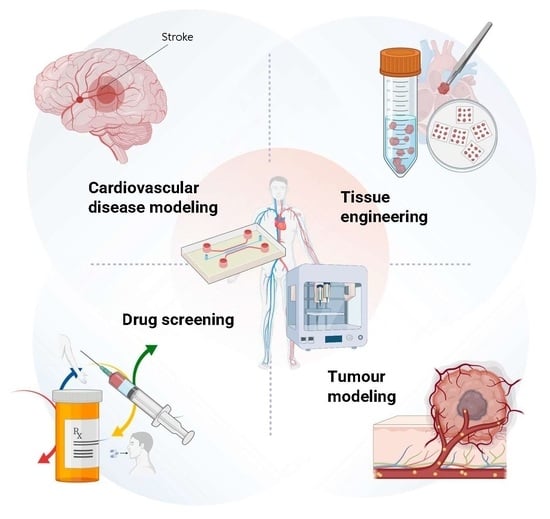
Engineered Vasculature for Cancer Research and Regenerative Medicine
Abstract
1. Introduction to Vascular Engineering: Strategies and Techniques
1.1. General Overview of Vascular Biology
1.2. Engineering 3-Dimensional In Vitro Vasculatures
1.2.1. Vasculatures in Organoids and 3D Cultures in Hydrogel
1.2.2. Conventional, Additive Manufacturing and Bioprinting of Vasculature Tubes
1.2.3. Vasculature-on-a-Chip
Creation of Vasculatures in Microfluidic Devices
Methods for Generating Fluid Flows and Performing Physical and Biochemical Sensing
2. Application of Engineered Vasculatures in Cancer Research and Drug Delivery
2.1. Angiogenesis and Tumor–Vasculature Interactions
2.2. Drug Efficacy and Toxicity Evaluation
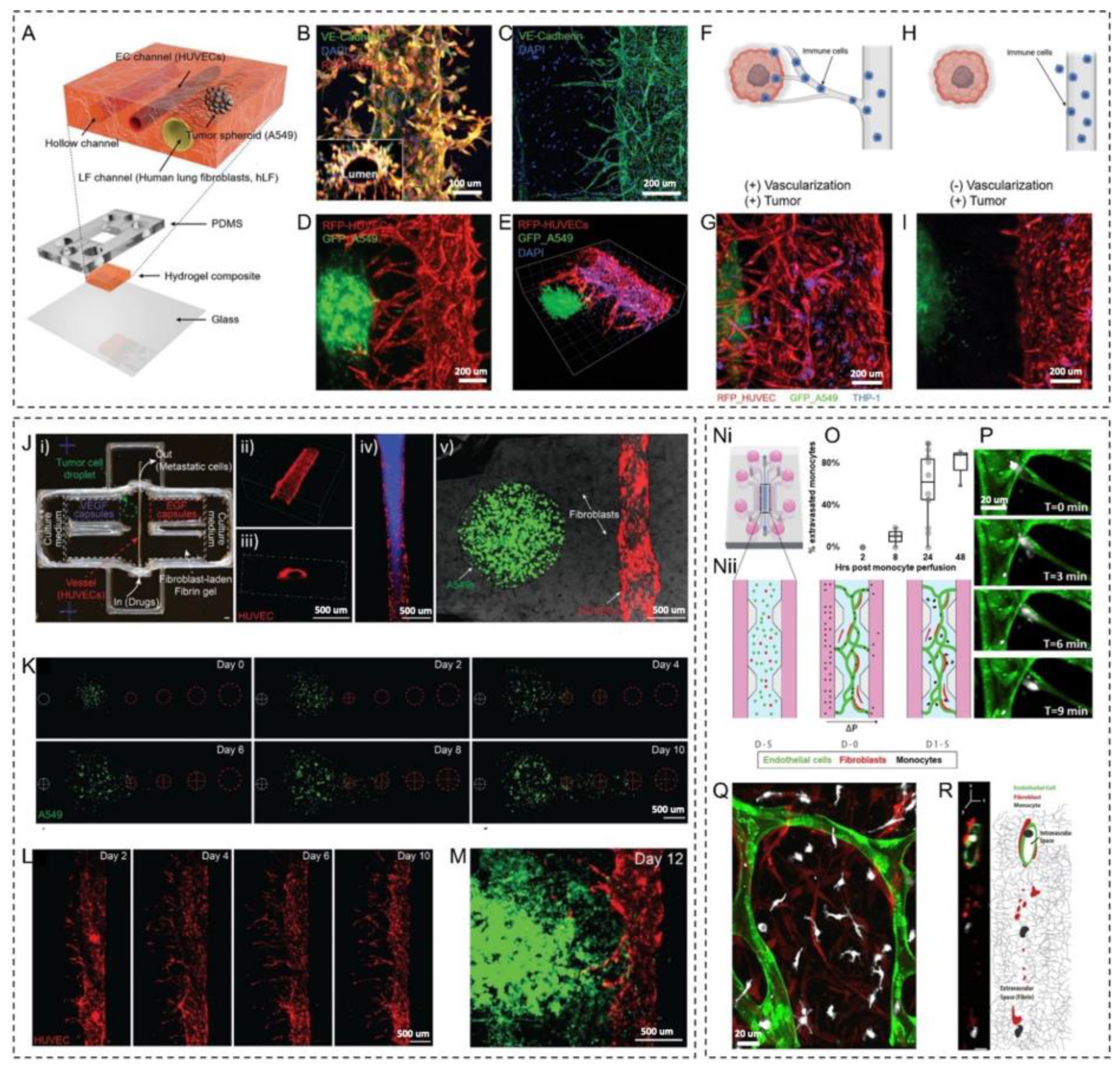
2.3. Metastasis and Immune Cell-Vasculature Interactions
2.4. High-Throughput Screening
2.5. Patient-Specific Model
3. Vascular Genesis and Angiogenesis for Tissue Engineering and Regenerative Medicine
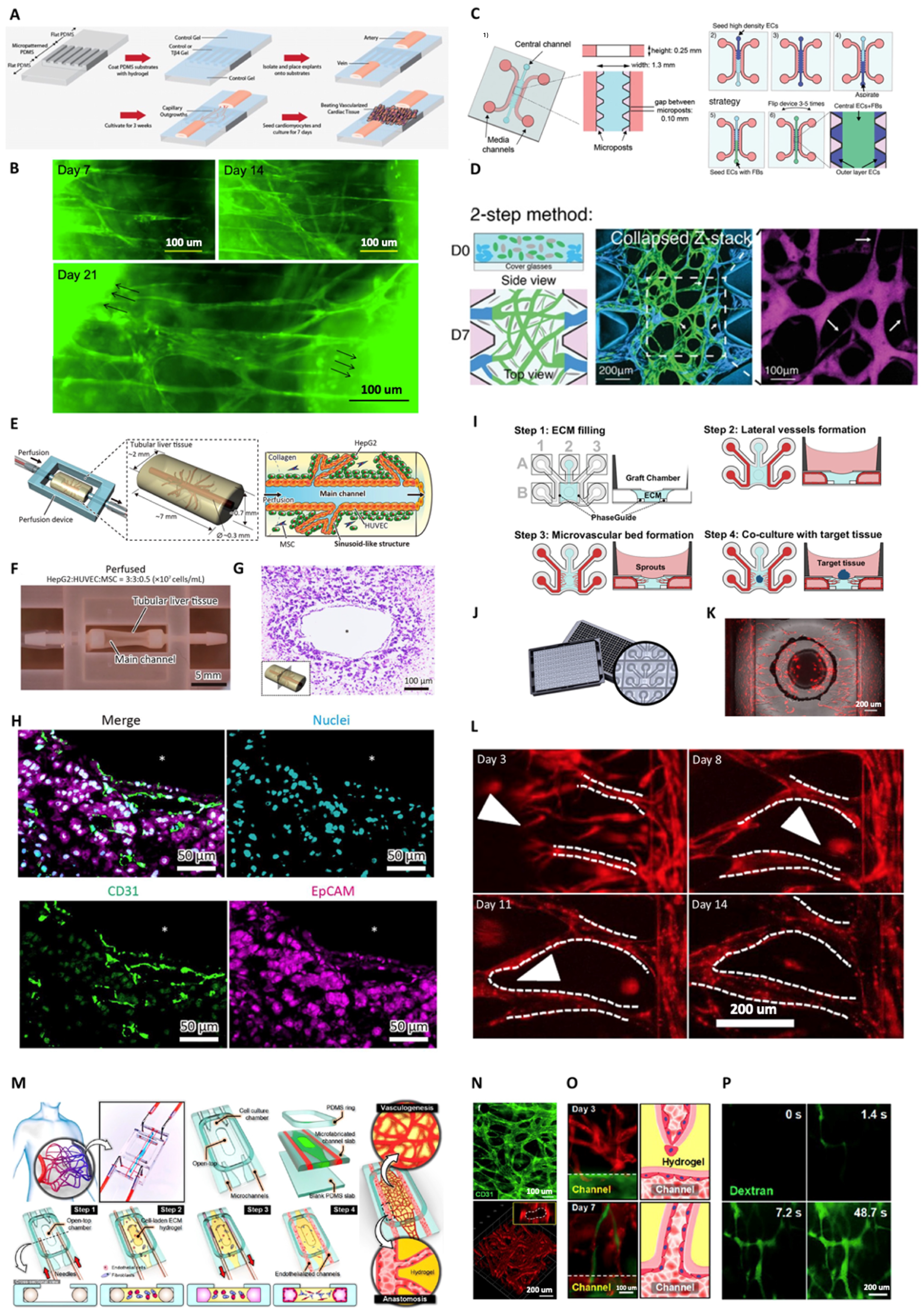
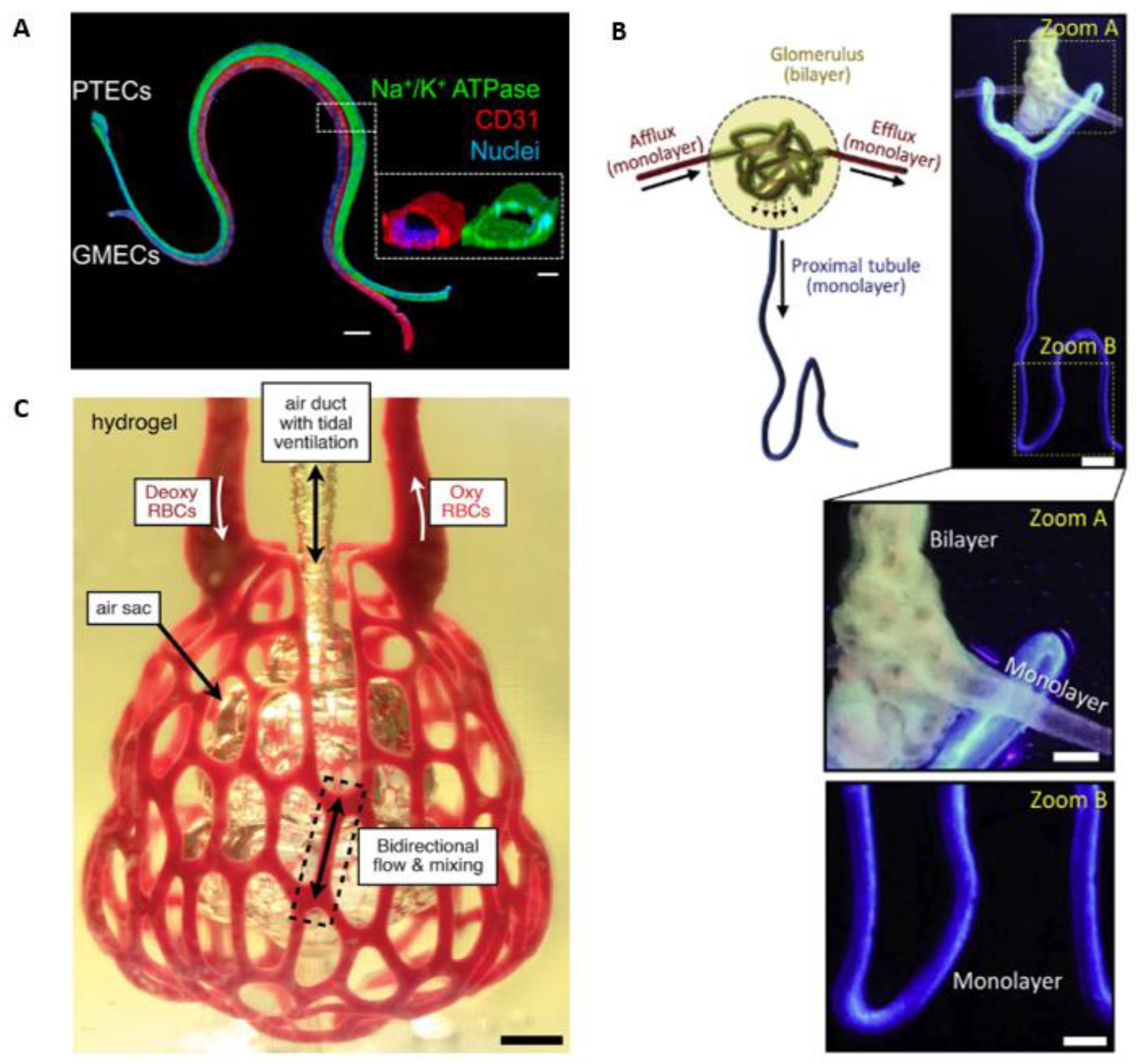
3.1. Strategies for Achieving Vessel Perfusability within In vitro Capillary Networks
3.2. Vascular Perfusion within Engineered 3D Tissues
3.3. Anastomosis of Graft Biofabricated Tissues
4. Vascularized Engineered Tissue: Challenge and Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashammakhi, N.; GhavamiNejad, A.; Tutar, R.; Fricker, A.; Roy, I.; Chatzistavrou, X.; Hoque Apu, E.; Nguyen, K.-L.; Ahsan, T.; Pountos, I. Highlights on Advancing Frontiers in Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 633–664. [Google Scholar] [CrossRef] [PubMed]
- Nourian Dehkordi, A.; Mirahmadi Babaheydari, F.; Chehelgerdi, M.; Raeisi Dehkordi, S. Skin Tissue Engineering: Wound Healing Based on Stem-Cell-Based Therapeutic Strategies. Stem Cell Res. Ther. 2019, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Walsh, C.; Yue, D.; Dardik, A.; Cheema, U. Current Advancements and Strategies in Tissue Engineering for Wound Healing: A Comprehensive Review. Adv. Wound Care 2017, 6, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-H.G.; Rumma, R.T.; Ozaki, C.K.; Edelman, E.R.; Chen, C.S. Vascular Tissue Engineering: Progress, Challenges, and Clinical Promise. Cell Stem Cell 2018, 22, 340–354. [Google Scholar] [CrossRef]
- Caddeo, S.; Boffito, M.; Sartori, S. Tissue Engineering Approaches in the Design of Healthy and Pathological in Vitro Tissue Models. Front. Bioeng. Biotechnol. 2017, 5, 40. [Google Scholar] [CrossRef]
- Perry, L.; Ben-Shaul, S.; Landau, S.; Levenberg, S. Co-Culture Systems for Vasculogenesis. In Vascularization for Tissue Engineering and Regenerative Medicine; Springer: Cham, Switzerland, 2021; pp. 385–413. [Google Scholar]
- Laschke, M.W.; Menger, M.D. Spheroids as Vascularization Units: From Angiogenesis Research to Tissue Engineering Applications. Biotechnol. Adv. 2017, 35, 782–791. [Google Scholar] [CrossRef]
- Kim, D.; Hwang, K.S.; Seo, E.U.; Seo, S.; Lee, B.C.; Choi, N.; Choi, J.; Kim, H.N. Vascularized Lung Cancer Model for Evaluating the Promoted Transport of Anticancer Drugs and Immune Cells in an Engineered Tumor Microenvironment. Adv. Healthc. Mater. 2022, 11, 2102581. [Google Scholar] [CrossRef]
- Fontana, F.; Marzagalli, M.; Sommariva, M.; Gagliano, N.; Limonta, P. In Vitro 3d Cultures to Model the Tumor Microenvironment. Cancers 2021, 13, 12. [Google Scholar] [CrossRef]
- Vitale, C.; Marzagalli, M.; Scaglione, S.; Dondero, A.; Bottino, C.; Castriconi, R. Tumor Microenvironment and Hydrogel-Based 3d Cancer Models for in Vitro Testing Immunotherapies. Cancers 2022, 14, 1013. [Google Scholar] [CrossRef]
- L’Heureux, N.; Paquet, S.; Labbe, R.; Germain, L.; Auger, F.A. A Completely Biological Tissue-Engineered Human Blood Vessel. FASEB J. 1998, 12, 47–56. [Google Scholar]
- Devillard, C.D.; Marquette, C.A. Vascular Tissue Engineering: Challenges and Requirements for an Ideal Large Scale Blood Vessel. Front. Bioeng. Biotechnol. 2021, 9, 913. [Google Scholar] [CrossRef]
- Pugsley, M.K.; Tabrizchi, R. The Vascular System: An Overview of Structure and Function. J. Pharmacol. Toxicol. Methods 2000, 44, 333–340. [Google Scholar] [CrossRef]
- Cleuren, A.C.A.; van der Ent, M.A.; Jiang, H.; Hunker, K.L.; Yee, A.; Siemieniak, D.R.; Molema, G.; Aird, W.C.; Ganesh, S.K.; Ginsburg, D. The in Vivo Endothelial Cell Translatome Is Highly Heterogeneous across Vascular Beds. Proc. Natl. Acad. Sci. USA 2019, 116, 23618–23624. [Google Scholar] [CrossRef]
- McMillan, D.B.; Harris, R.J. Chapter H–the Circulatory System. In An Atlas of Comparative Vertebrate Histology; McMillan, D.B., Harris, R.J., Eds.; Academic Press: San Diego, CA, USA, 2018; pp. 205–226. [Google Scholar]
- Santambrogio, L. Chapter Four–the Lymphatic Fluid. In International Review of Cell and Molecular Biology; Galluzzi, L., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 111–133. [Google Scholar]
- Mazurek, R.; Dave, J.M.; Chandran, R.R.; Misra, A.; Sheikh, A.Q.; Greif, D.M. Chapter Eight–Vascular Cells in Blood Vessel Wall Development and Disease. In Advances in Pharmacology; Khalil, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 323–350. [Google Scholar]
- Kolte, D.; McClung, J.A.; Aronow, W.S. Chapter 6–Vasculogenesis and Angiogenesis. In Translational Research in Coronary Artery Disease; Aronow, W.S., McClung, J.A., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 49–65. [Google Scholar]
- Semenza, G.L. Vasculogenesis, Angiogenesis, and Arteriogenesis: Mechanisms of Blood Vessel Formation and Remodeling. J. Cell. Biochem. 2007, 102, 840–847. [Google Scholar] [CrossRef]
- Marín-García, J. Chapter 9–Molecular Determinants of Cardiac Neovascularization. In Post-Genomic Cardiologyi, 2nd ed.; Marín-García, J., Ed.; Academic Press: Boston, MA, USA, 2014; pp. 279–303. [Google Scholar]
- Duran, C.L.; Howell, D.W.; Dave, J.M.; Smith, R.L.; Torrie, M.E.; Essner, J.J.; Bayless, K.J. Molecular Regulation of Sprouting Angiogenesis. Compr. Physiol. 2017, 8, 153–235. [Google Scholar]
- Ribatti, D. Angiogenesis. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 130–132. [Google Scholar]
- Castro, P.R.; Barbosa, A.S.; Pereira, J.M.; Ranfley, H.; Felipetto, M.; Gonçalves, C.A.X.; Paiva, I.R.; Berg, B.B.; Barcelos, L.S. Cellular and Molecular Heterogeneity Associated with Vessel Formation Processes. BioMed Res. Int. 2018, 2018, 6740408. [Google Scholar] [CrossRef]
- Folkman, J.; Haudenschild, C. Angiogenesis in Vitro. Nature 1980, 288, 551–556. [Google Scholar] [CrossRef]
- Wang, K.; Lin, R.-Z.; Melero-Martin, J.M. Bioengineering Human Vascular Networks: Trends and Directions in Endothelial and Perivascular Cell Sources. Cell. Mol. Life Sci. 2019, 76, 421–439. [Google Scholar] [CrossRef]
- Jambusaria, A.; Hong, Z.; Zhang, L.; Srivastava, S.; Jana, A.; Toth, P.T.; Dai, Y.; Malik, A.B.; Rehman, J. Endothelial Heterogeneity across Distinct Vascular Beds during Homeostasis and Inflammation. eLife 2020, 9, e51413. [Google Scholar] [CrossRef]
- Minami, T.; Muramatsu, M.; Kume, T. Organ/Tissue-Specific Vascular Endothelial Cell Heterogeneity in Health and Disease. Biol. Pharm. Bull. 2019, 42, 1609–1619. [Google Scholar] [CrossRef]
- Nguyen, J.; Lin, Y.-Y.; Gerecht, S. The Next Generation of Endothelial Differentiation: Tissue-Specific Ecs. Cell Stem Cell 2021, 28, 1188–1204. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, J.K.; Matuszcak, C.; Trepel, M.; Körbelin, J. Vascular Endothelial Cells: Heterogeneity and Targeting Approaches. Cells 2021, 10, 2712. [Google Scholar] [CrossRef] [PubMed]
