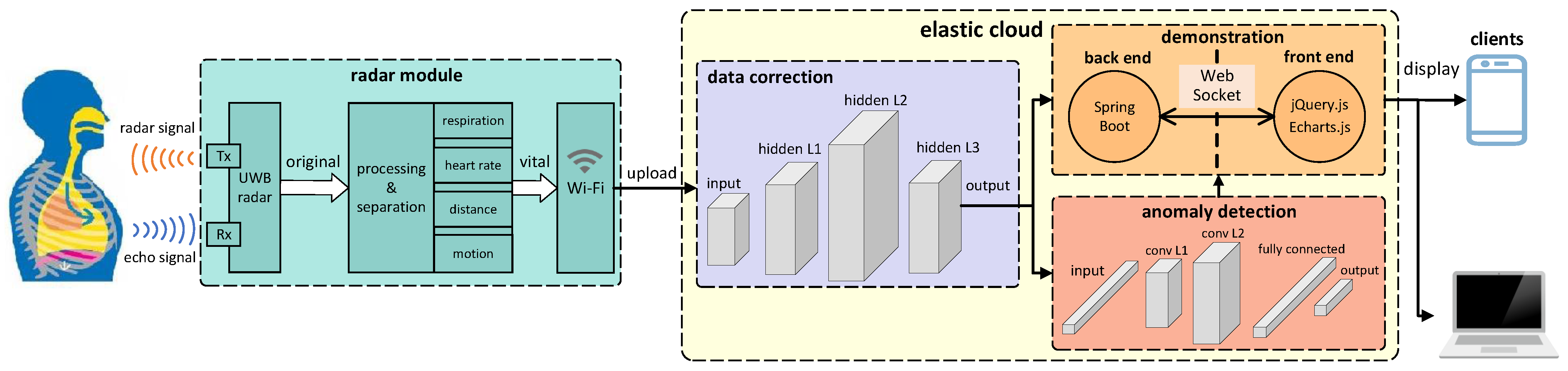
3. System Design
3.1. Preliminary
3.2. System Overview
3.3. Radar Module
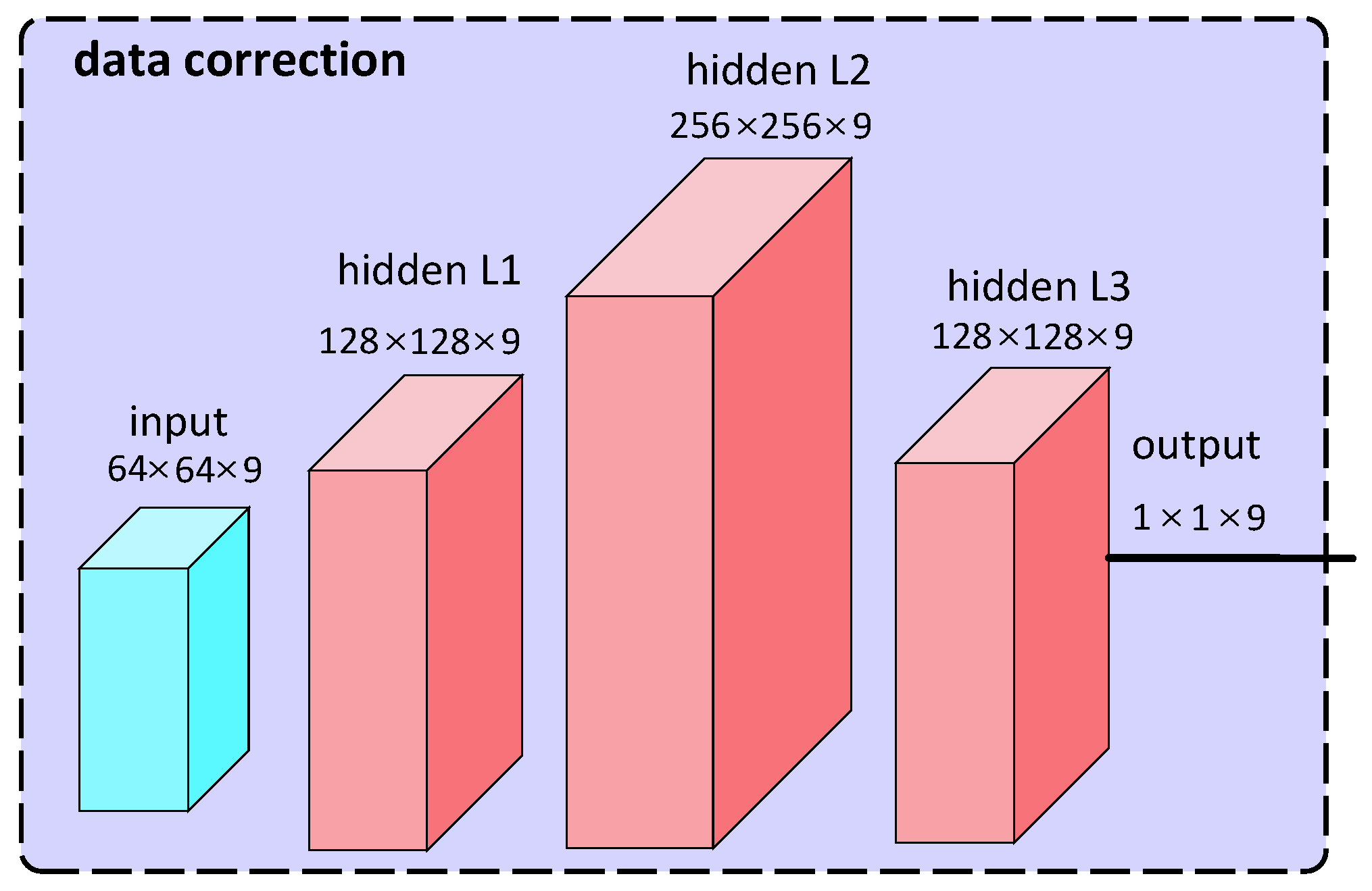
3.4. Data Correction
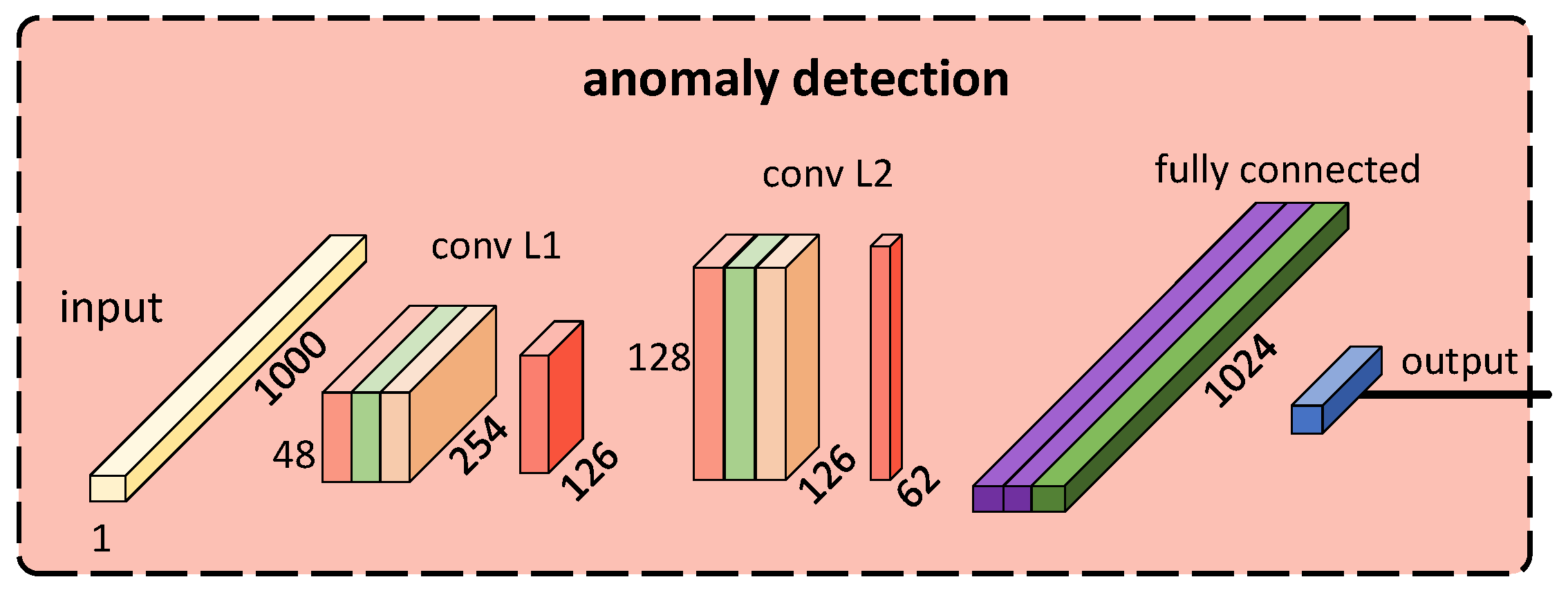
3.5. Anomaly Detection
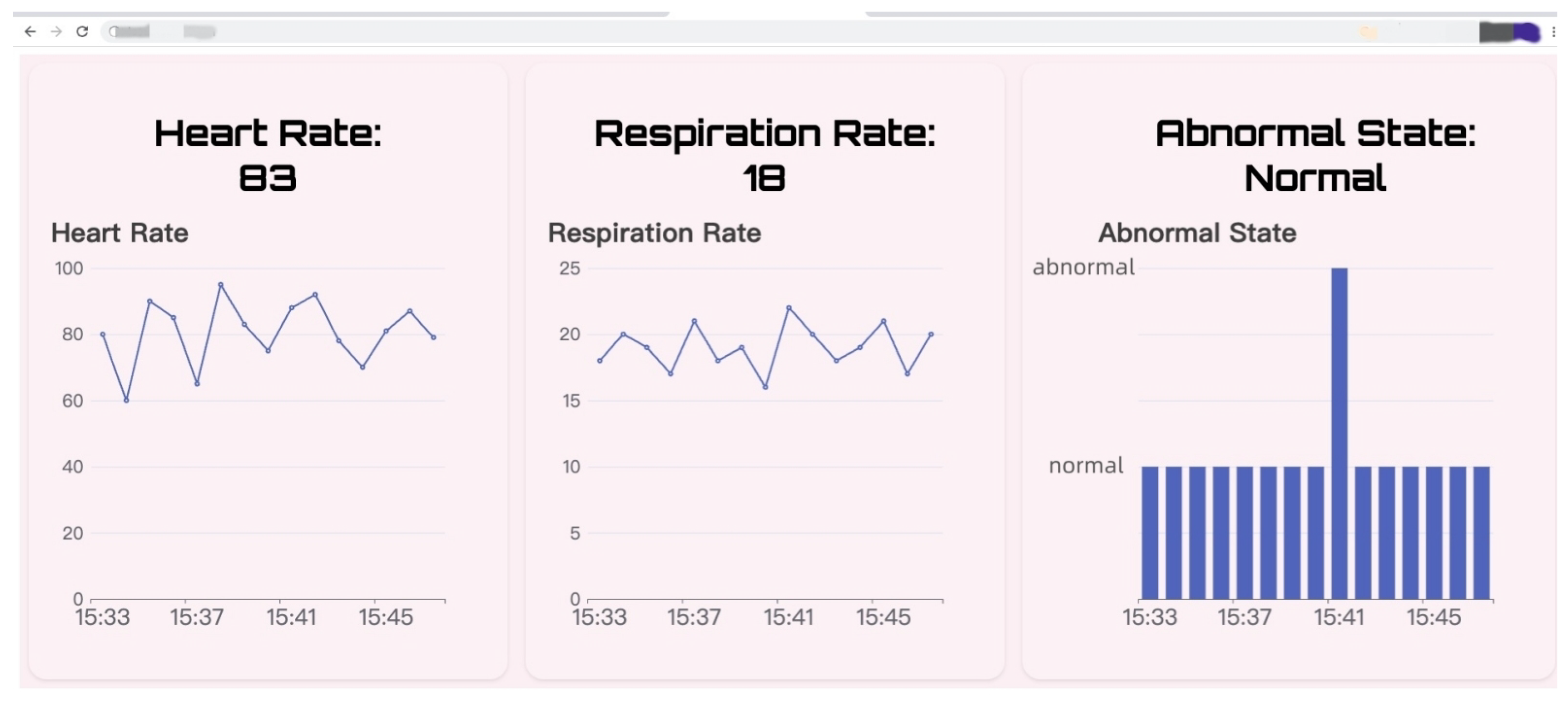
3.6. Demonstration
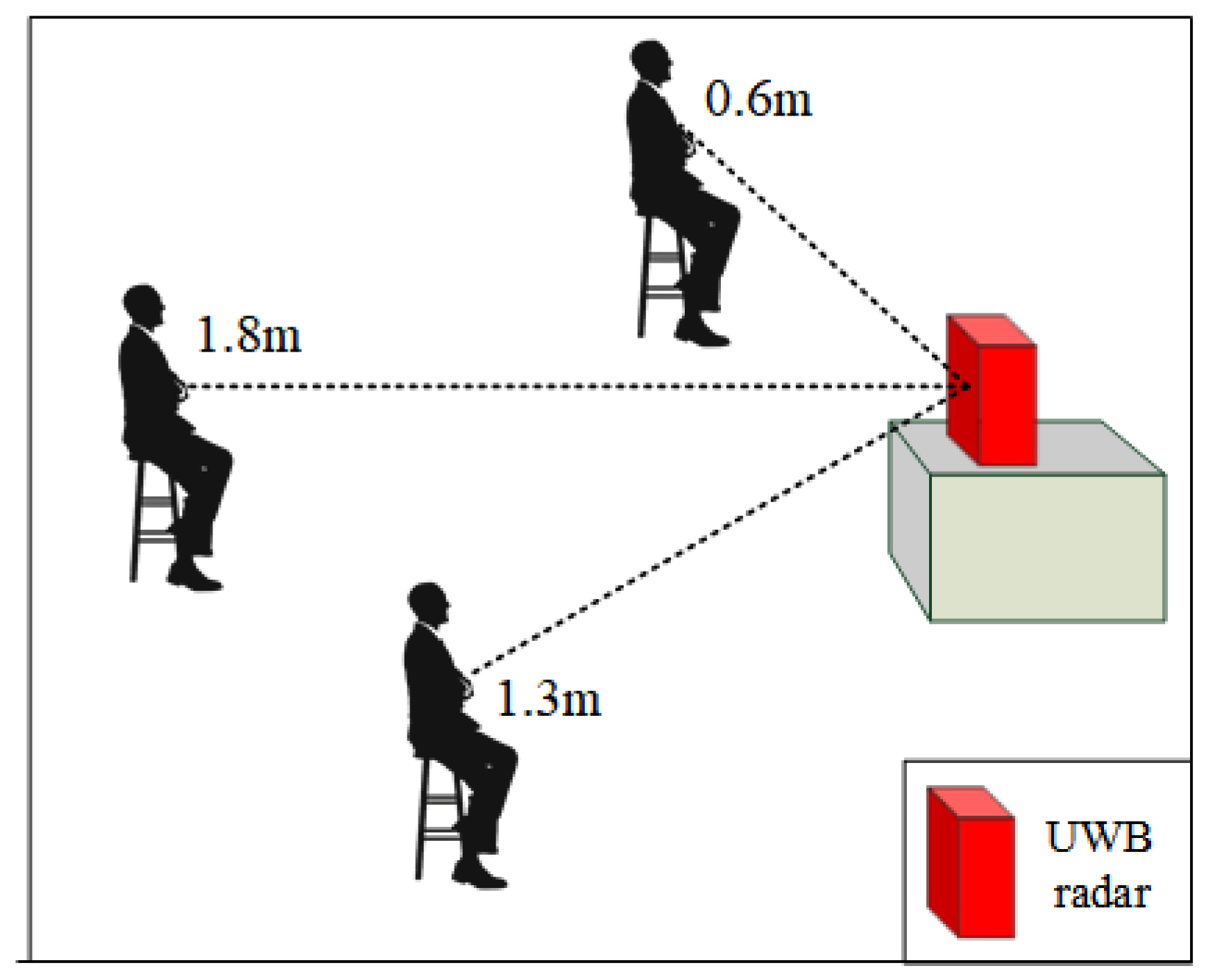
4. Evaluation
4.1. Data Correction Model
4.2. Anomaly Detection Model
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Topfer, F.; Oberhammer, J. Millimeter-wave tissue diagnosis: The most promising fields for medical applications. IEEE Microw. Mag. 2015, 16, 97–113. [Google Scholar] [CrossRef]
- Wang, C.; Xie, L.; Wang, W.; Chen, Y.; Bu, Y.; Lu, S. Rf-ecg: Heart rate variability assessment based on cots rfid tag array. In Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies; Association for Computing Machinery: New York, NY, USA, 2018; Volume 2, pp. 1–26. [Google Scholar]
- Yang, B.; Yu, C.; Dong, Y. Capacitively coupled electrocardiogram measuring system and noise reduction by singular spectrum analysis. IEEE Sens. J. 2016, 16, 3802–3810. [Google Scholar] [CrossRef]
- Kranjec, J.; Beguš, S.; Geršak, G.; Drnovšek, J. Non-contact heart rate and heart rate variability measurements: A review. Biomed. Signal Process. Control 2014, 13, 102–112. [Google Scholar] [CrossRef]
- Li, C.; Lubecke, V.M.; Boric-Lubecke, O.; Lin, J. A review on recent advances in Doppler radar sensors for noncontact healthcare monitoring. IEEE Trans. Microw. Theory Tech. 2013, 61, 2046–2060. [Google Scholar] [CrossRef]
