A Hemodynamic Pulse Wave Simulator Designed for Calibration of Local Pulse Wave Velocities Measurement for Cuffless Techniques
Abstract
1. Introduction
Our Contribution
2. Materials and Methods
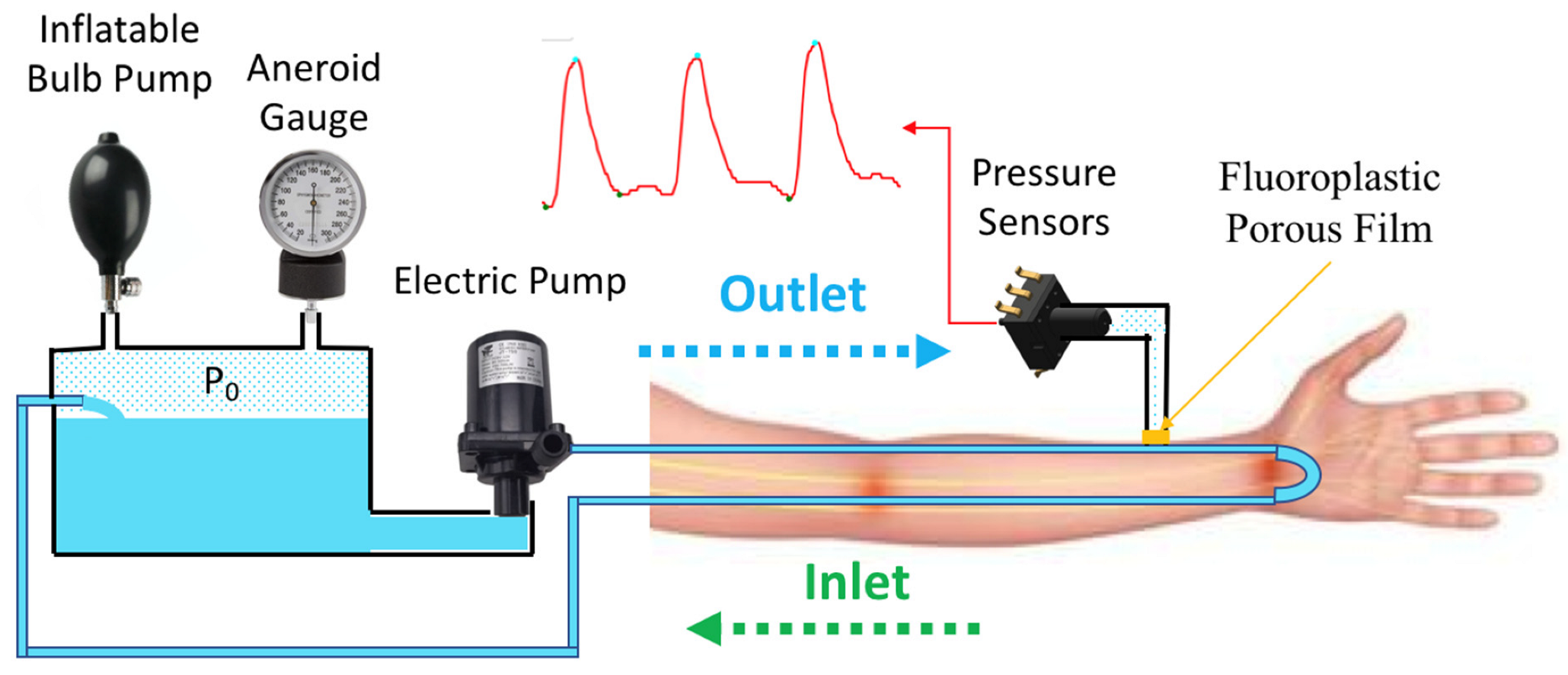
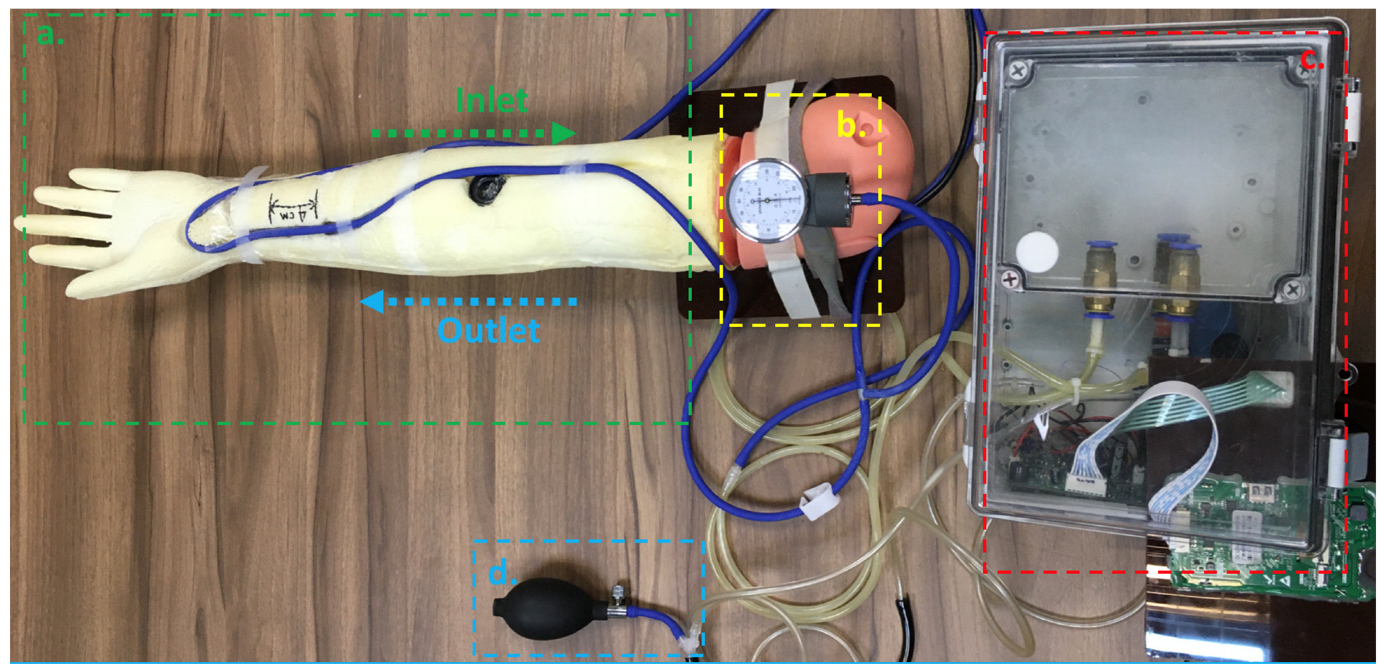
2.1. Design of Hemodynamic Simulator
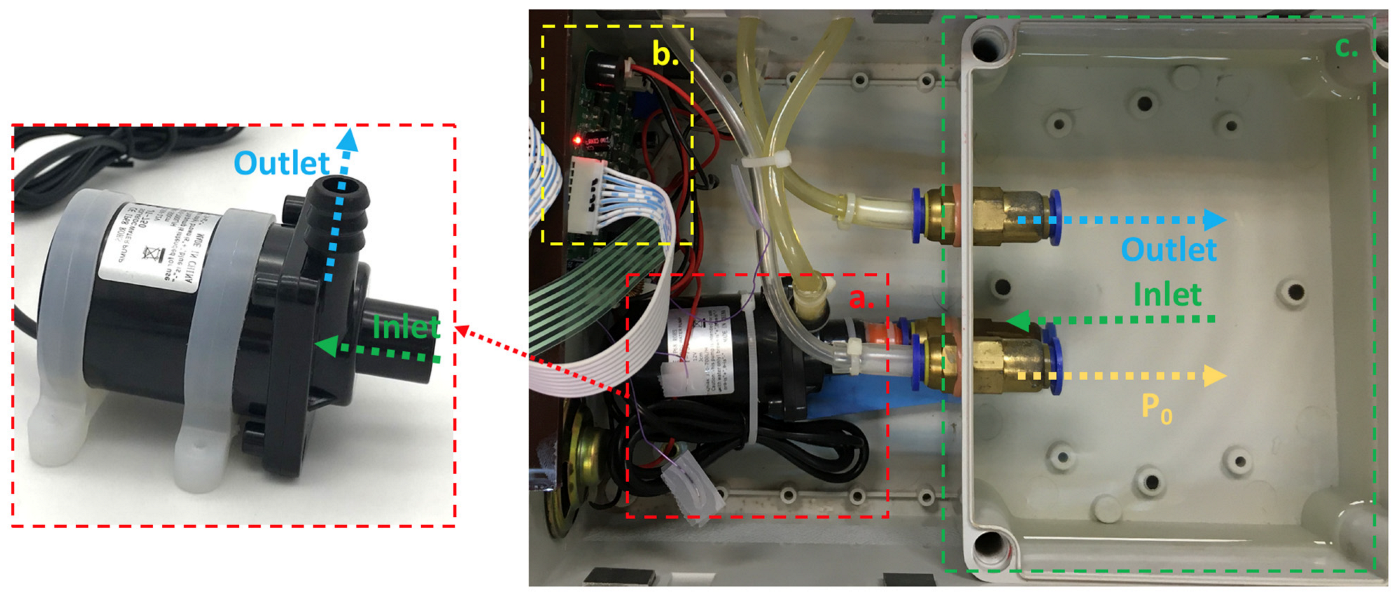
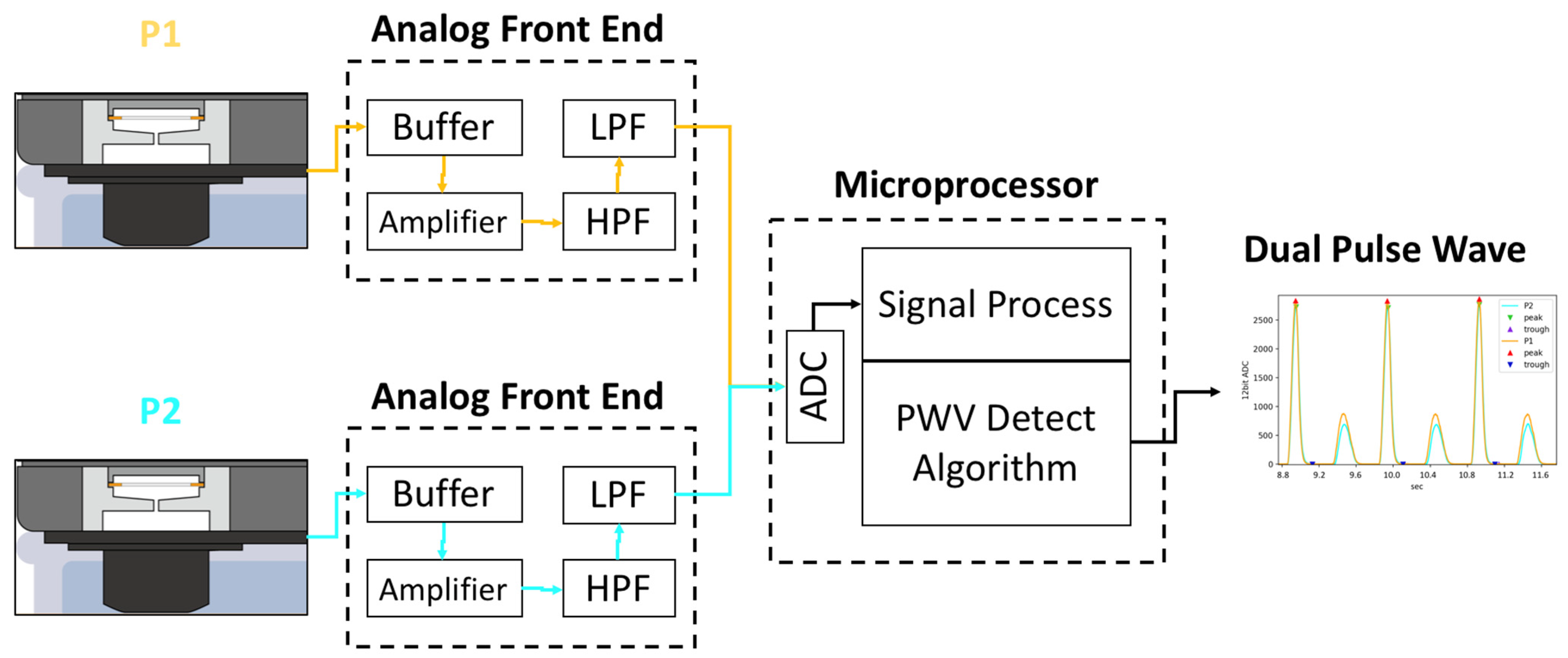
2.2. System Architecture of Hemodynamic Simulator
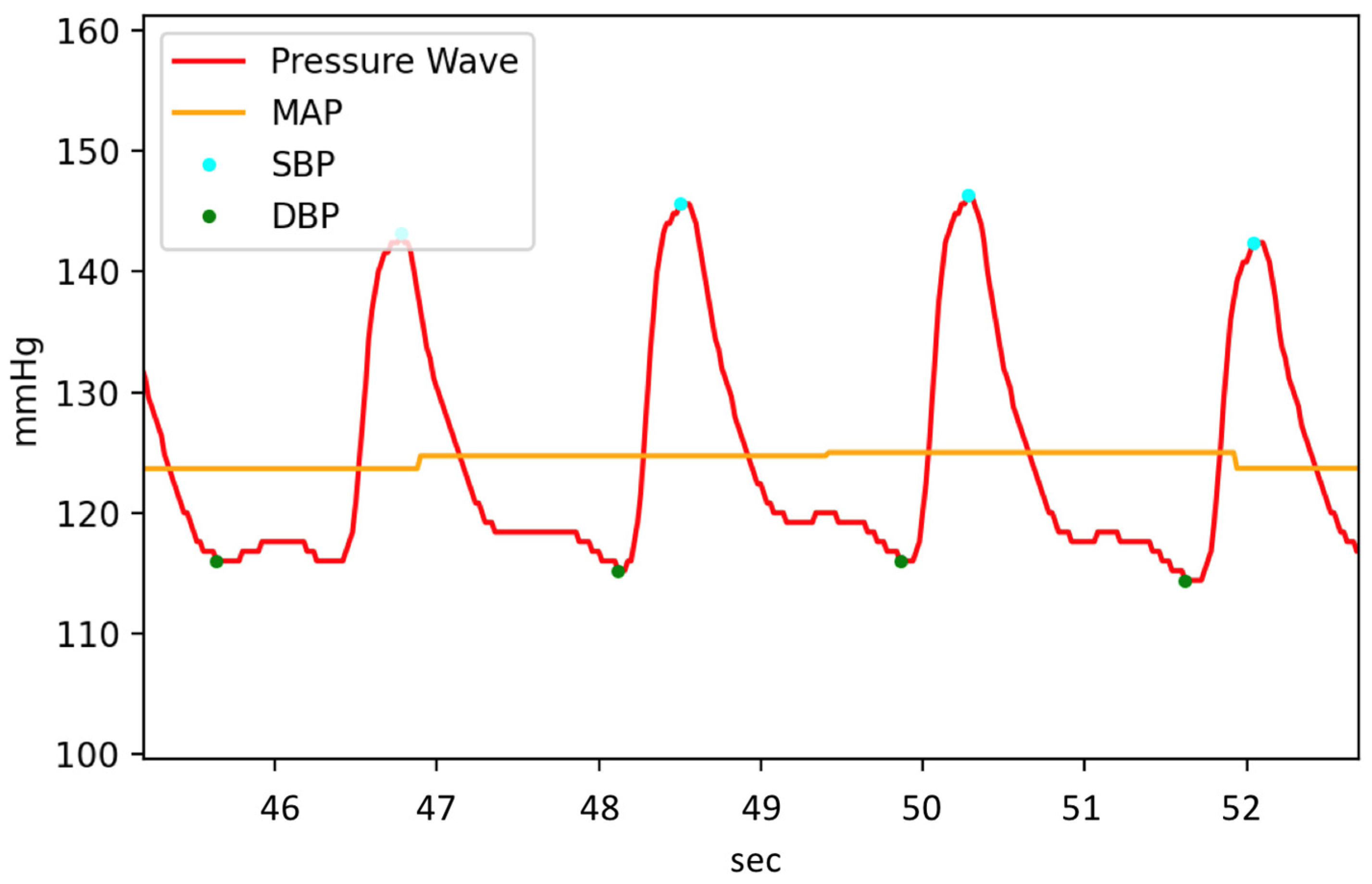
2.3. Characteristic of Hemodynamic Simulator
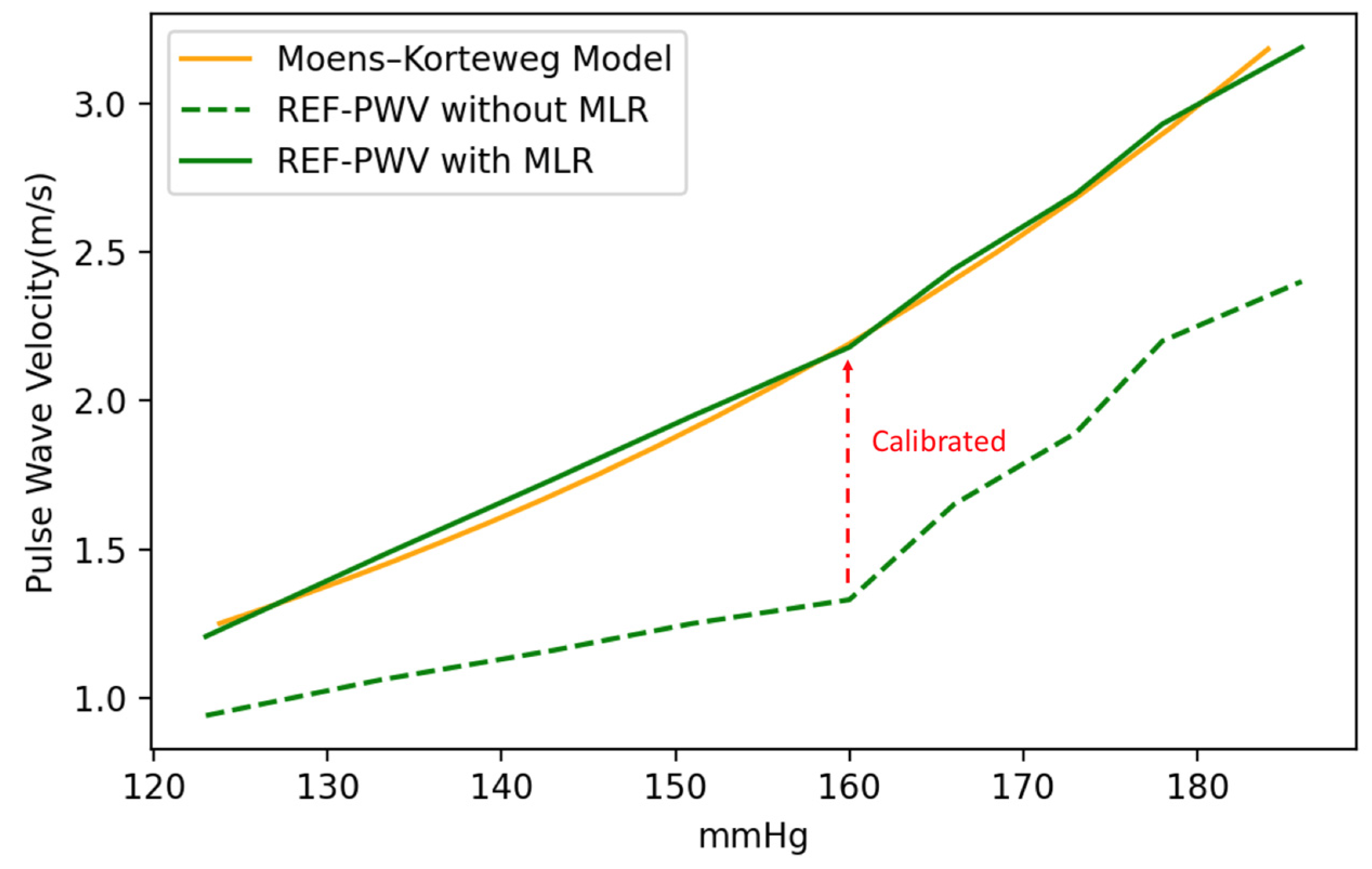
2.4. Experiment Setup of Validation for Pulse Wave Velocity
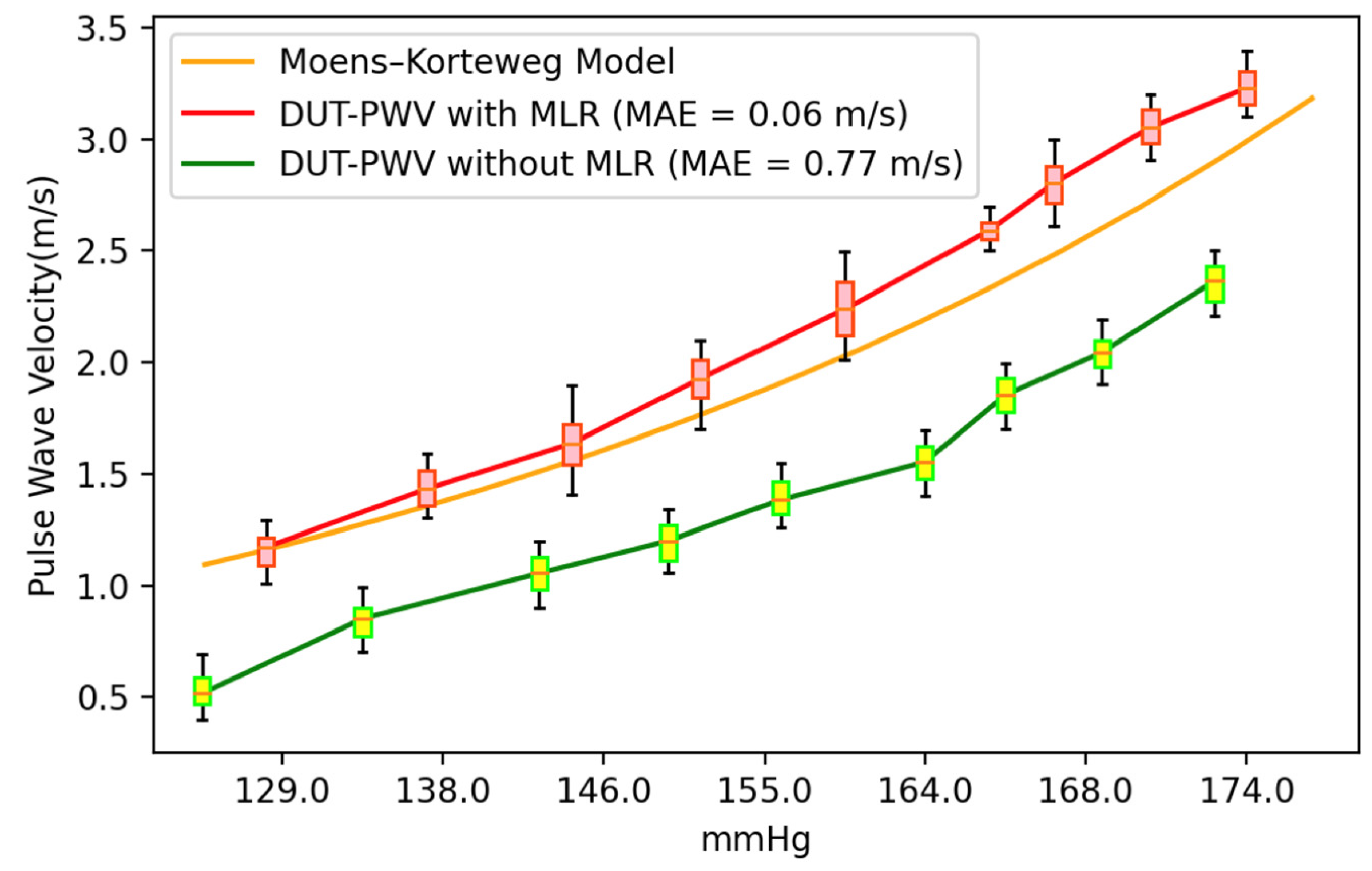
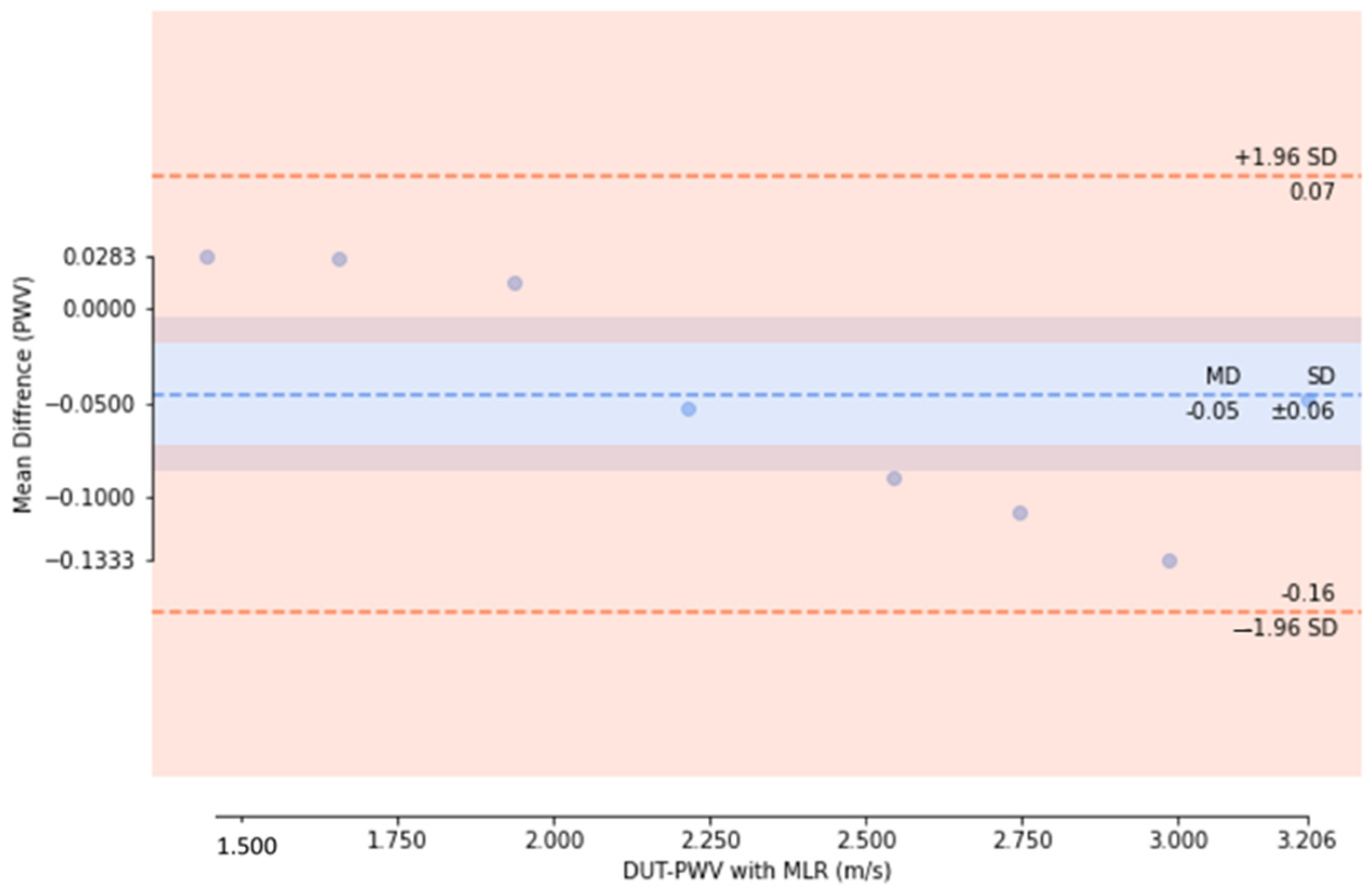
3. Results and Discussion
3.1. Experiment Analysis
3.2. Limitations and Future Works
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staessen, J.A.; Asmar, R.; De Buyzere, M.; Imai, Y.; Parati, G.; Shimada, K.; Stergiou, G.; Redón, J.; Verdecchia, P. Participants of