3D-Printed Microfluidic One-Way Valves and Pumps
Abstract
1. Introduction
2. Materials and Methods
2.1. Custom 3D Printer
2.2. Materials
2.3. Post-Print Processing
3. Results and Discussion
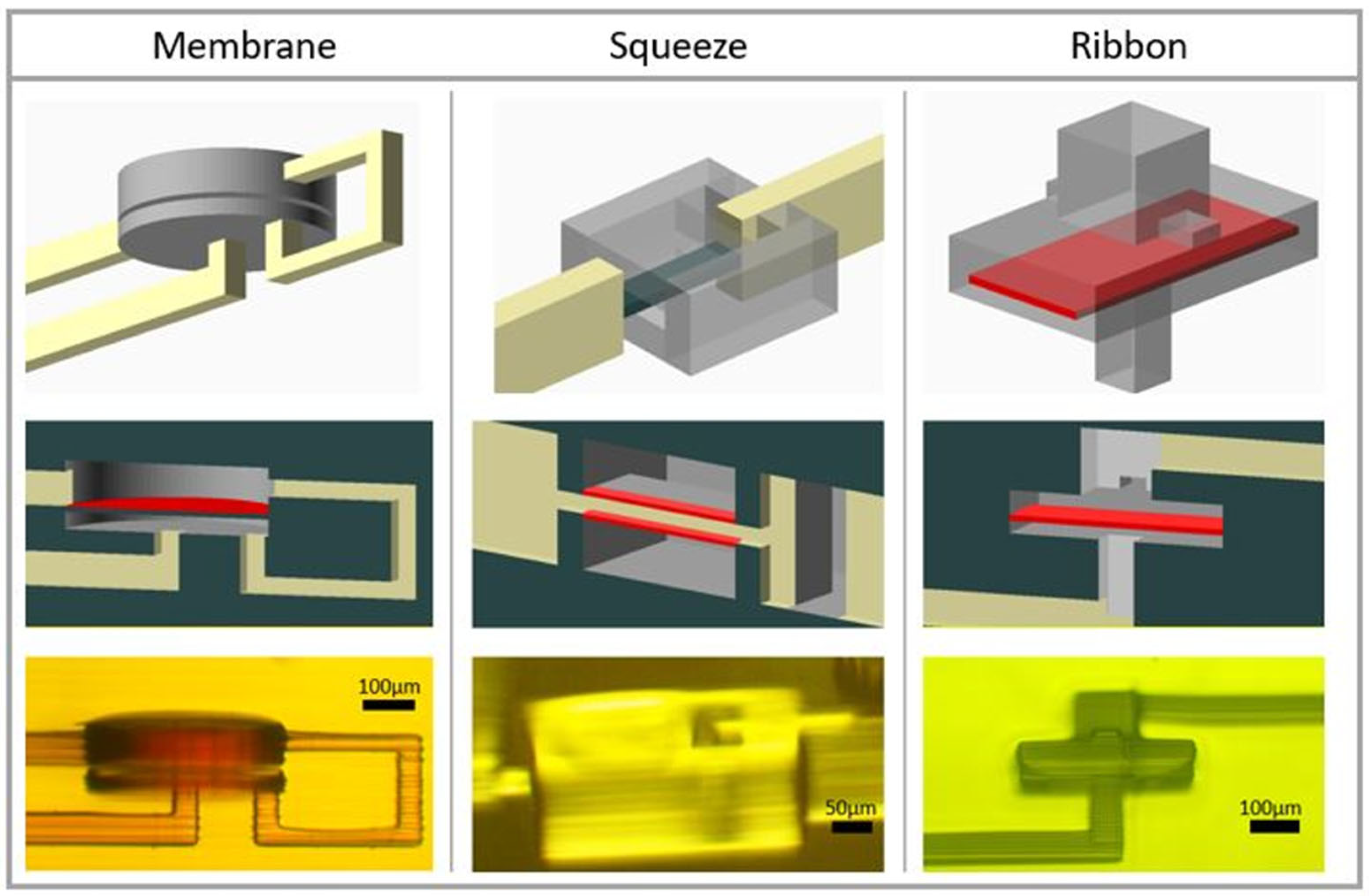
3.1. One-Way Valve Design
3.1.1. Membrane Valve
3.1.2. Squeeze Valve
3.1.3. Ribbon Valve
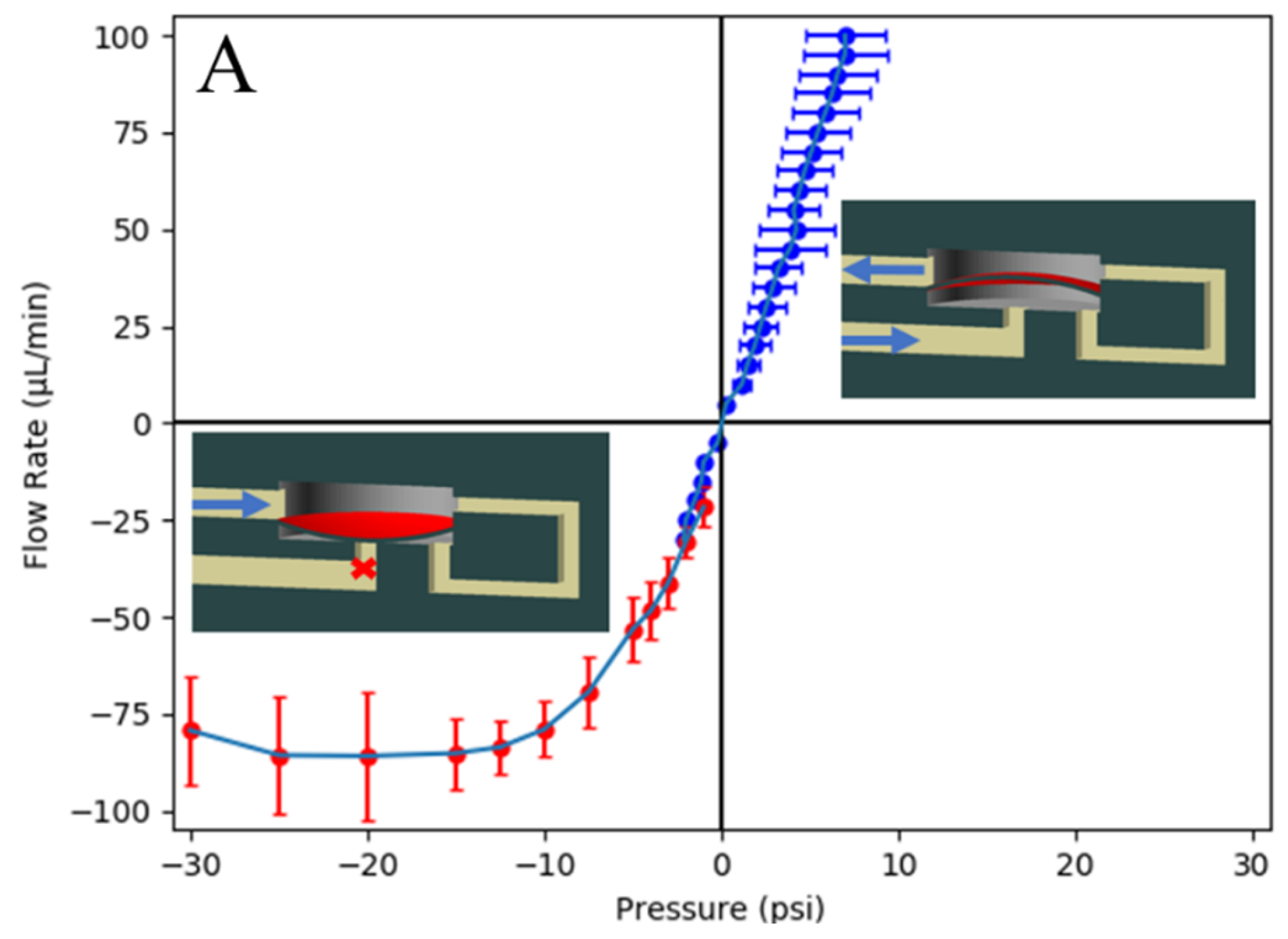
3.2. One-Way Valve Characterization
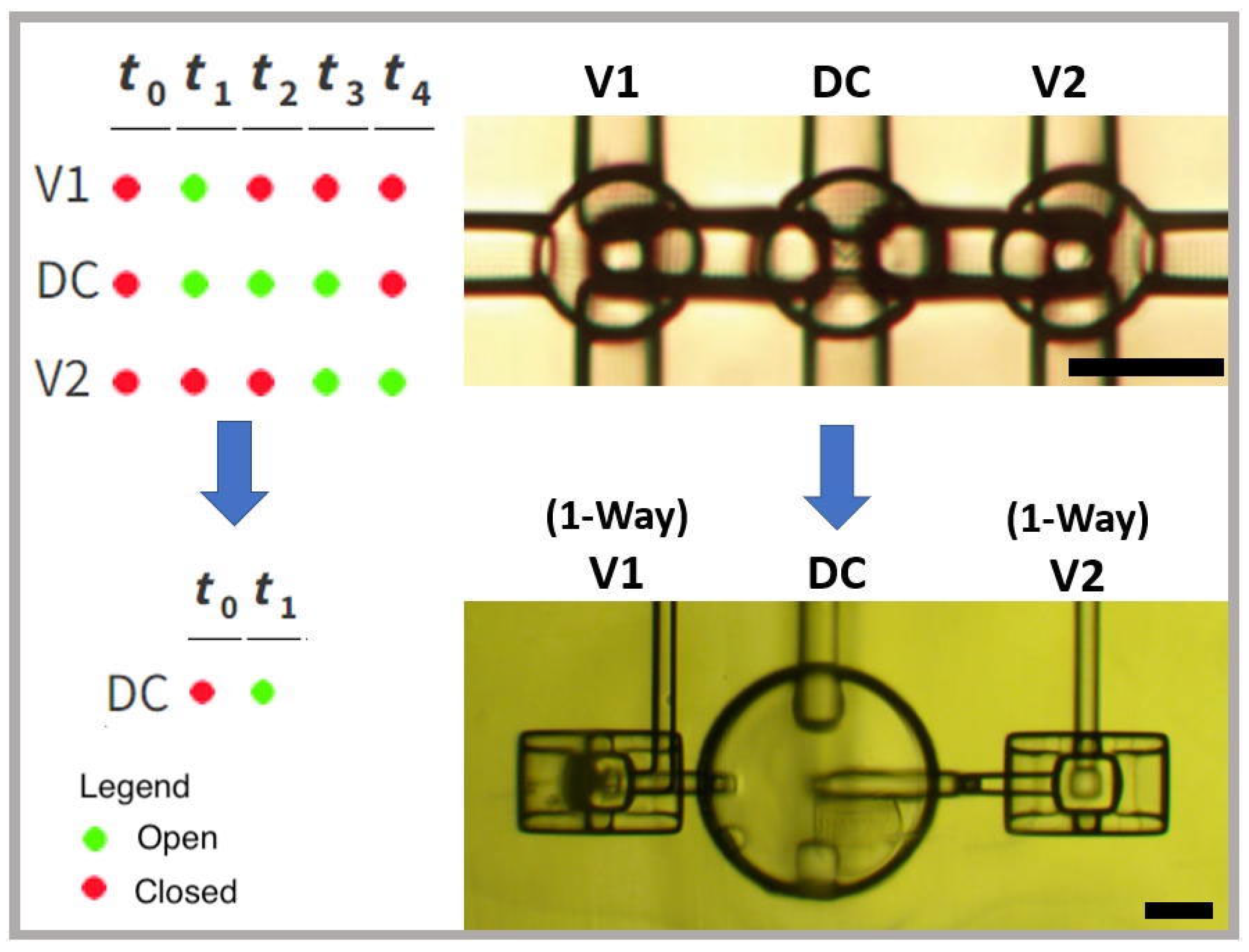
3.3. Single Pneumatic Control Pumps
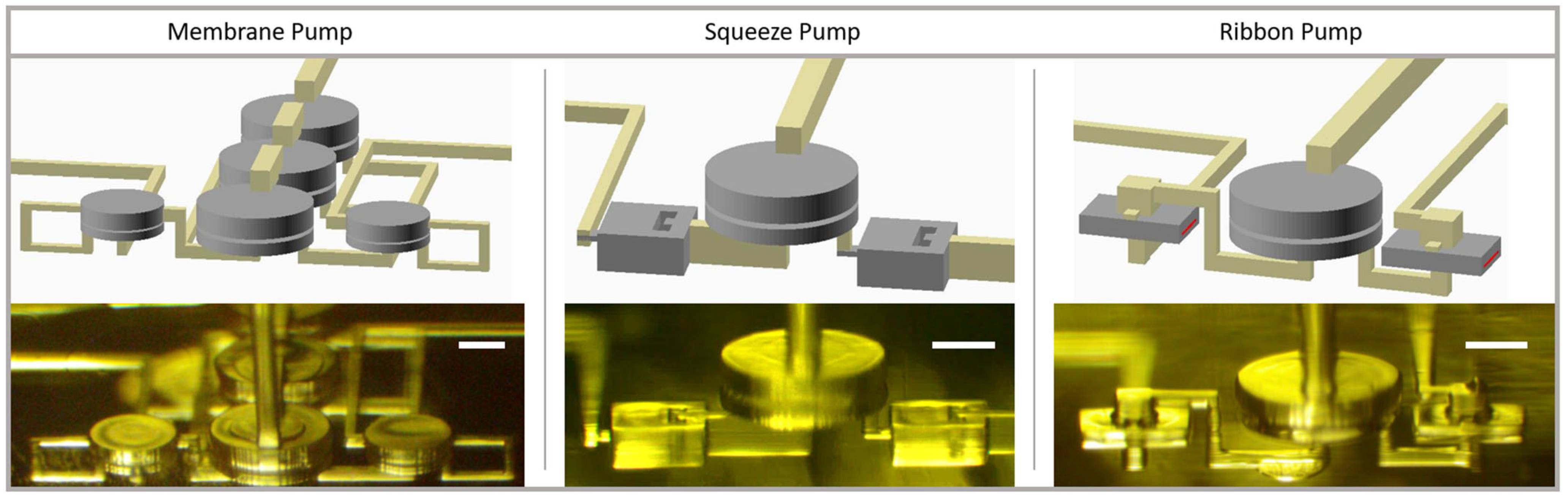
3.4. Pump Designs
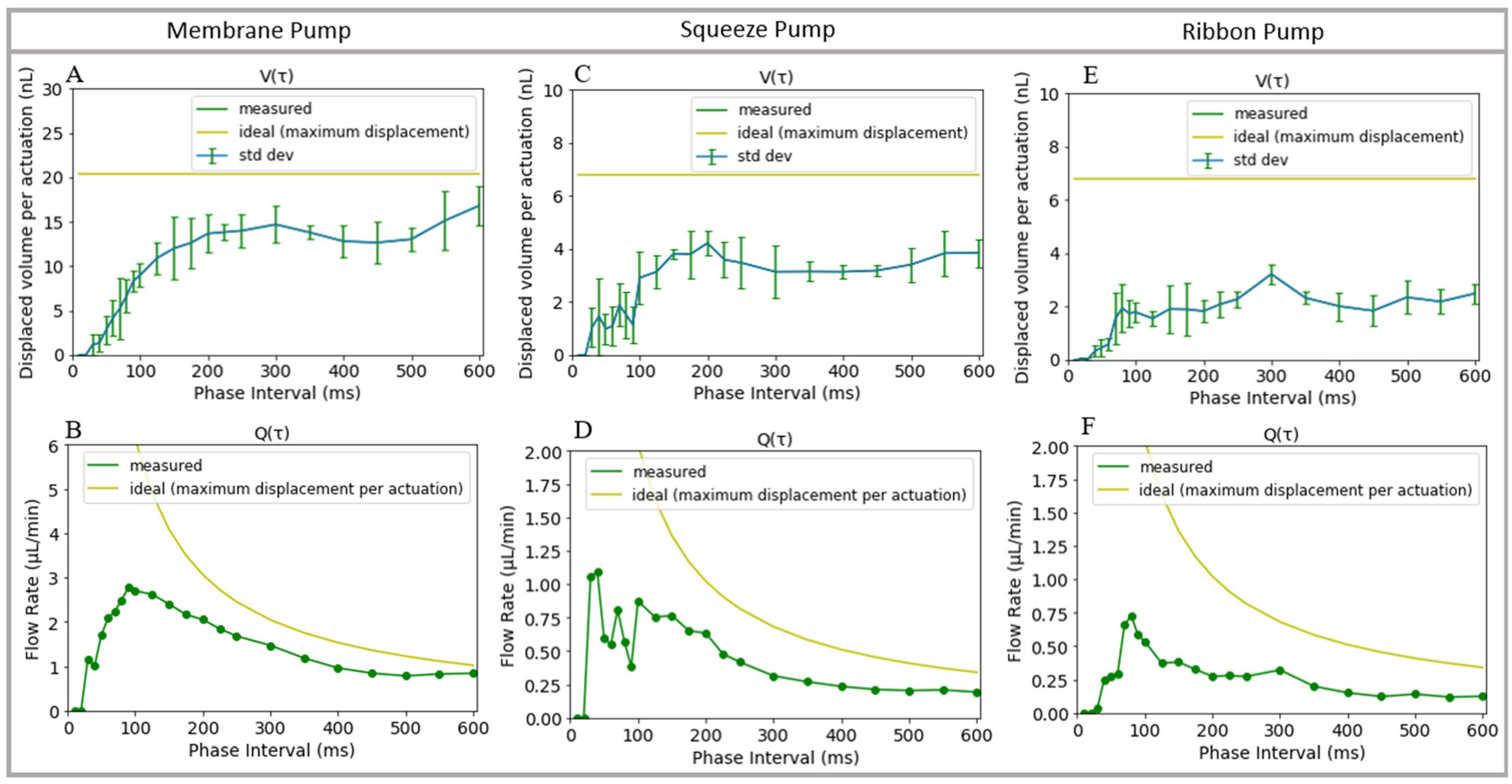
3.5. Pump Characterization
3.6. Single-Stage Diffusion Mixer
3.7. Mixer Design
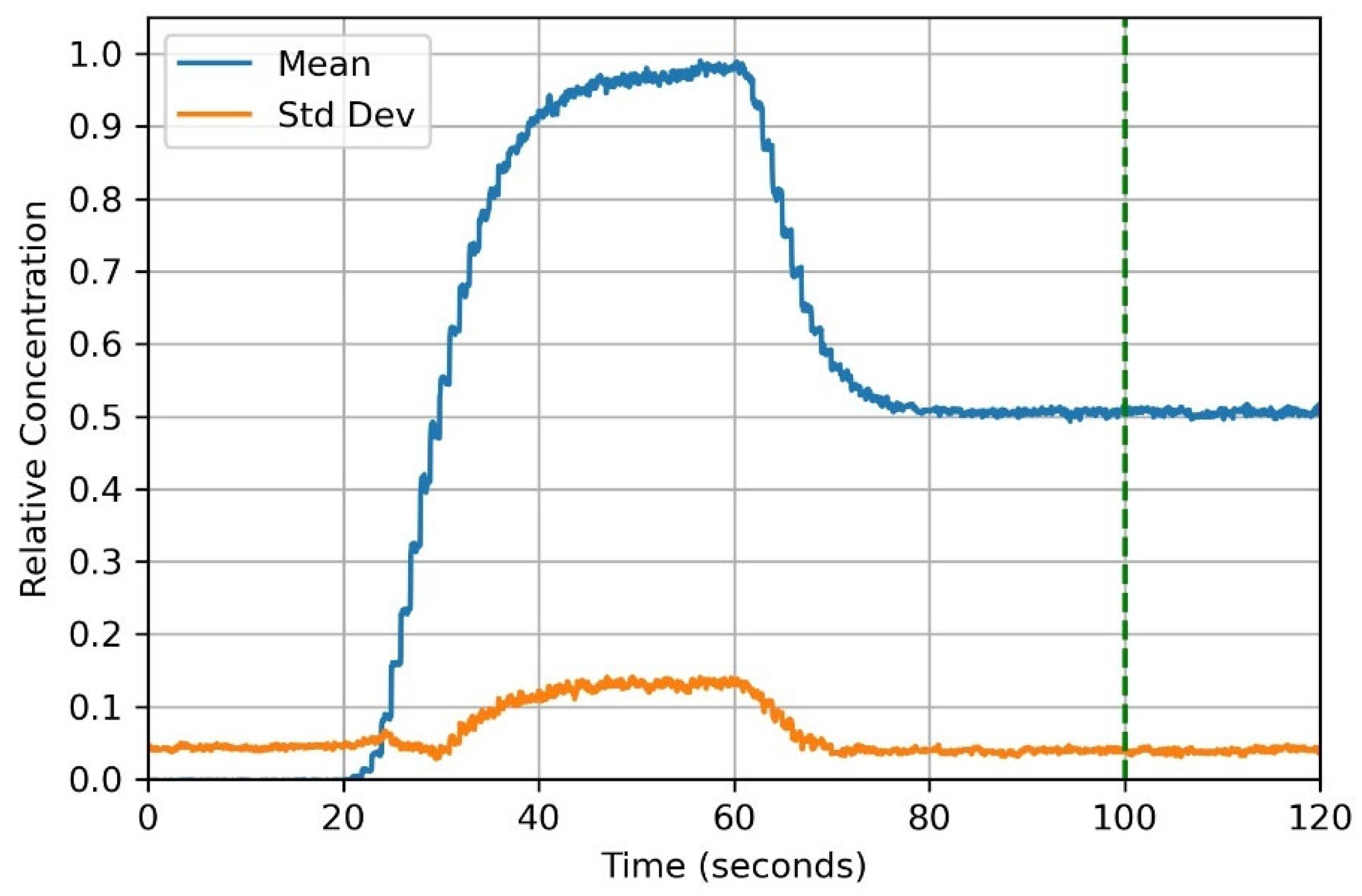
3.8. Mixer Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amini, A.; Guijt, R.M.; Themelis, T.; De Vos, J.; Eeltink, S. Recent Developments in Digital Light Processing 3D-Printing Techniques for Microfluidic Analytical Devices. J. Chromatogr. A 2023, 1692, 46384. [Google Scholar] [CrossRef]