- Urbanczyk, M.; Zbinden, A.; Schenke-Layland, K. Organ-Specific Endothelial Cell Heterogenicity and Its Impact on Regenerative Medicine and Biomedical Engineering Applications. Adv. Drug Deliv. Rev. 2022, 186, 114323. [Google Scholar] [CrossRef] [PubMed]
- Marcu, R.; Choi, Y.J.; Xue, J.; Fortin, C.L.; Wang, Y.; Nagao, R.J.; Xu, J.; MacDonald, J.W.; Bammler, T.K.; Murry, C.E. Human Organ-Specific Endothelial Cell Heterogeneity. iScience 2018, 4, 20–35. [Google Scholar] [CrossRef] [PubMed]
- Goveia, J.; Rohlenova, K.; Taverna, F.; Treps, L.; Conradi, L.-C.; Pircher, A.; Geldhof, V.; de Rooij, L.P.; Kalucka, J.; Sokol, L. An Integrated Gene Expression Landscape Profiling Approach to Identify Lung Tumor Endothelial Cell Heterogeneity and Angiogenic Candidates. Cancer Cell 2020, 37, 21–36.e13. [Google Scholar] [CrossRef]
- Sanz, E.; Bean, J.C.; Carey, D.P.; Quintana, A.; McKnight, G.S. Ribotag: Ribosomal Tagging Strategy to Analyze Cell-Type-Specific Mrna Expression in Vivo. Curr. Protoc. Neurosci. 2019, 88, e77. [Google Scholar] [CrossRef]
- Dellaquila, A.; Le Bao, C.; Letourneur, D.; Simon-Yarza, T. In Vitro Strategies to Vascularize 3d Physiologically Relevant Models. Adv. Sci. 2021, 8, 2100798. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Wu, S.; Wang, D.; Chu, C.; Hong, Y.; Tao, M.; Hu, H.; Xu, M.; Guo, X.; Liu, Y. Human Organoids in Basic Research and Clinical Applications. Signal Transduct. Target. Ther. 2022, 7, 1–17. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3d Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Schutgens, F.; Clevers, H. Human Organoids: Tools for Understanding Biology and Treating Diseases. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 211–234. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, Z.; Xiao, L.; Shi, T.; Xiao, H.; Wang, Y.; Li, Y.; Xue, F.; Zeng, W. Review on the Vascularization of Organoids and Organoids-on-a-C Hip. Front. Bioeng. Biotechnol. 2021, 9, 637048. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Ju, X.-C.; Li, Y.; Zeng, P.-M.; Wu, J.; Zhou, Y.-Y.; Shen, L.-B.; Dong, J.; Chen, Y.-J.; Luo, Z.-G. Generation of Vascularized Brain Organoids to Study Neurovascular Interactions. eLife 2022, 11, e76707. [Google Scholar] [CrossRef]
- Skylar-Scott, M.A.; Huang, J.Y.; Lu, A.; Ng, A.H.; Duenki, T.; Liu, S.; Nam, L.L.; Damaraju, S.; Church, G.M.; Lewis, J.A. Orthogonally Induced Differentiation of Stem Cells for the Programmatic Patterning of Vascularized Organoids and Bioprinted Tissues. Nat. Biomed. Eng. 2022, 6, 449–462. [Google Scholar] [CrossRef]
- Kim, J.W.; Nam, S.A.; Yi, J.; Kim, J.Y.; Lee, J.Y.; Park, S.Y.; Sen, T.; Choi, Y.M.; Lee, J.Y.; Kim, H.L. Kidney Decellularized Extracellular Matrix Enhanced the Vascularization and Maturation of Human Kidney Organoids. Adv. Sci. 2022, 9, 2103526. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The Design of Scaffolds for Use in Tissue Engineering. Part I. Traditional Factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef]
- Kinstlinger, I.S.; Saxton, S.H.; Calderon, G.A.; Ruiz, K.V.; Yalacki, D.R.; Deme, P.R.; Rosenkrantz, J.E.; Louis-Rosenberg, J.D.; Johansson, F.; Janson, K.D.; et al. Generation of Model Tissues with Dendritic Vascular Networks Via Sacrificial Laser-Sintered Carbohydrate Templates. Nat. Biomed. Eng. 2020, 4, 916–932. [Google Scholar] [CrossRef]
- Weinberg, C.B.; Bell, E. A Blood Vessel Model Constructed from Collagen and Cultured Vascular Cells. Science 1986, 231, 397–400. [Google Scholar] [CrossRef]
- Michael, P.; Francis, N.K. Yas Maghdouri-White, Nick Thayer. In 9–Additive Manufacturing for Biofabricated Medical Device Applications; Butterworth-Heinemann: Oxford, UK, 2018. [Google Scholar]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.H.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid Casting of Patterned Vascular Networks for Perfusable Engineered Three-Dimensional Tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef]
- Zhang, R.; Larsen, N.B. Stereolithographic Hydrogel Printing of 3d Culture Chips with Biofunctionalized Complex 3d Perfusion Networks. Lab Chip 2017, 17, 4273–4282. [Google Scholar] [CrossRef] [PubMed]
- Pedde, R.D.; Mirani, B.; Navaei, A.; Styan, T.; Wong, S.; Mehrali, M.; Thakur, A.; Mohtaram, N.K.; Bayati, A.; Dolatshahi-Pirouz, A.; et al. Emerging Biofabrication Strategies for Engineering Complex Tissue Constructs. Adv. Mater. 2017, 29, 1606061. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.K.; Kim, D.Y.; Ngo, H.; Lee, Y.; Seo, L.; Yoo, S.S.; Vincent, P.A.; Dai, G. Creating Perfused Functional Vascular Channels Using 3d Bio-Printing Technology. Biomaterials 2014, 35, 8092–8102. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3d Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Gungor-Ozkerim, P.S.; Zhang, Y.S.; Yue, K.; Zhu, K.; Liu, W.; Pi, Q.; Byambaa, B.; Dokmeci, M.R.; Shin, S.R.; et al. Direct 3d Bioprinting of Perfusable Vascular Constructs Using a Blend Bioink. Biomaterials 2016, 106, 58–68. [Google Scholar] [CrossRef]
- Grigoryan, B.; Sazer, D.W.; Avila, A.; Albritton, J.L.; Padhye, A.; Ta, A.H.; Greenfield, P.T.; Gibbons, D.L.; Miller, J.S. Development, Characterization, and Applications of Multi-Material Stereolithography Bioprinting. Sci. Rep. 2021, 11, 3171. [Google Scholar] [CrossRef]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular Networks and Functional Intravascular Topologies within Biocompatible Hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef]
- Szklanny, A.A.; Machour, M.; Redenski, I.; Chochola, V.; Goldfracht, I.; Kaplan, B.; Epshtein, M.; Simaan Yameen, H.; Merdler, U.; Feinberg, A.; et al. 3d Bioprinting of Engineered Tissue Flaps with Hierarchical Vessel Networks (Vesselnet) for Direct Host-to-Implant Perfusion. Adv. Mater. 2021, 33, e2102661. [Google Scholar] [CrossRef]
- Bertassoni, L.E.; Cecconi, M.; Manoharan, V.; Nikkhah, M.; Hjortnaes, J.; Cristino, A.L.; Barabaschi, G.; Demarchi, D.; Dokmeci, M.R.; Yang, Y.; et al. Hydrogel Bioprinted Microchannel Networks for Vascularization of Tissue Engineering Constructs. Lab Chip 2014, 14, 2202–2211. [Google Scholar] [CrossRef]
- Lin, N.Y.C.; Homan, K.A.; Robinson, S.S.; Kolesky, D.B.; Duarte, N.; Moisan, A.; Lewis, J.A. Renal Reabsorption in 3d Vascularized Proximal Tubule Models. Proc. Natl. Acad. Sci. USA 2019, 116, 5399–5404. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, D.H.; Lee, J.H.; Youn, Y.N. The Effect of Pulsatile Flow on Bmsc-Derived Endothelial-Like Cells in a Small-Sized Artificial Vessel Made by 3-Dimensional Bioprinting. Stem Cells Int. 2018, 2018, 7823830. [Google Scholar] [CrossRef]
- Figueiredo, L.; Le Visage, C.; Weiss, P.; Yang, J. Quantifying Oxygen Levels in 3d Bioprinted Cell-Laden Thick Constructs with Perfusable Microchannel Networks. Polymers 2020, 12, 1260. [Google Scholar] [CrossRef]
- Lee, V.K.; Lanzi, A.M.; Haygan, N.; Yoo, S.S.; Vincent, P.A.; Dai, G. Generation of Multi-Scale Vascular Network System within 3d Hydrogel Using 3d Bio-Printing Technology. Cell. Mol. Bioeng. 2014, 7, 460–472. [Google Scholar] [CrossRef]
- Kolesky, D.B.; Homan, K.A.; Skylar-Scott, M.A.; Lewis, J.A. Three-Dimensional Bioprinting of Thick Vascularized Tissues. Proc. Natl. Acad. Sci. USA 2016, 113, 3179–3184. [Google Scholar] [CrossRef]
- Schoneberg, J.; De Lorenzi, F.; Theek, B.; Blaeser, A.; Rommel, D.; Kuehne, A.J.C.; Kiessling, F.; Fischer, H. Engineering Biofunctional in Vitro Vessel Models Using a Multilayer Bioprinting Technique. Sci. Rep. 2018, 8, 10430. [Google Scholar] [CrossRef]
- Ji, S.; Almeida, E.; Guvendiren, M. 3d Bioprinting of Complex Channels within Cell-Laden Hydrogels. Acta Biomater. 2019, 95, 214–224. [Google Scholar] [CrossRef]