the 2001 Consensus Conference on Ambulatory Blood Pressure Monitoring: Task Force II: Blood pressure measurement and cardiovascular outcome. Blood Press. Monit. 2001, 6, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Dajani, H.R.; Rajan, S.; Lee, G.; Groza, V.Z. Uncertainty in blood pressure measurement estimated using ensemble-based recursive methodology. Sensors 2020, 20, 2108. [Google Scholar] [CrossRef]
- Islam, S.M.S.; Chow, C.K.; Daryabeygikhotbehsara, R.; Subedi, N.; Rawstorn, J.; Tegegne, T.; Karmakar, C.; Siddiqui, M.U.; Lambert, G.; Maddison, R. Wearable cuffless blood pressure monitoring devices: A systematic review and meta-analysis. Eur. Heart J. 2022, 3, 323–337. [Google Scholar] [CrossRef]
- Starzak, M.; Stanek, A.; Jakubiak, G.K.; Cholewka, A.; Cieślar, G. Arterial Stiffness Assessment by Pulse Wave Velocity in Patients with Metabolic Syndrome and Its Components: Is It a Useful Tool in Clinical Practice? Int. J. Environ. Res. Public Health 2022, 19, 10368. [Google Scholar] [CrossRef]
- Steptoe, A.; Smulyan, H.; Gribbin, B. Pulse wave velocity and blood pressure change: Calibration and applications. Psychophysiology 1976, 13, 488–493. [Google Scholar] [CrossRef]
- Muehlsteff, J.; Aubert, X.L.; Schuett, M. Cuffless estimation of systolic blood pressure for short effort bicycle tests: The prominent role of the pre-ejection period. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2006, 2006, 5088–5092. [Google Scholar]
- Martin, S.L.O.; Carek, A.M.; Kim, C.S.; Ashouri, H.; Inan, O.T.; Hahn, J.O.; Mukkamala, R. Weighing scale-based pulse transit time is a superior marker of blood pressure than conventional pulse arrival time. Sci. Rep. 2016, 6, 39273. [Google Scholar] [CrossRef] [PubMed]
- Mukkamala, R.; Hahn, J.O.; Inan, O.T.; Mestha, L.K.; Kim, C.S.; Töreyin, H.; Kyal, S. Toward ubiquitous blood pressure monitoring via pulse transit time: Theory and practice. IEEE. Trans. Biomed. Eng. 2015, 62, 1879–1901. [Google Scholar] [CrossRef]
