Comparison of Robot-Assisted and Manual Cannula Insertion in Simulated Big-Bubble Deep Anterior Lamellar Keratoplasty
Abstract
1. Introduction
2. Materials and Methods
2.1. Microscope and Intraoperative OCT
2.2. Robot
2.3. Instruments
2.4. Surgical Technique
2.5. Method
2.6. Parameters
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Krysik, K.; Wroblewska-Czajka, E.; Lyssek-Boron, A.; Wylegala, E.A.; Dobrowolski, D. Total penetrating keratoplasty: Indications, therapeutic approach, and long-term follow-up. J. Ophthalmol. 2018, 2018, 9580292. [Google Scholar] [CrossRef]
- Krysik, K.; Dobrowolski, D.; Lyssek-Boron, A.; Jankowska-Szmul, J.; Wylegala, E.A. Differences in surgical management of corneal perforations, measured over six years. J. Ophthalmol. 2017, 2017, 1582532. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Teichmann, K.D. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J. Cataract. Refract. Surg. 2002, 28, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Javadi, M.A.; Jafarinasab, M.R.; Karimian, F. Penetrating keratoplasty versus lamellar keratoplasty for mustard gas–induced keratitis. Cornea 2013, 32, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.C.; Murthy, S.I.; Vaddavalli, P.K.; Garg, P.; Ramappa, M.; Chaurasia, S.; Rathi, V.; Sangwan, V.S. Clinical outcomes and risk factors for graft failure after deep anterior lamellar keratoplasty and penetrating keratoplasty for macular corneal dystrophy. Cornea 2015, 34, 171–176. [Google Scholar] [CrossRef]
- Watson, S.L.; Ramsay, A.; Dart, J.K.; Bunce, C.; Craig, E. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus. Ophthalmology 2004, 111, 1676–1682. [Google Scholar] [CrossRef]
