Preconcentration of Fluorescent Dyes in Electromembrane Systems via Electrophoretic Migration
Abstract
1. Introduction
2. Materials and Methods
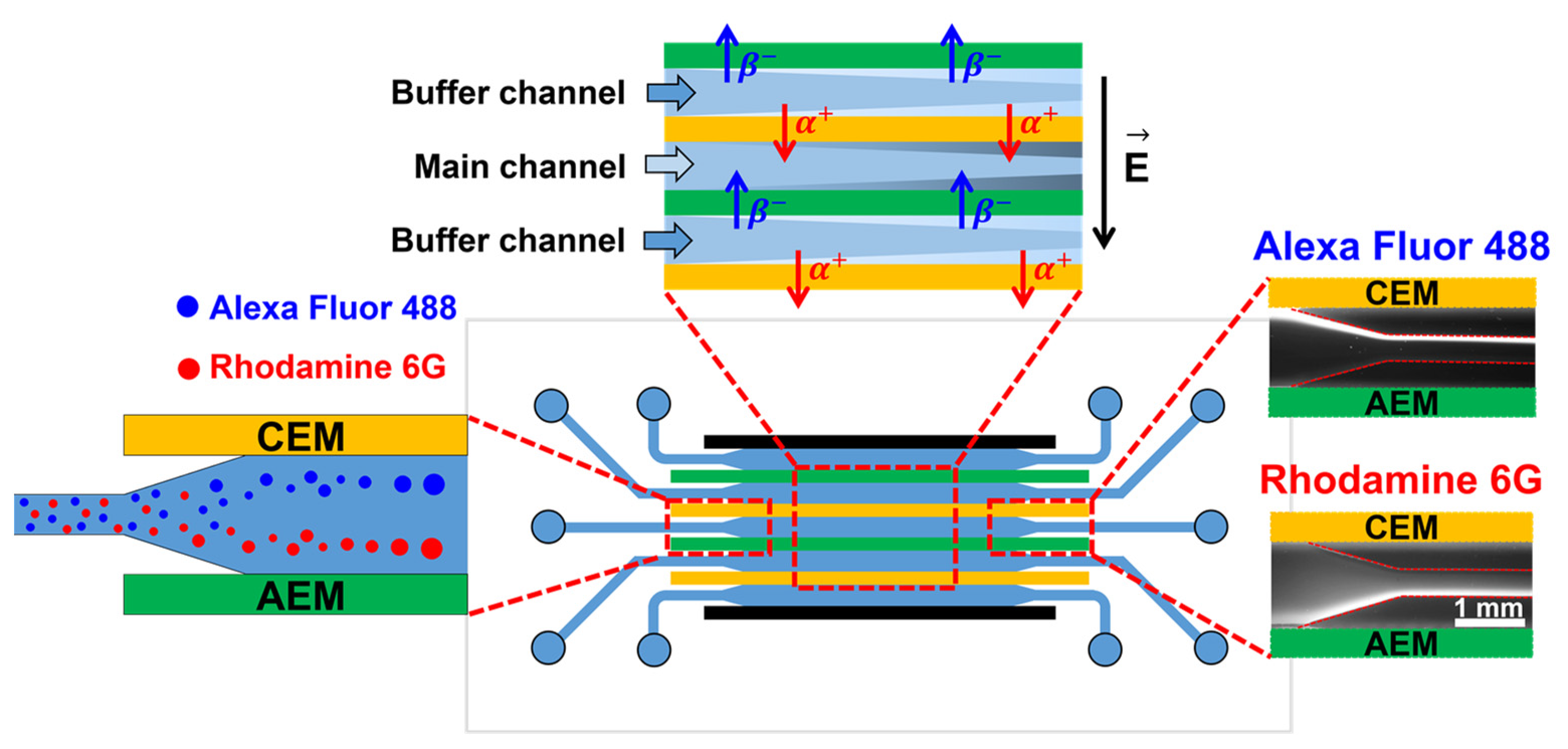
2.1. Theory
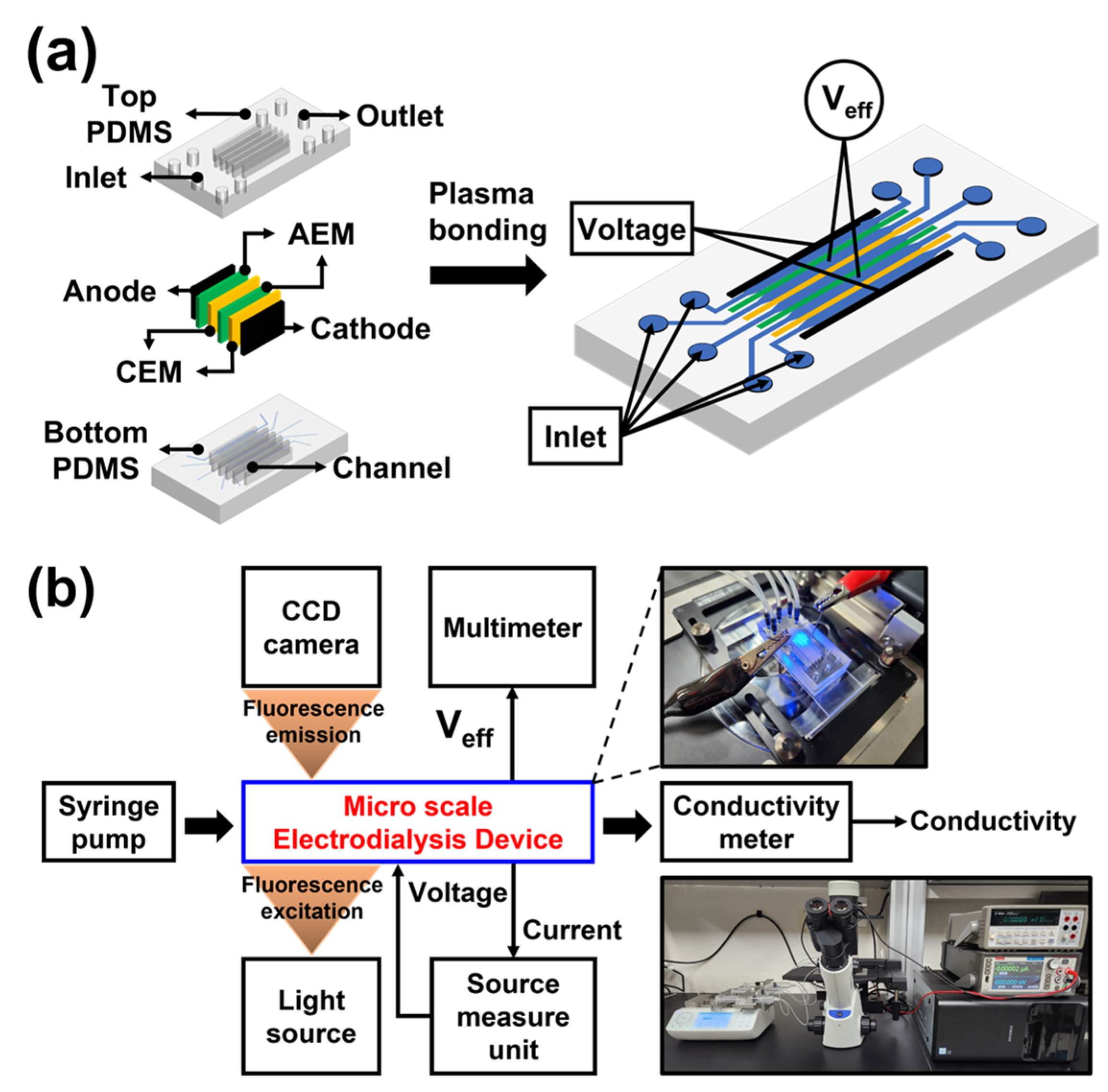
2.2. Device Fabrication and Experimental Setup
3. Results and Discussion
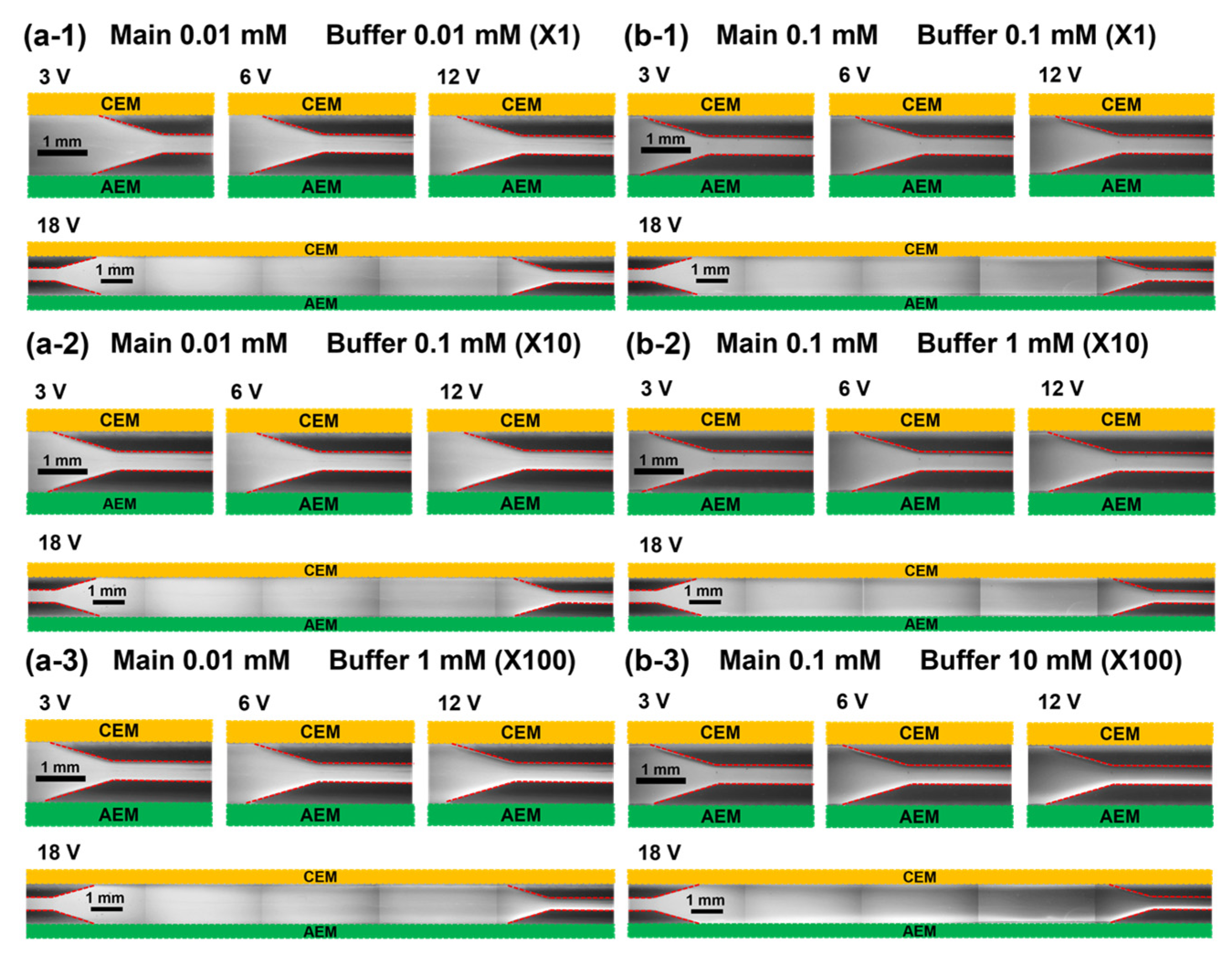
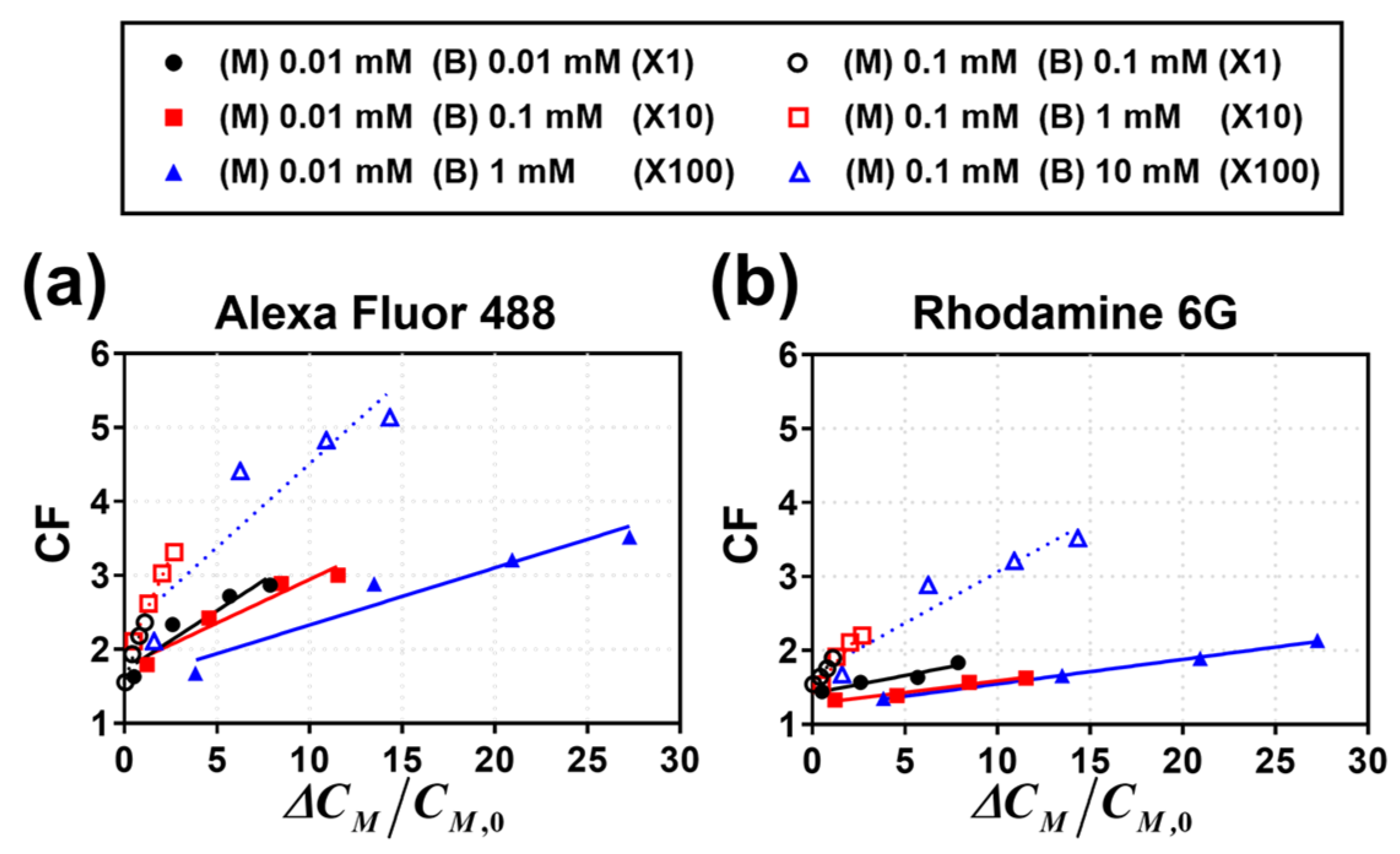
3.1. Visualization of Fluorescent Dye Preconcentration
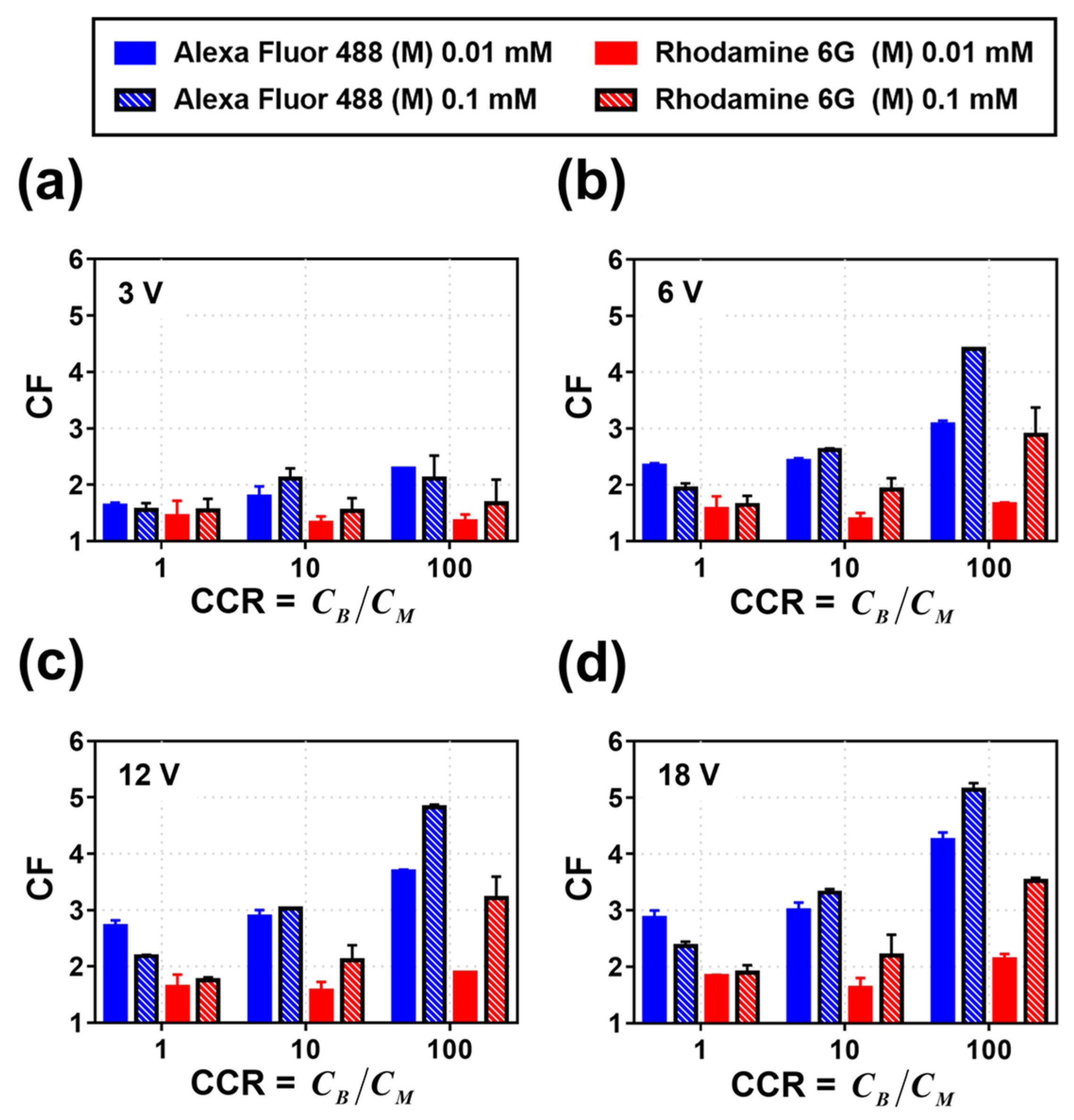
3.2. Calculation of CF
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lin, C.C.; Hsu, J.L.; Lee, G.B. Sample Preconcentration in Microfluidic Devices. Microfluid. Nanofluidics 2011, 10, 481–511. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A Critical Review on Solvent Extraction of Rare Earths from Aqueous Solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Astorga-Wells, J.; Swerdlow, H. Fluidic Preconcentrator Device for Capillary Electrophoresis of Proteins. Anal. Chem. 2003, 75, 5207–5212. [Google Scholar] [CrossRef]
- Song, S.; Singh, A.K.; Kirby, B.J. Electrophoretic Concentration of Proteins at Laser-Patterned Nanoporous Membranes in Microchips. Anal. Chem. 2004, 76, 4589–4592. [Google Scholar] [CrossRef]