- Liu, X.; Wang, X.; Zhang, L.; Sun, L.; Wang, H.; Zhao, H.; Zhang, Z.; Liu, W.; Huang, Y.; Ji, S.; et al. 3d Liver Tissue Model with Branched Vascular Networks by Multimaterial Bioprinting. Adv. Healthc. Mater. 2021, 10, e2101405. [Google Scholar] [CrossRef]
- Byambaa, B.; Annabi, N.; Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Jia, W.; Kazemzadeh-Narbat, M.; Shin, S.R.; Tamayol, A.; Khademhosseini, A. Bioprinted Osteogenic and Vasculogenic Patterns for Engineering 3d Bone Tissue. Adv. Healthc. Mater. 2017, 6, 1700015. [Google Scholar] [CrossRef]
- Hong, S.; Kim, J.S.; Jung, B.; Won, C.; Hwang, C. Coaxial Bioprinting of Cell-Laden Vascular Constructs Using a Gelatin-Tyramine Bioink. Biomater. Sci. 2019, 7, 4578–4587. [Google Scholar] [CrossRef]
- Millik, S.C.; Dostie, A.M.; Karis, D.G.; Smith, P.T.; McKenna, M.; Chan, N.; Curtis, C.D.; Nance, E.; Theberge, A.B.; Nelson, A. 3d Printed Coaxial Nozzles for the Extrusion of Hydrogel Tubes toward Modeling Vascular Endothelium. Biofabrication 2019, 11, 45009. [Google Scholar] [CrossRef]
- Attalla, R.; Ling, C.; Selvaganapathy, P. Fabrication and Characterization of Gels with Integrated Channels Using 3d Printing with Microfluidic Nozzle for Tissue Engineering Applications. Biomed. Microdevices 2016, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Y.; Akkouch, A.; Dababneh, A.; Dolati, F.; Ozbolat, I.T. In Vitro Study of Directly Bioprinted Perfusable Vasculature Conduits. Biomater. Sci. 2015, 3, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhu, W.; Huang, Y.; Liu, C.; Yu, Z.X.; Nowicki, M.; Miao, S.; Cheng, Y.; Zhou, X.; Lee, S.J.; et al. In Vitro and in Vivo Evaluation of 3d Bioprinted Small-Diameter Vasculature with Smooth Muscle and Endothelium. Biofabrication 2019, 12, 15004. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Lee, J.H.; Jang, J.; Lee, D.H.; Kong, J.-S.; Kim, B.S.; Choi, Y.-J.; Jang, W.B.; Hong, Y.J.; Kwon, S.-M.; et al. Tissue Engineered Bio-Blood-Vessels Constructed Using a Tissue-Specific Bioink and 3d Coaxial Cell Printing Technique: A Novel Therapy for Ischemic Disease. Adv. Funct. Mater. 2017, 27, 1700798. [Google Scholar] [CrossRef]
- Gao, G.; Park, J.Y.; Kim, B.S.; Jang, J.; Cho, D.W. Coaxial Cell Printing of Freestanding, Perfusable, and Functional in Vitro Vascular Models for Recapitulation of Native Vascular Endothelium Pathophysiology. Adv. Healthc. Mater. 2018, 7, e1801102. [Google Scholar] [CrossRef]
- Gao, G.; Kim, H.; Kim, B.S.; Kong, J.S.; Lee, J.Y.; Park, B.W.; Chae, S.; Kim, J.; Ban, K.; Jang, J.; et al. Tissue-Engineering of Vascular Grafts Containing Endothelium and Smooth-Muscle Using Triple-Coaxial Cell Printing. Appl. Physics Rev. 2019, 6, 41402. [Google Scholar] [CrossRef]
- Singh, N.K.; Han, W.; Nam, S.A.; Kim, J.W.; Kim, J.Y.; Kim, Y.K.; Cho, D.W. Three-Dimensional Cell-Printing of Advanced Renal Tubular Tissue Analogue. Biomaterials 2020, 232, 119734. [Google Scholar] [CrossRef]
- Orellano, I.; Thomas, A.; Herrera, A.; Brauer, E.; Wulsten, D.; Petersen, A.; Kloke, L.; Duda, G.N. Engineering Vascular Self-Assembly by Controlled 3d-Printed Cell Placement. Adv. Funct. Mater. 2022, 32, 2208325. [Google Scholar] [CrossRef]
- Ingber, D.E. Human Organs-on-Chips for Disease Modelling, Drug Development and Personalized Medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef]
- Kim, S.; Kim, W.; Lim, S.; Jeon, J.S. Vasculature-on-a-Chip for in Vitro Disease Models. Bioengineering 2017, 4, 8. [Google Scholar] [CrossRef]
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized Cancer on a Chip: The Effect of Perfusion on Growth and Drug Delivery of Tumor Spheroid. Biomaterials 2020, 229, 119547. [Google Scholar] [CrossRef]
- Sontheimer-Phelps, A.; Hassell, B.A.; Ingber, D.E. Modelling Cancer in Microfluidic Human Organs-on-Chips. Nature Rev. Cancer 2019, 19, 65–81. [Google Scholar] [CrossRef]
- Leung, C.M.; de Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S.; et al. A Guide to the Organ-on-a-Chip. Nat. Rev. Methods Prim. 2022, 2, 33. [Google Scholar] [CrossRef]
- Wang, Z.; Mithieux, S.M.; Weiss, A.S. Fabrication Techniques for Vascular and Vascularized Tissue Engineering. Adv. Healthc. Mater. 2019, 8, 1900742. [Google Scholar] [CrossRef]
- Fleischer, S.; Tavakol, D.N.; Vunjak-Novakovic, G. From Arteries to Capillaries: Approaches to Engineering Human Vasculature. Adv. Funct. Mater. 2020, 30, 1910811. [Google Scholar] [CrossRef]
- Skylar-Scott, M.A.; Uzel, S.G.; Nam, L.L.; Ahrens, J.H.; Truby, R.L.; Damaraju, S.; Lewis, J.A. Biomanufacturing of Organ-Specific Tissues with High Cellular Density and Embedded Vascular Channels. Sci. Adv. 2019, 5, eaaw2459. [Google Scholar] [CrossRef]
- Humayun, M.; Ayuso, J.M.; Park, K.Y.; Martorelli Di Genova, B.; Skala, M.C.; Kerr, S.C.; Knoll, L.J.; Beebe, D.J. Innate Immune Cell Response to Host-Parasite Interaction in a Human Intestinal Tissue Microphysiological System. Sci. Adv. 2022, 8, eabm8012. [Google Scholar] [CrossRef]
- Lugo-Cintrón, K.M.; Ayuso, J.M.; White, B.R.; Harari, P.M.; Ponik, S.M.; Beebe, D.J.; Gong, M.M.; Virumbrales-Muñoz, M. Matrix Density Drives 3d Organotypic Lymphatic Vessel Activation in a Microfluidic Model of the Breast Tumor Microenvironment. Lab Chip 2020, 20, 1586–1600. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H.; Chung, M.; Jeon, N.L. Engineering of Functional, Perfusable 3d Microvascular Networks on a Chip. Lab Chip 2013, 13, 1489–1500. [Google Scholar] [CrossRef]
- Hachey, S.J.; Movsesyan, S.; Nguyen, Q.H.; Burton-Sojo, G.; Tankazyan, A.; Wu, J.; Hoang, T.; Zhao, D.; Wang, S.; Hatch, M.M.; et al. An in Vitro Vascularized Micro-Tumor Model of Human Colorectal Cancer Recapitulates in Vivo Responses to Standard-of-Care Therapy. Lab Chip 2021, 21, 1333–1351. [Google Scholar] [CrossRef]
- Debbi, L.; Zohar, B.; Shuhmaher, M.; Shandalov, Y.; Goldfracht, I.; Levenberg, S. Integrating Engineered Macro Vessels with Self-Assembled Capillaries in 3d Implantable Tissue for Promoting Vascular Integration in-Vivo. Biomaterials 2022, 280, 121286. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, C.; Brady, E.; Zheng, Y.; Moore, E.; Stevens, K.R. Engineering the Multiscale Complexity of Vascular Networks. Nat. Rev. Mater. 2022, 7, 702–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kankala, R.K.; Ou, C.; Chen, A.; Yang, Z. Advances in Hydrogel-Based Vascularized Tissues for Tissue Repair and Drug Screening. Bioact. Mater. 2022, 9, 198–220. [Google Scholar] [CrossRef] [PubMed]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in Tissue Engineering: As Nature Intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef]
- Wang, H.-F.; Ran, R.; Liu, Y.; Hui, Y.; Zeng, B.; Chen, D.; Weitz, D.A.; Zhao, C.-X. Tumor-Vasculature-on-a-Chip for Investigating Nanoparticle Extravasation and Tumor Accumulation. ACS Nano 2018, 12, 11600–11609. [Google Scholar] [CrossRef]
- Gijzen, L.; Marescotti, D.; Raineri, E.; Nicolas, A.; Lanz, H.L.; Guerrera, D.; van Vught, R.; Joore, J.; Vulto, P.; Peitsch, M.C. An Intestine-on-a-Chip Model of Plug-and-Play Modularity to Study Inflammatory Processes. SLAS Technol. Transl. Life Sci. Innov. 2020, 25, 585–597. [Google Scholar] [CrossRef]
- Jain, R.K.; Stylianopoulos, T. Delivering Nanomedicine to Solid Tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef]
- Kim, S.; Chung, M.; Ahn, J.; Lee, S.; Jeon, N.L. Interstitial Flow Regulates the Angiogenic Response and Phenotype of Endothelial Cells in a 3d Culture Model. Lab Chip 2016, 16, 4189–4199. [Google Scholar] [CrossRef]
