Graphene Oxide Paper Manipulation of Micro-Reactor Drops
Abstract
1. Introduction
2. Materials and Methods
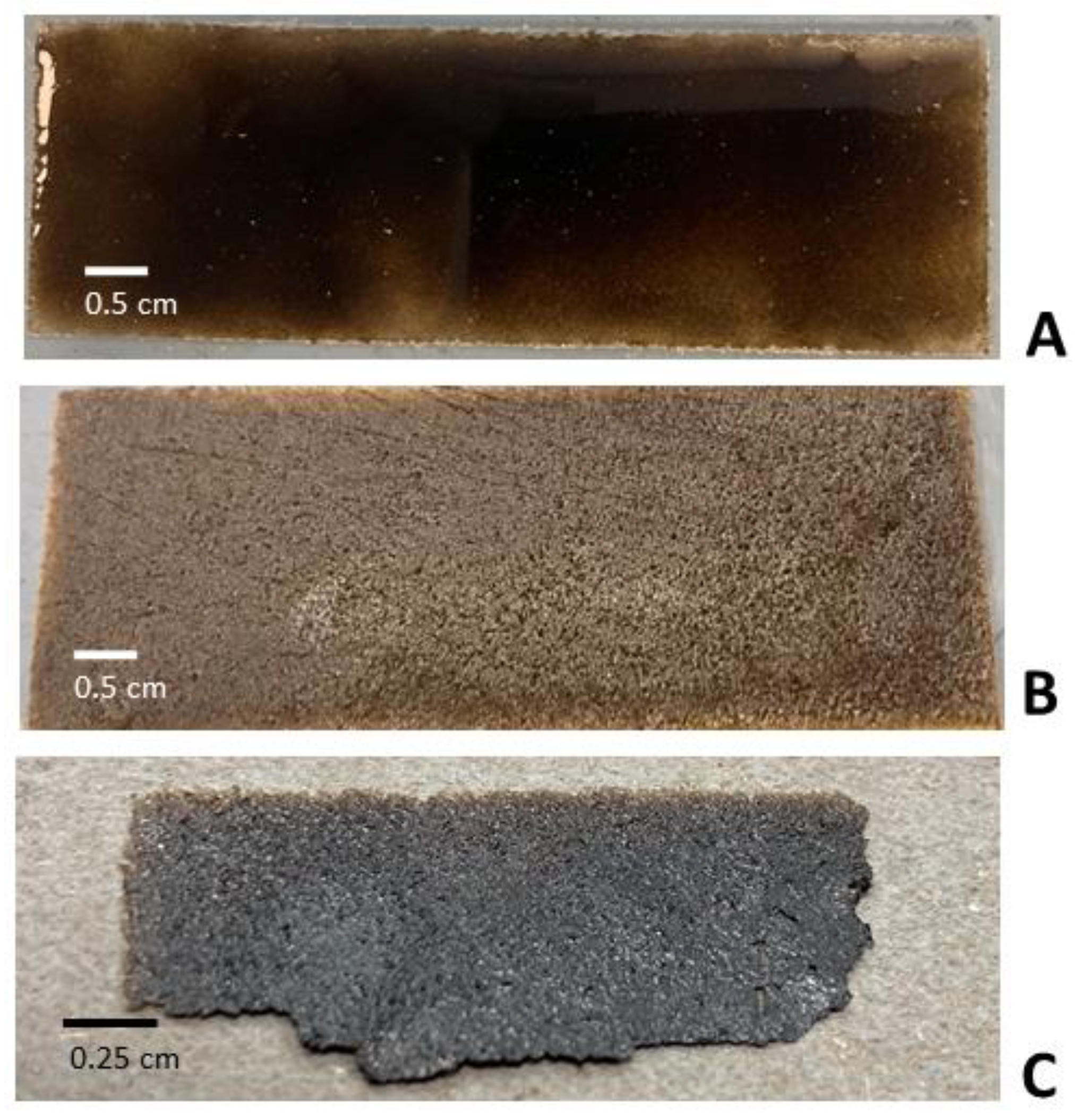
2.1. GO Paper Preparation
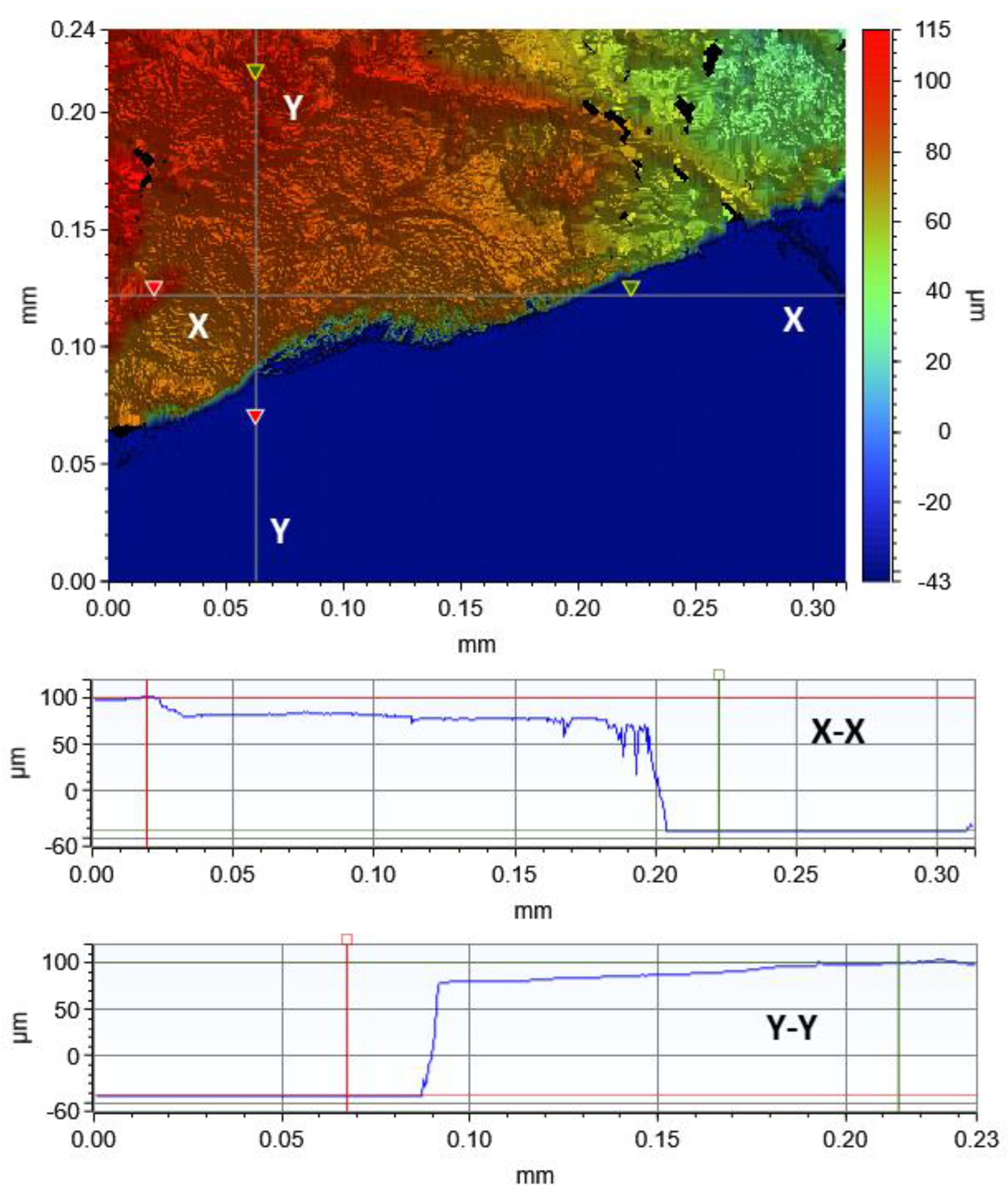
2.2. Thickness Characterization
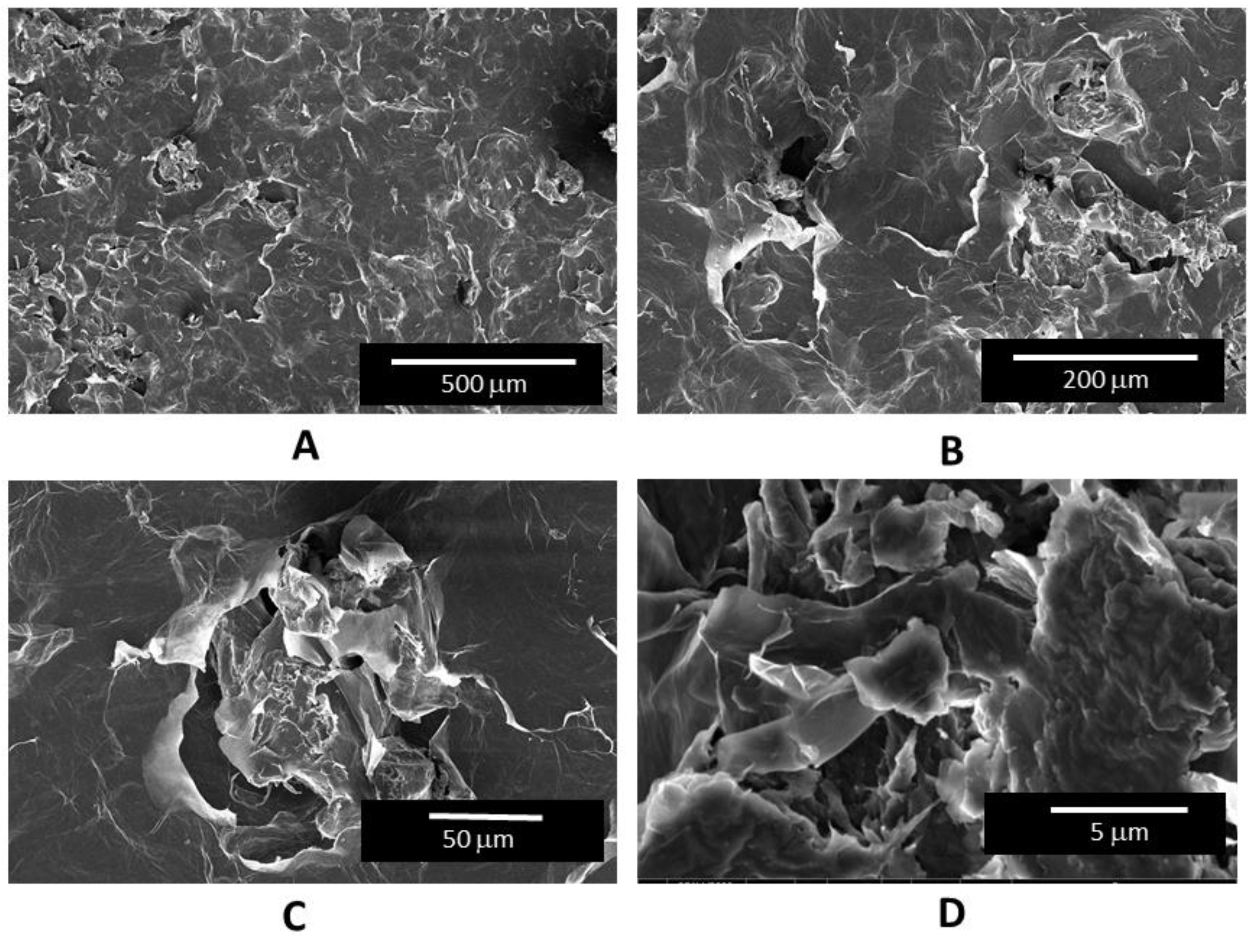
2.3. Scanning Electron Microscopy Characterization
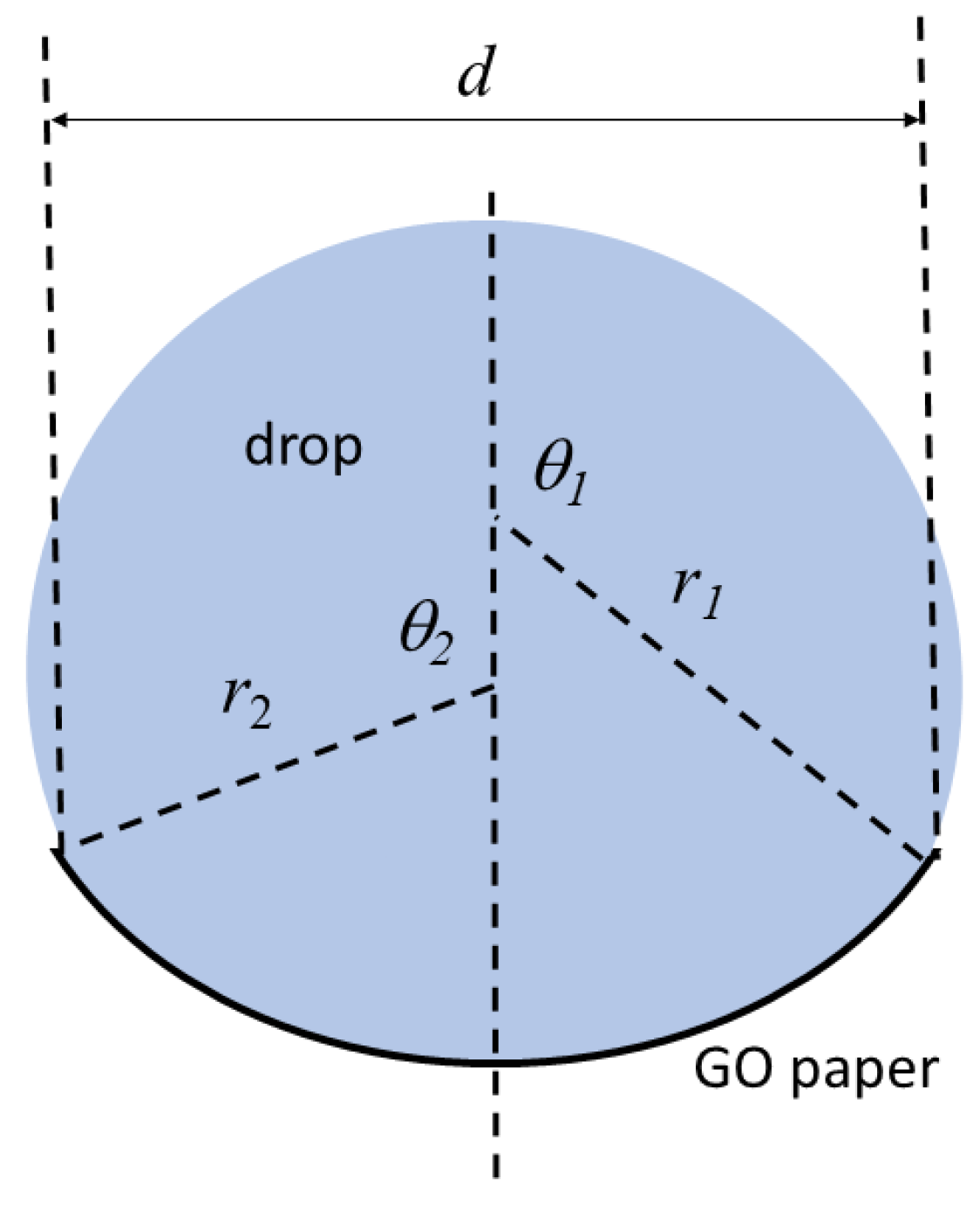
2.4. Wettability Characterization
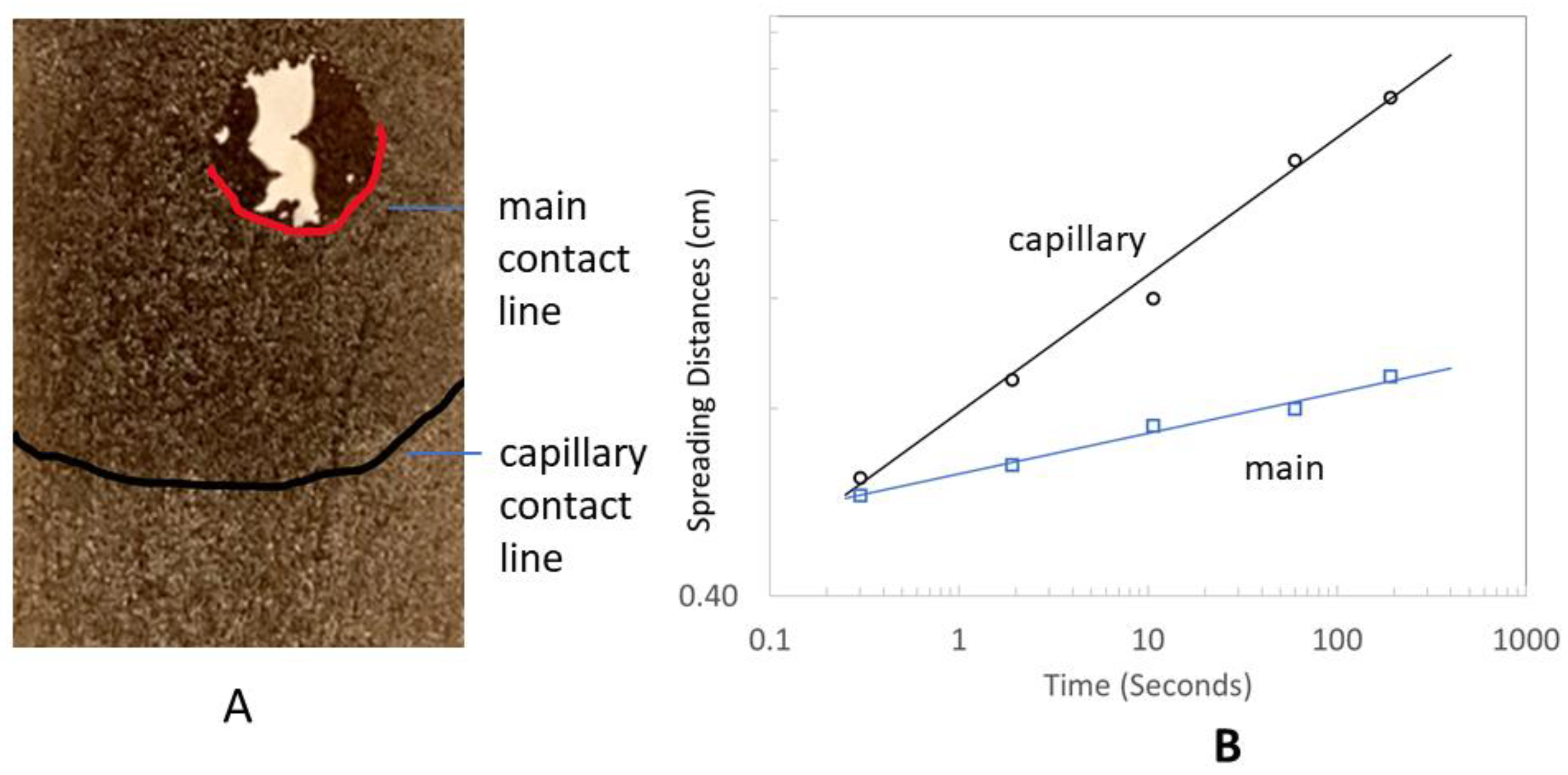
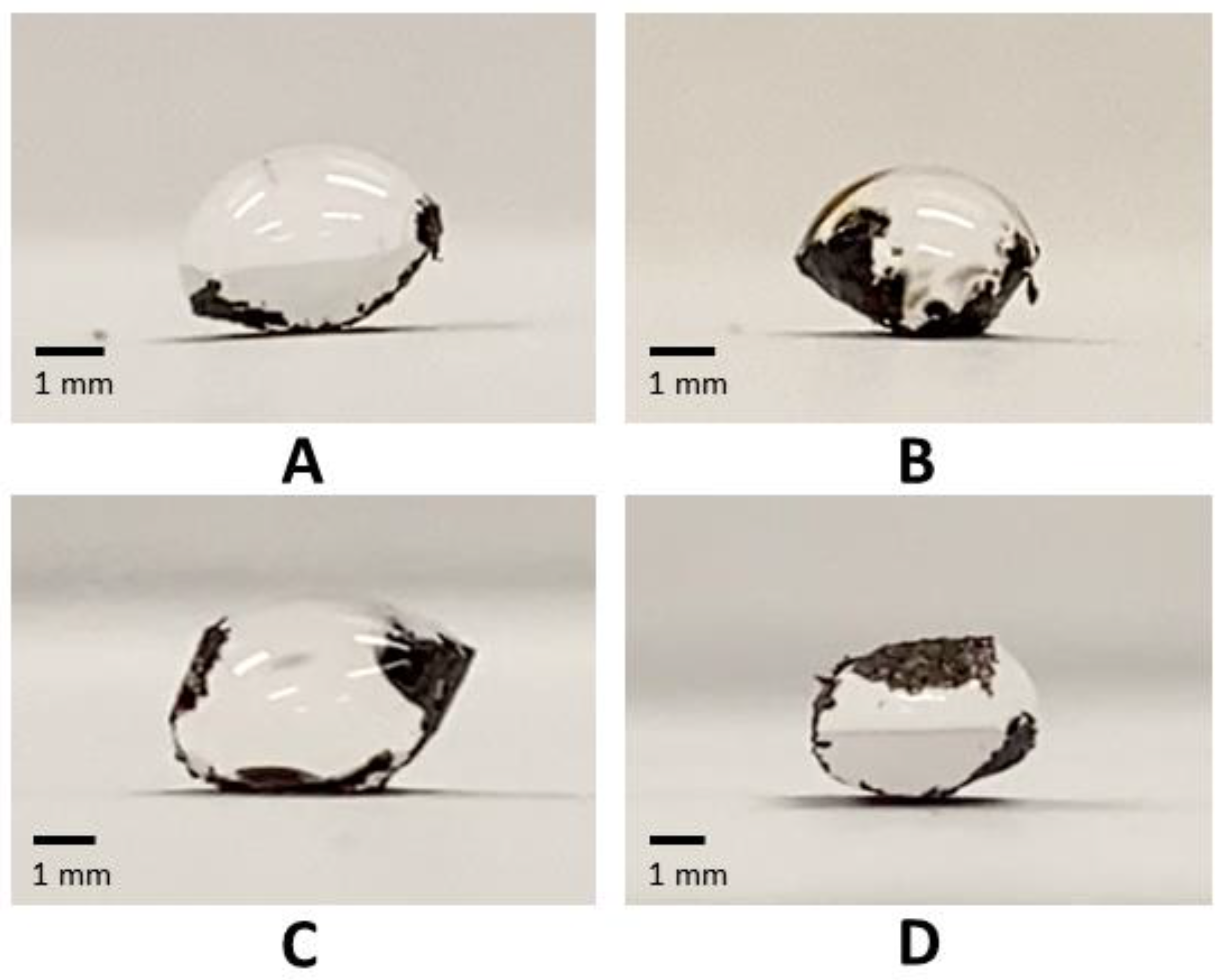
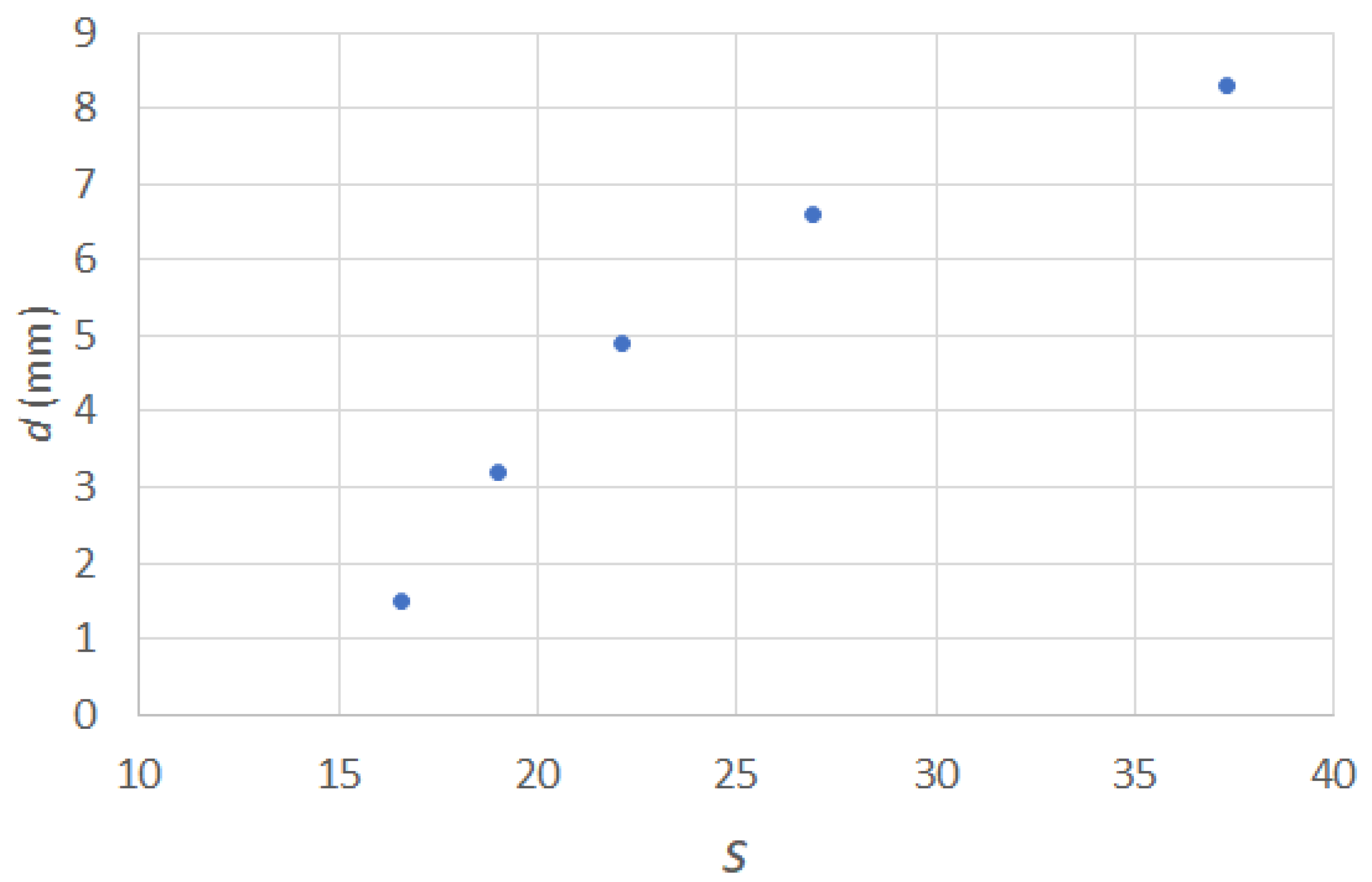
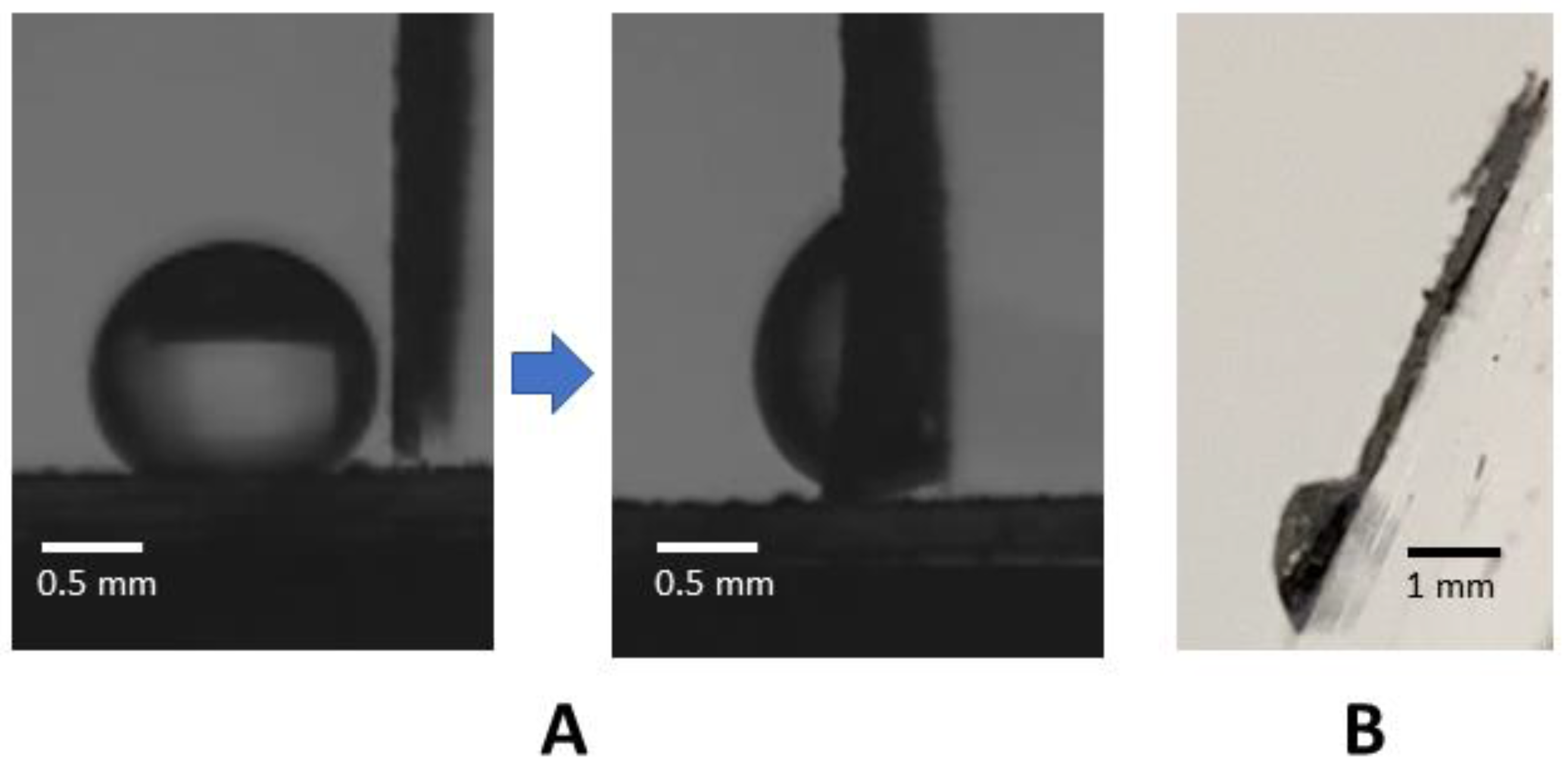
2.5. Elasto-Capillary Wrapping Behavior
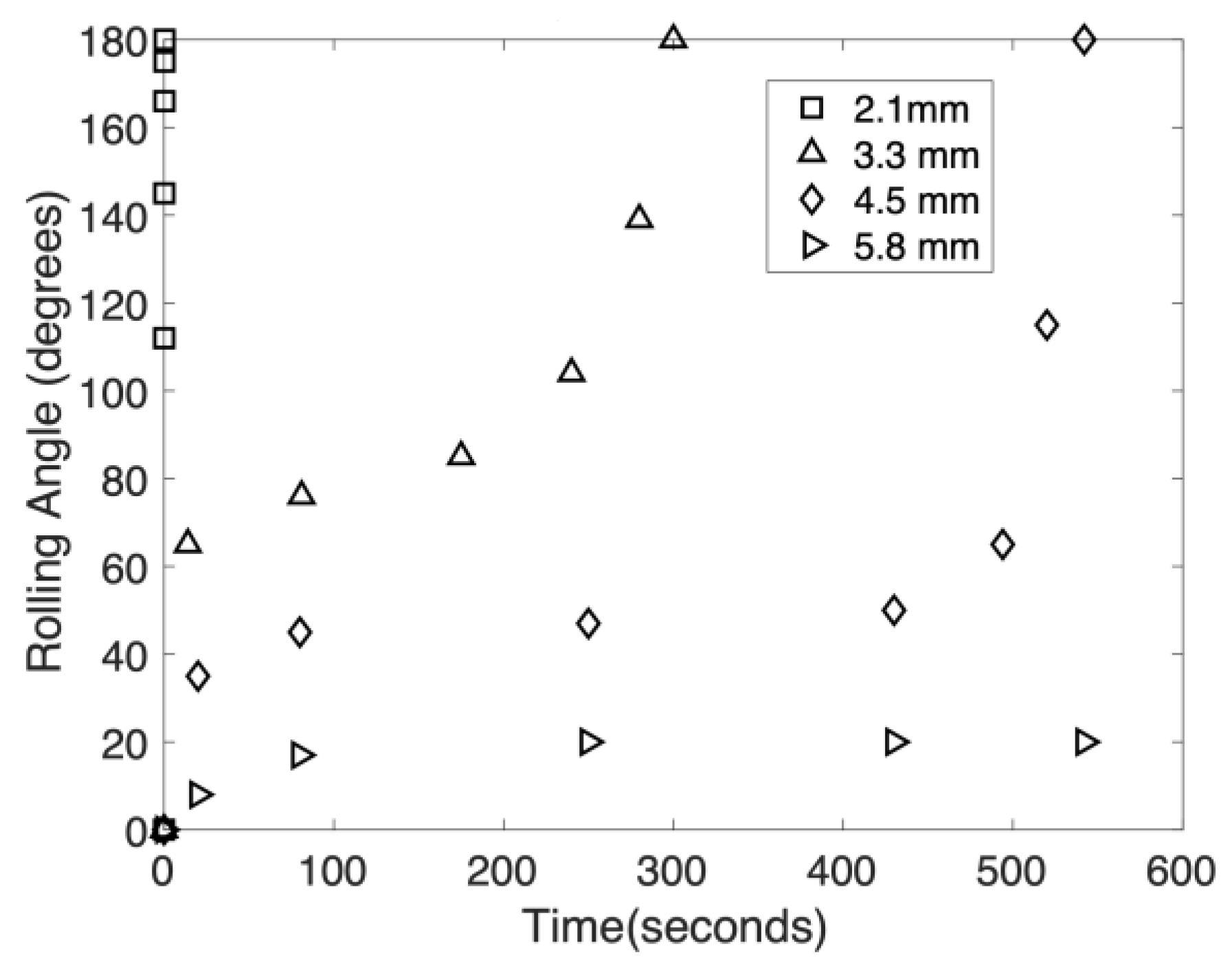
2.6. Edge Wrapping Behavior
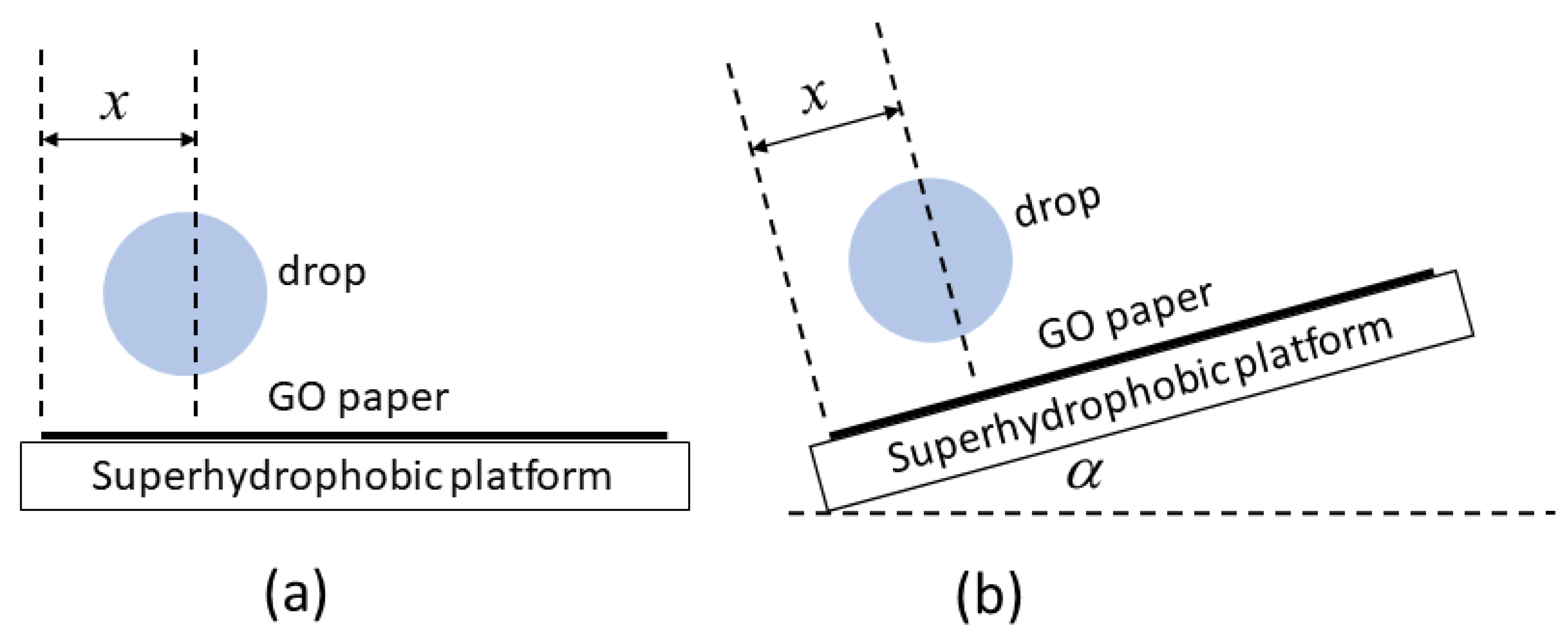
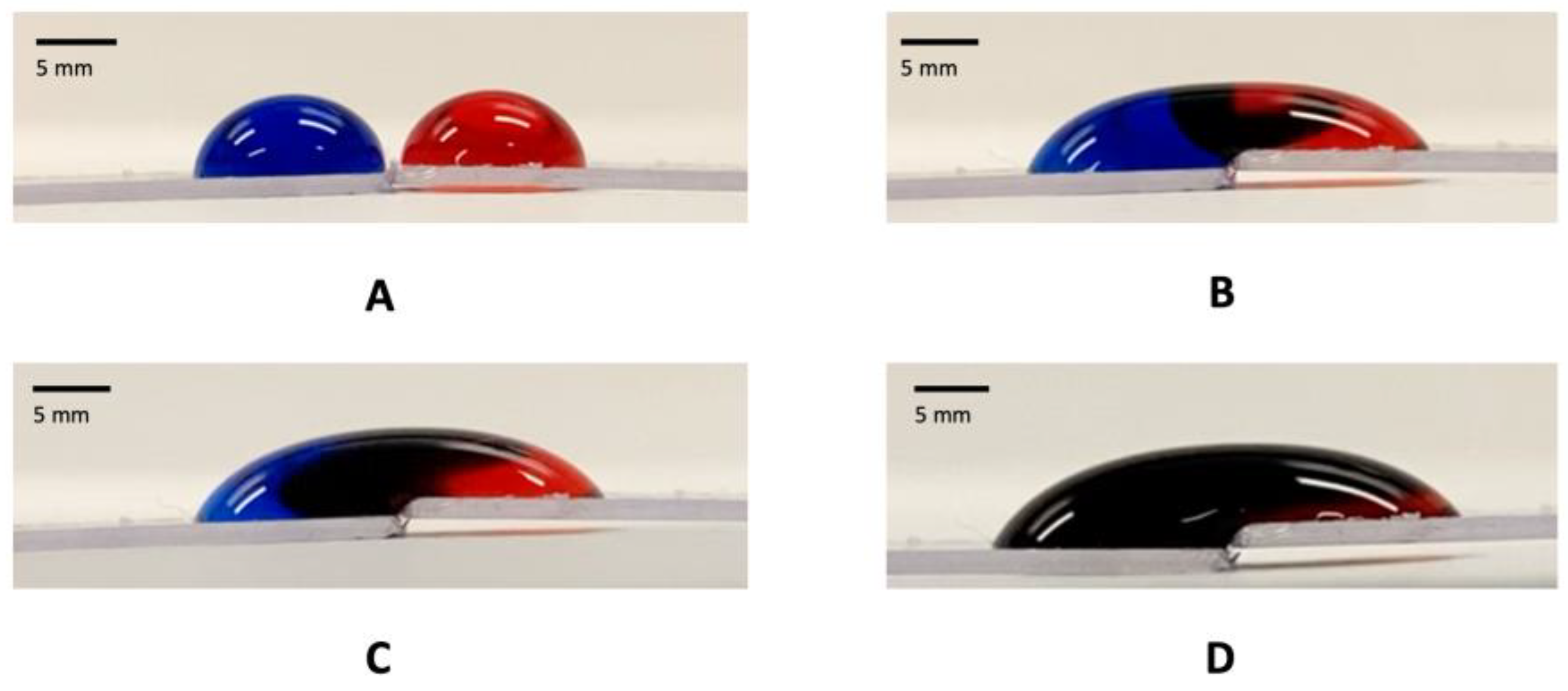
2.7. Transport and Interaction of Drops Wrapped in GO Paper
3. Results and Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaikh, K.A.; Ryu, K.S.; Goluch, E.D.; Liu, C. A modular microfluidic architecture for integrated biochemical analysis. Proc. Natl. Acad. Sci. USA 2005, 102, 9745–9750. [Google Scholar] [CrossRef]
- Baccouche, A.; Okumura, S.; Sieskind, R.; Henry, E.; Aubert-Kato, N.; Bredeche, N.; Bartolo, J.-F.; Taly, V.; Rondelez, Y.; Fujii, T.; et al. Massively parallel and multiparameter titration of biochemical assays with droplet microfluidics. Nat. Prot. 2017, 12, 1912–1932. [Google Scholar] [CrossRef]