- Nielsen, A.V.; Beauchamp, M.J.; Nordin, G.P.; Woolley, A.T. 3D Printed Microfluidics. Annu. Rev. Anal. Chem. 2020, 13, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Wang, F.; McAlpine, M.C. 3D Printed Microfluidics: Advances in Strategies, Integration, and Applications. Lab Chip 2023, 23, 1279–1299. [Google Scholar] [CrossRef] [PubMed]
- Noriega, J.L.S.; Chartrand, N.A.; Valdoz, J.C.; Cribbs, C.G.; Jacobs, D.A.; Poulson, D.; Viglione, M.S.; Woolley, A.T.; Van Ry, P.M.; Christensen, K.A.; et al. Spatially and Optically Tailored 3D Printing for Highly Miniaturized and Integrated Microfluidics. Nat. Commun. 2021, 12, 5509. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Qi, F.; Mao, H.; Li, S.; Zhu, Y.; Gong, J.; Wang, L.; Malmstadt, N.; Chen, Y. In-Situ Transfer Vat Photopolymerization for Transparent Microfluidic Device Fabrication. Nat. Commun. 2022, 13, 918. [Google Scholar] [CrossRef]
- Rogers, C.I.; Qaderi, K.; Woolley, A.T.; Nordin, G.P. 3D Printed Microfluidic Devices with Integrated Valves. Biomicrofluidics 2015, 9, 016501. [Google Scholar] [CrossRef]
- Gong, H.; Beauchamp, M.; Perry, S.; Woolley, A.T.; Nordin, G.P. Optical Approach to Resin Formulation for 3D Printed Microfluidics. RSC Adv. 2015, 5, 106621–106632. [Google Scholar] [CrossRef]
- Gong, H.; Bickham, B.P.; Woolley, A.T.; Nordin, G.P. Custom 3D Printer and Resin for 18 µm × 20 µm Microfluidic Flow Channels. Lab Chip 2017, 17, 2899–2909. [Google Scholar] [CrossRef]
- Au, A.K.; Lee, W.; Folch, A. Mail-Order Microfluidics: Evaluation of Stereolithography for the Production of Microfluidic Devices. Lab Chip 2014, 14, 1294–1301. [Google Scholar] [CrossRef]
- Au, A.K.; Bhattacharjee, N.; Horowitz, L.F.; Chang, T.C.; Folch, A. 3D-Printed Microfluidic Automation. Lab Chip 2015, 15, 1934–1941. [Google Scholar] [CrossRef]