- Zhang, B.; Montgomery, M.; Chamberlain, M.D.; Ogawa, S.; Korolj, A.; Pahnke, A.; Wells, L.A.; Massé, S.; Kim, J.; Reis, L.; et al. Biodegradable Scaffold with Built-in Vasculature for Organ-on-a-Chip Engineering and Direct Surgical Anastomosis. Nat. Mater. 2016, 15, 669–678. [Google Scholar] [CrossRef]
- Polacheck, W.J.; Li, R.; Uzel, S.G.; Kamm, R.D. Microfluidic Platforms for Mechanobiology. Lab Chip 2013, 13, 2252–2267. [Google Scholar] [CrossRef]
- Zhu, Y.; Mandal, K.; Hernandez, A.L.; Kawakita, S.; Huang, W.; Bandaru, P.; Ahadian, S.; Kim, H.-J.; Jucaud, V.; Dokmeci, M.R. State of the Art in Integrated Biosensors for Organ-on-a-Chip Applications. Curr. Opin. Biomed. Eng. 2021, 19, 100309. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Mousavi Shaegh, S.A.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-Integrated Organs-on-Chips Platform for Automated and Continual in Situ Monitoring of Organoid Behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef]
- Lee, S.; Ko, J.; Park, D.; Lee, S.-R.; Chung, M.; Lee, Y.; Jeon, N.L. Microfluidic-Based Vascularized Microphysiological Systems. Lab Chip 2018, 18, 2686–2709. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharm. 2018, 9, 6. [Google Scholar] [CrossRef]
- Khalil, A.S.; Jaenisch, R.; Mooney, D.J. Engineered Tissues and Strategies to Overcome Challenges in Drug Development. Adv. Drug Deliv. Rev. 2020, 158, 116–139. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning from 2d to 3d Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Shukla, S.J.; Huang, R.; Austin, C.P.; Xia, M. The Future of Toxicity Testing: A Focus on in Vitro Methods Using a Quantitative High-Throughput Screening Platform. Drug Discov. Today 2010, 15, 997–1007. [Google Scholar] [CrossRef]
- Mak, I.W.; Evaniew, N.; Ghert, M. Lost in Translation: Animal Models and Clinical Trials in Cancer Treatment. Am. J. Transl. Res. 2014, 6, 114–118. [Google Scholar]
- Whiteside, T.L. The Tumor Microenvironment and Its Role in Promoting Tumor Growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Martin, J.D.; Seano, G.; Jain, R.K. Normalizing Function of Tumor Vessels: Progress, Opportunities, and Challenges. Annu. Rev. Physiol. 2019, 81, 505–534. [Google Scholar] [CrossRef]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2d and Animal Models: Are 3d Cultures the Ideal Tool to Study Cancer-Microenvironment Interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]
- Brassard-Jollive, N.; Monnot, C.; Muller, L.; Germain, S. In Vitro 3d Systems to Model Tumor Angiogenesis and Interactions with Stromal Cells. Front. Cell Dev. Biol. 2020, 8, 594903. [Google Scholar] [CrossRef] [PubMed]
- Lovett, M.; Lee, K.; Edwards, A.; Kaplan, D.L. Vascularization Strategies for Tissue Engineering. Tissue Eng. Part B 2009, 15, 353. [Google Scholar] [CrossRef]
- Hong, S.; Jung, B.Y.; Hwang, C. Multilayered Engineered Tissue Sheets for Vascularized Tissue Regeneration. Tissue Eng. Regen. Med. 2017, 14, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Nah, S.-Y.; Lee, K.; Choi, N.; Kim, H.N. Triculture Model of in Vitro Bbb and Its Application to Study Bbb-Associated Chemosensitivity and Drug Delivery in Glioblastoma. Adv. Funct. Mater. 2022, 32, 2106860. [Google Scholar] [CrossRef]
- Kawakita, S.; Mandal, K.; Mou, L.; Mecwan, M.M.; Zhu, Y.; Li, S.; Sharma, S.; Hernandez, A.L.; Nguyen, H.T.; Maity, S. Organ-on-a-Chip Models of the Blood–Brain Barrier: Recent Advances and Future Prospects. Small 2022, 18, 2201401. [Google Scholar] [CrossRef]
- Truong, D.; Fiorelli, R.; Barrientos, E.S.; Melendez, E.L.; Sanai, N.; Mehta, S.; Nikkhah, M. A Three-Dimensional (3d) Organotypic Microfluidic Model for Glioma Stem Cells–Vascular Interactions. Biomaterials 2019, 198, 63–77. [Google Scholar] [CrossRef]
- Dey, M.; Ayan, B.; Yurieva, M.; Unutmaz, D.; Ozbolat, I.T. Studying Tumor Angiogenesis and Cancer Invasion in a Three-Dimensional Vascularized Breast Cancer Micro-Environment. Adv. Biol. 2021, 5, 2100090. [Google Scholar] [CrossRef]
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Dikici, S.; Claeyssens, F.; MacNeil, S. Bioengineering Vascular Networks to Study Angiogenesis and Vascularization of Physiologically Relevant Tissue Models in Vitro. ACS Biomater. Sci. Eng. 2020, 6, 3513–3528. [Google Scholar] [CrossRef]
- Song, J.; Miermont, A.; Lim, C.T.; Kamm, R.D. A 3d Microvascular Network Model to Study the Impact of Hypoxia on the Extravasation Potential of Breast Cell Lines. Sci. Rep. 2018, 8, 17949. [Google Scholar] [CrossRef]
- Carmeliet, P. Vegf as a Key Mediator of Angiogenesis in Cancer. Oncology 2005, 69 (Suppl. S3), 4–10. [Google Scholar] [CrossRef]
- Ando, Y.; Oh, J.M.; Zhao, W.; Tran, M.; Shen, K. Engineering a Vascularized Hypoxic Tumor Model for Therapeutic Assessment. Cells 2021, 10, 2201. [Google Scholar] [CrossRef]
- Massa, S.; Sakr, M.A.; Seo, J.; Bandaru, P.; Arneri, A.; Bersini, S.; Zare-Eelanjegh, E.; Jalilian, E.; Cha, B.H.; Antona, S.; et al. Bioprinted 3d Vascularized Tissue Model for Drug Toxicity Analysis. Biomicrofluidics 2017, 11, 44109. [Google Scholar] [CrossRef]
- Astashkina, A.I.; Jones, C.F.; Thiagarajan, G.; Kurtzeborn, K.; Ghandehari, H.; Brooks, B.D.; Grainger, D.W. Nanoparticle Toxicity Assessment Using an in Vitro 3-D Kidney Organoid Culture Model. Biomaterials 2014, 35, 6323–6331. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, Y.; Zhao, B.; Shao, T.; Shi, Q.; Zhou, N.; Cai, W. Encapsulation of Liver Microsomes into a Thermosensitive Hydrogel for Characterization of Drug Metabolism and Toxicity. Biomaterials 2013, 34, 9770–9778. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular Principles of Metastasis: A Hallmark of Cancer Revisited. Signal Transduct. Target 2020, 5, 28. [Google Scholar] [CrossRef]
- Welch, D.R.; Hurst, D.R. Defining the Hallmarks of Metastasis. Cancer Res. 2019, 79, 3011–3027. [Google Scholar] [CrossRef]
- Er, E.E.; Tello-Lafoz, M.; Huse, M. Mechanoregulation of Metastasis Beyond the Matrix. Cancer Res. 2022, 82, 3409–3419. [Google Scholar] [CrossRef]
- Paduch, R. The Role of Lymphangiogenesis and Angiogenesis in Tumor Metastasis. Cell Oncol. 2016, 39, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.P.; Naxerova, K.; Keller, L.; Pantel, K.; Witte, M. Molecular Mechanisms of Cancer Metastasis via the Lymphatic Versus the Blood Vessels. Clin. Exp. Metastasis 2022, 39, 159–179. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Dieterich, L.C.; Detmar, M. Multiple Roles of Lymphatic Vessels in Tumor Progression. Curr. Opin. Immunol. 2018, 53, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Lanitis, E.; Irving, M.; Coukos, G. Targeting the Tumor Vasculature to Enhance T Cell Activity. Curr. Opin. Immunol. 2015, 33, 55–63. [Google Scholar] [CrossRef]
- Hendry, S.A.; Farnsworth, R.H.; Solomon, B.; Achen, M.G.; Stacker, S.A.; Fox, S.B. The Role of the Tumor Vasculature in the Host Immune Response: Implications for Therapeutic Strategies Targeting the Tumor Microenvironment. Front. Immunol. 2016, 7, 621. [Google Scholar] [CrossRef]
- Schaaf, M.B.; Garg, A.D.; Agostinis, P. Defining the Role of the Tumor Vasculature in Antitumor Immunity and Immunotherapy. Cell Death Dis. 2018, 9, 115. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, X.; Li, J. Manipulation of Immune–Vascular Crosstalk: New Strategies Towards Cancer Treatment. Acta Pharm. Sin. B 2020, 10, 2018–2036. [Google Scholar] [CrossRef]
- Xiang, Y.; Miller, K.; Guan, J.; Kiratitanaporn, W.; Tang, M.; Chen, S. 3d Bioprinting of Complex Tissues in Vitro: State-of-the-Art and Future Perspectives. Arch. Toxicol. 2022, 96, 691–710. [Google Scholar] [CrossRef]