- Tsiamis, A.; Buchoux, A.; Mahon, S.T.; Walton, A.J.; Smith, S.; Clarke, D.J.; Stokes, A.A. Design and fabrication of a fully-integrated, miniaturised fluidic system for the analysis of enzyme kinetics. Micromachines 2023, 14, 537. [Google Scholar] [CrossRef] [PubMed]
- Riche, C.T.; Roberts, E.J.; Gupta, M.; Brutchey, R.L.; Malmstadt, N. Flow invariant droplet formation for stable parallel microreactors. Nat. Com. 2016, 7, 10780. [Google Scholar] [CrossRef] [PubMed]
- Skaltsounis, P.; Kokkoris, G.; Papaioannou, T.G.; Tserepi, A. Closed-loop microreactor on PCB for ultra-fast DNA amplification: Design and thermal validation. Micromachines 2023, 14, 172. [Google Scholar] [CrossRef]
- Jia, H.; Wong, Y.L.; Jian, A.; Tsoi, C.C.; Wang, M.; Li, W.; Zhang, W.; Sang, S.; Zhang, X. Microfluidic reactors for plasmonic photocatalysis using gold nanoparticles. Micromachines 2019, 10, 869. [Google Scholar] [CrossRef]
- Dressaire, E.; Sauret, A. Clogging of microfluidic systems. Soft Matter 2017, 13, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, K.; Aeken, W.V.; Mottaghi, M.; Kamyab, V.K.; Kuhn, S. Aggregation and clogging phenomena of rigid microparticles in microfluidics. Microfluid. Nanofluid. 2018, 22, 104. [Google Scholar] [CrossRef]
- Kim, J.; Kim, T.; Ji, I.; Hong, J. Digital microfluidic mixing via reciprocating motions of droplets driven by contact charge electrophoresis. Micromachines 2022, 13, 593. [Google Scholar] [CrossRef]
