Advances in MXene-Based Electrochemical (Bio)Sensors for Neurotransmitter Detection
Abstract
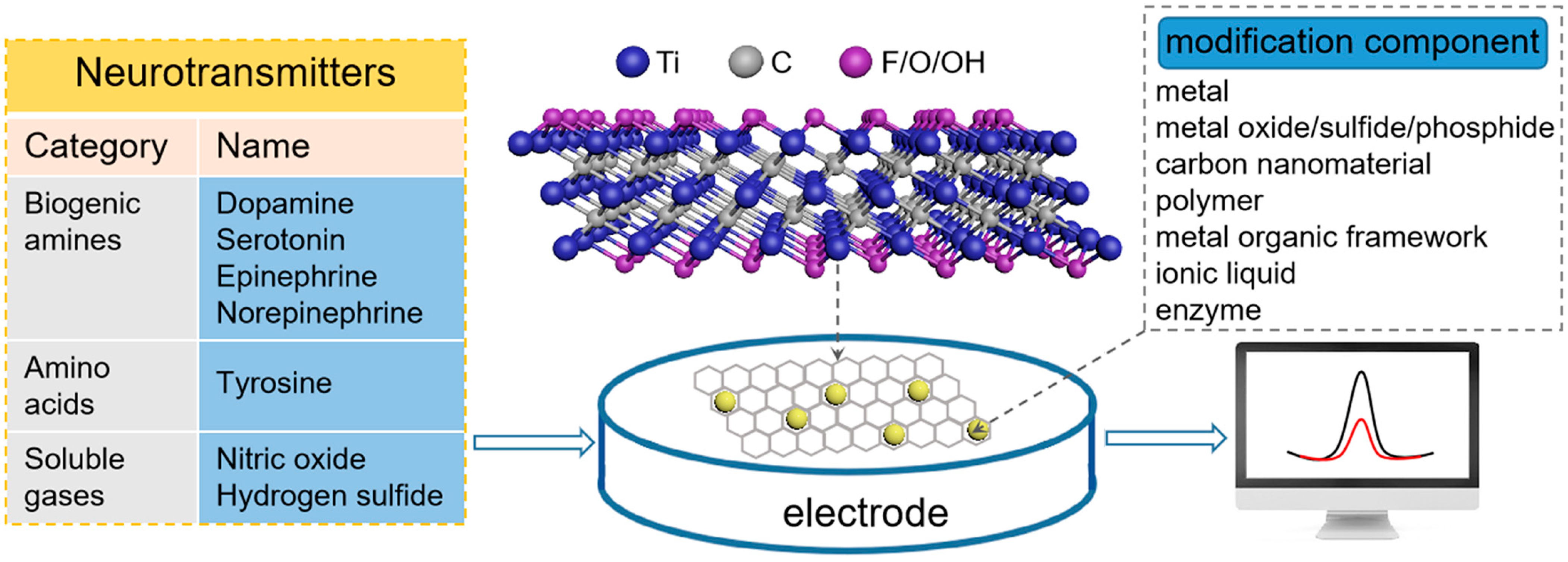
1. Introduction
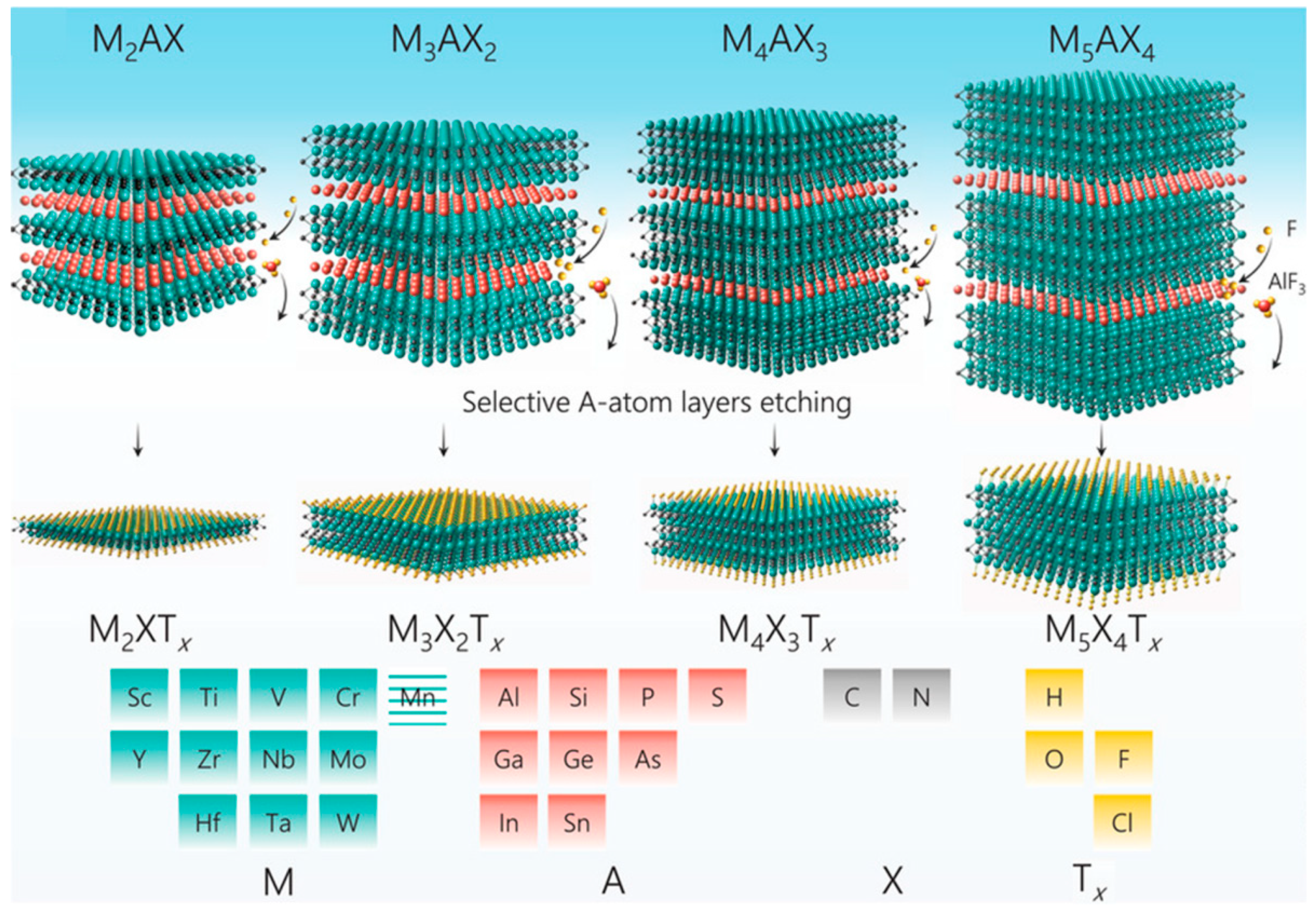
2. Brief Introduction to MXenes
2.1. Composition, Structure and Synthesis
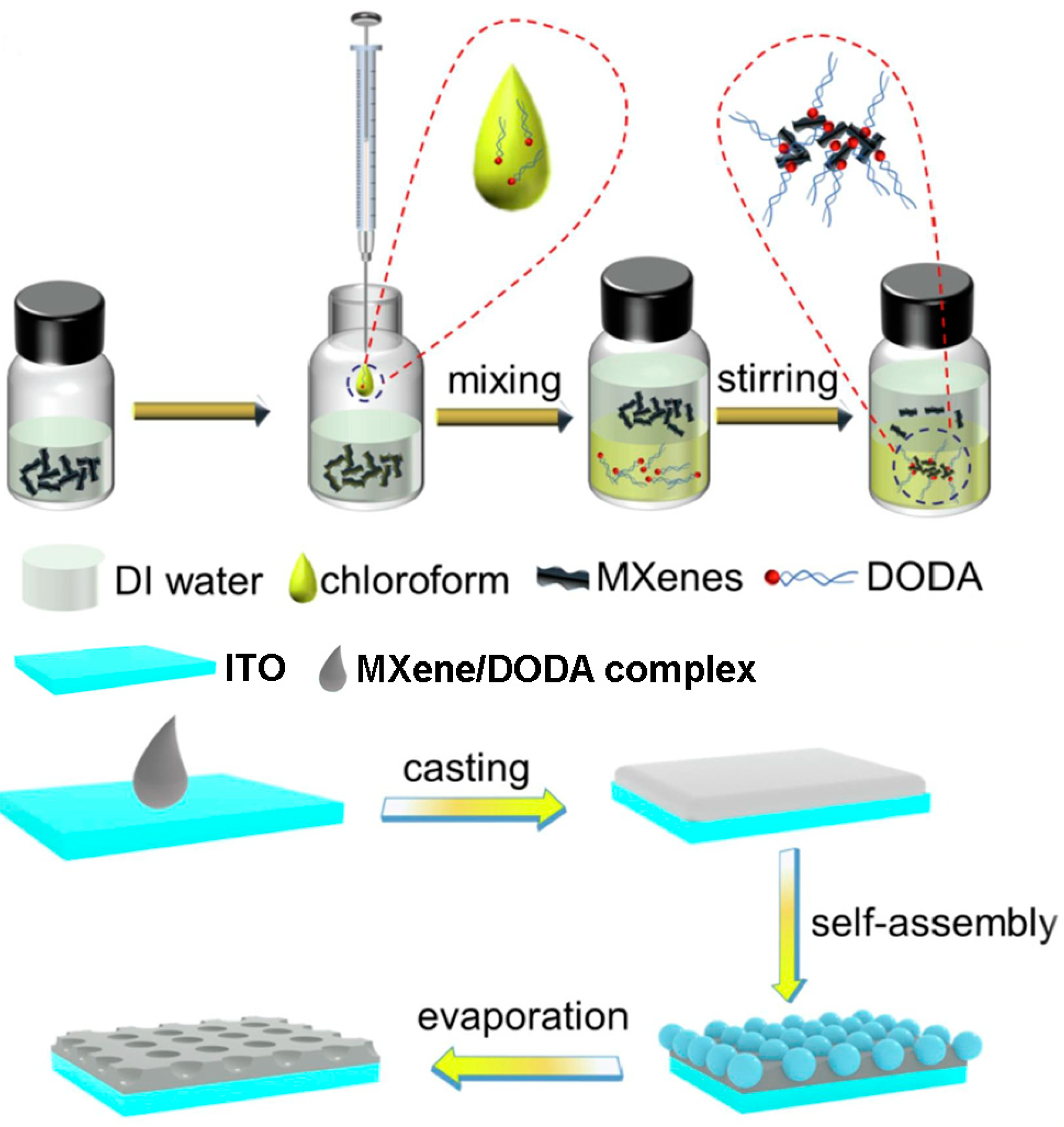
2.2. Properties, Delamination and Surface Modification/Functionalization Strategies
3. MXene-Based Electrochemical (Bio)Sensors for Neurotransmitter Detection
3.1. Biogenic Amines
3.1.1. DA
3.1.2. 5-HT
3.1.3. EP
3.1.4. NE
3.2. Amino Acids
3.3. Soluble Gases
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Baranwal, A.; Chandra, P. Clinical implications and electrochemical biosensing of monoamine neurotransmitters in body fluids, in vitro, in vivo, and ex vivo models. Biosens. Bioelectron. 2018, 121, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian-Ardakani, Z.; Hosu, O.; Cristea, C.; Mazloum-Ardakani, M.; Marrazza, G. Latest trends in electrochemical sensors for neurotransmitters: A review. Sensors 2019, 19, 2037. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.-B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef]
- Marrs, T.C.; Maynard, R.L. Neurotranmission systems as targets for toxicants: A review. Cell Biol. Toxicol. 2013, 29, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Feng, Z.-Y.; Li, D.; Jin, B.; Lan, Y.; Meng, L.-Y. Recent trends in carbon-based microelectrodes as electrochemical sensors for neurotransmitter detection: A review. Trends Anal. Chem. 2022, 148, 116541. [Google Scholar] [CrossRef]
- Madhurantakam, S.; Karnam, J.B.; Brabazon, D.; Takai, M.; Ahad, I.U.; Rayappan, J.B.B.; Krishnan, U.M. “Nano”: An emerging avenue in electrochemical detection of neurotransmitters. ACS Chem. Neurosci. 2020, 11, 4024–4047. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Soni, S.; Agrawal, P.; Balhara, Y.P.S.; Jain, U. Recent advancement in nanosensors for neurotransmitters detection: Present and future perspective. Process Biochem. 2020, 91, 241–259. [Google Scholar] [CrossRef]
- Xu, J.; Tao, J.; Su, L.; Wang, J.; Jiao, T. A critical review of carbon quantum dots: From synthesis toward applications in electrochemical biosensors for the determination of a depression-related neurotransmitter. Materials 2021, 14, 3987. [Google Scholar] [CrossRef]
- Hsine, Z.; Mlika, R.; Jaffrezic-Renault, N.; Korri-Youssoufi, H. Review—Recent progress in graphene based modified electrodes for electrochemical detection of dopamine. Chemosensors 2022, 10, 249. [Google Scholar] [CrossRef]
- Negahdary, M.; Akira Ameku, W.; Gomes Santos, B.; dos Santos Lima, I.; Gomes de Oliveira, T.; Carvalho França, M.; Angnes, L. Recent electrochemical sensors and biosensors for toxic agents based on screen-printed electrodes equipped with nanomaterials. Microchem. J. 2023, 185, 108281. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, H. Recent developments of flexible and stretchable electrochemical biosensors. Micromachines 2020, 11, 243. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Liu, Y.; Wang, W.; Ren, R.; Li, M.; Liu, D.; Liu, Y.; Liu, Y.; Pang, G. Electrochemical signal amplification strategies and their use in olfactory and taste evaluation. Biosensors 2022, 12, 566. [Google Scholar] [CrossRef] [PubMed]
- Nejad, F.G.; Tajik, S.; Beitollahi, H.; Sheikhshoaie, I. Magnetic nanomaterials based electrochemical (bio)sensors for food analysis. Talanta 2021, 228, 122075. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, M.K.; Mansha, M.; Alsharaa, A.; Jillani, S.M.S.; Bashee, C. Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: A review. TrAC Trends Anal. Chem. 2016, 76, 15–29. [Google Scholar] [CrossRef]