- Almeida, V.G.; Pereira, H.C.; Pereira, T.; Figueiras, E.; Borges, E.; Cardoso, J.M.R.; Correia, C. Piezoelectric probe for pressure waveform estimation in flexible tubes and its application to the cardiovascular system. Sens. Actuator A-Phys. 2011, 169, 217–226. [Google Scholar] [CrossRef]
- Lu, N.; Lu, C.; Yang, S.; Rogers, J. Highly sensitive skin-mountable strain gauges based entirely on elastomers. Adv. Funct. Mater. 2012, 22, 4044–4050. [Google Scholar] [CrossRef]
- Bae, J.H.; Jeon, Y.J.; Kim, J.Y.; Kim, J.U. New assessment model of pulse depth based on sensor displacement in pulse diagnostic devices. Evid. Based Complement. Altern. Med. 2013, 2013, 938641. [Google Scholar] [CrossRef]
- Fuiano, F.; Scorza, A.; Sciuto, S.A. Functional and Metrological Issues in Arterial Simulators for Biomedical Testing Applications: A Review. Metrology 2022, 2, 360–386. [Google Scholar] [CrossRef]
- Tamang, S.; Gurung, M.S.; Lhaden, T.; Penjore, T.; Tenzin, K. How reliable are the current Blood Pressure Measuring devices in Health Facilities of Bhutan? Bhutan Health J. 2020, 6, 11–17. [Google Scholar] [CrossRef]
- Yang, T.H.; Kim, J.U.; Kim, Y.M.; Koo, J.H.; Woo, S.Y. A new blood pulsation simulator platform incorporating cardiovascular physiology for evaluating radial pulse waveform. J. Healthc. Eng. 2019, 2019, 4938063. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Choi, J.; Hourlier-Fargette, A.; Xue, Y.; Chung, H.U.; Lee, J.Y.; Huang, Y. Relation between blood pressure and pulse wave velocity for human arteries. Proc. Natl. Acad. Sci. USA 2018, 115, 11144–11149. [Google Scholar] [CrossRef]
- Carreta, R. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles. J. Hypertens. 1998, 13, 553. [Google Scholar] [CrossRef]
- Fuiano, F.; Fiori, G.; Vurchio, F.; Scorza, A.; Sciuto, S.A. Transit Time Measurement of a Pressure Wave through an elastic tube based on LVDT sensors. In Proceedings of the 24th IMEKO TC4 International Symposium, Palermo, Italy, 6 February 2020; pp. 321–326. [Google Scholar]