- Jung, B.; Bharadwaj, R.; Santiago, J.G. Thousandfold Signal Increase Using Field-Amplified Sample Stacking for on-Chip Electrophoresis. Electrophoresis 2003, 24, 3476–3483. [Google Scholar] [CrossRef]
- Lian, D.S.; Zhao, S.J.; Li, J.; Li, B.L. Progress in Stacking Techniques Based on Field Amplification of Capillary Electrophoresis. Anal. Bioanal. Chem. 2014, 406, 6129–6150. [Google Scholar] [CrossRef]
- Jung, B.; Bharadwaj, R.; Santiago, J.G. On-Chip Millionfold Sample Stacking Using Transient Isotachophoresis. Anal. Chem. 2006, 78, 2319–2327. [Google Scholar] [CrossRef]
- Bottenus, D.; Jubery, T.Z.; Ouyang, Y.; Dong, W.J.; Dutta, P.; Ivory, C.F. 10000-Fold Concentration Increase of the Biomarker Cardiac Troponin in a Reducing Union Microfluidic Chip Using Cationic Isotachophoresis. Lab Chip 2011, 11, 890–898. [Google Scholar] [CrossRef]
- Smejkal, P.; Bottenus, D.; Breadmore, M.C.; Guijt, R.M.; Ivory, C.F.; Foret, F.; Macka, M. Microfluidic Isotachophoresis: A Review. Electrophoresis 2013, 34, 1493–1509. [Google Scholar] [CrossRef]
- Shim, J.; Dutta, P.; Ivory, C.F. Effects of Ampholyte Concentration on Protein Behavior in On-Chip Isoelectric Focusing. Electrophoresis 2008, 29, 1026–1035. [Google Scholar] [CrossRef]