- Omar, F.S.; Duraisamy, N.; Ramesh, K.; Ramesh, S. Conducting polymer and its composite materials based electrochemical sensor for nicotinamide adenine dinucleotide (NADH). Biosens. Bioelectron. 2016, 79, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Akiba, U.; Anzai, J.-I. Recent progress in nanomaterial-based electrochemical biosensors for cancer biomarkers: A review. Molecules 2017, 22, 1048. [Google Scholar] [CrossRef]
- Suhito, I.R.; Angeline, N.; Kim, T.-H. Nanomaterial-modified hybrid platforms for precise electrochemical detection of dopamine. BioChip J. 2019, 13, 20–29. [Google Scholar] [CrossRef]
- Sharifi, M.; Avadi, M.R.; Attar, F.; Dashtestani, F.; Ghorchian, H.; Rezayat, S.M.; Saboury, A.A.; Falahati, M. Cancer diagnosis using nanomaterials based electrochemical nanobiosensors. Biosens. Bioelectron. 2019, 126, 773–784. [Google Scholar] [CrossRef]
- Taheri, R.A.; Eskandari, K.; Negahdary, M. An electrochemical dopamine aptasensor using the modified Au electrode with spindle-shaped gold nanostructure. Microchem. J. 2018, 143, 243–251. [Google Scholar] [CrossRef]
- Decarli, N.O.; Zapp, E.; de Souza, B.S.; Santana, E.R.; Winiarski, J.P.; Vieira, I.C. Biosensor based on laccase-halloysite nanotube and imidazolium zwitterionic surfactant for dopamine determination. Biochem. Eng. J. 2022, 186, 108565. [Google Scholar] [CrossRef]
- Sharma, V.; Singh, P.; Kumar, A.; Gupta, N. Electrochemical detection of dopamine by using nickel supported carbon nanofibers modified screen printed electrode. Diam. Relat. Mater. 2023, 133, 109677. [Google Scholar] [CrossRef]
- Bala, K.; Sharma, D.; Gupta, N. Carbon-Nanotube-Based materials for electrochemical sensing of the neurotransmitter dopamine. ChemElectroChem 2019, 6, 274. [Google Scholar] [CrossRef]
- Sun, X.; Chen, C.; Xiong, C.; Zhang, C.; Zheng, X.; Wang, J.; Gao, X.; Yu, Z.-Q.; Wu, Y. Surface modification of MoS2 nanosheets by single Ni atom for ultrasensitive dopamine detection. Nano Res. 2023, 16, 917–924. [Google Scholar] [CrossRef]
- de Fátima Ulbrich, K.; Winiarski, J.P.; Jost, C.L.; de Campos, C.E.M. Mechanochemical synthesis of a Ni3-xTe2 nanocrystalline composite and its application for simultaneous electrochemical detection of dopamine and adrenaline. Compos. B Eng. 2020, 183, 107649. [Google Scholar] [CrossRef]
- Paul, J.; Moniruzzaman, M.; Kim, J. Framing of poly(arylene-ethynylene) around carbon nanotubes and iodine doping for the electrochemical detection of dopamine. Biosensors 2023, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Winiarski, J.P.; Tavares, B.F.; de Fátima Ulbrich, K.; de Campos, C.E.M.; Souza, A.A.U.; Souza, S.M.A.G.U.; Jost, C.L. Development of a multianalyte electrochemical sensor for depression biomarkers based on a waste of the steel industry for a sustainable and one-step electrode modification. Microchem. J. 2022, 175, 107141. [Google Scholar] [CrossRef]
- Wu, X.; Ma, P.; Sun, Y.; Du, F.; Song, D.; Xu, G. Application of MXene in electrochemical sensors: A review. Electroanalysis 2021, 33, 1827–1851. [Google Scholar] [CrossRef]
- Yang, M.; Lu, H.; Liu, S. Recent advances of MXene-based electrochemical immunosensors. Appl. Sci. 2022, 12, 5630. [Google Scholar] [CrossRef]
- Tran, V.A.; Tran, N.T.; Doan, V.D.; Nguyen, T.-Q.; Pham Thi, H.H.; Vo, G.N.L. Application prospects of MXenes materials modifications for sensors. Micromachines 2023, 14, 247. [Google Scholar] [CrossRef]