- Zannoli, R.; Corazza, I.; Branzi, A. Mechanical simulator of the cardiovascular system. Phys. Med. 2009, 25, 94–100. [Google Scholar] [CrossRef]
- Benbrahim, A.; Gilbert, J.L.; Milinazzo, B.B.; Warnock, D.F.; Dhara, S.; Gertler, J.P.; Abbott, W.M. A compliant tubular device to study the influences of wall strain and fluid shear stress on cells of the vascular wall. J. Vasc. Surg. 1994, 20, 184–194. [Google Scholar] [CrossRef]
- Hao, L.; Zhang, Q.; Chen, X.; Yao, Y.; Xu, L. Feasibility of waveform separation of central aortic pressure pulse based on lognormal flow wave approximation. Biomed. Signal Process. Control 2022, 77, 103784. [Google Scholar] [CrossRef]
- Domagała, Z.; Grzelak, J.; Pospiech, N.; Hunter, N.; Klekowski, J.; Lach, A.; Trzaska, M. Ultrasound evaluation of the radial artery in young adults—A pilot study. Ann. Anat. 2021, 238, 151763. [Google Scholar] [CrossRef]
- Pickering, T.G.; Hall, J.E.; Appel, L.J.; Falkner, B.E.; Graves, J.; Hill, M.N.; Jones, D.W.; Kurtz, T.; Sheps, S.G.; Roccella, E.J. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 2005, 45, 142–161. [Google Scholar]
- Mitchell, P.L.; Parlin, R.W.; Blackburn, H. Effect of vertical displacement of the arm on indirect blood-pressure measurement. N. Engl. J. Med. 1964, 271, 72–74. [Google Scholar] [CrossRef]
- DeMers, D.; Wachs, D. Physiology, Mean Arterial Pressure; StatPearls: Tampa, FL, USA, 2021. [Google Scholar]
- Vybornova, A.; Polychronopoulou, E.; Wurzner-Ghajarzadeh, A.; Fallet, S.; Sola, J.; Wuerzner, G. Blood pressure from the optical Aktiia Bracelet: A 1-month validation study using an extended ISO81060-2 protocol adapted for a cuffless wrist device. Blood Press. Monit. 2021, 26, 305. [Google Scholar] [CrossRef]
- ISO 81060-2:2018; Instrumentation, A. Non-Invasive Sphygmomanometers—Part 2: Clinical Validation of Automated Measurement Type. ISO: Geneva, Switzerland, 2018.