- Gadhvi, K.A.; Romano, V.; Cueto, L.F.V.; Aiello, F.; Day, A.C.; Allan, B.D. Deep anterior lamellar keratoplasty for keratoconus: Multisurgeon results. Am. J. Ophthalmol. 2019, 201, 54–62. [Google Scholar] [CrossRef]
- Javadi, M.A.; Feizi, S.; Yazdani, S.; Mirbabaee, F. Deep anterior lamellar keratoplasty versus penetrating keratoplasty for keratoconus: A clinical trial. Cornea 2010, 29, 365–371. [Google Scholar] [CrossRef]
- Razmju, H.; Shams, M.; Abtahi, M.A.; Abtahi, S.H. Comparison of deep lamellar keratoplasty and penetrating keratoplasty in patients with keratoconus: A clinical trial study. J. Isfahan Med. Sch. 2011, 29, 798–803. [Google Scholar]
- Scorcia, V.; Busin, M.; Lucisano, A.; Beltz, J.; Carta, A.; Scorcia, G. Anterior segment optical coherence tomography–guided big-bubble technique. Ophthalmology 2013, 120, 471–476. [Google Scholar] [CrossRef]
- Pasricha, N.D.; Shieh, C.; Carrasco-Zevallos, O.M.; Keller, B.; Cunefare, D.; Mehta, J.S.; Farsiu, S.; Izatt, J.A.; Toth, C.A.; Kuo, A.N. Needle depth and big bubble success in deep anterior lamellar keratoplasty: An ex vivo microscope-integrated OCT study. Cornea 2016, 35, 1471. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, R.C.; Bogoni, A.; Ghanem, V.C. Pachymetry-guided intrastromal air injection (“pachy-bubble”) for deep anterior lamellar keratoplasty: Results of the first 110 cases. Cornea 2015, 34, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Feizi, S.; Javadi, M.A.; Jamali, H.; Mirbabaee, F. Deep anterior lamellar keratoplasty in patients with keratoconus: Big-bubble technique. Cornea 2010, 29, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Fogla, R.; Padmanabhan, P. Results of deep lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am. J. Ophthalmol. 