- Kalambate, P.K.; Gadhari, N.S.; Li, X.; Rao, Z.; Navale, S.T.; Shen, Y.; Patil, V.R.; Huang, Y. Recent advances in MXene-based electrochemical sensors and biosensors. Trends Anal. Chem. 2019, 120, 115643. [Google Scholar] [CrossRef]
- Rajeev, R.; Thadathil, D.A.; Varghese, A. New horizons in surface topography modulation of MXenes for electrochemical sensing toward potential biomarkers of chronic disorders. Crit. Rev. Solid State Mater. Sci. 2022, 1–43. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L. Ti3C2Tx MXene as electrocatalyst for designing robust glucose biosensors. Adv. Mater. Technol. 2022, 7, 2200151. [Google Scholar] [CrossRef]
- Rizwan, K.; Rahdar, A.; Bilal, M.; Iqbal, H.M.N. MXene-Based electrochemical and biosensing platforms to detect toxic elements and pesticides pollutants from environmental matrices. Chemosphere 2022, 291, 132820. [Google Scholar] [CrossRef]
- Rhouati, A.; Berkani, M.; Vasseghian, Y.; Golzadeh, N. MXene-Based electrochemical sensors for detection of environmental pollutants: A comprehensive review. Chemosphere 2022, 291, 132921. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, P.-S.; Kim, S.-Y. Electrochemical sensing interfaces based on novel 2D-MXenes for monitoring environmental hazardous toxic compounds: A concise review. J. Ind. Eng. Chem. 2022, 109, 52–67. [Google Scholar] [CrossRef]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef]
- Wyatt, B.C.; Rosenkranz, A.; Anasori, B. 2D MXenes: Tunable mechanical and tribological properties. Adv. Mater. 2021, 33, 2007973. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Huang, Q. MXenes: Two-Dimensional building blocks for future materials and devices. ACS Nano 2021, 15, 5775–5780. [Google Scholar] [CrossRef] [PubMed]
- Tareen, A.K.; Khan, K.; Iqbal, M.; Zhang, Y.; Long, J.; Mahmood, A.; Mahmood, N.; Xie, Z.; Li, C.; Zhang, H. Recent advance in two-dimensional MXenes: New horizons in flexible batteries and supercapacitors technologies. Energy Storage Mater. 2022, 53, 783. [Google Scholar] [CrossRef]
- Xu, D.; Li, Z.; Li, L.; Wang, J. Insights into the photothermal conversion of 2D MXene nanomaterials: Synthesis, mechanism, and applications. Adv. Funct. Mater. 2020, 30, 2000712. [Google Scholar] [CrossRef]
- Park, H.; Kim, S.; Kim, S.; Kim, M.; Kang, Y.; Amirthalingam, S.; Lee, S.; Hwang, N.S.; Yang, K.; Kim, H.D. Bioactive inorganic compound MXene and its application in tissue engineering and regenerative medicine. J. Ind. Eng. Chem. 2023, 117, 38–53. [Google Scholar] [CrossRef]
- Panda, S.; Deshmukh, K.; Pasha, S.K.K.; Theerthagiri, J.; Manickam, S.; Choi, M.Y. MXene based emerging materials for supercapacitor applications: Recent advances, challenges, and future perspectives. Coord. Chem. Rev. 2022, 462, 214518. [Google Scholar] [CrossRef]
- Alwarappan, S.; Nesakumar, N.; Sun, D.; Hu, T.Y.; Li, C.-Z. 2D metal carbides and nitrides (MXenes) for sensors and biosensors. Biosens. Bioelectron. 2022, 205, 113943. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhou, D.; Yang, J.; Zhou, S.; Wang, H.; Yuan, X.; Liang, J.; Li, X.; Chen, Y.; Li, H. 2D single- and few-layered MXenes: Synthesis, applications and perspectives. J. Mater. Chem. A 2022, 10, 13651–13672. [Google Scholar] [CrossRef]
- Shukla, V. The tunable electric and magnetic properties of 2D MXenes and their potential applications. Mater. Adv. 2020, 1, 3104–3121. [Google Scholar] [CrossRef]