- Niu, J.C.; Zhou, T.; Niu, L.L.; Xie, Z.S.; Fang, F.; Yang, F.Q.; Wu, Z.Y. Simultaneous Pre-Concentration and Separation on Simple Paper-Based Analytical Device for Protein Analysis. Anal. Bioanal. Chem. 2018, 410, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Farmerie, L.; Rustandi, R.R.; Loughney, J.W.; Dawod, M. Recent Advances in Isoelectric Focusing of Proteins and Peptides. J. Chromatogr. A 2021, 1651, 462274. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Song, Y.A.; Han, J. Nanofluidic Concentration Devices for Biomolecules Utilizing Ion Concentration Polarization: Theory, Fabrication, and Applications. Chem. Soc. Rev. 2010, 39, 912–922. [Google Scholar] [CrossRef]
- Wang, Y.C.; Stevens, A.L.; Han, J. Million-Fold Preconcentration of Proteins and Peptides by Nanofluidic Filter. Anal. Chem. 2005, 77, 4293–4299. [Google Scholar] [CrossRef]
- Lee, J.H.; Song, Y.A.; Han, J. Multiplexed Proteomic Sample Preconcentration Device Using Surface-Patterned Ion-Selective Membrane. Lab Chip 2008, 8, 596–601. [Google Scholar] [CrossRef]
- Kim, M.; Kim, T. Integration of Nanoporous Membranes into Microfluidic Devices: Electrokinetic Bio-Sample Pre-Concentration. Analyst 2013, 138, 6007–6015. [Google Scholar] [CrossRef]
- Kwak, R.; Kang, J.Y.; Kim, T.S. Spatiotemporally Defining Biomolecule Preconcentration by Merging Ion Concentration Polarization. Anal. Chem. 2016, 88, 988–996. [Google Scholar] [CrossRef]