- Persaud, A.; Maus, A.; Strait, L.; Zhu, D. 3d Bioprinting with Live Cells. Eng. Regen. 2022, 3, 292–309. [Google Scholar] [CrossRef]
- Chen, E.P.; Toksoy, Z.; Davis, B.A.; Geibel, J.P. 3d Bioprinting of Vascularized Tissues for in Vitro and in Vivo Applications. Front. Bioeng. Biotechnol. 2021, 9, 664188. [Google Scholar] [CrossRef]
- Meng, F.; Meyer, C.M.; Joung, D.; Vallera, D.A.; McAlpine, M.C.; Panoskaltsis-Mortari, A. 3d Bioprinted in Vitro Metastatic Models Via Reconstruction of Tumor Microenvironments. Adv. Mater. 2019, 31, 1806899. [Google Scholar] [CrossRef]
- Coughlin, M.F.; Kamm, R.D. The Use of Microfluidic Platforms to Probe the Mechanism of Cancer Cell Extravasation. Adv. Healthc. Mater. 2020, 9, 1901410. [Google Scholar] [CrossRef]
- Boussommier-Calleja, A.; Atiyas, Y.; Haase, K.; Headley, M.; Lewis, C.; Kamm, R.D. The Effects of Monocytes on Tumor Cell Extravasation in a 3d Vascularized Microfluidic Model. Biomaterials 2019, 198, 180–193. [Google Scholar] [CrossRef]
- Kim, H.; Chung, H.; Kim, J.; Choi, D.H.; Shin, Y.; Kang, Y.G.; Kim, B.M.; Seo, S.U.; Chung, S.; and Seok, S.H. Macrophages-Triggered Sequential Remodeling of Endothelium-Interstitial Matrix to Form Pre-Metastatic Niche in Microfluidic Tumor Microenvironment. Adv. Sci. 2019, 6, 1900195. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Tumor Microenvironment Abnormalities: Causes, Consequences, and Strategies to Normalize. J. Cell. Biochem. 2007, 101, 937–949. [Google Scholar] [CrossRef]
- Ayuso, J.M.; Gong, M.M.; Skala, M.C.; Harari, P.M.; Beebe, D.J. Human Tumor-Lymphatic Microfluidic Model Reveals Differential Conditioning of Lymphatic Vessels by Breast Cancer Cells. Adv. Healthc. Mater. 2020, 9, 1900925. [Google Scholar] [CrossRef]
- Lee, S.; Kang, H.; Park, D.; Yu, J.; Koh, S.K.; Cho, D.; Kim, D.H.; Kang, K.S.; Jeon, N.L. Modeling 3d Human Tumor Lymphatic Vessel Network Using High-Throughput Platform. Adv. Biol. 2021, 5, 2000195. [Google Scholar] [CrossRef]
- Huh, D.; Kim, H.J.; Fraser, J.P.; Shea, D.E.; Khan, M.; Bahinski, A.; Hamilton, G.A.; Ingber, D.E. Microfabrication of Human Organs-on-Chips. Nat. Protoc. 2013, 8, 2135–2157. [Google Scholar] [CrossRef]
- Novak, R.; Didier, M.; Calamari, E.; Ng, C.F.; Choe, Y.; Clauson, S.L.; Nestor, B.A.; Puerta, J.; Fleming, R.; Firoozinezhad, S.J.; et al. Scalable Fabrication of Stretchable, Dual Channel, Microfluidic Organ Chips. J. Vis. Exp. 2018, 140, 58151. [Google Scholar]
- Azizgolshani, H.; Coppeta, J.R.; Vedula, E.M.; Marr, E.E.; Cain, B.P.; Luu, R.J.; Lech, M.P.; Kann, S.H.; Mulhern, T.J.; Tandon, V.; et al. High-Throughput Organ-on-Chip Platform with Integrated Programmable Fluid Flow and Real-Time Sensing for Complex Tissue Models in Drug Development Workflows. Lab Chip 2021, 21, 1454–1474. [Google Scholar] [CrossRef]
- Lanz, H.L.; Saleh, A.; Kramer, B.; Cairns, J.; Ng, C.P.; Yu, J.; Trietsch, S.J.; Hankemeier, T.; Joore, J.; Vulto, P.; et al. Therapy Response Testing of Breast Cancer in a 3d High-Throughput Perfused Microfluidic Platform. BMC Cancer 2017, 17, 709. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Schneider, S.; Loskill, P. High-Throughput Organ-on-a-Chip Systems: Current Status and Remaining Challenges. Curr. Opin. Biomed. Eng. 2018, 6, 33–41. [Google Scholar] [CrossRef]
- Parrish, J.; Lim, K.S.; Baer, K.; Hooper, G.J.; Woodfield, T.B.F. A 96-Well Microplate Bioreactor Platform Supporting Individual Dual Perfusion and High-Throughput Assessment of Simple or Biofabricated 3d Tissue Models. Lab Chip 2018, 18, 2757–2775. [Google Scholar] [CrossRef] [PubMed]
- Ragelle, H.; Dernick, K.; Khemais, S.; Keppler, C.; Cousin, L.; Farouz, Y.; Louche, C.; Fauser, S.; Kustermann, S.; Tibbitt, M.W.; et al. Human Retinal Microvasculature-on-a-Chip for Drug Discovery. Adv. Healthc. Mater. 2020, 9, 2001531. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Tung, Y.T.; Sim, E.; Chen, Y.C.; Lee, E.; Ferrer, M.; Song, M.J. Development of Human-Derived, Three-Dimensional Respiratory Epithelial Tissue Constructs with Perfusable Microvasculature on a High-Throughput Microfluidics Screening Platform. Biofabrication 2022, 14, 25012. [Google Scholar] [CrossRef]
- Junaid, A.; van Duinen, V.; Stam, W.; Dólleman, S.; Yang, W.; de Rijke, Y.; Endeman, H.; van Kooten, C.; Mashaghi, A.; de Boer, H.; et al. A Microfluidics-Based Screening Tool to Assess the Impact of Blood Plasma Factors on Microvascular Integrity. Adv. Biol. 2021, 5, 2100954. [Google Scholar] [CrossRef]
- Song, J.; Choi, H.; Koh, S.K.; Park, D.; Yu, J.; Kang, H.; Kim, Y.; Cho, D.; Jeon, N.L. High-Throughput 3d in Vitro Tumor Vasculature Model for Real-Time Monitoring of Immune Cell Infiltration and Cytotoxicity. Front. Immunol. 2021, 12, 733317. [Google Scholar] [CrossRef]
- Yu, J.; Lee, S.; Song, J.; Lee, S.-R.; Kim, S.; Choi, H.; Kang, H.; Hwang, Y.; Hong, Y.-K.; Jeon, N.L. Perfusable Micro-Vascularized 3d Tissue Array for High-Throughput Vascular Phenotypic Screening. Nano Converg. 2022, 9, 16. [Google Scholar] [CrossRef]
- van Duinen, V.; Zhu, D.; Ramakers, C.; van Zonneveld, A.J.; Vulto, P.; Hankemeier, T. Perfused 3d Angiogenic Sprouting in a High-Throughput in Vitro Platform. Angiogenesis 2019, 22, 157–165. [Google Scholar] [CrossRef]
- van Duinen, V.; Stam, W.; Mulder, E.; Famili, F.; Reijerkerk, A.; Vulto, P.; Hankemeier, T.; van Zonneveld, A.J. Robust and Scalable Angiogenesis Assay of Perfused 3d Human Ipsc-Derived Endothelium for Anti-Angiogenic Drug Screening. Int. J. Mol. Sci. 2020, 21, 4804. [Google Scholar] [CrossRef]
- Kim, C.; Kasuya, J.; Jeon, J.; Chung, S.; Kamm, R.D. A Quantitative Microfluidic Angiogenesis Screen for Studying Anti-Angiogenic Therapeutic Drugs. Lab Chip 2015, 15, 301–310. [Google Scholar] [CrossRef]
- Ashley, E.A. Towards Precision Medicine. Nat. Rev. Genet. 2016, 17, 507–522. [Google Scholar] [CrossRef]
- Moretti, A.; Bellin, M.; Welling, A.; Jung, C.B.; Lam, J.T.; Bott-Flügel, L.; Dorn, T.; Goedel, A.; Höhnke, C.; Hofmann, F.; et al. Patient-Specific Induced Pluripotent Stem-Cell Models for Long-Qt Syndrome. N. Engl. J. Med. 2010, 363, 1397–1409. [Google Scholar] [CrossRef]
- Bray, L.J.; Hutmacher, D.W.; Bock, N. Addressing Patient Specificity in the Engineering of Tumor Models. Front. Bioeng. Biotechnol. 2019, 7, 217. [Google Scholar] [CrossRef]
- Morgan, M.M.; Johnson, B.P.; Livingston, M.K.; Schuler, L.A.; Alarid, E.T.; Sung, K.E.; Beebe, D.J. Personalized in Vitro Cancer Models to Predict Therapeutic Response: Challenges and a Framework for Improvement. Pharm. Ther. 2016, 165, 79–92. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.C.; Yamanaka, S. Induced Pluripotent Stem Cell Technology: A Decade of Progress. Nat. Rev. Drug Discov. 2017, 16, 115–130. [Google Scholar] [CrossRef]
- Rowe, R.G.; Daley, G.Q. Induced Pluripotent Stem Cells in Disease Modelling and Drug Discovery. Nat. Rev. Genet. 2019, 20, 377–388. [Google Scholar] [CrossRef]
- Doss, M.X.; Sachinidis, A. Current Challenges of Ipsc-Based Disease Modeling and Therapeutic Implications. Cells 2019, 8, 403. [Google Scholar] [CrossRef]
- van der Meer, A.D.; Orlova, V.V.; ten Dijke, P.; van den Berg, A.; Mummery, C.L. Three-Dimensional Co-Cultures of Human Endothelial Cells and Embryonic Stem Cell-Derived Pericytes inside a Microfluidic Device. Lab Chip 2013, 13, 3562–3568. [Google Scholar] [CrossRef]
- Kurokawa, Y.K.; Yin, R.T.; Shang, M.R.; Shirure, V.S.; Moya, M.L.; George, S.C. Human Induced Pluripotent Stem Cell-Derived Endothelial Cells for Three-Dimensional Microphysiological Systems. Tissue Eng. Part C Methods 2017, 23, 474–484. [Google Scholar] [CrossRef]