- Krishnan, S.; Sharma, P.; Guoping, Z.; Woon, O.H. A UWB based localization system for indoor robot navigation. In Proceedings of the 2007 IEEE International Conference on Ultra-Wideband, Singapore, 24–26 September 2007; pp. 77–82. [Google Scholar]
- Alarifi, A.; Al-Salman, A.; Alsaleh, M.; Alnafessah, A.; Al-Hadhrami, S.; Al-Ammar, M.A.; Al-Khalifa, H.S. Ultra wideband indoor positioning technologies: Analysis and recent advances. Sensors 2016, 16, 707. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, K.; Hu, J. Real-time fire site map Construction based on the UWB/LiDAR. In Proceedings of the 2022 International Conference on Microwave and Millimeter Wave Technology (ICMMT), Harbin, China, 12–15 August 2022; pp. 1–3. [Google Scholar]
- Xia, X.; Xiong, L.; Huang, Y.; Lu, Y.; Gao, L.; Xu, N.; Yu, Z. Estimation on IMU yaw misalignment by fusing information of automotive onboard sensors. Mech. Syst. Signal Process. 2022, 162, 107993. [Google Scholar] [CrossRef]
- Xia, X.; Hashemi, E.; Xiong, L.; Khajepour, A. Autonomous Vehicle Kinematics and Dynamics Synthesis for Sideslip Angle Estimation Based on Consensus Kalman Filter. IEEE Trans. Control Syst. Technol. 2022, 31, 179–192. [Google Scholar] [CrossRef]
- Mokhtari, G.; Zhang, Q.; Hargrave, C.; Ralston, J.C. Non-wearable UWB sensor for human identification in smart home. IEEE Sens. J. 2017, 17, 3332–3340. [Google Scholar] [CrossRef]