- Cheow, L.F.; Han, J. Continuous Signal Enhancement for Sensitive Aptamer Affinity Probe Electrophoresis Assay Using Electrokinetic Concentration. Anal. Chem. 2011, 83, 7086–7093. [Google Scholar] [CrossRef]
- Choi, J.; Huh, K.; Moon, D.J.; Lee, H.; Son, S.Y.; Kim, K.; Kim, H.C.; Chae, J.H.; Sung, G.Y.; Kim, H.Y.; et al. Selective Preconcentration and Online Collection of Charged Molecules Using Ion Concentration Polarization. RSC Adv. 2015, 5, 66178–66184. [Google Scholar] [CrossRef]
- Choi, J.; Baek, S.; Kim, H.C.; Chae, J.H.; Koh, Y.; Seo, S.W.; Lee, H.; Kim, S.J. Nanoelectrokinetic Selective Preconcentration Based on Ion Concentration Polarization. Biochip J. 2020, 14, 100–109. [Google Scholar] [CrossRef]
- Marczak, S.; Richards, K.; Ramshani, Z.; Smith, E.; Senapati, S.; Hill, R.; Go, D.B.; Chang, H.C. Simultaneous Isolation and Preconcentration of Exosomes by Ion Concentration Polarization. Electrophoresis 2018, 39, 2029–2038. [Google Scholar] [CrossRef]
- Park, S.; Buhnik-Rosenblau, K.; Abu-Rjal, R.; Kashi, Y.; Yossifon, G. Periodic Concentration-Polarization-Based Formation of a Biomolecule Preconcentrate for Enhanced Biosensing. Nanoscale 2020, 12, 23586–23595. [Google Scholar] [CrossRef]
- Kwak, R.; Kim, S.J.; Han, J. Continuous-Flow Biomolecule and Cell Concentrator by Ion Concentration Polarization. Anal. Chem. 2011, 83, 7348–7355. [Google Scholar] [CrossRef]