- Zou, J.; Wu, J.; Wang, Y.; Deng, F.; Jiang, J.; Zhang, Y.; Liu, S.; Li, N.; Zhang, H.; Yu, J.; et al. Additive-Mediated intercalation and surface modification of MXenes. Chem. Soc. Rev. 2022, 51, 2972–2990. [Google Scholar] [CrossRef]
- Song, F.; Li, G.; Zhu, Y.; Wu, Z.; Xie, X.; Zhang, N. Rising from the horizon: Three-dimensional functional architectures assembled with MXene nanosheets. J. Mater. Chem. A 2020, 8, 18538–18559. [Google Scholar] [CrossRef]
- Huang, H.; Dong, C.H.; Feng, W.; Wang, Y.; Huang, B.C.; Chen, Y. Biomedical engineering of two-dimensional MXenes. Adv. Drug Deliv. Rev. 2022, 184, 114178. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Y.; Liu, Q. Electrochemical and optical biosensors based on multifunctional MXene nanoplatforms: Progress and prospects. Talanta 2021, 235, 122726. [Google Scholar] [CrossRef]
- Huang, H.; Jiang, R.; Feng, Y.; Ouyang, H.; Zhou, N.; Zhang, X.; Wei, Y. Recent development and prospects of surface modification and biomedical applications of MXenes. Nanoscale 2020, 12, 1325–1338. [Google Scholar] [CrossRef]
- Su, T.; Ma, X.; Tong, J.; Ji, H.; Qin, Z.; Wu, Z. Surface engineering of MXenes for energy and environmental applications. J. Mater. Chem. A 2022, 10, 10265–10296. [Google Scholar] [CrossRef]
- Yang, G.; Liu, F.; Zhao, J.; Fu, L.; Gu, Y.; Qu, L.; Zhu, C.; Zhu, J.; Lin, Y. MXenes-Based nanomaterials for biosensing and biomedicine. Coord. Chem. Rev. 2023, 479, 215002. [Google Scholar] [CrossRef]
- Noor, U.; Mughal, M.F.; Ahmed, T.; Farid, M.F.; Ammar, M.; Kulsum, U.; Saleem, A.; Naeem, M.; Khan, A.; Sharif, A.; et al. Synthesis and applications of MXene-based composites: A review. Nanotechnology 2023, 34, 262001. [Google Scholar] [CrossRef]
- Huang, W.; Hu, L.; Tang, Y.; Xie, Z.; Zhang, H. Recent advances in functional 2D MXene-based nanostructures for next-generation devices. Adv. Funct. Mater. 2020, 30, 2005223. [Google Scholar] [CrossRef]
- Shahzad, F.; Iqbal, A.; Zaidi, S.A.; Hwang, S.-W.; Koo, C.M. Nafion-stabilized two-dimensional transition metal carbide (MXene) as a high-performance electrochemical sensor for neurotransmitter. J. Ind. Eng. Chem. 2019, 79, 338–344. [Google Scholar] [CrossRef]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Pandey, R.P.; Gomez, T.; Jabbar, K.A.; Prenger, K.; Naguib, M.; Aïssa, B.; Mahmoud, K.A. Nb-based MXenes for efficient electrochemical sensing of small biomolecules in the anodic potential. Electrochem. Commun. 2020, 119, 106811. [Google Scholar] [CrossRef]
- Lian, M.; Shi, Y.; Zhang, W.; Zhao, J.; Chen, D. Nitrogen and sulfur co-doped Nb2C-MXene nanosheets for the ultrasensitive electrochemical detection dopamine under acidic conditions in gastric juice. J. Electroanal. Chem. 2022, 904, 115849. [Google Scholar] [CrossRef]
- Ankitha, M.; Shabana, N.; Arjun, A.M.; Rasheed, P.A. Facile chemical modification of Nb2CTx MXene with ethylene diamine for sensitive electrochemical detection of dopamine from human serum samples. Carbon Trends 2022, 9, 100232. [Google Scholar] [CrossRef]
- Arbabi, N.; Beitollahi, H. Ti3C2 nano layer modified screen printed electrode as a highly sensitive electrochemical sensor for the simultaneous determination of dopamine and tyrosine. Surf. Eng. Appl. Electrochem. 2022, 58, 13–19. [Google Scholar] [CrossRef]
- Xue, Y.; Zheng, Y.; Wang, E.; Yang, T.; Wang, H.; Hou, X. Ti3C2Tx (MXene)/Pt nanoparticle electrode for the accurate detection of DA coexisting with AA and UA. Dalton Trans. 2022, 51, 4549–4559. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, B.; Ding, A.; Weng, B.; Chen, J. Synthesis of MXene/DNA/Pd/Pt nanocomposite for sensitive detection of dopamine. J. Electroanal. Chem. 2018, 816, 189–194. [Google Scholar] [CrossRef]