- Rana, S.P.; Dey, M.; Ghavami, M.; Dudley, S. Non-contact human gait identification through IR-UWB edge-based monitoring sensor. IEEE Sens. J. 2019, 19, 9282–9293. [Google Scholar] [CrossRef]
- Walch, O.; Huang, Y.; Forger, D.; Goldstein, C. Sleep stage prediction with raw acceleration and photoplethysmography heart rate data derived from a consumer wearable device. Sleep 2019, 42, Zsz180. [Google Scholar] [CrossRef]
- Drew, B.J.; Califf, R.M.; Funk, M.; Kaufman, E.S.; Krucoff, M.W.; Laks, M.M.; Macfarlane, P.W.; Sommargren, C.; Swiryn, S.; Hare, G.F.V. Practice standards for electrocardiographic monitoring in hospital settings: An American Heart Association scientific statement from the Councils on Cardiovascular Nursing, Clinical Cardiology, and Cardiovascular Disease in the Young: Endorsed by the International Society of Computerized Electrocardiology and the American Association of Critical-Care Nurses. Circulation 2004, 110, 2721–2746. [Google Scholar]
- Mesleh, A.; Skopin, D.; Baglikov, S.; Quteishat, A. Heart rate extraction from vowel speech signals. J. Comput. Sci. Technol. 2012, 27, 1243–1251. [Google Scholar] [CrossRef]
- Poh, M.Z.; McDuff, D.J.; Picard, R.W. Non-contact, automated cardiac pulse measurements using video imaging and blind source separation. Opt. Express 2010, 18, 10762–10774. [Google Scholar] [CrossRef] [PubMed]