- Huang, H.; Huang, K.; Sun, Y.; Luo, D.; Wang, M.; Chen, T.; Li, M.; Duan, J.; Huang, L.; Dong, C. A digital microfluidic RT-qPCR platform for multiple detections of respiratory pathogens. Micromachines 2022, 13, 1650. [Google Scholar] [CrossRef]
- Song, Z.-R.; Zeng, J.; Zhou, J.-L.; Yan, B.-Y.; Gu, Z.; Wang, H.-F. Optimization of electrode patterns for an ITO-based digital microfluidic through the Finite Element simulation. Micromachines 2022, 13, 1563. [Google Scholar] [CrossRef]
- Vuong, T.; Cheong, B.H.-P.; Huynh, S.H.; Muradoglu, M.; Liew, O.W.; Ng, T.W. Drop transfer between superhydrophobic wells using air logic control. Lab A Chip 2015, 15, 991–995. [Google Scholar] [CrossRef]
- Keng, P.Y.; van Dam, R.M. Digital microfluidics–A new paradigm for radiochemistry. Mol. Imaging 2015, 14, 13–14. [Google Scholar] [CrossRef]
- Sathyanarayanan, G.; Haapala, M.; Kiiski, I.; Sikanen, T. Digital microfluidic immobilized cytochrome P450 reactors with integrated inkjet-printed microheaters for droplet-based drug metabolism research. Anal. Bioanal. Chem. 2018, 410, 6677–6687. [Google Scholar] [CrossRef]