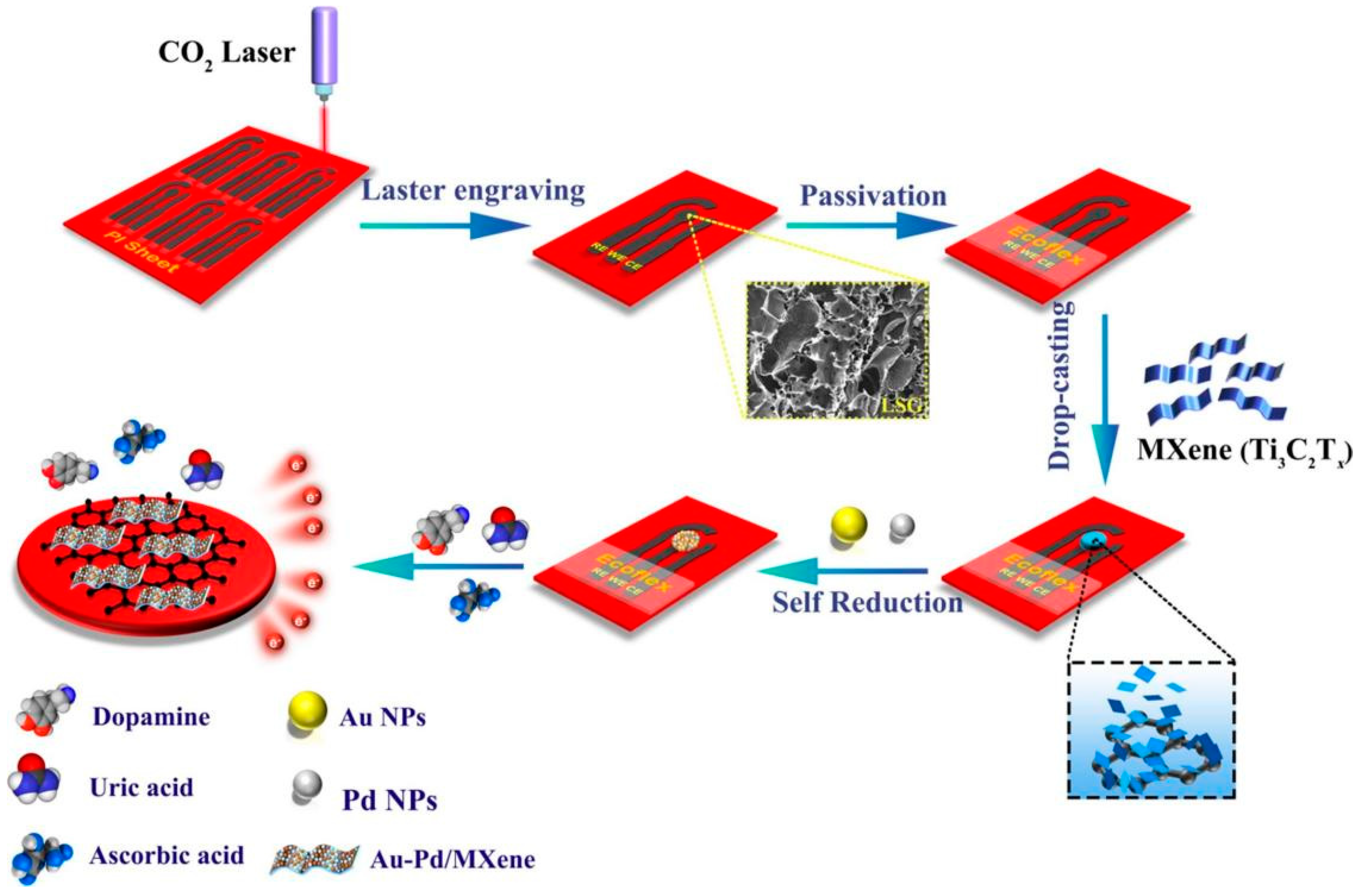
- Wang, Y.; Zhao, P.; Gao, B.; Yuan, M.; Yu, J.; Wang, Z.; Chen, X. Self-reduction of bimetallic nanoparticles on flexible MXene-graphene electrodes for simultaneous detection of ascorbic acid, dopamine, and uric acid. Microchem. J. 2023, 185, 108177. [Google Scholar] [CrossRef]
- Shetty, S.S.; El-Demellawi, J.K.; Khan, Y.; Hedhili, M.N.; Arul, P.; Mani, V.; Alshareef, H.N.; Salama, K.N. Iron single-atom catalysts on MXenes for ultrasensitive monitoring of adrenal tumor markers and cellular dopamine. Adv. Mater. Technol. 2023, 8, 2202069. [Google Scholar] [CrossRef]
- Cao, M.; Liu, S.; Liu, S.; Tong, Z.; Wang, X.; Xu, X. Preparation of ZnO/Ti3C2Tx/Nafion/Au electrode. Microchem. J. 2022, 175, 107068. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, K.; Mei, L.; Yang, X.; Shi, Y.; Wu, X.; Li, X.; Miao, M.; Zhang, S. SnO2 quantum dots-functionalized Ti3C2 MXene nanosheets for electrochemical determination of dopamine in body fluids. Microchim. Acta 2022, 189, 451. [Google Scholar] [CrossRef]
- Arif, N.; Gul, S.; Sohail, M.; Rizwan, S.; Iqbal, M. Synthesis and characterization of layered Nb2C MXene/ZnS nanocomposites for highly selective electrochemical sensing of dopamine. Ceram. Int. 2021, 47, 2388–2396. [Google Scholar] [CrossRef]
- Ankitha, M.; Shabana, N.; Arjun, A.M.; Muhsin, P.; Rasheed, P.A. Ultrasensitive electrochemical detection of dopamine from human serum samples by Nb2CTx-MoS2 heterostructures. Microchem. J. 2023, 187, 108424. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Ge, W. Porous NiCoP nanowire arrays on the surface of Ti3C2Tx-modified carbon cloth for sensitive determination of dopamine. ACS Appl. Nano Mater. 2023, 6, 2035–2047. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, Q.; Li, X.; Wu, L.; Yu, A.; Lai, G.; Fu, L.; Wei, Q.; Dai, D.; Jia, N.; et al. A double-deck structure of reduced graphene oxide modified porous Ti3C2Tx electrode towards ultrasensitive and simultaneous detection of dopamine and uric acid. Biosensors 2021, 11, 462. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, C.; Yang, Y.; Han, J.; Huang, W.; Zhou, J.; Zhang, Y. Anti-biofouling Ti3C2TX MXene-holey graphene modified electrode for dopamine sensing in complex biological fluids. Talanta 2022, 247, 123614. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Li, C.; Han, J.; Huang, W.; Zhou, J.; Yang, Y. Hydrophilic antifouling 3D porous MXene/holey graphene nanocomposites for electrochemical determination of dopamine. Microchem. J. 2022, 181, 107713. [Google Scholar] [CrossRef]
- Ni, M.; Chen, J.; Wang, C.; Wang, Y.; Huang, L.; Xiong, W.; Zhao, P.; Xie, Y.; Fei, J. A high-sensitive dopamine electrochemical sensor based on multilayer Ti3C2 MXene, graphitized multi-walled carbon nanotubes and ZnO nanospheres. Microchem. J. 2022, 178, 107410. [Google Scholar] [CrossRef]
- Wang, G.; Yang, Z.; Wu, L.; Wang, J.; Liu, X. Studies on improved stability and electrochemical activity of titanium carbide MXene-polymer nanocomposites. J. Electroanal. Chem. 2021, 900, 115708. [Google Scholar] [CrossRef]
- You, Q.; Guo, Z.; Zhang, R.; Chang, Z.; Ge, M.; Mei, Q.; Dong, W.-F. Simultaneous recognition of dopamine and uric acid in the presence of ascorbic acid via an intercalated MXene/PPy nanocomposite. Sensors 2021, 21, 3069. [Google Scholar] [CrossRef]