- Poh, M.Z.; McDuff, D.J.; Picard, R.W. Advancements in noncontact, multiparameter physiological measurements using a webcam. IEEE Trans. Biomed. Eng. 2010, 58, 7–11. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, C.; Wang, B.; Wu, M.; Bugos, D.; Zhang, H.; Liu, K.R. SMARS: Sleep monitoring via ambient radio signals. IEEE Trans. Mob. Comput. 2019, 20, 217–231. [Google Scholar] [CrossRef]
- Wu, C.; Yang, Z.; Zhou, Z.; Liu, X.; Liu, Y.; Cao, J. Non-invasive detection of moving and stationary human with WiFi. IEEE J. Sel. Areas Commun. 2015, 33, 2329–2342. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, F.; Wu, C.; Wang, B.; Liu, K.R. ViMo: Multiperson vital sign monitoring using commodity millimeter-wave radio. IEEE Internet Things J. 2020, 8, 1294–1307. [Google Scholar] [CrossRef]
- Obeid, D.; Sadek, S.; Zaharia, G.; El Zein, G. Multitunable microwave system for touchless heartbeat detection and heart rate variability extraction. Microw. Opt. Technol. Lett. 2010, 52, 192–198. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.Y.; Choi, Y.W.; Park, H.K.; Cho, S.H.; Cho, S.H.; Lim, Y.H. A novel non-contact heart rate monitor using impulse-radio ultra-wideband (IR-UWB) radar technology. Sci. Rep. 2018, 8, 13053. [Google Scholar] [CrossRef]
- Varanini, M.; Berardi, P.C.; Conforti, F.; Micalizzi, M.; Neglia, D.; Macerata, A. Cardiac and respiratory monitoring through non-invasive and contactless radar technique. In Proceedings of the 2008 Computers in Cardiology, Bologna, Italy, 14–17 September 2008; pp. 149–152. [Google Scholar]