2006, 141, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Al-Torbak, A.A.; Al-Motowa, S.; Al-Assiri, A.; Al-Kharashi, S.; Al-Shahwan, S.; Al-Mezaine, H.; Teichmann, K. Deep anterior lamellar keratoplasty for keratoconus. Cornea 2006, 25, 408–412. [Google Scholar]
- Michieletto, P.; Balestrazzi, A.; Balestrazzi, A.; Mazzotta, C.; Occhipinti, I.; Rossi, T. Factors predicting unsuccessful big bubble deep lamellar anterior keratoplasty. Ophthalmologica 2006, 220, 379–382. [Google Scholar] [CrossRef]
- Koçluk, Y.; Sukgen, E.A.; Burcu, A. Comparison of outcomes in patients who underwent deep anterior lamellar keratoplasty and those converted to penetrating keratoplasty. Turk. J. Ophthalmol. 2017, 47, 63. [Google Scholar] [CrossRef]
- Tao, Y.K.; LaBarbera, M.; Ehlers, J.P.; Srivastava, S.K.; Dupps, W.J., Jr. Image-guided modified deep anterior lamellar keratoplasty (DALK) corneal transplant using intraoperative optical coherence tomography. In Proceedings of the Ophthalmic Technologies XXV; SPIE: Bellingham, DC, USA, 2015; Volume 9307, pp. 11–14. [Google Scholar]
- Roodaki, H.; di San Filippo, C.A.; Zapp, D.; Navab, N.; Eslami, A. A surgical guidance system for big-bubble deep anterior lamellar keratoplasty. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2016: 19th International Conference, Athens, Greece, 17–21 October 2016; Proceedings, Part I 19. Springer: Berlin/Heidelberg, Germany, 2016; pp. 378–385. [Google Scholar]
- Draelos, M.; Keller, B.; Tang, G.; Kuo, A.; Hauser, K.; Izatt, J. Real-time image-guided cooperative robotic assist device for deep anterior lamellar keratoplasty. In Proceedings of the 2018 IEEE International Conference on Robotics and Automation (ICRA), Brisbane, Australia, 21–25 May 2018; pp. 4013–4018. [Google Scholar]