- Vila Cuenca, M.; Cochrane, A.; van den Hil, F.E.; de Vries, A.A.F.; Lesnik Oberstein, S.A.J.; Mummery, C.L.; Orlova, V.V. Engineered 3d Vessel-on-Chip Using Hipsc-Derived Endothelial- and Vascular Smooth Muscle Cells. Stem Cell Rep. 2021, 16, 2159–2168. [Google Scholar] [CrossRef] [PubMed]
- Natividad-Diaz, S.L.; Browne, S.; Jha, A.K.; Ma, Z.; Hossainy, S.; Kurokawa, Y.K.; George, S.C.; Healy, K.E. A Combined Hipsc-Derived Endothelial Cell and in Vitro Microfluidic Platform for Assessing Biomaterial-Based Angiogenesis. Biomaterials 2019, 194, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Salmon, I.; Grebenyuk, S.; Abdel Fattah, A.R.; Rustandi, G.; Pilkington, T.; Verfaillie, C.; Ranga, A. Engineering Neurovascular Organoids with 3d Printed Microfluidic Chips. Lab Chip 2022, 22, 1615–1629. [Google Scholar] [CrossRef] [PubMed]
- Tatla, A.S.; Justin, A.W.; Watts, C.; Markaki, A.E. A Vascularized Tumoroid Model for Human Glioblastoma Angiogenesis. Sci. Rep. 2021, 11, 19550. [Google Scholar] [CrossRef] [PubMed]
- Shirure, V.S.; Bi, Y.; Curtis, M.B.; Lezia, A.; Goedegebuure, M.M.; Goedegebuure, S.P.; Aft, R.; Fields, R.C.; George, S.C. Tumor-on-a-Chip Platform to Investigate Progression and Drug Sensitivity in Cell Lines and Patient-Derived Organoids. Lab Chip 2018, 18, 3687–3702. [Google Scholar] [CrossRef]
- Seiler, K.M.; Bajinting, A.; Alvarado, D.M.; Traore, M.A.; Binkley, M.M.; Goo, W.H.; Lanik, W.E.; Ou, J.; Ismail, U.; Iticovici, M.; et al. Patient-Derived Small Intestinal Myofibroblasts Direct Perfused, Physiologically Responsive Capillary Development in a Microfluidic Gut-on-a-Chip Model. Sci. Rep. 2020, 10, 3842. [Google Scholar] [CrossRef]
- Nothdurfter, D.; Ploner, C.; Coraça-Huber, D.C.; Wilflingseder, D.; Müller, T.; Hermann, M.; Hagenbuchner, J.; Ausserlechner, M.J. 3d Bioprinted, Vascularized Neuroblastoma Tumor Environment in Fluidic Chip Devices for Precision Medicine Drug Testing. Biofabrication 2022, 14, 35002. [Google Scholar] [CrossRef]
- Amsterdam, E.A.; Wenger, N.K.; Brindis, R.G.; Casey, D.E.; Ganiats, T.G.; Holmes, D.R.; Jaffe, A.S.; Jneid, H.; Kelly, R.F.; Kontos, M.C.; et al. 2014 Aha/Acc Guideline for the Management of Patients with Non-St-Elevation Acute Coronary Syndromes: Executive Summary. Circulation 2014, 130, 2354–2394. [Google Scholar] [CrossRef]
- Albers, G.W.; Lansberg, M.G.; Brown, S.; Jadhav, A.P.; Haussen, D.C.; Martins, S.O.; Rebello, L.C.; Demchuk, A.M.; Goyal, M.; Ribo, M.; et al. Assessment of Optimal Patient Selection for Endovascular Thrombectomy Beyond 6 Hours after Symptom Onset: A Pooled Analysis of the Aurora Database. JAMA Neurol. 2021, 78, 1064–1071. [Google Scholar] [CrossRef]
- Antonopoulos, C.N.; Mylonas, S.N.; Moulakakis, K.G.; Sergentanis, T.N.; Sfyroeras, G.S.; Lazaris, A.M.; Kakisis, J.D.; Vasdekis, S.N. A Network Meta-Analysis of Randomized Controlled Trials Comparing Treatment Modalities for De Novo Superficial Femoral Artery Occlusive Lesions. J. Vasc. Surg. 2017, 65, 234–245.e11. [Google Scholar] [CrossRef]
- Jongkind, V.; Akkersdijk, G.J.M.; Yeung, K.K.; Wisselink, W. A Systematic Review of Endovascular Treatment of Extensive Aortoiliac Occlusive Disease. J. Vasc. Surg. 2010, 52, 1376–1383. [Google Scholar] [CrossRef]
- Kang, J.L.; Patel, V.I.; Conrad, M.F.; LaMuraglia, G.M.; Chung, T.K.; Cambria, R.P. Common Femoral Artery Occlusive Disease: Contemporary Results Following Surgical Endarterectomy. J. Vasc. Surg. 2008, 48, 872–877.e1. [Google Scholar] [CrossRef]
- Arthurs, Z.M.; Titus, J.; Bannazadeh, M.; Eagleton, M.J.; Srivastava, S.; Sarac, T.P.; Clair, D.G. A Comparison of Endovascular Revascularization with Traditional Therapy for the Treatment of Acute Mesenteric Ischemia. J. Vasc. Surg. 2011, 53, 698–704, discussion 04-5. [Google Scholar] [CrossRef]
- Lim, S.; Halandras, P.M.; Bechara, C.; Aulivola, B.; Crisostomo, P. Contemporary Management of Acute Mesenteric Ischemia in the Endovascular Era. Vasc. Endovasc. Surg. 2019, 53, 42–50. [Google Scholar] [CrossRef]
- Algoet, M.; Janssens, S.; Himmelreich, U.; Gsell, W.; Pusovnik, M.; Van den Eynde, J.; Oosterlinck, W. Myocardial Ischemia-Reperfusion Injury and the Influence of Inflammation. Trends Cardiovasc. Med. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Madeddu, P. Therapeutic Angiogenesis and Vasculogenesis for Tissue Regeneration. In Cardiovascular Genomics; Rai, M.K., Julian, F., Paton, R., Kasparov, S., Katovich, M.J., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 305–323. [Google Scholar]
- Cooke, J.P.; Losordo, D.W. Modulating the Vascular Response to Limb Ischemia: Angiogenic and Cell Therapies. Circ. Res. 2015, 116, 1561–1578. [Google Scholar] [CrossRef]
- Iyer, S.R.; Annex, B.H. Therapeutic Angiogenesis for Peripheral Artery Disease: Lessons Learned in Translational Science. JACC Basic Transl. Sci. 2017, 2, 503–512. [Google Scholar] [CrossRef]
- Cooke, J.P.; Meng, S. Vascular Regeneration in Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1627–1634. [Google Scholar] [CrossRef]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef]
- Herron, L.A.; Hansen, C.S.; Abaci, H.E. Engineering Tissue-Specific Blood Vessels. Bioeng. Transl. Med. 2019, 4, e10139. [Google Scholar] [CrossRef]
- Stan, R.V. Endothelial Stomatal and Fenestral Diaphragms in Normal Vessels and Angiogenesis. J. Cell. Mol. Med. 2007, 11, 621–643. [Google Scholar] [CrossRef] [PubMed]
- Nolan, D.J.; Ginsberg, M.; Israely, E.; Palikuqi, B.; Poulos, M.G.; James, D.; Ding, B.S.; Schachterle, W.; Liu, Y.; Rosenwaks, Z.; et al. Molecular Signatures of Tissue-Specific Microvascular Endothelial Cell Heterogeneity in Organ Maintenance and Regeneration. Dev. Cell 2013, 26, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.K.; Canfield, S.G.; Shusta, E.V.; Palecek, S.P. Concise Review: Tissue-Specific Microvascular Endothelial Cells Derived from Human Pluripotent Stem Cells. Stem Cells 2014, 32, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Levenberg, S.; Golub, J.S.; Amit, M.; Itskovitz-Eldor, J.; Langer, R. Endothelial Cells Derived from Human Embryonic Stem Cells. Proc. Natl. Acad. Sci. USA 2002, 99, 4391–4396. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wang, T.-M.; Young, T.-H.; Lai, Y.-K.; Yen, M.-L. The Critical Role of Ecm Proteins within the Human Msc Niche in Endothelial Differentiation. Biomaterials 2013, 34, 4223–4234. [Google Scholar] [CrossRef]
- Gu, M. Efficient Differentiation of Human Pluripotent Stem Cells to Endothelial Cells. Curr. Protoc. Hum. Genet. 2018, 98, e64. [Google Scholar] [CrossRef]
- Sobrino, A.; Phan, D.T.; Datta, R.; Wang, X.; Hachey, S.J.; Romero-López, M.; Gratton, E.; Lee, A.P.; George, S.C.; Hughes, C.C. 3d Microtumors in Vitro Supported by Perfused Vascular Networks. Sci. Rep. 2016, 6, 31589. [Google Scholar] [CrossRef]
- Chiu, L.L.; Montgomery, M.; Liang, Y.; Liu, H.; Radisic, M. Perfusable Branching Microvessel Bed for Vascularization of Engineered Tissues. Proc. Natl. Acad. Sci. USA 2012, 109, E3414–E3423. [Google Scholar] [CrossRef]
- Whisler, J.A.; Chen, M.B.; Kamm, R.D. Control of Perfusable Microvascular Network Morphology Using a Multiculture Microfluidic System. Tissue Eng. Part C Methods 2014, 20, 543–552. [Google Scholar] [CrossRef]
- Jeon, J.S.; Bersini, S.; Gilardi, M.; Dubini, G.; Charest, J.L.; Moretti, M.; Kamm, R.D. Human 3d Vascularized Organotypic Microfluidic Assays to Study Breast Cancer Cell Extravasation. Proc. Natl. Acad. Sci. USA 2015, 112, 214–219. [Google Scholar] [CrossRef]
- Chen, M.B.; Whisler, J.A.; Frose, J.; Yu, C.; Shin, Y.; Kamm, R.D. On-Chip Human Microvasculature Assay for Visualization and Quantification of Tumor Cell Extravasation Dynamics. Nat. Protoc. 2017, 12, 865–880. [Google Scholar] [CrossRef]