- Fletcher, R.; Han, J. Low-cost differential front-end for Doppler radar vital sign monitoring. In Proceedings of the 2009 IEEE MTT-S International Microwave Symposium Digest, Boston, MA, USA, 7–12 June 2009; pp. 1325–1328. [Google Scholar]
- Kendall, M.G. Rank Correlation Methods; American Psychological Association: Washington, DC, USA, 1948. [Google Scholar]
- Malik, M.; Bigger, J.T.; Camm, A.J.; Kleiger, R.E.; Malliani, A.; Moss, A.J.; Schwartz, P.J. Heart rate variability: Standards of measurement, physiological interpretation, and clinical use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Reed, M.J.; Robertson, C.E.; Addison, P.S. Heart rate variability measurements and the prediction of ventricular arrhythmias. QJM Int. J. Med. 2005, 98, 87–95. [Google Scholar] [CrossRef]
- Appelhans, B.M.; Luecken, L.J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef]
- Xiao, M.; Yan, H.; Song, J.; Yang, Y.; Yang, X. Sleep stages classification based on heart rate variability and random forest. Biomed. Signal Process. Control 2013, 8, 624–633. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov, P.C.; Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a new research resource for complex physiologic signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef] [PubMed]
- Irurzun, I.M.; Garavaglia, L.; Defeo, M.M.; Maill, J.T. RR interval time series from healthy subjects (version 1.0.0). PhysioNet 2000. [Google Scholar] [CrossRef]