- Draelos, M.; Tang, G.; Keller, B.; Kuo, A.; Hauser, K.; Izatt, J.A. Optical coherence tomography guided robotic needle insertion for deep anterior lamellar keratoplasty. IEEE Trans. Biomed. Eng. 2019, 67, 2073–2083. [Google Scholar] [CrossRef]
- Nanavaty, M.A.; Vijjan, K.S.; Yvon, C. Deep anterior lamellar keratoplasty: A surgeon’s guide. J. Curr. Ophthalmol. 2018, 30, 297–310. [Google Scholar] [CrossRef]
- Steven, P.; Le Blanc, C.; Lankenau, E.; Krug, M.; Oelckers, S.; Heindl, L.M.; Gehlsen, U.; Huettmann, G.; Cursiefen, C. Optimising deep anterior lamellar keratoplasty (DALK) using intraoperative online optical coherence tomography (iOCT). Br. J. Ophthalmol. 2014, 98, 900–904. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, H.J.; Tsaousis, K.T.; Tabin, G.C. Salvaging deep anterior lamellar keratoplasty with microbubble incision technique in failed “big bubble” cases: An update study. Eur. J. Ophthalmol. 2016, 26, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yang, J.; Huang, K.; Lee, Z.; Lee, X. A comparison of biomechanical properties between human and porcine cornea. J. Biomech. 2001, 34, 533–537. [Google Scholar] [CrossRef] [PubMed]
- De Benito-Llopis, L.; Mehta, J.S.; Angunawela, R.I.; Ang, M.; Tan, D.T. Intraoperative anterior segment optical coherence tomography: A novel assessment tool during deep anterior lamellar keratoplasty. Am. J. Ophthalmol. 2014, 157, 334–341. [Google Scholar] [CrossRef] [PubMed]

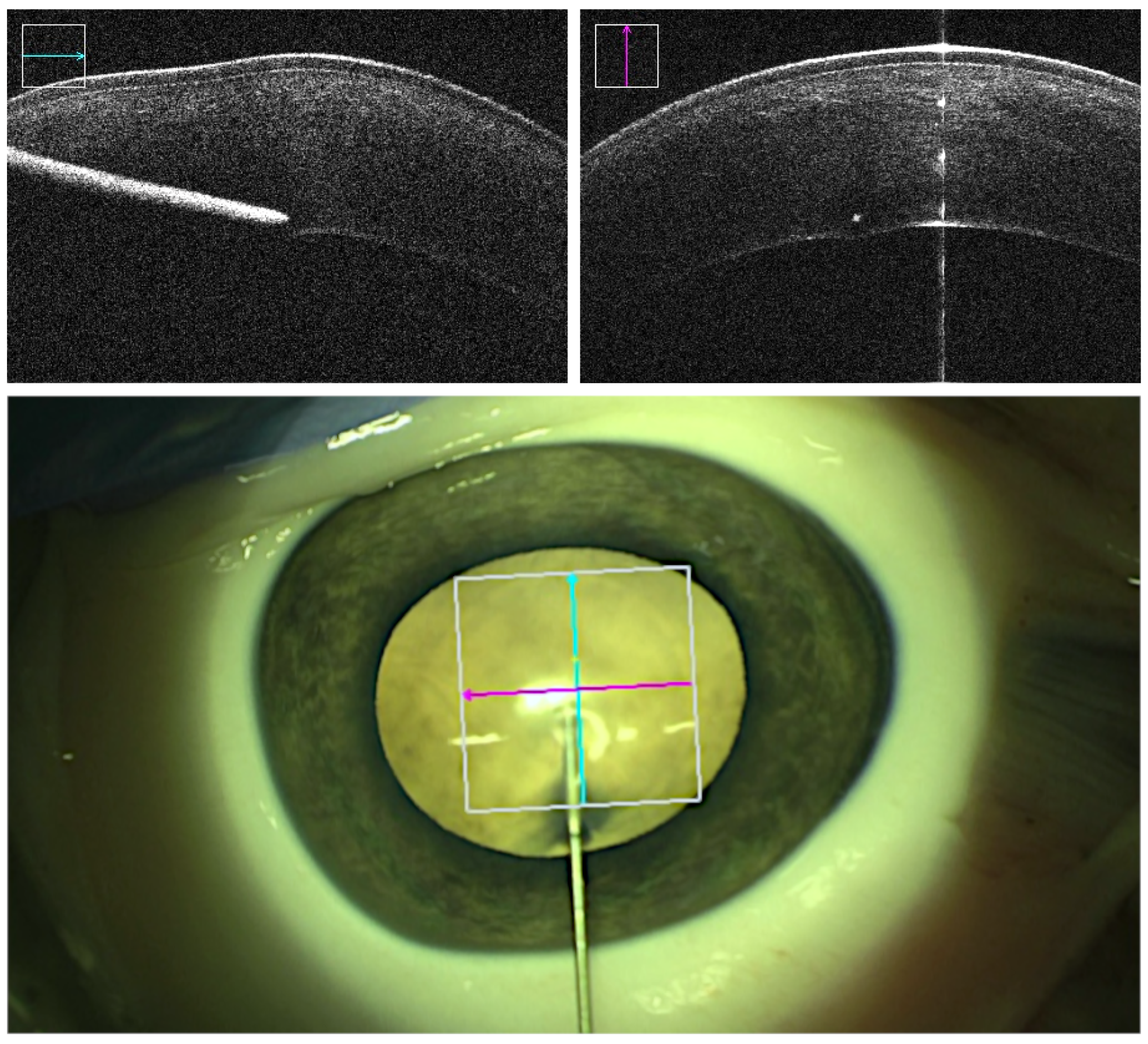
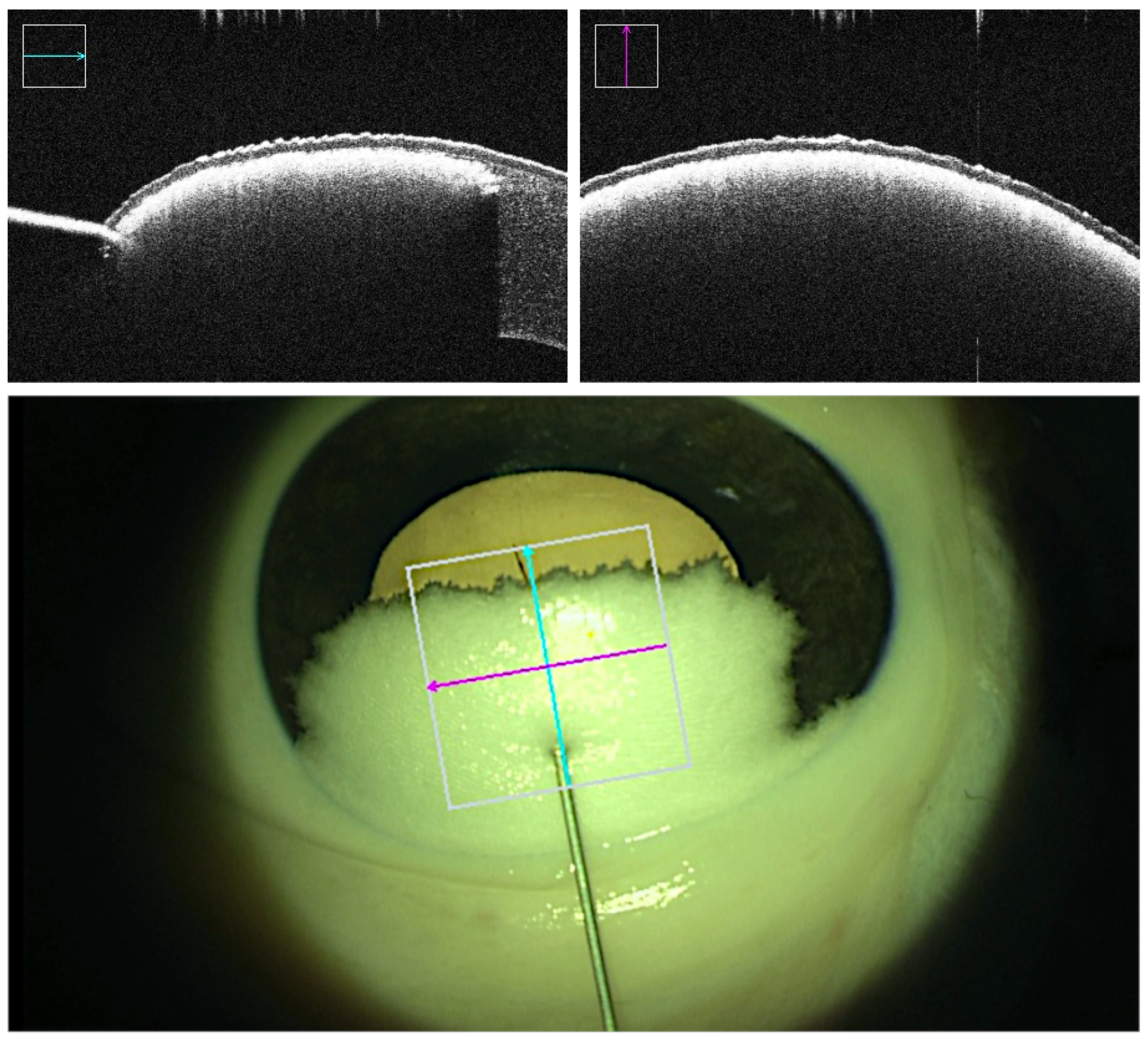

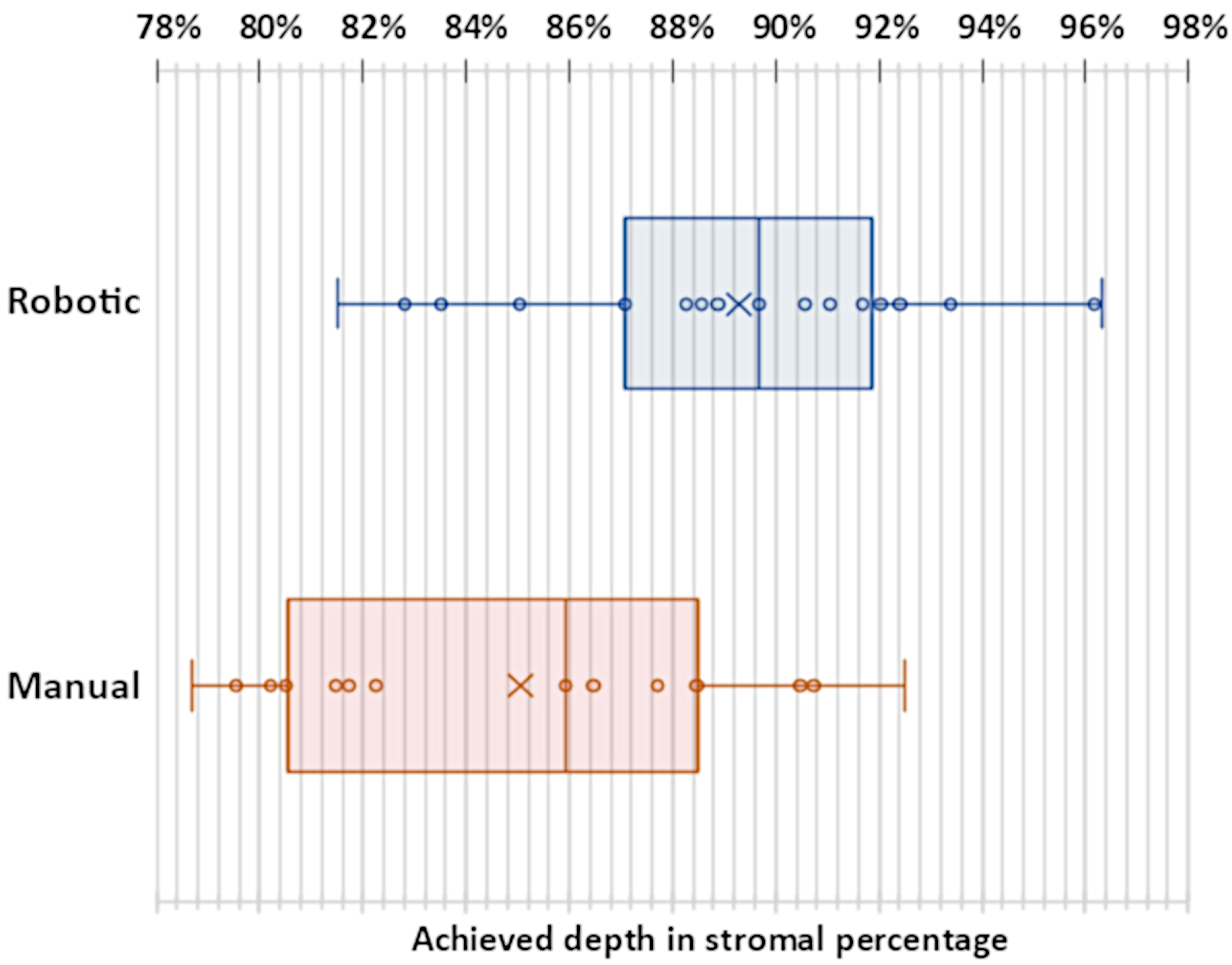