- Das, A.; Weise, C.; Polack, M.; Urban, R.D.; Krafft, B.; Hasan, S.; Westphal, H.; Warias, R.; Schmidt, S.; Gulder, T.; et al. On-the-fly mass spectrometry in digital microfluidics enabled by a microspray hole: Toward multidimensional reaction monitoring in automated synthesis platforms. J. Am. Chem. Soc. 2022, 144, 10353–10360. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.H.; Vadivelu, R.; Jin, J.; Sreejith, K.R.; Singh, P.; Nguyen, N.-K.; Nguyen, N.-T. Liquid marble-based digital microfluidics–fundamentals and applications. Lab Chip 2021, 21, 1199–1216. [Google Scholar] [CrossRef] [PubMed]
- Azizian, P.; Mohammadrashidi, M.; Azimi, A.A.; Bijarchi, M.A.; Shafii, M.B.; Nasiri, R. Magnetically driven manipulation of nonmagnetic liquid marbles: Billiards with liquid marbles. Micromachines 2023, 14, 49. [Google Scholar] [CrossRef]
- Lin, E.S.; Song, Z.; Ong, J.W.; Abid, H.A.; Liew, O.W.; Ng, T.W. Liquid marble microbioreactor aeration facilitated by on-demand electrolysis. Results Chem. 2022, 4, 100334. [Google Scholar] [CrossRef]
- Jebrail, M.J.; Bartsch, M.S.; Patel, K.D. Digital microfluidics: A versatile tool for applications in chemistry, biology and medicine. Lab Chip 2012, 12, 2452–2463. [Google Scholar] [CrossRef]
- Geng, H.; Cho, S.K. Antifouling digital microfluidics using lubricant infused porous film. Lab Chip 2019, 19, 2275–2283. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Martinez, A.W.; Whitesides, G.M. Understanding wax printing: A simple micropatterning process for paper-based microfluidics. Anal. Chem. 2009, 81, 7091–7095. [Google Scholar] [CrossRef] [PubMed]