- Cho, Y.; Yoon, J.; Lim, D.W.; Kim, J.; Lee, J.H.; Chung, S. Ion Concentration Polarization for Pre-Concentration of Biological Samples without pH Change. Analyst 2016, 141, 6510–6514. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Nam, H.; Lee, E.; Huh, J.W.; Kim, Y.M.; Jeon, H.; Lim, G. Ion Concentration Polarization-Based Multifunctional Microfluidic Device for Analysis of Intracellular Components. Sens. Actuators B Chem. 2022, 371, 132576. [Google Scholar] [CrossRef]
- Kim, W.; Park, S.; Kim, K.; Kim, S.J. Experimental Verification of Simultaneous Desalting and Molecular Preconcentration by Ion Concentration Polarization. Lab Chip 2017, 17, 3841–3850. [Google Scholar] [CrossRef]
- Kim, K.; Kim, W.; Lee, H.; Kim, S.J. Stabilization of Ion Concentration Polarization Layer Using Micro Fin Structure for High-Throughput Applications. Nanoscale 2017, 9, 3466–3475. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Oh, J.; Kwon, S.; Kim, K.; Kim, S.J. Quantifying the PH Shift Induced by Selective Anodic Electrochemical Reactions in the Ion Concentration Polarization Phenomenon. Lab Chip 2019, 19, 1359–1369. [Google Scholar] [CrossRef]