- Shallan, A.I.; Smejkal, P.; Corban, M.; Guijt, R.M.; Breadmore, M.C. Cost-Effective Three-Dimensional Printing of Visibly Transparent Microchips within Minutes. Anal. Chem. 2014, 86, 3124–3130. [Google Scholar] [CrossRef]
- Gross, B.C.; Erkal, J.L.; Lockwood, S.Y.; Chen, C.; Spence, D.M. Evaluation of 3D Printing and Its Potential Impact on Biotechnology and the Chemical Sciences. Anal. Chem. 2014, 86, 3240–3253. [Google Scholar] [CrossRef]
- Leslie, D.C.; Easley, C.J.; Seker, E.; Karlinsey, J.M.; Utz, M.; Begley, M.R.; Landers, J.P. Frequency-Specific Flow Control in Microfluidic Circuits with Passive Elastomeric Features. Nat. Phys. 2009, 5, 231–235. [Google Scholar] [CrossRef]
- Xia, H.; Wu, J.; Zheng, J.; Zhang, J.; Wang, Z. Nonlinear Microfluidics: Device Physics, Functions, and Applications. Lab Chip 2021, 21, 1241–1268. [Google Scholar] [CrossRef]
- Loverich, J.; Kanno, I.; Kotera, H. Single-Step Replicable Microfluidic Check Valve for Rectifying and Sensing Low Reynolds Number Flow. Microfluid. Nanofluidics 2006, 3, 427–435. [Google Scholar] [CrossRef]
- Kim, J.; Baek, J.; Lee, K.; Park, Y.; Sun, K.; Lee, T.; Lee, S. Photopolymerized Check Valve and Its Integration into a Pneumatic Pumping System for Biocompatible Sample Delivery. Lab Chip 2006, 6, 1091. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, M.J.; Gong, H.; Woolley, A.T.; Nordin, G.P. 3D printed microfluidic features using dose control in X, Y, and Z dimensions. Micromachines 2018, 9, 326. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Bhattacharjee, N.; Folch, A. 3D-Printed Quake-Style Microvalves and Micropumps. Lab Chip 2018, 18, 1207–1214. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.-T.; Truong, T.-Q.; Wong, K.-K.; Ho, S.-S.; Low, C.L.-N. Micro Check Valves for Integration into Polymeric Microfluidic Devices. J. Micromech. Microeng. 2003, 14, 69–75. [Google Scholar] [CrossRef]