- Abadian, A.; Jafarabadi-Ashtiani, S. Paper-based digital microfluidics. Microfluid. Nanofluid. 2014, 16, 989–995. [Google Scholar] [CrossRef]
- Ruecha, N.; Lee, J.; Chae, H.; Cheong, H.; Soum, V.; Preechakasedkit, P.; Chailapakul, O.; Tanev, G.; Madsen, J.; Rodthongkum, N.; et al. Paper-based digital microfluidic chip for multiple electrochemical assay operated by a wireless portable control system. Adv. Mater. Technol. 2017, 2, 1600267. [Google Scholar] [CrossRef]
- Yafia, M.; Foudeh, A.M.; Tabrizian, M.; Najjaran, H. Low-cost graphene-based digital microfluidic system. Micromachines 2020, 11, 880. [Google Scholar] [CrossRef]
- Chai, D.; Jiang, J.; Fan, Y. Low-cost digital microfluidic approach on thin and pliable polymer films. Instrum. Sci. Technol. 2022, 50, 496–506. [Google Scholar] [CrossRef]
- Li, X.Y.; Cheong, B.H.-P.; Somers, A.; Liew, O.W.; Ng, T.W. Surface-scribed transparency-based microplates. Langmuir 2013, 29, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.; Yun, X.; Qiu, L.; Sun, Y.; Tang, B.; He, Z.; Xiao, J.; Chung, D.C.K.; Ng, T.W.; Yan, H.; et al. A dynamic graphene oxide network enables spray printing of colloidal gels for high-performance micro-supercapacitors. Adv. Mater. 2019, 31, 1804434. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Y.; Hu, M.; Xi, S.; Liu, R.; Cheng, M.; Dong, Y. Potential universal engineering component: Tetracycline response nanoswitch Based on triple helix-graphene oxide. Micromachines 2022, 13, 2119. [Google Scholar] [CrossRef]