- Wustoni, S.; Saleh, A.; El-Demellawi, J.K.; Koklu, A.; Hama, A.; Druet, V.; Wehbe, N.; Zhang, Y.; Inal, S. MXene improves the stability and electrochemical performance of electropolymerized PEDOT films. APL Mater. 2020, 8, 121105. [Google Scholar] [CrossRef]
- Amara, U.; Mehran, M.T.; Sarfaraz, B.; Mahmood, K.; Hayat, A.; Nasir, M.; Riaz, S.; Nawaz, M.H. Perylene diimide/MXene-modified graphitic pencil electrode-based electrochemical sensor for dopamine detection. Microchim. Acta 2021, 188, 230. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, F.; Sun, Y.; Yu, K.; Guo, W.; Qu, F. A 2D/2D NiCo-MOF/Ti3C2 heterostructure for the simultaneous detection of acetaminophen, dopamine and uric acid by differential pulse voltammetry. Dalton Trans. 2021, 50, 16593–16600. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Xing, Y.; Liu, G.; Hou, S. Electrochemical sensor based on Ti3C2 membrane doped with UIO-66-NH2 for dopamine. Microchim. Acta 2022, 189, 141. [Google Scholar] [CrossRef]
- Amara, U.; Sarfraz, B.; Mahmood, K.; Mehran, M.T.; Muhammad, N.; Hayat, A.; Nawaz, M.H. Fabrication of ionic liquid stabilized MXene interface for electrochemical dopamine detection. Microchim. Acta 2022, 189, 64. [Google Scholar] [CrossRef]
- Wagh, M.D.; Renuka, H.; Kumar, P.S.; Amreen, K.; Kumar, S.S.; Goel, S. Integrated microfluidic device with MXene enhanced laser-induced graphene bioelectrode for sensitive and selective electroanalytical detection of dopamine. IEEE Sens. J. 2022, 22, 14620–14627. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, Y.; Han, Y.; Xu, K.; Yao, S.; Shi, L.; Zhu, M. 3D porous structure assembled from MXene via breath figure method for electrochemical detection of dopamine. Chem. Eng. J. 2023, 452, 139414. [Google Scholar] [CrossRef]
- Wan, M.; Jimu, A.; Yang, H.; Zhou, J.; Dai, X.; Zheng, Y.; Ou, J.; Yang, Y.; Liu, J.; Wang, L. MXene quantum dots enhanced 3D-printed electrochemical sensor for the highly sensitive detection of dopamine. Microchem. J. 2023, 184, 108180. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Chen, Y.; Yang, J.; Dong, J.; Lu, X. l-cysteine-terminated triangular silver nanoplates/MXene nanosheets are used as electrochemical biosensors for efficiently detecting 5-hydroxytryptamine. Anal. Chem. 2021, 93, 16655–16663. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Tian, L.; Su, M.; Cao, X.; Jiang, Q.; Huo, X.; Yu, C. Real-time monitoring of 5-HT release from cells based on MXene hybrid single-walled carbon nanotubes modified electrode. Anal. Bioanal. Chem. 2022, 414, 7967–7976. [Google Scholar] [CrossRef]
- Su, M.; Lan, H.; Tian, L.; Jiang, M.; Cao, X.; Zhu, C.; Yu, C. Ti3C2Tx-reduced graphene oxide nanocomposite-based electrochemical sensor for serotonin in human biofluids. Sens. Actuators B Chem. 2022, 367, 132019. [Google Scholar] [CrossRef]
- Shankar, S.S.; Shereema, R.M.; Rakhi, R.B. Electrochemical determination of adrenaline using MXene/graphite composite paste electrodes. ACS Appl. Mater. Interfaces 2018, 10, 43343–43351. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Yue, H.; Gao, X.; Huang, S.; Zhang, X.; Yu, Y.; Zhang, H.; Zhang, H. Electrochemical determination of epinephrine based on Ti3C2Tx MXene-reduced graphene oxide/ITO electrode. J. Electroanal. Chem. 2021, 895, 115425. [Google Scholar] [CrossRef]
- Chen, S.; Shi, M.; Xu, Q.; Xu, J.; Duan, X.; Gao, Y.; Lu, L.; Gao, F.; Wang, X.; Yu, Y. Ti3C2Tx MXene/nitrogen-doped reduced graphene oxide composite: A high-performance electrochemical sensing platform for adrenaline detection. Nanotechnology 2021, 32, 265501. [Google Scholar] [CrossRef]