- Wan, Z.; Zhong, A.X.; Zhang, S.; Pavlou, G.; Coughlin, M.F.; Shelton, S.E.; Nguyen, H.T.; Lorch, J.H.; Barbie, D.A.; Kamm, R.D. A Robust Method for Perfusable Microvascular Network Formation in Vitro. Small Methods 2022, 6, 2200143. [Google Scholar] [CrossRef]
- Mori, N.; Akagi, Y.; Imai, Y.; Takayama, Y.; Kida, Y.S. Fabrication of Perfusable Vascular Channels and Capillaries in 3d Liver-Like Tissue. Sci. Rep. 2020, 10, 5646. [Google Scholar] [CrossRef]
- Bonanini, F.; Kurek, D.; Previdi, S.; Nicolas, A.; Hendriks, D.; de Ruiter, S.; Meyer, M.; Clapés Cabrer, M.; Dinkelberg, R.; García, S.B.; et al. In Vitro Grafting of Hepatic Spheroids and Organoids on a Microfluidic Vascular Bed. Angiogenesis 2022, 25, 455–470. [Google Scholar] [CrossRef]
- Paek, J.; Park, S.E.; Lu, Q.; Park, K.T.; Cho, M.; Oh, J.M.; Kwon, K.W.; Yi, Y.S.; Song, J.W.; Edelstein, H.I.; et al. Microphysiological Engineering of Self-Assembled and Perfusable Microvascular Beds for the Production of Vascularized Three-Dimensional Human Microtissues. ACS Nano 2019, 13, 7627–7643. [Google Scholar] [CrossRef]
- Hajal, C.; Offeddu, G.S.; Shin, Y.; Zhang, S.; Morozova, O.; Hickman, D.; Knutson, C.G.; and Kamm, R.D. Engineered Human Blood–Brain Barrier Microfluidic Model for Vascular Permeability Analyses. Nat. Protoc. 2022, 17, 95–128. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Lim, J.; Choi, H.; Kang, H.; Jeon, N.L.; Son, Y. Human Bone Marrow-Derived Mesenchymal Stem Cells Play a Role as a Vascular Pericyte in the Reconstruction of Human Bbb on the Angiogenesis Microfluidic Chip. Biomaterials 2021, 279, 121210. [Google Scholar] [CrossRef]
- Rouwkema, J.; Koopman, B.; Blitterswijk, C.; Dhert, W.; Malda, J. Supply of Nutrients to Cells in Engineered Tissues. Biotechnol. Genet. Eng. Rev. 2010, 26, 163–178. [Google Scholar] [CrossRef]
- Jung, A.; Faltermeier, R.; Rothoerl, R.; Brawanski, A. A Mathematical Model of Cerebral Circulation and Oxygen Supply. J. Math. Biol. 2005, 51, 491–507. [Google Scholar] [CrossRef]
- Traore, M.A.; George, S.C. Tissue Engineering the Vascular Tree. Tissue Eng. Part B Rev. 2017, 23, 505–514. [Google Scholar] [CrossRef]
- Feng, B.; Jinkang, Z.; Zhen, W.; Jianxi, L.; Jiang, C.; Jian, L.; Guolin, M.; Xin, D. The Effect of Pore Size on Tissue Ingrowth and Neovascularization in Porous Bioceramics of Controlled Architecture in Vivo. Biomed. Mater. 2011, 6, 15007. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Du, Y.; Yang, G.; Hu, X.; Wang, L.; Liu, B.; Wang, J.; Zhang, S. Delivering Proangiogenic Factors from 3d-Printed Polycaprolactone Scaffolds for Vascularized Bone Regeneration. Adv. Healthc. Mater. 2020, 9, 2000727. [Google Scholar] [CrossRef] [PubMed]
- Kuss, M.A.; Wu, S.; Wang, Y.; Untrauer, J.B.; Li, W.; Lim, J.Y.; Duan, B. Prevascularization of 3d Printed Bone Scaffolds by Bioactive Hydrogels and Cell Co-Culture. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Redd, M.A.; Zeinstra, N.; Qin, W.; Wei, W.; Martinson, A.; Wang, Y.; Wang, R.K.; Murry, C.E.; Zheng, Y. Patterned Human Microvascular Grafts Enable Rapid Vascularization and Increase Perfusion in Infarcted Rat Hearts. Nat. Commun. 2019, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Park, H.-J.; Kim, S.-W.; Kim, H.; Park, J.Y.; Na, S.J.; Kim, H.J.; Park, M.N.; Choi, S.H.; Park, S.H. 3d Printed Complex Tissue Construct Using Stem Cell-Laden Decellularized Extracellular Matrix Bioinks for Cardiac Repair. Biomaterials 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Bang, S.; Tahk, D.; Choi, Y.H.; Lee, S.; Lim, J.; Lee, S.R.; Kim, B.S.; Kim, H.N.; Hwang, N.S.; Jeon, N.L. 3d Microphysiological System-Inspired Scalable Vascularized Tissue Constructs for Regenerative Medicine. Adv. Funct. Mater. 2022, 32, 2105475. [Google Scholar] [CrossRef]
- Joshi, A.; Choudhury, S.; Gugulothu, S.B.; Visweswariah, S.S.; Chatterjee, K. Strategies to Promote Vascularization in 3d Printed Tissue Scaffolds: Trends and Challenges. Biomacromolecules 2022, 23, 2730–2751. [Google Scholar] [CrossRef]
- van Mulken, T.J.M.; Schols, R.M.; Scharmga, A.M.J.; Winkens, B.; Cau, R.; Schoenmakers, F.B.F.; Qiu, S.S.; van der Hulst, R.; MicroSurgical Robot Research, G. First-in-Human Robotic Supermicrosurgery Using a Dedicated Microsurgical Robot for Treating Breast Cancer-Related Lymphedema: A Randomized Pilot Trial. Nat. Commun. 2020, 11, 757. [Google Scholar] [CrossRef]
- Seki, Y.; Kajikawa, A. Fundamental and Essential Techniques for Supermicrosurgical Lymphaticovenular Anastomosis: The Art of Isao Koshima’s Supermicrosurgery. Plast. Aesthet. Res. 2021, 8, 44. [Google Scholar] [CrossRef]
- Chlupáč, J.; Filová, E.; Bačáková, L. Blood Vessel Replacement: 50 Years of Development and Tissue Engineering Paradigms in Vascular Surgery. Physiol. Res. 2009, 58 (Suppl. S2), S119–S140. [Google Scholar] [CrossRef]
- Wang, X.; Lin, P.; Yao, Q.; Chen, C. Development of Small-Diameter Vascular Grafts. World J. Surg. 2007, 31, 682–689. [Google Scholar] [CrossRef]
- Aslani, S.; Kabiri, M.; HosseinZadeh, S.; Hanaee-Ahvaz, H.; Taherzadeh, E.S.; Soleimani, M. The Applications of Heparin in Vascular Tissue Engineering. Microvasc. Res. 2020, 131, 104027. [Google Scholar] [CrossRef]
- Hoenig, M.R.; Campbell, G.R.; Rolfe, B.E.; Campbell, J.H. Tissue-Engineered Blood Vessels: Alternative to Autologous Grafts? Arter. Thromb. Vasc. Biol. 2005, 25, 1128–1134. [Google Scholar] [CrossRef]
- Yoneyama, T.; Ito, M.; Sugihara, K.; Ishihara, K.; Nakabayashi, N. Small Diameter Vascular Prosthesis with a Nonthrombogenic Phospholipid Polymer Surface: Preliminary Study of a New Concept for Functioning in the Absence of Pseudo- or Neointima Formation. Artif. Organs 2000, 24, 23–28. [Google Scholar] [CrossRef]
- Radke, D.; Jia, W.; Sharma, D.; Fena, K.; Wang, G.; Goldman, J.; Zhao, F. Tissue Engineering at the Blood-Contacting Surface: A Review of Challenges and Strategies in Vascular Graft Development. Adv. Health Mater. 2018, 7, e1701461. [Google Scholar] [CrossRef]
- Machour, M.S.A.A.; Levenberg, S. Fabrication of Engineered Vascular Flaps Using 3d Printing Technologies. J. Vis. Exp. 2022, 183. [Google Scholar]
- Wu, Y.; Ravnic, D.J.; Ozbolat, I.T. Intraoperative Bioprinting: Repairing Tissues and Organs in a Surgical Setting. Trends Biotechnol. 2020, 38, 594–605. [Google Scholar] [CrossRef]
- Villalona, G.A.; Udelsman, B.; Duncan, D.R.; McGillicuddy, E.; Sawh-Martinez, R.F.; Hibino, N.; Painter, C.; Mirensky, T.; Erickson, B.; Shinoka, T. Cell-Seeding Techniques in Vascular Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 341–350. [Google Scholar] [CrossRef]
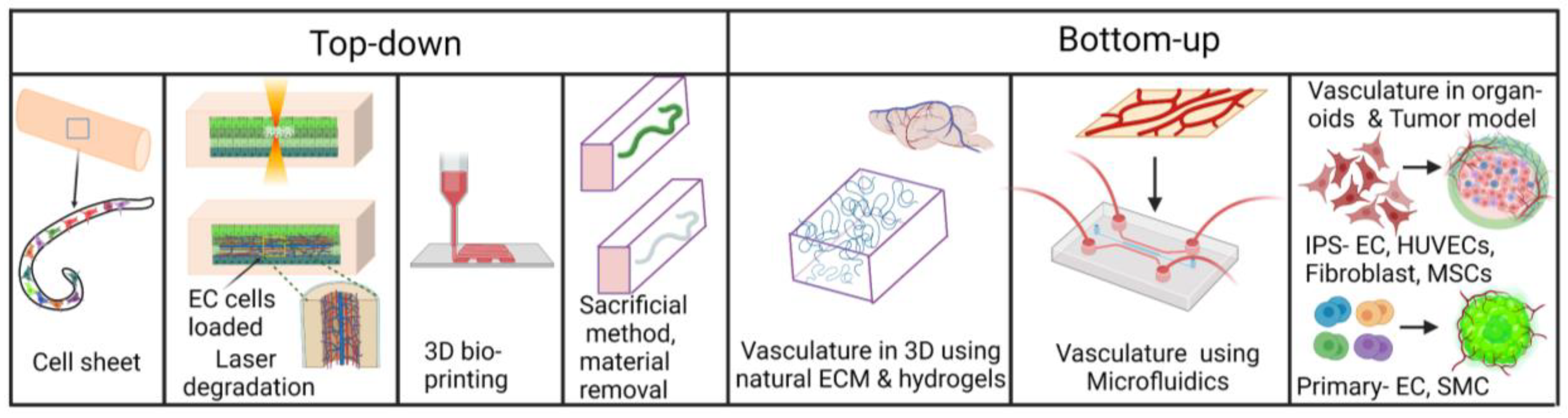
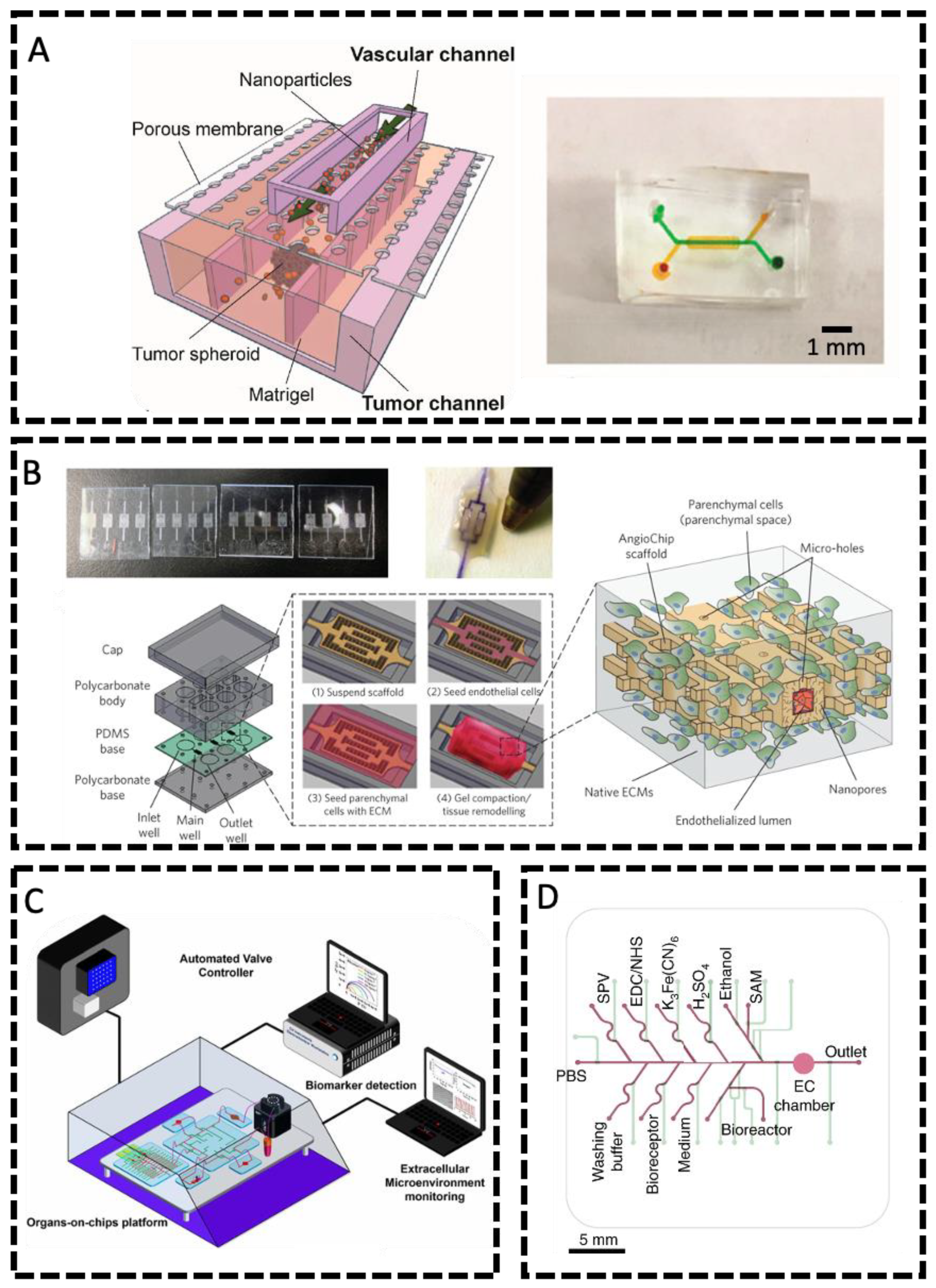
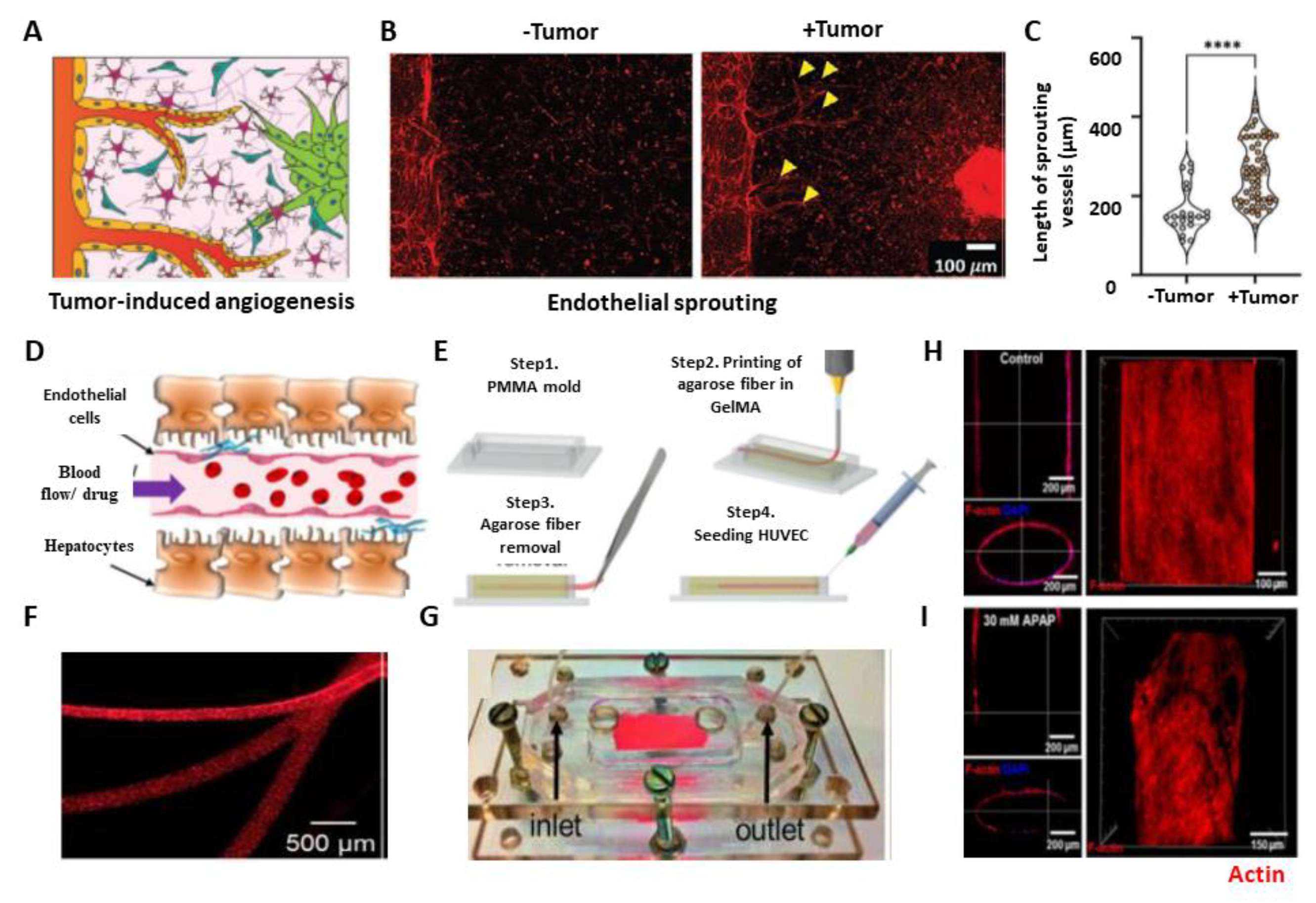
| Manufacturing Method | EC | Associated Cell Type(s) | Desired Organ | Biomaterial | Fugitive Ink | Flow and/or Static Culture | Microvascular Integration | Ref. |
|---|---|---|---|---|---|---|---|---|
| Conventional manufactured sacrificial templates (CMST): Annular mold | Bovine aortic ECs | Bovine smooth muscle cells, and adventitial fibroblasts | Only vasculature | Collagen | / | Static | No | Weinberg & Bell (1986) [45] |
| CMST: Sheet rolling | Human umbilical vein endothelial cells (HUVECs) | Human vascular smooth muscle cells and human skin fibroblasts | Only vasculature | Cell sheets produced by smooth muscle cells and fibroblasts | / | Flow | No | L’heureux et al. (1998) [11] |