- Liu, L.; Li, Q.; Shi, H.; Gao, L. Thrombin determination using graphene oxide sensors with co-assisted amplification. Micromachines 2022, 13, 1435. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Gong, T.; Zhang, K.; Lee, C. Graphene oxide papers with high water adsorption capacity for air dehumidification. Sci. Rep. 2017, 7, 9761. [Google Scholar] [CrossRef] [PubMed]
- Suo, Y.; Zhang, L.; Li, Y.; Wu, Y.; Zhang, J.; Wen, Q. Ultra-thin terahertz deflection device based on laser direct writing graphene oxide paper. Micromachines 2022, 13, 686. [Google Scholar] [CrossRef] [PubMed]
- Py, C.; Reverdy, P.; Doppler, L.; Bico, J.; Roman, B.; Baroud, C.N. Capillary origami: Spontaneous wrapping of a droplet with an elastic sheet. Phys. Rev. Lett. 2007, 98, 156103. [Google Scholar] [CrossRef]
- De Langre, E.; Baroud, C.N.; Reverdy, P. Energy criteria for elasto-capillary wrapping. J. Fluid Struct. 2010, 26, 205–217. [Google Scholar] [CrossRef]
- Schneider, A.; Traut, N.; Hamburger, M. Analysis and optimization of relevant parameters of blade coating and gravure printing processes for the fabrication of highly efficient organic solar cells. Sol. Energy Mater. Sol. Cells 2014, 126, 149–154. [Google Scholar] [CrossRef]