- Chen, S.; Shi, M.; Yang, J.; Yu, Y.; Xu, Q.; Xu, J.; Duan, X.; Gao, Y.; Lu, L. MXene/carbon nanohorns decorated with conductive molecularly imprinted poly(hydroxymethyl-3,4-ethylenedioxythiophene) for voltammetric detection of adrenaline. Microchim. Acta 2021, 188, 420. [Google Scholar] [CrossRef]
- Boobphahom, S.; Siripongpreda, T.; Zhang, D.; Qin, J.; Rattanawaleedirojn, P.; Rodthongkum, N. TiO2/MXene-PVA/GO hydrogel-based electrochemical sensor for neurological disorder screening via urinary norepinephrine detection. Microchim. Acta 2021, 188, 387. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.; Li, S.; Yang, J.; Dong, J.; Lu, X. MXene/CNTs/Cu-MOF electrochemical probe for detecting tyrosine. Carbon 2022, 199, 110–118. [Google Scholar] [CrossRef]
- Tan, Y.; Yang, L.; Zhai, D.; Sun, L.; Zhai, S.; Zhou, W.; Wang, X.; Deng, W.-Q.; Wu, H. MXene-derived metal-organic framework@MXene heterostructures toward electrochemical NO sensing. Small 2022, 18, 2204942. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; He, L.; Li, P.; Li, X.; Zhang, P. A direct electrochemical H2S sensor based on Ti3C2Tx MXene. ChemElectroChem 2021, 8, 3658. [Google Scholar] [CrossRef]
- Elugoke, S.E.; Adekunle, A.S.; Fayemi, O.E.; Mamba, B.B.; Sherif, E.-S.M.; Ebenso, E.E. Carbon-Based quantum dots for electrochemical detection of monoamine neurotransmitters—Review. Biosensors 2020, 10, 162. [Google Scholar] [CrossRef]
- Renjini, S.; Abraham, P.; Kumary, V.A.; Chithra, P.G.; Sreevalsan, K. Review—Progress on carbon-based electrochemical sensors for epinephrine and norepinephrine. J. Electrochem. Soc. 2022, 169, 046519. [Google Scholar]
- Negut, C.C.; Stefan-van Staden, R.I. Review—Recent trends in supramolecular recognition of dopamine, tyrosine, and tryptophan, using electrochemical sensors. J. Electrochem. Soc. 2021, 168, 067517. [Google Scholar] [CrossRef]
- Asif, M.; Aziz, A.; Wang, Z.; Ashraf, G.; Wang, J.; Luo, H.; Chen, X.; Xiao, F.; Liu, H. Hierarchical CNTs@CuMn layered double hydroxide nanohybrid with enhanced electrochemical performance in H2S detection from live cells. Anal. Chem. 2019, 91, 3912–3920. [Google Scholar] [CrossRef]
- Xu, H.; Shang, H.; Liu, Q.; Wang, C.; Di, J.; Chen, C.; Jin, L.; Du, Y. Dual mode electrochemical-photoelectrochemical sensing platform for hydrogen sulfide detection based on the inhibition effect of titanium dioxide/bismuth tungstate/silver heterojunction. J. Colloid Interface Sci. 2021, 581, 323–333. [Google Scholar] [CrossRef] [PubMed]
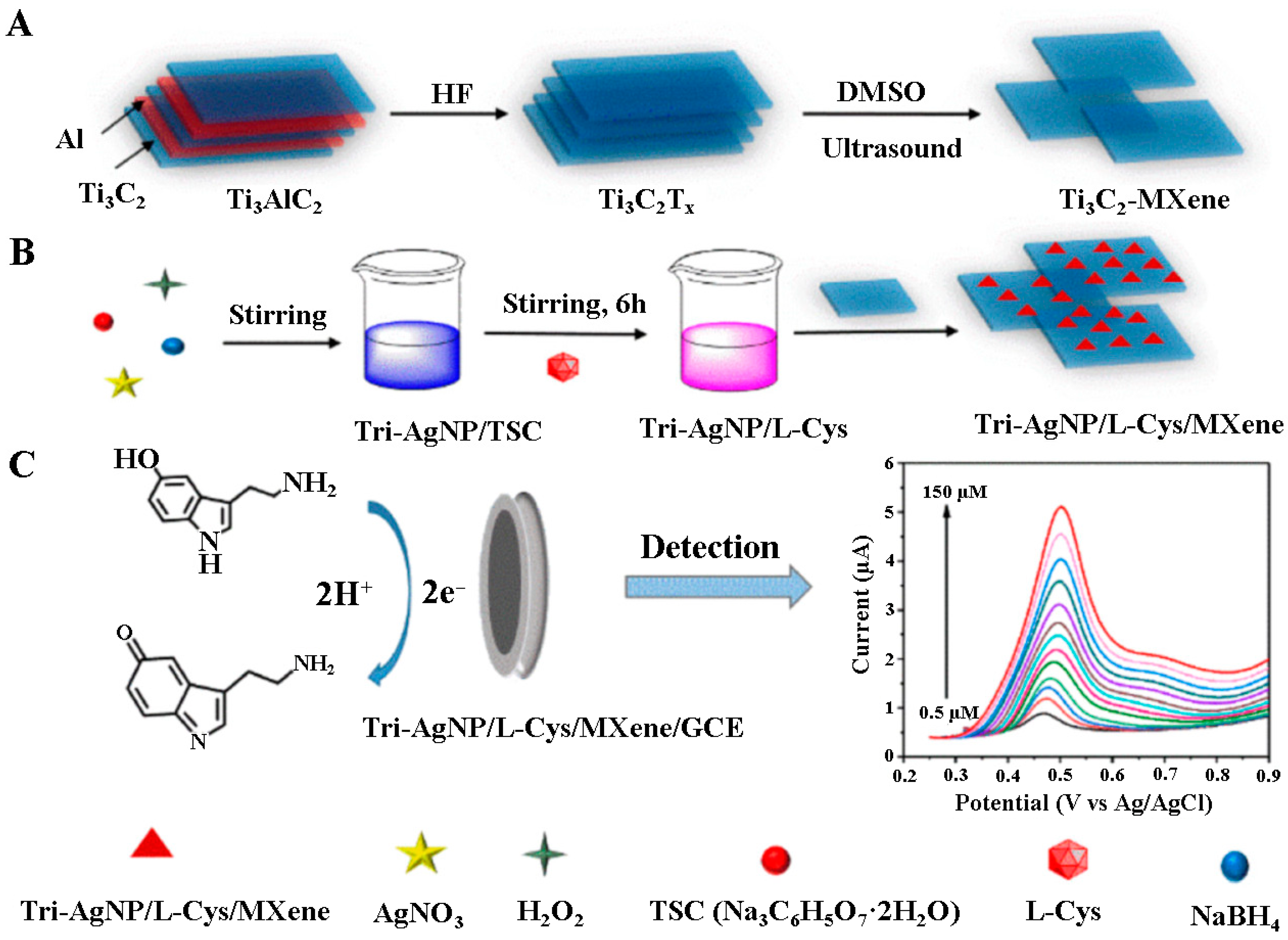
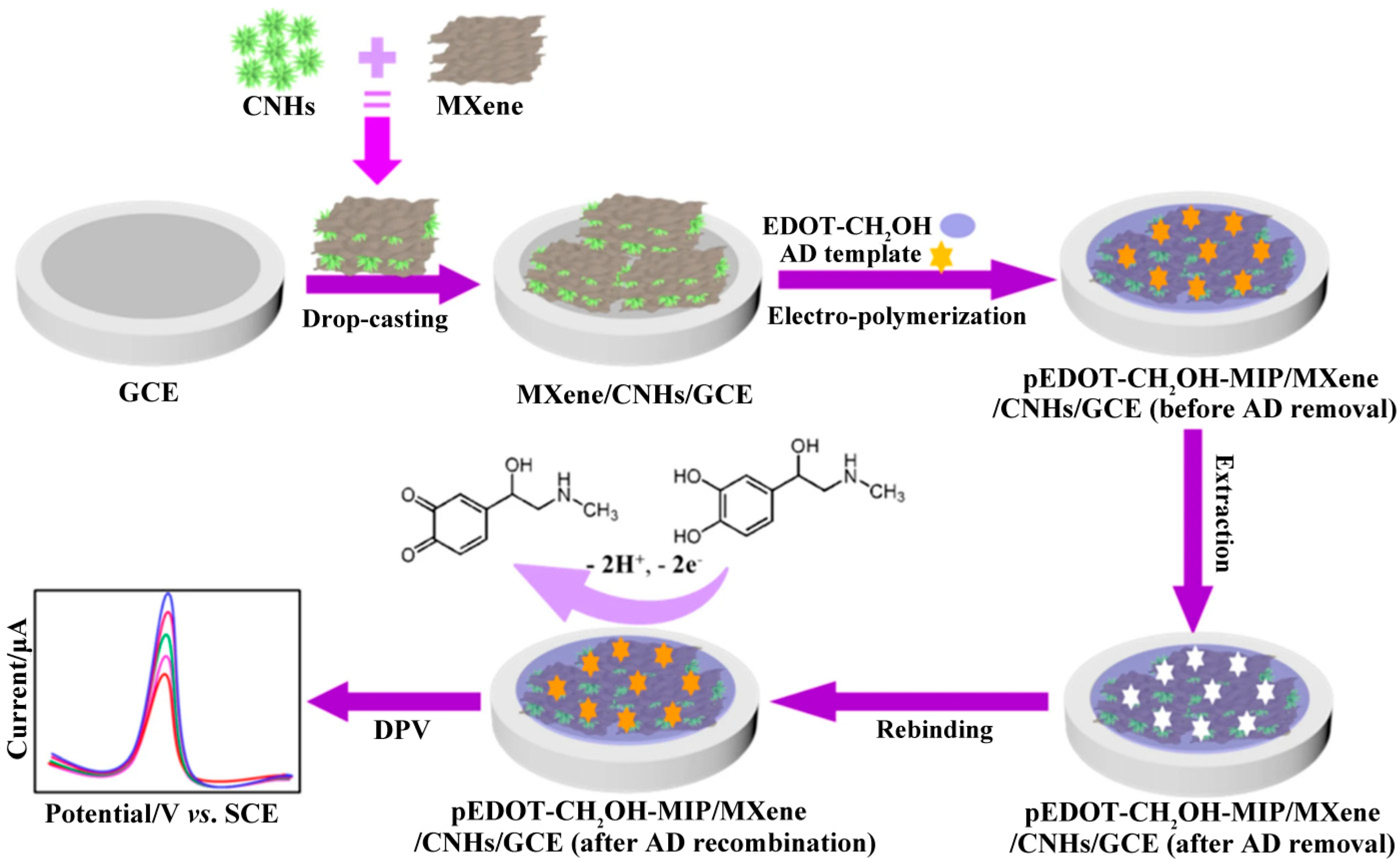

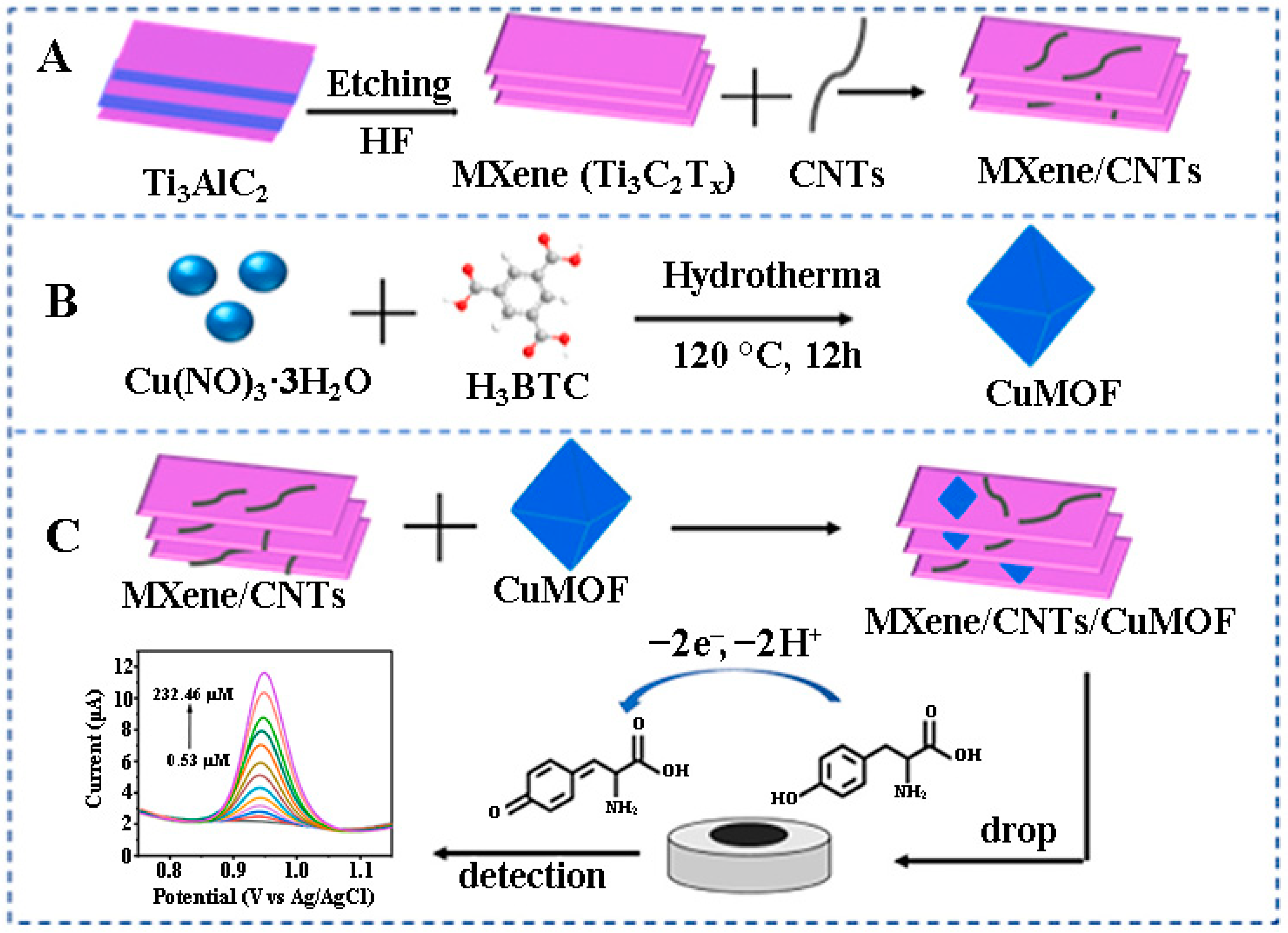
| Analyte [ref.] | Working Electrode | Etchant for MXene | Analytical Method | Limit of Detection | Linear Range | Real Sample |
|---|---|---|---|---|---|---|
| DA [55] | Nafion-stabilized Ti3C2Tx/GCE | LiF + HCl | Amperometry | 3 nM | 0.015–10 μM | urine and serum |
| DA [56] | Ti-C-Tx/GCE | HF | DPV | 0.06 μM | 0.5–50 μM | urine |
| DA [57] | Nb4C3Tx/GCE | HF | DPV | 29 nM | 50 nM–1 μM | / |
| DA [58] | NS-Nb2C/Nafion/GCE | HF | DPV | 0.12 µM | 0.4–90 μM | simulated gastric juice |
| DA [59] | EDA@Nb2CTx/CC | HF | Amperometry | 300 pM | 1 nM–100 µM | serum |
| DA & Tyr [60] | Ti3C2/SPE | HF | DPV | DA: 0.15 µM | DA: 0.5–600.0 μM | DA tablet and urine |
| DA [61] | Ti3C2/PtNPs/GCE | HF | DPV | 0.48/0.38 µM | 5–180 µM | urine |
| DA [62] | Ti3C2Tx/DNA/Pd/Pt@GCE | LiF + HCl | Amperometry | 30 nM | 0.2–1000 μM | serum |
| DA [63] | Au-Pd/Ti3C2Tx/LSGE | LiF + HCl | DPV | 0.13 µM | 12–240 µM | urine and sweat |