- Chappel, E. A Review of Passive Constant Flow Regulators for Microfluidic Applications. Appl. Sci. 2020, 10, 8858. [Google Scholar] [CrossRef]
- Gong, H.; Woolley, A.T.; Nordin, G.P. High Density 3D Printed Microfluidic Valves, Pumps, and Multiplexers. Lab Chip 2016, 16, 2450–2458. [Google Scholar] [CrossRef]
- Kim, J.H.; Lau, K.T.; Shepherd, R.; Wu, Y.; Wallace, G.; Diamond, D. Performance Characteristics of a Polypyrrole Modified Polydimethylsiloxane (PDMS) Membrane Based Microfluidic Pump. Sens. Actuators A Phys. 2008, 148, 239–244. [Google Scholar] [CrossRef]
- Gong, H.; Woolley, A.T.; Nordin, G.P. 3D Printed Selectable Dilution Mixer Pumps. Biomicrofluidics 2019, 13, 014106. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Iovenitti, P.; Harvey, E.; Masood, S. Optimizing Layout of Obstacles for Enhanced Mixing in Microchannels. Smart Mater. Struct. 2002, 11, 662–667. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinnen, H.; Viglione, M.; Munro, T.R.; Woolley, A.T.; Nordin, G.P. 3D-Printed Microfluidic One-Way Valves and Pumps. Micromachines 2023, 14, 1286. https://doi.org/10.3390/mi14071286
Hinnen H, Viglione M, Munro TR, Woolley AT, Nordin GP. 3D-Printed Microfluidic One-Way Valves and Pumps. Micromachines. 2023; 14(7):1286. https://doi.org/10.3390/mi14071286
Chicago/Turabian StyleHinnen, Hunter, Matthew Viglione, Troy R. Munro, Adam T. Woolley, and Gregory P. Nordin. 2023. "3D-Printed Microfluidic One-Way Valves and Pumps" Micromachines 14, no. 7: 1286. https://doi.org/10.3390/mi14071286
APA StyleHinnen, H., Viglione, M., Munro, T. R., Woolley, A. T., & Nordin, G. P. (2023). 3D-Printed Microfluidic One-Way Valves and Pumps. Micromachines, 14(7), 1286. https://doi.org/10.3390/mi14071286