- Garavaglia, L.; Gulich, D.; Defeo, M.M.; Maill, J.T.; Irurzun, I.M. The Effect of Age on the Heart Rate Variability of Healthy Subjects. PLoS ONE 2021, 16, e0255894. [Google Scholar] [CrossRef] [PubMed]






| Category | Frequency (HZ) | Amplitude (mm) |
|---|---|---|
| respiration | 0.1–0.3 | 4–12 |
| heartbeat | 1–2 | 0.2–0.5 |
| Abnormal Segment | Average RR Interval (ms) | Latency Value k | Detection Result |
|---|---|---|---|
| 1 | 350 | 7 | success |
| 2 | 315 | 6 | success |
| 3 | 450 | 7 | success |
| 4 | 350 | 5 | success |
| 5 | 299.5 | 1 | success |
| 6 | 502 | 8 | success |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, Z.; Lu, S.; He, Y.; Liu, X.; Fang, J. Nmr-VSM: Non-Touch Motion-Robust Vital Sign Monitoring via UWB Radar Based on Deep Learning. Micromachines 2023, 14, 1479. https://doi.org/10.3390/mi14071479
Yuan Z, Lu S, He Y, Liu X, Fang J. Nmr-VSM: Non-Touch Motion-Robust Vital Sign Monitoring via UWB Radar Based on Deep Learning. Micromachines. 2023; 14(7):1479. https://doi.org/10.3390/mi14071479
Chicago/Turabian StyleYuan, Zhonghang, Shuaibing Lu, Yi He, Xuetao Liu, and Juan Fang. 2023. "Nmr-VSM: Non-Touch Motion-Robust Vital Sign Monitoring via UWB Radar Based on Deep Learning" Micromachines 14, no. 7: 1479. https://doi.org/10.3390/mi14071479
APA StyleYuan, Z., Lu, S., He, Y., Liu, X., & Fang, J. (2023). Nmr-VSM: Non-Touch Motion-Robust Vital Sign Monitoring via UWB Radar Based on Deep Learning. Micromachines, 14(7), 1479. https://doi.org/10.3390/mi14071479