| DA [64] | Fe-SACs/Ti3C2Tx/LSGE | LiF + HCl | SWV | 1.0 nM | 10 nM–200 µM | serum and urine |
| DA [65] | ZnO/Ti3C2Tx/Nafion/Au | LiF + HCl | Amperometry | 0.076 μM | 0.1–1200 μM | serum |
| DA [66] | SnO2QDs@Ti3C2/GCE | HF | CV | 2.0 nM | 0.004–8.0 µM | serum and urine |
| DA [67] | Nb2C-ZnS/GCE | HF | DPV | 1.39 μM | 0.09–0.82 mM | / |
| DA [68] | Nb2CTx@MoS2/CC | HF | Amperometry | 0.23 fM | 1 fM–10 µM | serum |
| DA [69] | CC/Ti3C2Tx/NiCoP/Pt | LiF + HCl | Amperometry | 0.18 nM | 0.17–784.55 μM | / |
| DA [70] | Ti3C2Tx/rGO/GCE | / | DPV | 9.5 nM | 0.1–100 μM | serum |
| DA [71] | Ti3C2Tx-ERHG/GCE | LiF + HCl | Amperometry | 0.044 μM | 0.2–125 μM | serum and aCSF |
| DA [72] | S-Ti3C2Tx/HG/GCE | LiF + HCl | DPV Amperometry | DPV: 0.06 µM Amperometry: 0.058 µM | DPV: 0.5–50 µM Amperometry: 0.1–55 µM; 55–255 µM | aCSF |
| DA [73] | Ti3C2/G-MWCNTs/ZnO/GCE | HF | DPV | 3.2 nM | 0.01–30 μM | serum |
| DA [74] | Ti3C2-PVP/GCE Ti3C2-PVA/GCE Ti3C2-PAM/GCE | HF | DPV | 20 µM 30 µM 10 µM | 100–450 µM 200–550 µM 150–400 µM | / |
| DA [75] | Ti3C2Tx/PPy/GCE | LiF + HCl | DPV | 0.37 µM | 12.5–125 µM | / |
| DA [76] | PEDOT:PSS:Ti3C2/Au | / | DPV | / | 1–100 μM | / |
| DA [77] | PDI-Ti3C2/GPE | HF | DPV | 240 nM | 100–1000 μM | serum |
| DA [78] | NiCo-MOF/Ti3C2/CCE | / | DPV | 0.004 μM | 0.01–300 μM | serum and urine |
| DA [79] | Ti3C2@UIO-66-NH2/GCE | HF | DPV | 0.81 fM | 1–250 fM | serum |
| DA [80] | IL-Ti3C2Cl2/GPE | CuCl2 | Amperometry | 702 nM | 10–2000 μM | serum |
| DA [81] | Laccase-Ti3C2-LIG | HF | SWV | 0.47 nM | 1 nM–10 μM | serum and synthetic urine |
| DA [82] | Ti3C2Tx/ITO | / | DPV | 36.8 nM | 0–10 μM; 10–50 μM | urine |
| DA [83] | MQDs@3DE | HF | CV | 0.003 µM | 0.01–20 µM | DA hydrochloride injection |
| 5-HT [84] | Tri-AgNP/l-Cys/Ti3C2Tx/GCE | HF | DPV | 0.08 μM | 0.5–150 μM | serum |
| 5-HT [85] | Ti3C2Tx-SWCNTs/GCE | HF | Amperometry | 1.5 nM | 4 nM–103.2 µM | secretion from cultured living cells |
| 5-HT [86] | Ti3C2Tx-rGO/GCE | / | DPV | 10 nM | 0.025–147 µM | blood plasma |
| EP [87] | Ti2CTx/GCPE | HF | Amperometry | 9.5 nM | 0.02–10 μM; 10–100 μM | epinephrine tartrate injection |
| EP [88] | rGO/Ti3C2Tx/ITO | LiF + HCl | DPV | 3.5 nM | 1–60 μM | urine |
| EP [89] | Ti3C2Tx/N-rGO/GCE | / | DPV | 3.0 nM | 0.01–90 μM | urine |
| EP [90] | pEDOT-CH2OH-MIP/Ti3C2Tx/CNHs/GCE | / | DPV | 0.3 nM | 1.0 nM–60.0 μM | urine |
| NE [91] | TiO2/Ti3C2-PVA/GO hydrogel/SPCE | LiF + HCl | Amperometry | 8 nM | 0.01–1.0; 1.0–60.0 μM | urine |
| Tyr [92] | Ti3C2Tx/CNTs/CuMOF/GCE | HF | DPV | 0.19 μM | 0.53 μM–232.46 μM | serum |
| NO [93] | MOF@V2CTx/SPE | HF | CV | / | 1–13 µM | / |
| H2S [94] | Ti3C2Tx/GCE | HF | CV | 16.0 nM | 100 nM–300 µM | bovine serum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Wang, L.; Lu, H.; Dong, Q. Advances in MXene-Based Electrochemical (Bio)Sensors for Neurotransmitter Detection. Micromachines 2023, 14, 1088. https://doi.org/10.3390/mi14051088
Yang M, Wang L, Lu H, Dong Q. Advances in MXene-Based Electrochemical (Bio)Sensors for Neurotransmitter Detection. Micromachines. 2023; 14(5):1088. https://doi.org/10.3390/mi14051088
Chicago/Turabian StyleYang, Meiqing, Lu Wang, Haozi Lu, and Qizhi Dong. 2023. "Advances in MXene-Based Electrochemical (Bio)Sensors for Neurotransmitter Detection" Micromachines 14, no. 5: 1088. https://doi.org/10.3390/mi14051088
APA StyleYang, M., Wang, L., Lu, H., & Dong, Q. (2023). Advances in MXene-Based Electrochemical (Bio)Sensors for Neurotransmitter Detection. Micromachines, 14(5), 1088. https://doi.org/10.3390/mi14051088