- Tsai, P.-T.; Tsai, C.-Y.; Wang, C.M.; Chang, Y.F.; Meng, H.-F.; Chen, Z.K.; Lin, H.W.; Zan, H.-W.; Horng, S.F.; Lai, Y.C.; et al. High-efficiency polymer solar cells by blade coating in chlorine-free solvents. Org. Electron. 2014, 15, 893–903. [Google Scholar] [CrossRef]
- Zhao, H.P.; Wang, Y.C.; Li, B.W.; Feng, X.Q. Improvement of the peeling strength of thin films by a bioinspired hierarchical interface. Int. J. Appl. Mech. 2013, 15, 1350012. [Google Scholar] [CrossRef]
- Tirumkudulu, M.S.; Russel, W.B. Cracking in drying latex films. Langmuir 2005, 21, 4938–4948. [Google Scholar] [CrossRef]
- Fischer, S.B.; Koos, E. Influence of drying conditions on the stress and weight development of capillary suspensions. J. Am. Ceram. Soc. 2021, 104, 1255–1270. [Google Scholar] [CrossRef]
- Song, Z.; Lin, E.S.; Zhu, J.; Ong, J.W.; Abid, H.A.; Uddin, M.H.; Liew, O.W.; Ng, T.W. Sustained graphene oxide coated superhydrophilicity and superwetting using humidity control. Colloids Surf. A 2021, 613, 126097. [Google Scholar] [CrossRef]
- Song, Z.; Lin, E.S.; Zhu, J.; Ong, J.W.; Abid, H.A.; Uddin, M.H.; Liew, O.W.; Ng, T.W. Fog harvesting with highly wetting and nonwetting vertical strips. Langmuir 2022, 38, 1845–1852. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Jiang, J.-W.; Park, H.S. Negative Poisson’s ratio in graphene oxide. Nanoscale 2017, 9, 4007–4012. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical properties of monolayer graphene oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef] [PubMed]