| Additive manufactured sacrificial templates (AMST): Stereolithography (SLA) | / | Red blood cells, Human lung epithelial cells Additionally, Human lung fibroblasts | Lung | 6 kDa Polyethylene glycol diacrylate (PEGDA) or a mixture of methacrylated gelatin (GelMA) and 6 kDa PEGDA | 6 kDa PEGDA or mixture of GelMA and 6 kDa PEGDA | Flow | No | Grigoryan et al. (2019) [54] |
| AMST: Stereolithography (SLA) | HUVECs | Human colorectal adenocarcinoma cells | Vasculature and associated single cells | PEGDA: 0.7 kDa Additionally, GelMA added after templating | PEGDA: 0.7 kDa | Flow and static | No | Zhang et al. (2017) [48] |
| AMST: Selective Laser Sintering | / | Hepatic aggregates consisting of primary rat hepatocytes and human dermal fibroblasts | Liver | Agarose | Isomalt and cornstarch | Flow and static | No | Kinstlinger et al. (2020) [44] |
| AMST: Selective Laser Sintering | HUVECs | IMR—90 lung fibroblasts | Vasculature and associated single cells | GelMA | Isomalt and cornstarch | Flow and static | No | Kinstlinger et al. (2020) [44] |
| AMST: 3D printing | HUVECs | C3H/10T 1/2 cells and Human Embryonic Kidney cells | Vasculature and associated single cells | Fibrin | Carbohydrate glass | Flow and static | Yes | Miller et al. (2012) [47] |
| AMST: 3D (Bio)printing | Human adipose microvascular ECs | Dental pulp stem cells | Tissue flap, transplantable with direct anastomosis | PLLA–PLGA blend and recombinant human collagen methacrylate | butanediol vinyl alcohol copolymer | Static | Yes | Szklanny et al. (2021) [55] |
| AMST: 3D (Bio)printing | HUVECs | Mouse calvarial pre-osteoblasts cells | Vasculature and associated single cells | GelMA | Agarose | Static | No | Bertassonia et al. [56] |
| AMST: 3D (Bio)printing | Glomerular microvascular ECs | Proximal tubular epithelial cells | Kidney | Gelatin-fibrin blend | Pluronic F-127 with high-molecular weight PEO | Flow | No | Lin et al. (2019) [57] |
| Indirect bioprinting | HUVECs | human neonatal dermal fibroblasts (HNDFs) | Vasculature and associated single cells | GelMA | Pluronic F-127 | Static | No | Kolesky et al. (2014) [51] |
| Indirect bioprinting | / | Rabbit bone marrow-derived mesenchymal stem cells | Only vasculature | Sodium alginate, medium viscosity | polycaprolactone | Static and flow | No | Lee et al. (2018) [58] |
| Indirect bioprinting | / | Sheep primary bone marrow stromal cells | Vasculature and associated single cells | Silanized Hydroxypropylmethylcellulose | Gelatin | Static and flow | No | Figueiredo et al. (2020) [59] |
| Indirect bioprinting | HUVECs | / | Only vasculature | Collagen | Gelatin | Static and flow | Yes | Lee et al. (2014) [50] |
| Indirect bioprinting | HUVECs | Human lung fibroblasts | Vasculature and associated single cells | Collagen and fibrin | Gelatin | Static and flow | Yes | Lee et al. (2014) [60] |
| Indirect bioprinting | HUVECs | Human bone-marrow-derived mesenchymal stem cells (hMSCs) and human neonatal dermal fibroblasts (HNDFs), | Bone | Fibrin-gelatin blend | Pluronic F-127 | Flow | No | Kolesky et al. (2016) [61] |
| Indirect bioprinting | HUVECs | Normal Human Dermal Fibroblasts and Human umbilical artery smooth muscle cells (HUASMC) | Only vasculature | Fibrin and Fibrin-collagen blend | Gelatin | Flow | No | Schöneberg et al. (2018) [62] |
| Indirect bioprinting | HUVECs | Human mesenchymal stem cells | Vasculature and associated single cells | Methacrylated alginate and methacrylated hyaluronic acid (HAMA) | Pluronic F-127 | Static | No | Ji et al. (2019) [63] |
| Indirect bioprinting | HUVECs | HepG2, Human foreskin fibroblasts, and human umbilical cord MSCs | Liver, transplantable with direct anastomosis | GelMA–fibrin blend | Gelatin | Flow | Yes | Liu et al. (2021) [64] |
| Indirect bioprinting | HUVECs | Bone-marrow-derived human mesenchymal stem cells | Bone | High-stiffness GelMA | Low stiffness GelMA | Flow | Yes | Byambaa et al. (2017) [65] |
| Indirect bioprinting | HUVECs | Human dermal neonatal fibroblasts | Only vasculature | Gelatin–poly(ethylene glycol)–tyramine mixed with horse radish peroxidase | Gelatin −H2O2 blend | Static | No | Hong et al. (2019) [66] |
| Indirect bioprinting | HUVECs | / | Only vasculature | Pluronic F-127-BUM (with collagen 1 additive) | Pluronic F-127 | Static | No | Millik et al. (2019) [67] |
| Indirect bioprinting | HUVECs | / | Only vasculature | Sodium alginate | CaCl2 | Static | No | Attalla et al. (2016) [68] |
| Indirect bioprinting | HUVECs | Human mesenchymal stem cells | Only vasculature | GelMA-Alginate—4-arm poly(ethylene glycol)-tetra-acrylate (PEGTA) blend | CaCl2 | Static | No | Jia et al. (2016) [52] |
| Indirect bioprinting | / | Primary human umbilical vein smooth muscle cells | Only vasculature | Sodium alginate | CaCl2 | Flow and static | No | Zhang et al. (2015) [69] |
| Direct bioprinting | HUVECs | Human Coronary Artery Smooth Muscle Cells and Human bone marrow-derived mesenchymal stem cells | Vasculature and associated single cells | Catechol-functionalized GelMA | Pluronic F-127 | Flow and static | No | Cui et al. (2020) [70] |
| Direct bioprinting | HUVECs | / | Only vasculature | Vascular-tissue-derived dECM–sodium alginate blend | Pluronic F-127—CaCl2 Blend | Static | No | Gao et al. (2017) [71] |
| Direct bioprinting | HUVECs | Human HL-60 cell line | Vasculature and airway inflammation modelling | Vascular-tissue-derived decellularized ECM–sodium alginate blend | Pluronic F-127—CaCl2 Blend | Flow and static | Yes | Gao et al. (2018) [72] |
| Direct bioprinting | HUVECs | Human aortic smooth muscle cells | Only vasculature | Porcine aorta derived decellularized ECM–sodium alginate blend | Pluronic F-127—CaCl2 Blend | Flow and static | No | Gao et al. (2019) [73] |
| Direct bioprinting | HUVECs | Renal proximal tubular epithelial cells | Kidney | Kidney-derived decellularized ECM–sodium alginate blend | Pluronic F-127—CaCl2 Blend | Flow and static | No | Singh et al. (2020) [74] |
| Multimaterial SLA | HUVECs | Human bone marrow-derived mesenchymal stem cells and human dermal fibroblasts | Only vasculature | GelMA | HAMA | Static | Yes | Orellano et al. (2022) [75] |
| Organ | Disease | Treatment | Ref |
|---|---|---|---|
| Heart | Acute coronary syndrome |
| [175] |
| Brain | Ischemic stroke |
| [176] |
| Limb | Critical limb ischemia |
| [177,178,179] |
| Bowel | Acute mesenteric occlusion |
| [180,181] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.T.; Peirsman, A.; Tirpakova, Z.; Mandal, K.; Vanlauwe, F.; Maity, S.; Kawakita, S.; Khorsandi, D.; Herculano, R.; Umemura, C.; et al. Engineered Vasculature for Cancer Research and Regenerative Medicine. Micromachines 2023, 14, 978. https://doi.org/10.3390/mi14050978
Nguyen HT, Peirsman A, Tirpakova Z, Mandal K, Vanlauwe F, Maity S, Kawakita S, Khorsandi D, Herculano R, Umemura C, et al. Engineered Vasculature for Cancer Research and Regenerative Medicine. Micromachines. 2023; 14(5):978. https://doi.org/10.3390/mi14050978
Chicago/Turabian StyleNguyen, Huu Tuan, Arne Peirsman, Zuzana Tirpakova, Kalpana Mandal, Florian Vanlauwe, Surjendu Maity, Satoru Kawakita, Danial Khorsandi, Rondinelli Herculano, Christian Umemura, and et al. 2023. "Engineered Vasculature for Cancer Research and Regenerative Medicine" Micromachines 14, no. 5: 978. https://doi.org/10.3390/mi14050978
APA StyleNguyen, H. T., Peirsman, A., Tirpakova, Z., Mandal, K., Vanlauwe, F., Maity, S., Kawakita, S., Khorsandi, D., Herculano, R., Umemura, C., Yilgor, C., Bell, R., Hanson, A., Li, S., Nanda, H. S., Zhu, Y., Najafabadi, A. H., Jucaud, V., Barros, N., ... Khademhosseini, A. (2023). Engineered Vasculature for Cancer Research and Regenerative Medicine. Micromachines, 14(5), 978. https://doi.org/10.3390/mi14050978