- Kim, D.; Ihm, S.; Park, S.; Yu, Y.; Kwak, R. Concentric Ion Concentration Polarization Desalination for Efficient En-Bloc Preconcentration and Desalination. Desalination 2021, 499, 114810. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.; Kim, S.; Kim, B.; Lim, G. Continuous Separation of Submicron-Scale Oil Droplets in Aqueous Electrolyte by Electrophoretic Migration. Sens. Actuators B Chem. 2021, 344, 130145. [Google Scholar] [CrossRef]
- Kwak, R.; Pham, V.S.; Kim, B.; Chen, L.; Han, J. Enhanced Salt Removal by Unipolar Ion Conduction in Ion Concentration Polarization Desalination. Sci. Rep. 2016, 6, 25349. [Google Scholar] [CrossRef]
- Kim, B.; Kwak, R.; Kwon, H.J.; Pham, V.S.; Kim, M.; Al-Anzi, B.; Lim, G.; Han, J. Purification of High Salinity Brine by Multi-Stage Ion Concentration Polarization Desalination. Sci. Rep. 2016, 6, 31850. [Google Scholar] [CrossRef]
- Wuzhang, J.; Song, Y.; Sun, R.; Pan, X.; Li, D. Electrophoretic Mobility of Oil Droplets in Electrolyte and Surfactant Solutions. Electrophoresis 2015, 36, 2489–2497. [Google Scholar] [CrossRef]
- Kim, B.; Choi, S.; Pham, V.S.; Kwak, R.; Han, J. Energy Efficiency Enhancement of Electromembrane Desalination Systems by Local Flow Redistribution Optimized for the Asymmetry of Cation/Anion Diffusivity. J. Membr. Sci. 2017, 524, 280–287. [Google Scholar] [CrossRef]