- Antkowiak, A.; Audoly, B.; Josserand, C.; Neukirch, S.; Rivetti, M. Instant fabrication and selection of folded structures using drop impact. Proc. Natl. Acad. Sci. USA 2011, 108, 10400–10404. [Google Scholar] [CrossRef]
- Jin, J.; Ooi, C.H.; Dao, D.V.; Nguyen, N.-T. Liquid marble coalescence via vertical collision. Soft Matter 2018, 14, 4160–4168. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Lin, E.S.; Uddin, M.H.; Abid, H.A.; Ong, J.W.; Ng, T.W. Graphene Oxide Paper Manipulation of Micro-Reactor Drops. Micromachines 2023, 14, 1306. https://doi.org/10.3390/mi14071306
Song Z, Lin ES, Uddin MH, Abid HA, Ong JW, Ng TW. Graphene Oxide Paper Manipulation of Micro-Reactor Drops. Micromachines. 2023; 14(7):1306. https://doi.org/10.3390/mi14071306
Chicago/Turabian StyleSong, Zhixiong, Eric Shen Lin, Md Hemayet Uddin, Hassan Ali Abid, Jian Wern Ong, and Tuck Wah Ng. 2023. "Graphene Oxide Paper Manipulation of Micro-Reactor Drops" Micromachines 14, no. 7: 1306. https://doi.org/10.3390/mi14071306
APA StyleSong, Z., Lin, E. S., Uddin, M. H., Abid, H. A., Ong, J. W., & Ng, T. W. (2023). Graphene Oxide Paper Manipulation of Micro-Reactor Drops. Micromachines, 14(7), 1306. https://doi.org/10.3390/mi14071306