- Hu, J.R.; Martin, G.; Iyengar, S.; Kovell, L.C.; Plante, T.B.; Helmond, N.V.; Dart, R.A.; Brady, T.M.; Turkson-Ocran, R.N.; Juraschek, S.P. Validating cuffless continuous blood pressure monitoring devices. Cardiovasc. Digit. Health J. 2023, 4, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Shi, P.; Hu, S.; Yu, H. A revised point-to-point calibration approach with adaptive errors correction to weaken initial sensitivity of cuff-less blood pressure estimation. Sensors 2020, 20, 2205. [Google Scholar] [PubMed]
- Grumann, M.; Steigert, J.; Riegger, L.; Moser, I.; Enderle, B.; Riebeseel, K.; Ducrée, J.J.B.M. Sensitivity enhancement for colorimetric glucose assays on whole blood by on-chip beam-guidance. Biomed. Microdevices 2006, 8, 209–214. [Google Scholar] [CrossRef]
- Samimi, H.; Dajani, H.R. Cuffless Blood Pressure Estimation Using Calibrated Cardiovascular Dynamics in the Photoplethysmogram. Bioengineering 2022, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Bank, A.J.; Wilson, R.F.; Kubo, S.H.; Holte, J.E.; Dresing, T.J.; Wang, H. Direct effects of smooth muscle relaxation and contraction on in vivo human brachial artery elastic properties. Circ. Res. 1995, 77, 1008–1016. [Google Scholar] [CrossRef]
- Kao, Y.H.; Tu, T.Y.; Chao, P.C.P.; Lee, Y.P.; Wey, C.L. Optimizing a new cuffless blood pressure sensor via a solid–fluid-electric finite element model with consideration of varied mis-positionings. Microsyst. Technol. 2016, 22, 1437–1447. [Google Scholar] [CrossRef]
- Proença, J.; Muehlsteff, J.; Aubert, X.; Carvalho, P. Is pulse transit time a good indicator of blood pressure changes during short physical exercise in a young population? In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; pp. 598–601. [Google Scholar]
- Guo, C.Y.; Huang, C.H.; Chang, C.C.; Wang, K.J.; Hsieh, T.L. Combining Local PWV and Quantified Arterial Changes for Calibration-Free Cuffless Blood Pressure Estimation: A Clinical Validation. IEEE Sens. J. 2022, 23, 658–668. [Google Scholar] [CrossRef]
- Guo, C.Y.; Chang, H.C.; Wang, K.J.; Hsieh, T.L. An Arterial Compliance Sensor for Cuffless Blood Pressure Estimation Based on Piezoelectric and Optical Signals. Micromachines 2022, 13, 1327. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.Y.; Chang, C.C.; Wang, K.J.; Hsieh, T.L. Assessment of a calibration-free method of cuffless blood pressure measurement: A pilot study. IEEE J. Transl. Eng. Health Med. 2022, 99, 2168–2372. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; Yu, H.; Chen, Z.; Zhang, Y.; Zhang, L.; Zhang, K. A category-based calibration approach with fault tolerance for air monitoring sensors. IEEE Sens. J. 2020, 20, 10756–10765. [Google Scholar] [CrossRef]
- Haddad, S.; Boukhayma, A.; Caizzone, A. Continuous PPG-based blood pressure monitoring using multi-linear regression. IEEE J. Biomed. Health Inform. 2021, 26, 2096–2105. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xu, W.; Lyu, W.; Tan, F.; Yu, C.; Dong, B. High Fidelity MZI-BCG Sensor With Homodyne Demodulation for Unobtrusive HR and BP Monitoring. IEEE Sens. J. 2020, 22, 7798–7807. [Google Scholar] [CrossRef]
- Tang, Q.; Huang, L.; Pan, Z. Multiple linear regression model for vascular aging assessment based on radial artery pulse wave. Eur. J. Integr. Med. 2019, 28, 92–97. [Google Scholar] [CrossRef]
- Grubbs, F.E. Errors of measurement, precision, accuracy and the statistical comparison of measuring instruments. Technometrics 1973, 15, 53–66. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Yan, B.P.; Zhang, Y.T.; Poon, C.C. An armband wearable device for overnight and cuff-less blood pressure measurement. IEEE. Trans. Biomed. Eng. 2014, 61, 2179–2186. [Google Scholar] [CrossRef]
- Hughes, D.J.; Babbs, C.F.; Geddes, L.A.; Bourland, J.D. Measurements of Young’s modulus of elasticity of the canine aorta with ultrasound. Ultrason. Imaging 1979, 1, 356–367. [Google Scholar] [CrossRef]
- Nabeel, P.M.; Jayaraj, J.; Mohanasankar, S. Single-source PPG-based local pulse wave velocity measurement: A potential cuffless blood pressure estimation technique. Physiol. Meas. 2017, 38, 2122. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, C.-Y.; Perng, J.-W.; Chen, L.-C.; Hsieh, T.-L. A Hemodynamic Pulse Wave Simulator Designed for Calibration of Local Pulse Wave Velocities Measurement for Cuffless Techniques. Micromachines 2023, 14, 1218. https://doi.org/10.3390/mi14061218
Guo C-Y, Perng J-W, Chen L-C, Hsieh T-L. A Hemodynamic Pulse Wave Simulator Designed for Calibration of Local Pulse Wave Velocities Measurement for Cuffless Techniques. Micromachines. 2023; 14(6):1218. https://doi.org/10.3390/mi14061218
Chicago/Turabian StyleGuo, Cheng-Yan, Jau-Woei Perng, Li-Ching Chen, and Tung-Li Hsieh. 2023. "A Hemodynamic Pulse Wave Simulator Designed for Calibration of Local Pulse Wave Velocities Measurement for Cuffless Techniques" Micromachines 14, no. 6: 1218. https://doi.org/10.3390/mi14061218
APA StyleGuo, C.-Y., Perng, J.-W., Chen, L.-C., & Hsieh, T.-L. (2023). A Hemodynamic Pulse Wave Simulator Designed for Calibration of Local Pulse Wave Velocities Measurement for Cuffless Techniques. Micromachines, 14(6), 1218. https://doi.org/10.3390/mi14061218