| Successful Manual (74%) | Failed Manual (26%) | Successful Robotic (83%) | Failed Robotic (17%) | ||
|---|---|---|---|---|---|
| Achieved depth (stromal percentage) | Mean STD | 85.05% 4.52% | 89.29% 4.03% | ||
| Needle entry angle (degree) | Mean STD | 23.45° 8.80° | 28.61° 9.13° | 23.35° 6.48° | 32.44° 5.73° |
| Needle tunnel length (millimeters) | Mean STD | 1.82 mm 0.27 mm | 1.72 mm 0.45 mm | ||
| Operation duration (seconds) | Mean STD | 212 s 96 s | 260 s 96 s |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Jablonka, A.-M.; Maierhofer, N.A.; Roodaki, H.; Eslami, A.; Maier, M.; Nasseri, M.A.; Zapp, D. Comparison of Robot-Assisted and Manual Cannula Insertion in Simulated Big-Bubble Deep Anterior Lamellar Keratoplasty. Micromachines 2023, 14, 1261. https://doi.org/10.3390/mi14061261
Zhao Y, Jablonka A-M, Maierhofer NA, Roodaki H, Eslami A, Maier M, Nasseri MA, Zapp D. Comparison of Robot-Assisted and Manual Cannula Insertion in Simulated Big-Bubble Deep Anterior Lamellar Keratoplasty. Micromachines. 2023; 14(6):1261. https://doi.org/10.3390/mi14061261
Chicago/Turabian StyleZhao, Yinzheng, Anne-Marie Jablonka, Niklas A. Maierhofer, Hessam Roodaki, Abouzar Eslami, Mathias Maier, Mohammad Ali Nasseri, and Daniel Zapp. 2023. "Comparison of Robot-Assisted and Manual Cannula Insertion in Simulated Big-Bubble Deep Anterior Lamellar Keratoplasty" Micromachines 14, no. 6: 1261. https://doi.org/10.3390/mi14061261
APA StyleZhao, Y., Jablonka, A.-M., Maierhofer, N. A., Roodaki, H., Eslami, A., Maier, M., Nasseri, M. A., & Zapp, D. (2023). Comparison of Robot-Assisted and Manual Cannula Insertion in Simulated Big-Bubble Deep Anterior Lamellar Keratoplasty. Micromachines, 14(6), 1261. https://doi.org/10.3390/mi14061261