- Jang, J.; Kim, M.; Shin, J.; Yang, D.; Kim, M.; Kim, B. Experimental Study on Ion Transport in Microfluidic Electrodialysis Using Partially Masked Ion Exchange Membranes. Micromachines 2022, 13, 356. [Google Scholar] [CrossRef]
- Milanova, D.; Chambers, R.D.; Bahga, S.S.; Santiago, J.G. Electrophoretic Mobility Measurements of Fluorescent Dyes Using On-Chip Capillary Electrophoresis. Electrophoresis 2011, 32, 3286–3294. [Google Scholar] [CrossRef] [PubMed]
- Khurana, T.K.; Santiago, J.G. Sample Zone Dynamics in Peak Mode Isotachophoresis. Anal. Chem. 2008, 80, 6300–6307. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Kim, B. Preconcentration of Fluorescent Dyes in Electromembrane Systems via Electrophoretic Migration. Micromachines 2023, 14, 398. https://doi.org/10.3390/mi14020398
Kim M, Kim B. Preconcentration of Fluorescent Dyes in Electromembrane Systems via Electrophoretic Migration. Micromachines. 2023; 14(2):398. https://doi.org/10.3390/mi14020398
Chicago/Turabian StyleKim, Minsung, and Bumjoo Kim. 2023. "Preconcentration of Fluorescent Dyes in Electromembrane Systems via Electrophoretic Migration" Micromachines 14, no. 2: 398. https://doi.org/10.3390/mi14020398
APA StyleKim, M., & Kim, B. (2023). Preconcentration of Fluorescent Dyes in Electromembrane Systems via Electrophoretic Migration. Micromachines, 14(2), 398. https://doi.org/10.3390/mi14020398






